Baseline Hemostatic Biomarker Assessment Identifies Breast Cancer Patients at High Risk for Venous Thromboembolism During Chemotherapy
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Blood Collection and Plasma Preparation
2.3. Biomarker Assessment
2.4. Study Outcomes
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Population
3.2. Primary and Secondary Outcomes
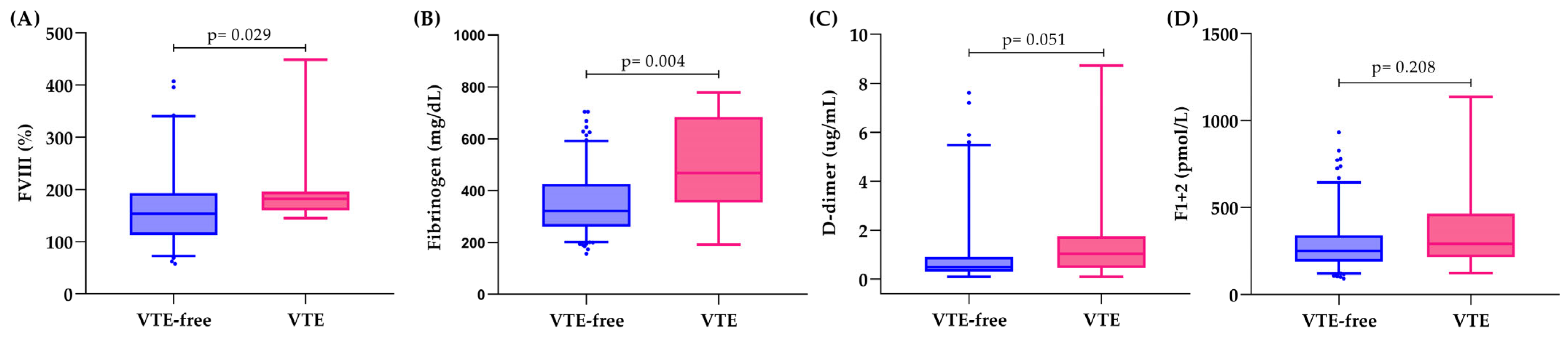
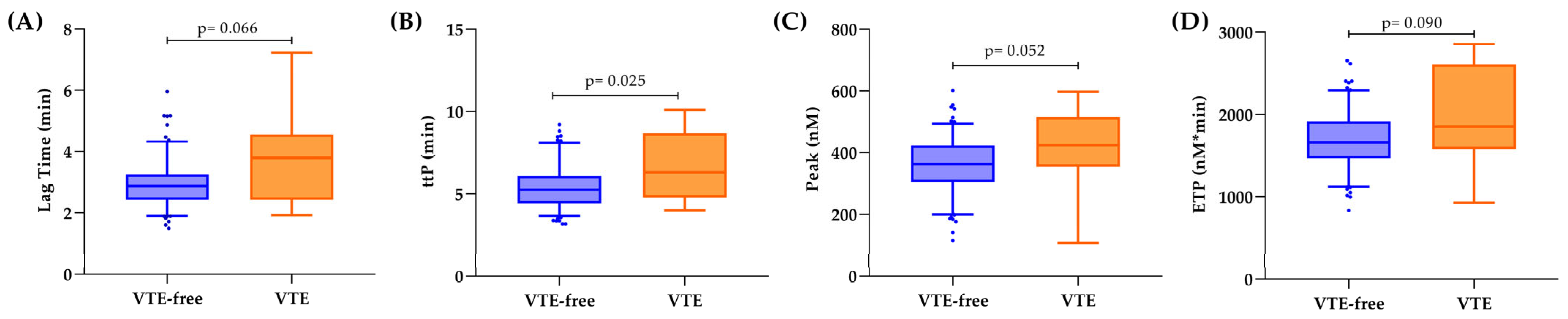
3.3. Hemostatic Biomarkers and Thrombin Generation in Relation to VTE
3.4. Predictors of 12-Month VTE
3.5. Development of a Risk Assessment Model
3.6. Predictors of 12-Month Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- ECIS—European Cancer Information System. Breast Cancer in the EU. Available online: https://ecis.jrc.ec.europa.eu (accessed on 7 August 2025).
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Benitez Fuentes, J.D.; Morgan, E.; de Luna Aguilar, A.; Mafra, A.; Shah, R.; Giusti, F.; Vignat, J.; Znaor, A.; Musetti, C.; Yip, C.H.; et al. Global Stage Distribution of Breast Cancer at Diagnosis: A Systematic Review and Meta-Analysis. JAMA Oncol. 2024, 10, 71–78. [Google Scholar] [CrossRef]
- Liao, L. Inequality in breast cancer: Global statistics from 2022 to 2050. Breast 2025, 79, 103851. [Google Scholar] [CrossRef] [PubMed]
- Claessens, A.K.M.; Ibragimova, K.I.E.; Geurts, S.M.E.; Bos, M.; Erdkamp, F.L.G.; Tjan-Heijnen, V.C.G. The role of chemotherapy in treatment of advanced breast cancer: An overview for clinical practice. Crit. Rev. Oncol. Hematol. 2020, 153, 102988. [Google Scholar] [CrossRef]
- Xiong, X.; Zheng, L.W.; Ding, Y.; Chen, Y.F.; Cai, Y.W.; Wang, L.P.; Huang, L.; Liu, C.C.; Shao, Z.M.; Yu, K.D. Breast cancer: Pathogenesis and treatments. Signal. Transduct. Target. Ther. 2025, 10, 49. [Google Scholar] [CrossRef]
- Coles, C.E.; Earl, H.; Anderson, B.O.; Barrios, C.H.; Bienz, M.; Bliss, J.M.; Cameron, D.A.; Cardoso, F.; Cui, W.; Francis, P.A.; et al. The Lancet Breast Cancer Commission. Lancet 2024, 403, 1895–1950. [Google Scholar] [CrossRef] [PubMed]
- Probert, J.; Dodwell, D.; Broggio, J.; Charman, J.; Dowsett, M.; Kerr, A.; McGale, P.; Taylor, C.; Darby, S.C.; Mannu, G.S. Ki67 and breast cancer mortality in women with invasive breast cancer. JNCI Cancer Spectr. 2023, 7, pkad054. [Google Scholar] [CrossRef]
- Walker, A.J.; West, J.; Card, T.R.; Crooks, C.; Kirwan, C.C.; Grainge, M.J. When are breast cancer patients at highest risk of venous thromboembolism? A cohort study using English health care data. Blood 2016, 127, 849–857, quiz 953. [Google Scholar] [CrossRef] [PubMed]
- Brand, J.S.; Hedayati, E.; Bhoo-Pathy, N.; Bergh, J.; Hall, P.; Humphreys, K.; Ludvigsson, J.F.; Czene, K. Time-dependent risk and predictors of venous thromboembolism in breast cancer patients: A population-based cohort study. Cancer 2017, 123, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Londero, A.P.; Bertozzi, S.; Cedolini, C.; Neri, S.; Bulfoni, M.; Orsaria, M.; Mariuzzi, L.; Uzzau, A.; Risaliti, A.; Barillari, G. Incidence and Risk Factors for Venous Thromboembolism in Female Patients Undergoing Breast Surgery. Cancers 2022, 14, 988. [Google Scholar] [CrossRef]
- Levine, M.N.; Gent, M.; Hirsh, J.; Arnold, A.; Goodyear, M.D.; Hryniuk, W.; De Pauw, S. The thrombogenic effect of anticancer drug therapy in women with stage II breast cancer. N. Engl. J. Med. 1988, 318, 404–407. [Google Scholar] [CrossRef] [PubMed]
- Falanga, A.; Marchetti, M.; Russo, L. The mechanisms of cancer-associated thrombosis. Thromb. Res. 2015, 135 (Suppl. S1), S8–S11. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.T.; Gorzelanny, C.; Gebhardt, C.; Pantel, K.; Schneider, S.W. Interplay between coagulation and inflammation in cancer: Limitations and therapeutic opportunities. Cancer Treat Rev. 2022, 102, 102322. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, C.C.; Blower, E.L. Contemporary breast cancer treatment-associated thrombosis. Thromb. Res. 2022, 213 (Suppl. S1), S8–S15. [Google Scholar] [CrossRef] [PubMed]
- Moik, F.; Ay, C. Venous and arterial thromboembolism in patients with cancer treated with targeted anti-cancer therapies. Thromb. Res. 2022, 213 (Suppl. S1), S58–S65. [Google Scholar] [CrossRef]
- Nasser, N.J.; Fox, J.; Agbarya, A. Potential Mechanisms of Cancer-Related Hypercoagulability. Cancers 2020, 12, 566. [Google Scholar] [CrossRef]
- Faiz, A.S.; Guo, S.; Kaveney, A.; Philipp, C.S. Risk of venous thromboembolism and endocrine therapy in older women with breast cancer in the United States. Blood Coagul. Fibrinolysis 2021, 32, 373–381. [Google Scholar] [CrossRef]
- Khan, U.T.; Walker, A.J.; Baig, S.; Card, T.R.; Kirwan, C.C.; Grainge, M.J. Venous thromboembolism and mortality in breast cancer: Cohort study with systematic review and meta-analysis. BMC Cancer 2017, 17, 747. [Google Scholar] [CrossRef]
- Gervaso, L.; Montero, A.J.; Jia, X.; Khorana, A.A. Venous thromboembolism in breast cancer patients receiving cyclin-dependent kinase inhibitors. J. Thromb. Haemost. 2020, 18, 162–168. [Google Scholar] [CrossRef]
- Falanga, A.; Ay, C.; Di Nisio, M.; Gerotziafas, G.; Jara-Palomares, L.; Langer, F.; Lecumberri, R.; Mandala, M.; Maraveyas, A.; Pabinger, I.; et al. Venous thromboembolism in cancer patients: ESMO Clinical Practice Guideline. Ann. Oncol. 2023, 34, 452–467. [Google Scholar] [CrossRef]
- Lyman, G.H.; Carrier, M.; Ay, C.; Di Nisio, M.; Hicks, L.K.; Khorana, A.A.; Leavitt, A.D.; Lee, A.Y.Y.; Macbeth, F.; Morgan, R.L.; et al. American Society of Hematology 2021 guidelines for management of venous thromboembolism: Prevention and treatment in patients with cancer. Blood Adv. 2021, 5, 927–974. [Google Scholar] [CrossRef]
- Farge, D.; Frere, C.; Connors, J.M.; Khorana, A.A.; Kakkar, A.; Ay, C.; Munoz, A.; Brenner, B.; Prata, P.H.; Brilhante, D.; et al. 2022 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer, including patients with COVID-19. Lancet Oncol. 2022, 23, e334–e347. [Google Scholar] [CrossRef]
- Gerotziafas, G.T.; Taher, A.; Abdel-Razeq, H.; AboElnazar, E.; Spyropoulos, A.C.; El Shemmari, S.; Larsen, A.K.; Elalamy, I.; Group, C.-C.W. A Predictive Score for Thrombosis Associated with Breast, Colorectal, Lung, or Ovarian Cancer: The Prospective COMPASS-Cancer-Associated Thrombosis Study. Oncologist 2017, 22, 1222–1231. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; Kuderer, N.M.; Culakova, E.; Lyman, G.H.; Francis, C.W. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008, 111, 4902–4907. [Google Scholar] [CrossRef]
- Pabinger, I.; van Es, N.; Heinze, G.; Posch, F.; Riedl, J.; Reitter, E.M.; Di Nisio, M.; Cesarman-Maus, G.; Kraaijpoel, N.; Zielinski, C.C.; et al. A clinical prediction model for cancer-associated venous thromboembolism: A development and validation study in two independent prospective cohorts. Lancet Haematol. 2018, 5, e289–e298. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.R.; Samarawickrema, I.; Naunton, M.; Peterson, G.M.; Yip, D.; Newman, P.; Mortazavi, R. Models for predicting venous thromboembolism in ambulatory patients with lung cancer: A systematic review and meta-analysis. Thromb. Res. 2024, 234, 120–133. [Google Scholar] [CrossRef]
- van Es, N.; Ventresca, M.; Di Nisio, M.; Zhou, Q.; Noble, S.; Crowther, M.; Briel, M.; Garcia, D.; Lyman, G.H.; Macbeth, F.; et al. The Khorana score for prediction of venous thromboembolism in cancer patients: An individual patient data meta-analysis. J. Thromb. Haemost. 2020, 18, 1940–1951. [Google Scholar] [CrossRef] [PubMed]
- Falanga, A.; Santoro, A.; Labianca, R.; De Braud, F.; Gasparini, G.; D’Alessio, A.; Barni, S.; Iacoviello, L.; Group, H.S. Hypercoagulation screening as an innovative tool for risk assessment, early diagnosis and prognosis in cancer: The HYPERCAN study. Thromb. Res. 2016, 140 (Suppl. S1), S55–S59. [Google Scholar] [CrossRef]
- Giaccherini, C.; Marchetti, M.; Masci, G.; Verzeroli, C.; Russo, L.; Celio, L.; Sarmiento, R.; Gamba, S.; Tartari, C.J.; Diani, E.; et al. Thrombotic biomarkers for risk prediction of malignant disease recurrence in patients with early stage breast cancer. Haematologica 2020, 105, 1704–1711. [Google Scholar] [CrossRef]
- Marchetti, M.; Castoldi, E.; Spronk, H.M.; van Oerle, R.; Balducci, D.; Barbui, T.; Rosing, J.; Ten Cate, H.; Falanga, A. Thrombin generation and activated protein C resistance in patients with essential thrombocythemia and polycythemia vera. Blood 2008, 112, 4061–4068. [Google Scholar] [CrossRef]
- Gomez-Rosas, P.; Giaccherini, C.; Russo, L.; Verzeroli, C.; Gamba, S.; Tartari, C.J.; Bolognini, S.; Ticozzi, C.; Schieppati, F.; Barcella, L.; et al. A New Risk Prediction Model for Venous Thromboembolism and Death in Ambulatory Lung Cancer Patients. Cancers 2023, 15, 4588. [Google Scholar] [CrossRef]
- Giaccherini, C.; Verzeroli, C.; Russo, L.; Gamba, S.; Tartari, C.J.; Bolognini, S.; Schieppati, F.; Ticozzi, C.; Sarmiento, R.; Celio, L.; et al. Thrombin Generation and D-Dimer for Prediction of Disease Progression and Mortality in Patients with Metastatic Gastrointestinal Cancer. Cancers 2022, 14, 4347. [Google Scholar] [CrossRef] [PubMed]
- Carrier, M.; Khorana, A.A.; Zwicker, J.I.; Lyman, G.H.; Le Gal, G.; Lee, A.Y. Subcommittee on Haemostasis and Malignancy for the SSCof the ISTH. Venous thromboembolism in cancer clinical trials: Recommendation for standardized reporting and analysis. J. Thromb. Haemost. 2012, 10, 2599–2601. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, C.C.; McDowell, G.; McCollum, C.N.; Byrne, G.J. Incidence of venous thromboembolism during chemotherapy for breast cancer: Impact on cancer outcome. Anticancer. Res. 2011, 31, 2383–2388. [Google Scholar]
- Kirwan, C.C.; McDowell, G.; McCollum, C.N.; Kumar, S.; Byrne, G.J. Early changes in the haemostatic and procoagulant systems after chemotherapy for breast cancer. Br. J. Cancer 2008, 99, 1000–1006. [Google Scholar] [CrossRef]
- Mulder, F.I.; Horváth-Puhó, E.; van Es, N.; van Laarhoven, H.W.M.; Pedersen, L.; Moik, F.; Ay, C.; Büller, H.R.; Sørensen, H.T. Venous thromboembolism in cancer patients: A population-based cohort study. Blood 2021, 137, 1959–1969. [Google Scholar] [CrossRef] [PubMed]
- Chaari, M.; Ayadi, I.; Rousseau, A.; Lefkou, E.; Van Dreden, P.; Sidibe, F.; Ketatni, H.; Galea, V.; Khaterchi, A.; Bouzguenda, R.; et al. Impact of breast cancer stage, time from diagnosis and chemotherapy on plasma and cellular biomarkers of hypercoagulability. BMC Cancer 2014, 14, 991. [Google Scholar] [CrossRef]
- Vormittag, R.; Simanek, R.; Ay, C.; Dunkler, D.; Quehenberger, P.; Marosi, C.; Zielinski, C.; Pabinger, I. High factor VIII levels independently predict venous thromboembolism in cancer patients: The cancer and thrombosis study. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 2176–2181. [Google Scholar] [CrossRef]
- Tafur, A.J.; Dale, G.; Cherry, M.; Wren, J.D.; Mansfield, A.S.; Comp, P.; Rathbun, S.; Stoner, J.A. Prospective evaluation of protein C and factor VIII in prediction of cancer-associated thrombosis. Thromb. Res. 2015, 136, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liang, X.; Wang, S.; Wang, Y.; Qin, L.; Chen, D.; Jiang, Y.; Zhang, H. Analysis of the Risk Factors for Elevated D-Dimer Level After Breast Cancer Surgery: A Multicenter Study Based on Nursing Follow-Up Data. Front. Oncol. 2022, 12, 772726. [Google Scholar] [CrossRef] [PubMed]
- Ay, C.; Dunkler, D.; Simanek, R.; Thaler, J.; Koder, S.; Marosi, C.; Zielinski, C.; Pabinger, I. Prediction of venous thromboembolism in patients with cancer by measuring thrombin generation: Results from the Vienna Cancer and Thrombosis Study. J. Clin. Oncol. 2011, 29, 2099–2103. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, M.; Giaccherini, C.; Masci, G.; Verzeroli, C.; Russo, L.; Celio, L.; Sarmiento, R.; Gamba, S.; Tartari, C.J.; Diani, E.; et al. Thrombin generation predicts early recurrence in breast cancer patients. J. Thromb. Haemost. 2020, 18, 2220–2231. [Google Scholar] [CrossRef]
- Gomez-Rosas, P.; Pesenti, M.; Verzeroli, C.; Giaccherini, C.; Russo, L.; Sarmiento, R.; Masci, G.; Celio, L.; Minelli, M.; Gamba, S.; et al. Validation of the Role of Thrombin Generation Potential by a Fully Automated System in the Identification of Breast Cancer Patients at High Risk of Disease Recurrence. TH Open 2021, 5, e56–e65. [Google Scholar] [CrossRef]
- Dielis, A.W.; Castoldi, E.; Spronk, H.M.; van Oerle, R.; Hamulyák, K.; Ten Cate, H.; Rosing, J. Coagulation factors and the protein C system as determinants of thrombin generation in a normal population. J. Thromb. Haemost. 2008, 6, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Kremers, R.M.; Wagenvoord, R.J.; Hemker, H.C. The effect of fibrin(ogen) on thrombin generation and decay. Thromb. Haemost. 2014, 112, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, R.K.; Sørensen, H.T.; Pedersen, L.; Jacobsen, J.; Lash, T.L. Tamoxifen treatment and risk of deep venous thrombosis and pulmonary embolism: A Danish population-based cohort study. Cancer 2009, 115, 4442–4449. [Google Scholar] [CrossRef]
- Davey, M.G.; Hynes, S.O.; Kerin, M.J.; Miller, N.; Lowery, A.J. Ki-67 as a Prognostic Biomarker in Invasive Breast Cancer. Cancers 2021, 13, 4455. [Google Scholar] [CrossRef]
- Yerushalmi, R.; Woods, R.; Ravdin, P.M.; Hayes, M.M.; Gelmon, K.A. Ki67 in breast cancer: Prognostic and predictive potential. Lancet Oncol. 2010, 11, 174–183. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, H.; Zhong, G.; Fu, Z.; Peng, Y.; Yao, T. Development and Validation of a Nomogram to Predict the Probability of Venous Thromboembolism in Patients with Epithelial Ovarian Cancer. Clin. Appl. Thromb. Hemost. 2022, 28, 10760296221095558. [Google Scholar] [CrossRef]
- Shaker, H.; Bundred, N.J.; Landberg, G.; Pritchard, S.A.; Albadry, H.; Nicholson, S.L.; Harries, L.J.; Heah, J.Y.E.; Castle, J.; Kirwan, C.C. Breast cancer stromal clotting activation (Tissue Factor and thrombin): A pre-invasive phenomena that is prognostic in invasion. Cancer Med. 2020, 9, 1768–1778. [Google Scholar] [CrossRef]
- Marchetti, M.; Russo, L.; Balducci, D.; Falanga, A. All trans-retinoic acid modulates the procoagulant activity of human breast cancer cells. Thromb. Res. 2011, 128, 368–374. [Google Scholar] [CrossRef]
- Marchetti, M.; Vignoli, A.; Russo, L.; Balducci, D.; Pagnoncelli, M.; Barbui, T.; Falanga, A. Endothelial capillary tube formation and cell proliferation induced by tumor cells are affected by low molecular weight heparins and unfractionated heparin. Thromb. Res. 2008, 121, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Falanga, A.; Tartari, C.J.; Marchetti, M. Microparticles in tumor progression. Thromb. Res. 2012, 129 (Suppl. S1), S132–S136. [Google Scholar] [CrossRef]
- Rupa-Matysek, J.; Lembicz, M.; Rogowska, E.K.; Gil, L.; Komarnicki, M.; Batura-Gabryel, H. Evaluation of risk factors and assessment models for predicting venous thromboembolism in lung cancer patients. Med. Oncol. 2018, 35, 63. [Google Scholar] [CrossRef]
- Xu, Q.; Li, X.; Yuan, Y.; Hu, Z.; Liang, G.; Wang, Y.; Zhang, W.; Liu, Y.; Wang, W.; Lei, H. Development and validation of a predictive risk tool for VTE in women with breast cancer under chemotherapy: A cohort study in China. Breast Cancer 2025, 32, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qiang, W.M.; Wang, Y.; Wang, X.Y. Development and validation of a risk assessment nomogram for venous thromboembolism associated with hospitalized postoperative Chinese breast cancer patients. J. Adv. Nurs. 2021, 77, 473–483. [Google Scholar] [CrossRef]
- Chew, H.K.; Wun, T.; Harvey, D.J.; Zhou, H.; White, R.H. Incidence of venous thromboembolism and the impact on survival in breast cancer patients. J. Clin. Oncol. 2007, 25, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, H.T.; Pedersen, L.; van Es, N.; Buller, H.R.; Horvath-Puho, E. Impact of venous thromboembolism on the mortality in patients with cancer: A population-based cohort study. Lancet Reg. Health Eur. 2023, 34, 100739. [Google Scholar] [CrossRef]
- Krüger-Genge, A.; Köhler, S.; Laube, M.; Haileka, V.; Lemm, S.; Majchrzak, K.; Kammerer, S.; Schulz, C.; Storsberg, J.; Pietzsch, J.; et al. Anti-Cancer Prodrug Cyclophosphamide Exerts Thrombogenic Effects on Human Venous Endothelial Cells Independent of CYP450 Activation-Relevance to Thrombosis. Cells 2023, 12, 1965. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saphner, T.; Tormey, D.C.; Gray, R. Venous and arterial thrombosis in patients who received adjuvant therapy for breast cancer. J. Clin. Oncol. 1991, 9, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Seng, S.; Liu, Z.; Chiu, S.K.; Proverbs-Singh, T.; Sonpavde, G.; Choueiri, T.K.; Tsao, C.K.; Yu, M.; Hahn, N.M.; Oh, W.K.; et al. Risk of venous thromboembolism in patients with cancer treated with Cisplatin: A systematic review and meta-analysis. J. Clin. Oncol. 2012, 30, 4416–4426. [Google Scholar] [CrossRef] [PubMed]
- Goodnough, L.T.; Saito, H.; Manni, A.; Jones, P.K.; Pearson, O.H. Increased incidence of thromboembolism in stage IV breast cancer patients treated with a five-drug chemotherapy regimen. A study of 159 patients. Cancer 1984, 54, 1264–1268. [Google Scholar] [CrossRef] [PubMed]
- Nalluri, S.R.; Chu, D.; Keresztes, R.; Zhu, X.; Wu, S. Risk of venous thromboembolism with the angiogenesis inhibitor bevacizumab in cancer patients: A meta-analysis. JAMA 2008, 300, 2277–2285. [Google Scholar] [CrossRef] [PubMed]
- Zhan, P.; Wang, Q.; Qian, Q.; Yu, L.K. Risk of venous thromboembolism with the erythropoiesis-stimulating agents (ESAs) for the treatment of cancer-associated anemia: A meta-analysis of randomized control trials. Chin. Clin. Oncol. 2012, 1, 19. [Google Scholar] [CrossRef] [PubMed]
- Du, X.L.; Zhang, Y.; Hardy, D. Associations between hematopoietic growth factors and risks of venous thromboembolism, stroke, ischemic heart disease and myelodysplastic syndrome: Findings from a large population-based cohort of women with breast cancer. Cancer Causes Control 2016, 27, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Mackey, J.R.; Ramos-Vazquez, M.; Lipatov, O.; McCarthy, N.; Krasnozhon, D.; Semiglazov, V.; Manikhas, A.; Gelmon, K.A.; Konecny, G.E.; Webster, M.; et al. Primary results of ROSE/TRIO-12, a randomized placebo-controlled phase III trial evaluating the addition of ramucirumab to first-line docetaxel chemotherapy in metastatic breast cancer. J. Clin. Oncol. 2015, 33, 141–148. [Google Scholar] [CrossRef] [PubMed]

| Overall Cohort (n = 189) | VTE-Free (n = 175) | 1 Year-VTE (n = 14) | p-Value | |
|---|---|---|---|---|
| Female sex, n (%) | 185 (98) | 172 (98) | 13 (93) | 0.271 |
| Age, years, mean (SD) | 60 (12.5) | 60 (12.6) | 59 (10.7) | 0.725 |
| BMI, kg/m2, mean (SD) | 26 (5.4) | 26 (5.4) | 26 (5.3) | 0.910 |
| BMI ≥ 35 kg/m2, n (%) | 12 (6) | 11 (6) | 1 (7) | 0.629 |
| ECOG, n (%) | ||||
| • 0 | 114 (60) | 110 (63) | 4 (29) | 0.131 |
| • 1 | 50 (27) | 43 (25) | 6 (43) | |
| • 2 | 16 (9) | 15 (9) | 1 (7) | |
| Smoking, n (%) | ||||
| • Active | 15 (8) | 12 (7) | 3 (21) | 0.020 |
| • Previous | 21 (11) | 17 (8) | 3 (21) | |
| Comorbidities ≥ 1, n (%) | 102 (54) | 96 (55) | 6 (43) | 0.619 |
| • Diabetes | 18 (10) | 17 (10) | 1 (7) | 0.701 |
| • Hypertension | 64 (34) | 59 (34) | 5 (36) | 0.512 |
| • Dyslipidemia | 12 (6) | 12 (7) | 0 (0) | - |
| • Cardiopathy | 1 (0.5) | 1 (0.6) | 0 (0) | - |
| Central venous catheter, n (%) | 24 (13) | 23 (13) | 1 (7) | 0.641 |
| Antithrombotic therapy, n (%) | ||||
| • Antiplatelet drugs | 5 (3) | 4 (2) | 1 (7) | 0.264 |
| • Anticoagulants * | 4 (2) | 4 (2) | 0 (0) | - |
| Histological subtypes, n (%) | ||||
| • Ductal | 156 (83) | 145 (83) | 11 (79) | 0.852 |
| • Lobular | 16 (9) | 14 (8) | 2 (14) | |
| • Not classified | 17 (9) | 16 (9) | 1 (7) | |
| Molecular subtype, n (%) | ||||
| • Luminal A | 27 (14) | 25 (14) | 2 (14) | 0.957 |
| • Luminal B | 69 (37) | 64 (37) | 5 (36) | |
| • Luminal B HER2-positive | 50 (27) | 46(26) | 4 (29) | |
| • HER 2-positive | 17 (9) | 16 (9) | 1 (7) | |
| • Triple-negative | 21 (11) | 19 (11) | 2 (14) | |
| • Not classified | 5 (3) | 3 (2) | 2 (14) | |
| Chemotherapy, n (%) | ||||
| • Anthracycline | 37 (20) | 34 (19) | 3 (21) | 0.680 |
| • Taxane | 118 (62) | 109 (62) | 9 (64) | |
| • Anthracycline/taxane | 15 (8) | 15 (9) | 0 (0) | |
| • Other | 16 (9) | 14 (8) | 2 (14) | |
| Immunotherapy first-line, n (%) | 81 (43) | 72 (41) | 8 (57) | 0.219 |
| Endocrine therapy, n (%) | ||||
| • Tamoxifen | 100 (53) | 93 (53) | 7 (50) | 0.473 |
| • LHRH antagonists | 21 (11) | 19 (11) | 2 (14) | |
| • Aromatase inhibitors | 80 (42) | 75 (43) | 5 (36) | |
| Blood count, median (IQR) | ||||
| • Leukocytes, 109/L | 6.75 (5.22–8.39) | 6.70 (5.10–8.30) | 6.50 (5.50–8.94) | 0.949 |
| • Hemoglobin, g/dL | 13.0 (12.0–13.8) | 13.0 (12.0–13.8) | 12.5 (11.3–13.4) | 0.112 |
| • Hematocrit, % | 39.3 (36.4–42.1) | 39.1 (36.5–42.1) | 39 (34.1–40.3) | 0.240 |
| • Platelets, 109/L | 257 (209–307) | 256 (209–305) | 271 (222–356) | 0.456 |
| Blood count KRS cut-off, n (%) | ||||
| • Leukocyte >11 × 109/L | 15 (8) | 14 (8) | 1 (7) | 0.995 |
| • Hemoglobin, <10 g/dL | 7 (4) | 5 (3) | 2 (14) | 0.093 |
| • Platelets, >350 × 109/L | 24 (13) | 20 (11) | 4 (29) | 0.083 |
| Variables | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| Clinical and Tumor Characteristics | SHR | 95% CI | p-Value | SHR | 95% CI | p-Value |
| Age, years | 0.991 | 0.957–1.028 | 0.635 | |||
| BMI kg/m2 | 0.996 | 0.913–1.088 | 0.933 | |||
| Leukocytes, ×109/L | 0.972 | 0.809–1.168 | 0.765 | |||
| Hemoglobin, g/dL | 0.786 | 0.629–0.983 | 0.034 | 0.787 | 0.585–1.060 | 0.114 |
| Platelets, ×109/L | 1.003 | 0.997–1.009 | 0.334 | |||
| Comorbidities ≥ 1 | 1.165 | 0.405–3.347 | 0.777 | |||
| Active smoking | 3.603 | 0.909–4.284 | 0.068 | |||
| Histological subtypes | ||||||
| • Ductal (vs. lobular) | 1.979 | 0.187–2.897 | 0.570 | |||
| Ki67 % | 1.021 | 1.000–1.043 | 0.049 | 1.055 | 1.009–1.103 | 0.017 |
| Ki67 positivity | 1.537 | 0.363–6.506 | 0.559 | |||
| Estrogen receptor | 0.998 | 0.984–1.011 | 0.730 | |||
| Estrogen receptor positivity | 1.063 | 0.293–3.860 | 0.926 | |||
| Progesterone receptor | 0.992 | 0.976–1.008 | 0.329 | |||
| Progesterone receptor positivity | 0.764 | 0.250–2.331 | 0.636 | |||
| HER2 status | 1.517 | 0.534–4.311 | 0.434 | |||
| ECOG ≥ 2 | 1.755 | 0.398–7.743 | 0.458 | |||
| Molecular subtype | ||||||
| • Triple-negative (vs. other) | 1.336 | 0.290–6.147 | 0.710 | |||
| Central venous catheter | 1.222 | 0.226–6.609 | 0.815 | |||
| Chemotherapy | ||||||
| • Anthracycline-based | 0.724 | 0.201–2.607 | 0.621 | |||
| • Taxane-based | 0.684 | 0.230–2.034 | 0.495 | |||
| • Antracycline/taxane | - | - | - | |||
| Immunotherapy first line | 1.672 | 0.581–4.815 | 0.341 | |||
| Endocrine therapy | ||||||
| • Tamoxifen | 0.834 | 0.296–2.355 | 0.732 | |||
| • LHRH antagonists | 1.249 | 0.292–5.349 | 0.765 | |||
| • Aromatase inhibitors | 0.722 | 0.245–2.128 | 0.555 | |||
| Biomarkers | ||||||
| F1 + 2, pmol/L | 1.001 | 0.999–1.002 | 0.294 | |||
| D-dimer, µg/mL | 1.238 | 1.026–1.495 | 0.026 | 0.991 | 0.629–1.560 | 0.968 |
| Fibrinogen, mg/dL | 1.007 | 1.003–1.010 | <0.001 | 1.008 | 1.001–1.017 | 0.048 |
| FVIII, % | 1.007 | 1.001–1.014 | 0.028 | 1.015 | 1.001–1.030 | 0.034 |
| TG lag time, min | 1.438 | 1.112–1.858 | 0.006 | 0.474 | 0.098–2.278 | 0.351 |
| TG peak, nM | 1.001 | 0.998–1.004 | 0.250 | |||
| TG ttP, min | 1.716 | 1.266–2.327 | 0.020 | 1.484 | 0.933–5.005 | 0.156 |
| TG ETP, nM*min | 1.001 | 1.000–1.002 | 0.034 | 1.000 | 0.998–1.002 | 0.980 |
| Variables | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| Clinical and Tumor Characteristics | HR | 95% CI | p-Value | HR | 95% CI | p-Value |
| Age, years | 1.007 | 0.976–1.038 | 0.665 | |||
| BMI kg/m2 | 0.950 | 0.873–1.034 | 0.237 | |||
| Leukocytes, ×109/L | 1.145 | 1.011–1.296 | 0.033 | 1.150 | 1.000–1.322 | 0.050 |
| Hemoglobin, g/dL | 1.030 | 0.787–1.347 | 0.831 | |||
| Platelets, ×109/L | 1.002 | 0.997–1.007 | 0.341 | |||
| Cardiovascular risk factors ≥ 1 | 0.670 | 0.304–1.476 | 0.320 | |||
| Active smoking | 1.074 | 0.240–4.799 | 0.926 | |||
| Histological subtypes | ||||||
| • Ductal (vs. Lobular) | 1.118 | 0.263–4.753 | 0.880 | |||
| Ki67 % | 1.009 | 0.991–1.028 | 0.317 | |||
| Ki67 positivity | 1.215 | 0.415–3.555 | 0.722 | |||
| Estrogen receptor % | 0.990 | 0.980–1.000 | 0.052 | |||
| Estrogen receptor positivity | 0.575 | 0.308–1.072 | 0.082 | |||
| Progesterone receptor % | 0.979 | 0.962–0.996 | 0.016 | |||
| Progesterone receptor positivity | 0.541 | 0.295–0.992 | 0.047 | |||
| HER2 status | 0.660 | 0.272–1.605 | 0.360 | |||
| ECOG ≥ 2 | 4.616 | 1.830–11.64 | 0.001 | |||
| Molecular subtype | ||||||
| • Triple-negative (vs. other) | 3.318 | 1.848–5.958 | <0.001 | 3.988 | 2.059–7.724 | <0.001 |
| Central venous catheter | 0.643 | 0.231–1.786 | 0.397 | |||
| Chemotherapy | ||||||
| • Anthracycline-based | 0.550 | 0.154–1.968 | 0.358 | |||
| • Taxane-based | 0.809 | 0.160–4.094 | 0.798 | |||
| • Antracycline/taxane | 0.407 | 0.185–0.893 | 0.025 | |||
| Immunotherapy first line | 0.284 | 0.107–0.753 | 0.011 | |||
| Endocrine therapy | ||||||
| • Tamoxifen | 0.249 | 0.117–0.530 | <0.001 | |||
| • LHRH antagonists | 0.104 | 0.002–4.400 | 0.236 | |||
| • Aromatase inhibitors | 0.160 | 0.048–0.536 | 0.003 | |||
| Biomarkers | ||||||
| F1 + 2, pmol/L | 1.000 | 0.999–1.002 | 0.207 | |||
| D-dimer, µg/mL | 1.257 | 1.060–1.491 | 0.008 | |||
| Fibrinogen, mg/dL | 1.003 | 1.001–1.006 | 0.009 | |||
| FVIII, % | 1.008 | 1.004–1.014 | <0.001 | 1.010 | 1.005–1.016 | <0.001 |
| TG lag time, min | 1.160 | 0.865–1.556 | 0.323 | |||
| TG peak, nM | 1.001 | 0.999–1.003 | 0.213 | |||
| TG ttP, min | 1.033 | 0.821–1.299 | 0.780 | |||
| TG ETP, nM*min | 1.000 | 0.999–1.001 | 0.341 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchetti, M.; Gomez-Rosas, P.; Russo, L.; Tartari, C.J.; Bolognini, S.; Ticozzi, C.; Romeo, D.; Schieppati, F.; Barcella, L.; Sarmiento, R.; et al. Baseline Hemostatic Biomarker Assessment Identifies Breast Cancer Patients at High Risk for Venous Thromboembolism During Chemotherapy. Cancers 2025, 17, 2712. https://doi.org/10.3390/cancers17162712
Marchetti M, Gomez-Rosas P, Russo L, Tartari CJ, Bolognini S, Ticozzi C, Romeo D, Schieppati F, Barcella L, Sarmiento R, et al. Baseline Hemostatic Biomarker Assessment Identifies Breast Cancer Patients at High Risk for Venous Thromboembolism During Chemotherapy. Cancers. 2025; 17(16):2712. https://doi.org/10.3390/cancers17162712
Chicago/Turabian StyleMarchetti, Marina, Patricia Gomez-Rosas, Laura Russo, Carmen Julia Tartari, Silvia Bolognini, Chiara Ticozzi, Debora Romeo, Francesca Schieppati, Luca Barcella, Roberta Sarmiento, and et al. 2025. "Baseline Hemostatic Biomarker Assessment Identifies Breast Cancer Patients at High Risk for Venous Thromboembolism During Chemotherapy" Cancers 17, no. 16: 2712. https://doi.org/10.3390/cancers17162712
APA StyleMarchetti, M., Gomez-Rosas, P., Russo, L., Tartari, C. J., Bolognini, S., Ticozzi, C., Romeo, D., Schieppati, F., Barcella, L., Sarmiento, R., Masci, G., Gasparini, G., De Braud, F., Tondini, C., Santoro, A., Petrelli, F., Giuliani, F., D’Alessio, A., Labianca, R., & Falanga, A., on behalf of the HYPERCAN Investigators. (2025). Baseline Hemostatic Biomarker Assessment Identifies Breast Cancer Patients at High Risk for Venous Thromboembolism During Chemotherapy. Cancers, 17(16), 2712. https://doi.org/10.3390/cancers17162712











