The Surgical Imprint: How Operative Trauma May Shape Radiation Tolerance After Prostatectomy
Simple Summary
Abstract
1. Introduction
2. Complication Profile of Open Versus Minimally Invasive Prostatectomy
2.1. Contemporary Comparative Data
2.2. Tissue-Level Effects of Surgical Approach in Radical Prostatectomy
2.2.1. Surgical Trauma and Local Microenvironment Changes
2.2.2. Cytokine Signaling and Fibrotic Response Post-Radiotherapy
2.2.3. Systemic Immune Modulation: Open vs. Minimally Invasive Surgery
2.2.4. Periprostatic Adipose Tissue: Histological and Dosimetric Consequences
2.2.5. Interaction Between Surgical Technique, ADT, and Radiation Toxicity
3. Toxicity Profile of Post-Prostatectomy Radiotherapy Delivered with Modern Techniques
3.1. Impact of Advanced Radiotherapy Techniques on Post-Prostatectomy Toxicity
3.2. Radiation-Induced Tissue Injury: Mechanisms of Cellular and Microenvironmental Damage
3.3. Site-Specific Sequelae in Pelvic Organs at Risk
4. Surgical Technique as a Determinant of Acute Toxicity After Salvage Radiotherapy
4.1. Clinical Evidence from Our Multicenter Study
- Patients who had undergone open prostatectomy experienced significantly higher rates of GI (41.8%) and GU (35.9%) toxicity than those treated with laparoscopic or robotic surgery (18.9% and 12.2%, respectively).
- Among open-surgery patients, CTV-to-PTV margins ≥ 10 mm markedly increased the risk (up to 70.4% for both GI and GU).
- In patients with minimally invasive surgery, toxicity remained low overall, but EQD2 dose, comorbidity burden, and ADT type modestly modulated GI toxicity.
- In patients undergoing ORP and irradiated with a CTV-PTV margin < 10 mm, the use of a more extensive lymphadenectomy (≥15 resected nodes) was associated with an increase in both GI and GU toxicity.
- Among men who underwent minimally invasive surgery and received EQD2 < 70 Gy (N = 85), the crude rate of acute GI toxicity was higher with LHRH agonists (18.9%) than in patients without ADT or treated with high-dose bicalutamide. This comparison is unadjusted and based on a small subgroup with heterogeneous agents; it is therefore hypothesis-generating and not part of the primary inferences.
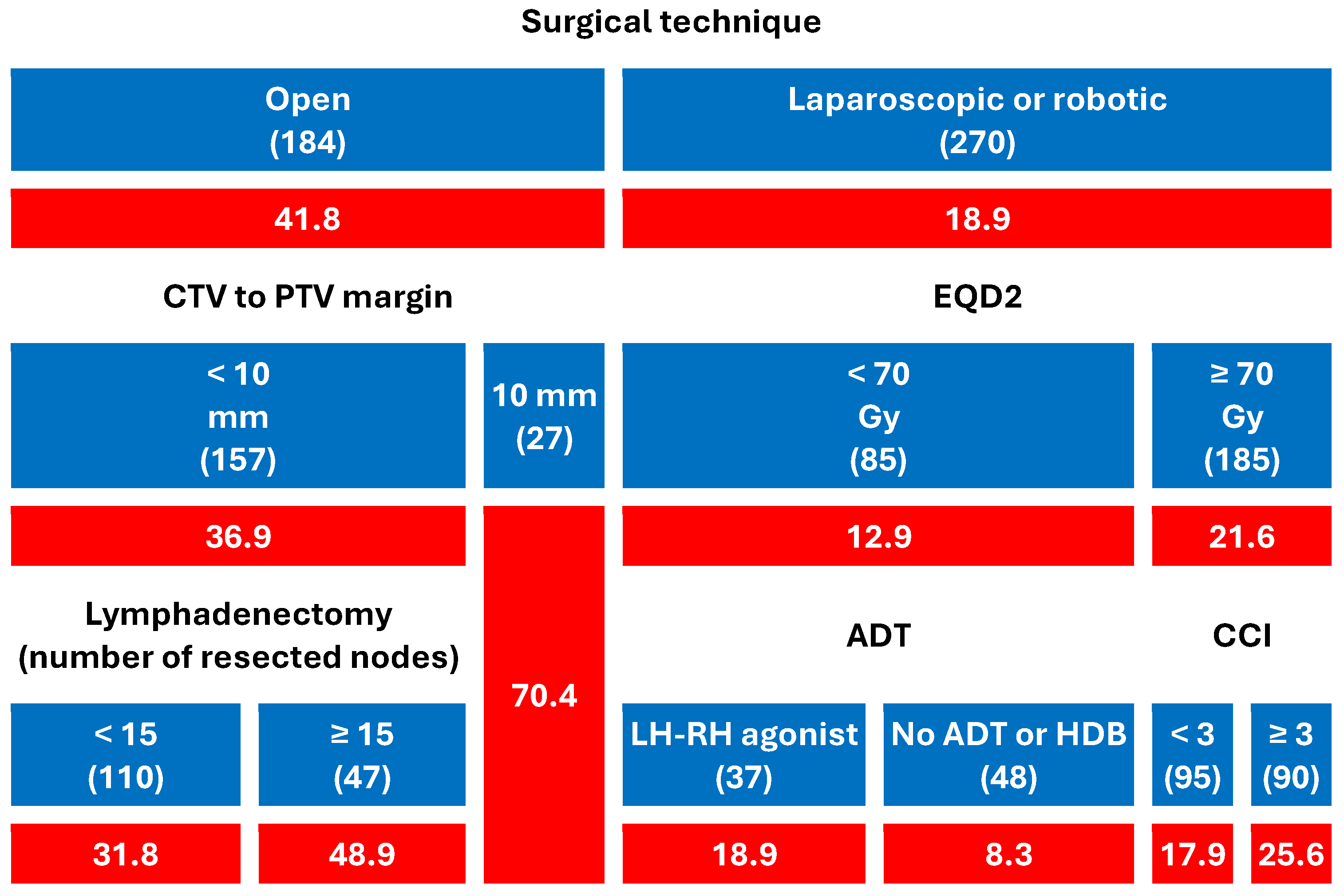

4.2. Why Surgical Approach Dominated Every Other Predictor of Acute Toxicity
4.3. CTV–PTV Margin: A Biological Amplifier
4.4. Why Beam-Delivery Platform (IMRT vs. 3D-CRT) Looked Neutral
- Surgical/biological dominance. The “surgical imprint” (Section 3.1) and the associated margin size (Section 3.2) together determine how much vulnerable tissue enters the high-dose region; once that volume is set, further modulation of fluence (IMRT vs. 3D-CRT) contributes a smaller incremental change, too small to surface in LASSO selection.
- Tight contemporary constraints. In our centers, rectum and bladder dose-volume constraints were respected in almost every plan, regardless of technique. When constraints are uniformly achieved, technique differences become statistically less relevant.
4.5. An Integrated Mechanistic Model
5. Discussion
5.1. Unexpected Predictive Power of Surgical Technique
5.2. A Novel and Biologically Grounded Hypothesis
5.3. Future Validation Strategies
5.4. Planning Margins and Tissue Susceptibility
5.5. Clinical Implications for Risk Stratification and Planning
5.6. A Possible “Natural Spacer” Effect of Minimally Invasive Surgery
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Brunckhorst, O.; Darraugh, J.; Eberli, D.; De Meerleer, G.; De Santis, M.; Farolfi, A.; et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer-2024 Update. Part I: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2024, 86, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Eastham, J.A.; Auffenberg, G.B.; Barocas, D.A.; Chou, R.; Crispino, T.; Davis, J.W.; Eggener, S.; Horwitz, E.M.; Kane, C.J.; Kirkby, E.; et al. Clinically Localized Prostate Cancer: AUA/ASTRO Guideline. Part III: Principles of Radiation and Future Directions. J. Urol. 2022, 208, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, E.M.; Srinivas, S.; Adra, N.; An, Y.; Barocas, D.; Bitting, R.; Bryce, A.; Chapin, B.; Cheng, H.H.; D’Amico, A.V.; et al. Prostate Cancer, Version 4.2023, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2023, 21, 1067–1096. [Google Scholar] [CrossRef] [PubMed]
- Thompson, I.M.; Tangen, C.M.; Paradelo, J.; Lucia, M.S.; Miller, G.; Troyer, D.; Messing, E.; Forman, J.; Chin, J.; Swanson, G.; et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: Long-term followup of a randomized clinical trial. J. Urol. 2009, 181, 956–962. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bolla, M.; van Poppel, H.; Tombal, B.; Vekemans, K.; Da Pozzo, L.; de Reijke, T.M.; Verbaeys, A.; Bosset, J.F.; van Velthoven, R.; Colombel, M.; et al. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: Long-term results of a randomised controlled trial (EORTC trial 22911). Lancet 2012, 380, 2018–2027. [Google Scholar] [CrossRef] [PubMed]
- Sidhom, M.A.; Kneebone, A.B.; Lehman, M.; Wiltshire, K.L.; Millar, J.L.; Mukherjee, R.K.; Shakespeare, T.P.; Tai, K.H. Post-prostatectomy radiation therapy: Consensus guidelines of the Australian and New Zealand Radiation Oncology Genito-Urinary Group. Radiother. Oncol. 2008, 88, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Sargos, P.; Chabaud, S.; Latorzeff, I.; Magné, N.; Benyoucef, A.; Supiot, S.; Pasquier, D.; Abdiche, M.S.; Gilliot, O.; Graff-Cailleaud, P.; et al. Adjuvant radiotherapy versus early salvage radiotherapy plus short-term androgen deprivation therapy in men with localised prostate cancer after radical prostatectomy (GETUG-AFU 17): A randomised, phase 3 trial. Lancet Oncol. 2020, 21, 1341–1352. [Google Scholar] [CrossRef] [PubMed]
- Petersen, P.M.; Cook, A.D.; Sydes, M.R.; Clarke, N.; Cross, W.; Kynaston, H.; Logue, J.; Neville, P.; Patient Representative; Payne, H.; et al. Salvage Radiation Therapy After Radical Prostatectomy: Analysis of Toxicity by Dose-Fractionation in the RADICALS-RT Trial. Int. J. Radiat. Oncol. Biol. Phys. 2023, 117, 624–629. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sandhu, A.; Sethi, R.; Rice, R.; Wang, J.Z.; Marcus, L.; Salem, C.; Downs, T.; Parsons, J.K.; Millard, F.; Pawlicki, T.; et al. Prostate bed localization with image-guided approach using on-board imaging: Reporting acute toxicity and implications for radiation therapy planning following prostatectomy. Radiother. Oncol. 2008, 88, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Hodgins, T.; Forde, E. A systematic review of prostate bed motion and anisotropic margins in post-prostatectomy external beam radiotherapy. Tech. Innov. Patient Support Radiat. Oncol. 2024, 32, 100287. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tomita, N.; Uchiyama, K.; Mizuno, T.; Imai, M.; Sugie, C.; Ayakawa, S.; Niwa, M.; Matsui, T.; Otsuka, S.; Manabe, Y.; et al. Impact of advanced radiotherapy techniques and dose intensification on toxicity of salvage radiotherapy after radical prostatectomy. Sci. Rep. 2020, 10, 114. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- von Ahlen, C.; Geissler, A.; Vogel, J. Comparison of the effectiveness of open, laparoscopic, and robotic-assisted radical prostatectomies based on complication rates: A retrospective observational study with administrative data from Switzerland. BMC Urol. 2024, 24, 215. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, C.J.; Chen, C.X.; Liu, Y.; Wen, Z.; Li, H.Y.; Huang, H.T.; Yang, X.S. Comparative analysis of perioperative outcomes in obese patients undergoing robot-assisted radical prostatectomy (RARP) versus open radical prostatectomy (ORP): A systematic review and meta-analysis. J. Robot. Surg. 2024, 18, 248. [Google Scholar] [CrossRef] [PubMed]
- Deodato, F.; Macchia, G.; Duhanxhiu, P.; Mammini, F.; Cavallini, L.; Ntreta, M.; Zamfir, A.A.; Buwenge, M.; Cellini, F.; Ciabatti, S.; et al. A Multicenter Machine Learning-Based Predictive Model of Acute Toxicity in Prostate Cancer Patients Undergoing Salvage Radiotherapy (ICAROS Study). Cancers 2025, 17, 2142. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, J.; Hu, K.; Wang, Y.; Wu, Y.; Bao, E.; Wang, J.; Tan, C.; Tang, T. Robot-assisted versus open radical prostatectomy: A systematic review and meta-analysis of prospective studies. J. Robot. Surg. 2023, 17, 2617–2631. [Google Scholar] [CrossRef]
- Ilic, D.; Evans, S.M.; Allan, C.A.; Jung, J.H.; Murphy, D.; Frydenberg, M. Laparoscopic and robotic-assisted versus open radical prostatectomy for the treatment of localised prostate cancer. Cochrane Database Syst. Rev. 2017, 9, CD009625. [Google Scholar] [CrossRef]
- Nahas, W.C.; Rodrigues, G.J.; Rodrigues Gonçalves, F.A.; Sawczyn, G.V.; Barros, G.G.; Cardili, L.; Guglielmetti, G.B.; Fazoli, A.J.C.; Cordeiro, M.D.; Cassão, V.D.A.; et al. Perioperative, Oncological, and Functional Outcomes Between Robot-Assisted Laparoscopic Prostatectomy and Open Radical Retropubic Prostatectomy: A Randomized Clinical Trial. J. Urol. 2024, 212, 32–40. [Google Scholar] [CrossRef]
- Coughlin, G.D.; Yaxley, J.W.; Chambers, S.K.; Occhipinti, S.; Samaratunga, H.; Zajdlewicz, L.; Teloken, P.; Dunglison, N.; Williams, S.; Lavin, M.F.; et al. Robot-assisted laparoscopic prostatectomy versus open radical retropubic prostatectomy: 24-month outcomes from a randomised controlled study. Lancet Oncol. 2018, 19, 1051–1060. [Google Scholar] [CrossRef]
- Lantz, A.; Bock, D.; Akre, O.; Angenete, E.; Bjartell, A.; Carlsson, S.; Modig, K.K.; Nyberg, M.; Kollberg, K.S.; Steineck, G.; et al. Functional and Oncological Outcomes After Open Versus Robot-assisted Laparoscopic Radical Prostatectomy for Localised Prostate Cancer: 8-Year Follow-up. Eur. Urol. 2021, 80, 650–660. [Google Scholar] [CrossRef]
- Muaddi, H.; Hafid, M.E.; Choi, W.J.; Lillie, E.; de Mestral, C.; Nathens, A.; Stukel, T.A.; Karanicolas, P.J. Clinical Outcomes of Robotic Surgery Compared to Conventional Surgical Approaches (Laparoscopic or Open): A Systematic Overview of Reviews. Ann. Surg. 2021, 273, 467–473. [Google Scholar] [CrossRef]
- Baghaie, L.; Haxho, F.; Leroy, F.; Lewis, B.; Wawer, A.; Minhas, S.; Harless, W.W.; Szewczuk, M.R. Contemporaneous Perioperative Inflammatory and Angiogenic Cytokine Profiles of Surgical Breast, Colorectal, and Prostate Cancer Patients: Clinical Implications. Cells 2023, 12, 2767. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tang, F.; Tie, Y.; Tu, C.; Wei, X. Surgical trauma-induced immunosuppression in cancer: Recent advances and the potential therapies. Clin. Transl. Med. 2020, 10, 199–223. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bosas, P.; Zaleskis, G.; Dabkevičiene, D.; Dobrovolskiene, N.; Mlynska, A.; Tikuišis, R.; Ulys, A.; Pašukoniene, V.; Jarmalaitė, S.; Jankevičius, F. Immunophenotype Rearrangement in Response to Tumor Excision May Be Related to the Risk of Biochemical Recurrence in Prostate Cancer Patients. J. Clin. Med. 2021, 10, 3709. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kopčalić, K.; Matić, I.Z.; Besu, I.; Stanković, V.; Bukumirić, Z.; Stanojković, T.P.; Stepanović, A.; Nikitović, M. Circulating levels of IL-6 and TGF-β1 in patients with prostate cancer undergoing radiotherapy: Associations with acute radiotoxicity and fatigue symptoms. BMC Cancer 2022, 22, 1167. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lu, Q.; Liang, Y.; Tian, S.; Jin, J.; Zhao, Y.; Fan, H. Radiation-Induced Intestinal Injury: Injury Mechanism and Potential Treatment Strategies. Toxics 2023, 11, 1011. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Quinto, D.; Reis, S.T.; Zampolli, L.J.; Pimenta, R.; Guimarães, V.R.; Viana, N.I.; Dos Santos, G.A.; Gimenez, M.P.; Leite, K.R.; Zampolli, H.; et al. Robotically assisted laparoscopic radical prostatectomy induces lower tissue trauma than radical retropubic prostatectomy. J. Robot. Surg. 2021, 15, 147–151. [Google Scholar] [CrossRef] [PubMed]
- AlZaim, I.; Al-Saidi, A.; Hammoud, S.H.; Darwiche, N.; Al-Dhaheri, Y.; Eid, A.H.; El-Yazbi, A.F. Thromboinflammatory Processes at the Nexus of Metabolic Dysfunction and Prostate Cancer: The Emerging Role of Periprostatic Adipose Tissue. Cancers 2022, 14, 1679. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McDowell, J.A.; Kosmacek, E.A.; Baine, M.J.; Adebisi, O.; Zheng, C.; Bierman, M.M.; Myers, M.S.; Chatterjee, A.; Liermann-Wooldrik, K.T.; Lim, A.; et al. Exogenous APN protects normal tissues from radiation-induced oxidative damage and fibrosis in mice and prostate cancer patients with higher levels of APN have less radiation-induced toxicities. Redox Biol. 2024, 73, 103219. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tiberi, D.; Gruszczynski, N.; Meissner, A.; Delouya, G.; Taussky, D. Influence of body mass index and periprostatic fat on rectal dosimetry in permanent seed prostate brachytherapy. Radiat. Oncol. 2014, 9, 93. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carrie, C.; Hasbini, A.; de Laroche, G.; Richaud, P.; Guerif, S.; Latorzeff, I.; Supiot, S.; Bosset, M.; Lagrange, J.L.; Beckendorf, V.; et al. Salvage radiotherapy withor without short-term hormone therapy for rising prostate-specific antigen concentration after radical prostatectomy (GETUG-AFU 16): A randomised, multicentre, open-label phase 3 trial. Lancet Oncol. 2016, 17, 747–756, Erratum in: Lancet Oncol. 2016, 17, e223. Erratum in: Lancet Oncol. 2019, 20, e293. [Google Scholar] [CrossRef]
- Shipley, W.U.; Seiferheld, W.; Lukka, H.R.; Major, P.P.; Heney, N.M.; Grignon, D.J.; Sartor, O.; Patel, M.P.; Bahary, J.P.; Zietman, A.L.; et al. NRG Oncology RTOG. Radiation with or without Antiandrogen Therapy in Recurrent Prostate Cancer. N. Engl. J. Med. 2017, 376, 417–428. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alongi, F.; Fiorino, C.; Cozzarini, C.; Broggi, S.; Perna, L.; Cattaneo, G.M.; Calandrino, R.; Di Muzio, N. IMRT significantly reduces acute toxicity of whole-pelvis irradiation in patients treated with postoperative adjuvant or salvage radiotherapy after radical prostatectomy. Radiother. Oncol. 2009, 93, 207–212. [Google Scholar] [CrossRef]
- Goenka, A.; Magsanoc, J.M.; Pei, X.; Schechter, M.; Kollmeier, M.; Cox, B.; Scardino, P.T.; Eastham, J.A.; Zelefsky, M.J. Improved toxicity profile following high-dose post-prostatectomy salvage radiation therapy with intensity-modulated radiation therapy. Eur. Urol. 2011, 60, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Shimoyachi, N.; Yoshioka, Y.; Sasamura, K.; Yonese, J.; Yamamoto, S.; Yuasa, T.; Soyano, T.; Kozuka, T.; Oguchi, M. Comparison between dose-escalated IMRT and 3D-CRT for salvage radiation therapy after prostatectomy. Adv. Radiat. Oncol. 2021, 6, 100753. [Google Scholar] [CrossRef] [PubMed]
- Byun, S.J.; Kim, Y.S.; Ahn, H.; Kim, C.-S. Image-guided, whole-pelvic IMRT for biochemical recurrence after radical prostatectomy in high-risk patients. PLoS ONE 2018, 13, e0190479. [Google Scholar] [CrossRef]
- Picardi, C.; Perret, I.; Miralbell, R.; Zilli, T. Hypofractionated radiotherapy for prostate cancer in the postoperative setting: What is the evidence so far? Cancer Treat. Rev. 2018, 62, 91–96. [Google Scholar] [CrossRef]
- Pollack, A.; Karrison, T.G.; Balogh, A.G.; Gomella, L.G.; Low, D.A.; Bruner, D.W.; Wefel, J.S.; Martin, A.-G.; Michalski, J.M.; Angyalfi, S.J. Addition of androgen deprivation therapy and pelvic lymph-node treatment to prostate-bed salvage radiotherapy (NRG Oncology/RTOG 0534 SPPORT): Phase-III trial. Lancet 2022, 399, 1886–1901. [Google Scholar] [CrossRef]
- Mukai, Y.; Omura, M.; Minagawa, Y.; Mase, M.; Nishikawa, Y.; Miura, I.; Hata, M. Long-term outcomes of salvage radiotherapy using TomoTherapy with image guidance for postoperative prostate cancer patients. Cancer Diagn Progn 2025, 5, 189–197. [Google Scholar] [CrossRef]
- Santivasi, W.L.; Xia, F. Ionizing radiation-induced DNA damage, response, and repair. Antioxid. Redox Signal. 2014, 21, 251–259. [Google Scholar] [CrossRef]
- Wei, J.; Wang, B.; Wang, H.; Meng, L.; Zhao, Q.; Li, X.; Xin, Y.; Jiang, X. Radiation-Induced Normal Tissue Damage: Oxidative Stress and Epigenetic Mechanisms. Oxid. Med. Cell. Longev. 2019, 2019, 3010342. [Google Scholar] [CrossRef]
- Zhou, L.; Zhu, J.; Liu, Y.; Zhou, P.; Gu, Y. Mechanisms of radiation-induced tissue damage and response. MedComm 2024, 5, e725. [Google Scholar] [CrossRef]
- Farhood, B.; Khodamoradi, E.; Hoseini-Ghahfarokhi, M.; Motevaseli, E.; Mirtavoos-Mahyari, H.; Musa, A.E.; Najafi, M. TGF-β in radiotherapy: Mechanisms of tumor resistance and normal tissues injury. Pharmacol. Res. 2020, 155, 104745. [Google Scholar] [CrossRef]
- Barcellos-Hoff, M.H. The radiobiology of TGFβ. Semin. Cancer Biol. 2022, 86 Pt 3, 857–867. [Google Scholar] [CrossRef] [PubMed]
- De Ruysscher, D.; Niedermann, G.; Burnet, N.G.; Siva, S.; Lee, A.W.M.; Hegi-Johnson, F. Radiotherapy toxicity. Nat. Rev. Dis. Primers 2019, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Hubenak, J.R.; Zhang, Q.; Branch, C.D.; Kronowitz, S.J. Mechanisms of injury to normal tissue after radiotherapy: A review. Plast. Reconstr. Surg. 2014, 133, 49e–56e. [Google Scholar] [CrossRef]
- Neckonoff, E.; Anderson, C.B. Advancements in Understanding and Managing Radiation Cystitis: A Comprehensive Review. Curr. Urol. Rep. 2024, 26, 1. [Google Scholar] [CrossRef]
- Mari, A.; D’aNdrea, D.; Abufaraj, M.; Foerster, B.; Kimura, S.; Shariat, S.F. Genetic determinants for chemo- and radiotherapy resistance in bladder cancer. Transl. Androl. Urol. 2017, 6, 1081–1089. [Google Scholar] [CrossRef]
- Stansborough, R.L.; Al-Dasooqi, N.; Bateman, E.H.; Keefe, D.M.K.; Gibson, R.J. Radiotherapy-induced gut toxicity: Involvement of matrix metalloproteinases and the intestinal microvasculature. Int. J. Radiat. Biol. 2016, 92, 241–248. [Google Scholar] [CrossRef]
- Tonneau, M.; Elkrief, A.; Pasquier, D.; Del Socorro, T.P.; Chamaillard, M.; Bahig, H.; Routy, B. The role of the gut microbiome on radiation therapy efficacy and gastrointestinal complications: A systematic review. Radiother. Oncol. 2021, 156, 1–9. [Google Scholar] [CrossRef]
- Marano, L.; Verre, L.; Carbone, L.; Poto, G.E.; Fusario, D.; Venezia, D.F.; Calomino, N.; Kaźmierczak-Siedlecka, K.; Polom, K.; Marrelli, D.; et al. Current Trends in Volume and Surgical Outcomes in Gastric Cancer. J. Clin. Med. 2023, 12, 2708. [Google Scholar] [CrossRef]
- Leow, J.J.; Chang, S.L.; Meyer, C.P.; Trinh, Q.D. Systematic Review of the Volume–Outcome Relationship for Radical Prostatectomy. Eur. Urol. Focus. 2018, 4, 614–620. [Google Scholar] [CrossRef]
- Trinh, Q.-D.; Bjartell, A.; Freedland, S.J.; Hollenbeck, B.K.; Hu, J.C.; Shariat, S.F.; Sun, M.; Vickers, A.J. A Systematic Review of the Volume–Outcome Relationship for Radical Prostatectomy. Eur. Urol. 2013, 64, 786–798. [Google Scholar] [CrossRef]
- Vickers, A.J.; Bianco, F.J.; Serio, A.M.; Eastham, J.A.; Schrag, D.; Klein, E.A.; Reuther, A.M.; Kattan, M.W.; Pontes, J.E.; Scardino, P.T. The Surgical Learning Curve for Prostate Cancer Control After Radical Prostatectomy. J. Natl. Cancer Inst. 2007, 99, 1171–1177. [Google Scholar] [CrossRef]
- Fracalanza, S.; Ficarra, V.; Cavalleri, S.; Galfano, A.; Novara, G.; Mangano, A.; Plebani, M.; Artibani, W. Is robotically assisted laparoscopic radical prostatectomy less invasive than retropubic radical prostatectomy? Results from a prospective, unrandomized, comparative study. BJU Int. 2008, 101, 1145–1149. [Google Scholar] [CrossRef]
- Martinschek, A.; Stumm, L.; Ritter, M.; Heinrich, E.; Bolenz, C.; Trojan, L. Prospective, Controlled Study of Invasiveness and Post-Aggression Metabolism in Patients Undergoing Robotic-Assisted Radical Prostatectomy. Urol. Int. 2017, 99, 201–206. [Google Scholar] [CrossRef]
- Stone, H.B.; Coleman, C.N.; Anscher, M.S.; McBride, W.H. Effects of radiation on normal tissue: Consequences and mechanisms. Lancet Oncol. 2003, 4, 529–536. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morganti, A.G.; Macchia, G.; Mammini, F.; Zamfir, A.A.; Buwenge, M.; Cellini, F.; Bianchi, L.; Schiavina, R.; Brunocilla, E.; Deodato, F.; et al. The Surgical Imprint: How Operative Trauma May Shape Radiation Tolerance After Prostatectomy. Cancers 2025, 17, 2685. https://doi.org/10.3390/cancers17162685
Morganti AG, Macchia G, Mammini F, Zamfir AA, Buwenge M, Cellini F, Bianchi L, Schiavina R, Brunocilla E, Deodato F, et al. The Surgical Imprint: How Operative Trauma May Shape Radiation Tolerance After Prostatectomy. Cancers. 2025; 17(16):2685. https://doi.org/10.3390/cancers17162685
Chicago/Turabian StyleMorganti, Alessio G., Gabriella Macchia, Filippo Mammini, Arina A. Zamfir, Milly Buwenge, Francesco Cellini, Lorenzo Bianchi, Riccardo Schiavina, Eugenio Brunocilla, Francesco Deodato, and et al. 2025. "The Surgical Imprint: How Operative Trauma May Shape Radiation Tolerance After Prostatectomy" Cancers 17, no. 16: 2685. https://doi.org/10.3390/cancers17162685
APA StyleMorganti, A. G., Macchia, G., Mammini, F., Zamfir, A. A., Buwenge, M., Cellini, F., Bianchi, L., Schiavina, R., Brunocilla, E., Deodato, F., & Cilla, S. (2025). The Surgical Imprint: How Operative Trauma May Shape Radiation Tolerance After Prostatectomy. Cancers, 17(16), 2685. https://doi.org/10.3390/cancers17162685








