Prehabilitation Prior to Chemotherapy in Humans: A Review of Current Evidence and Future Directions
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
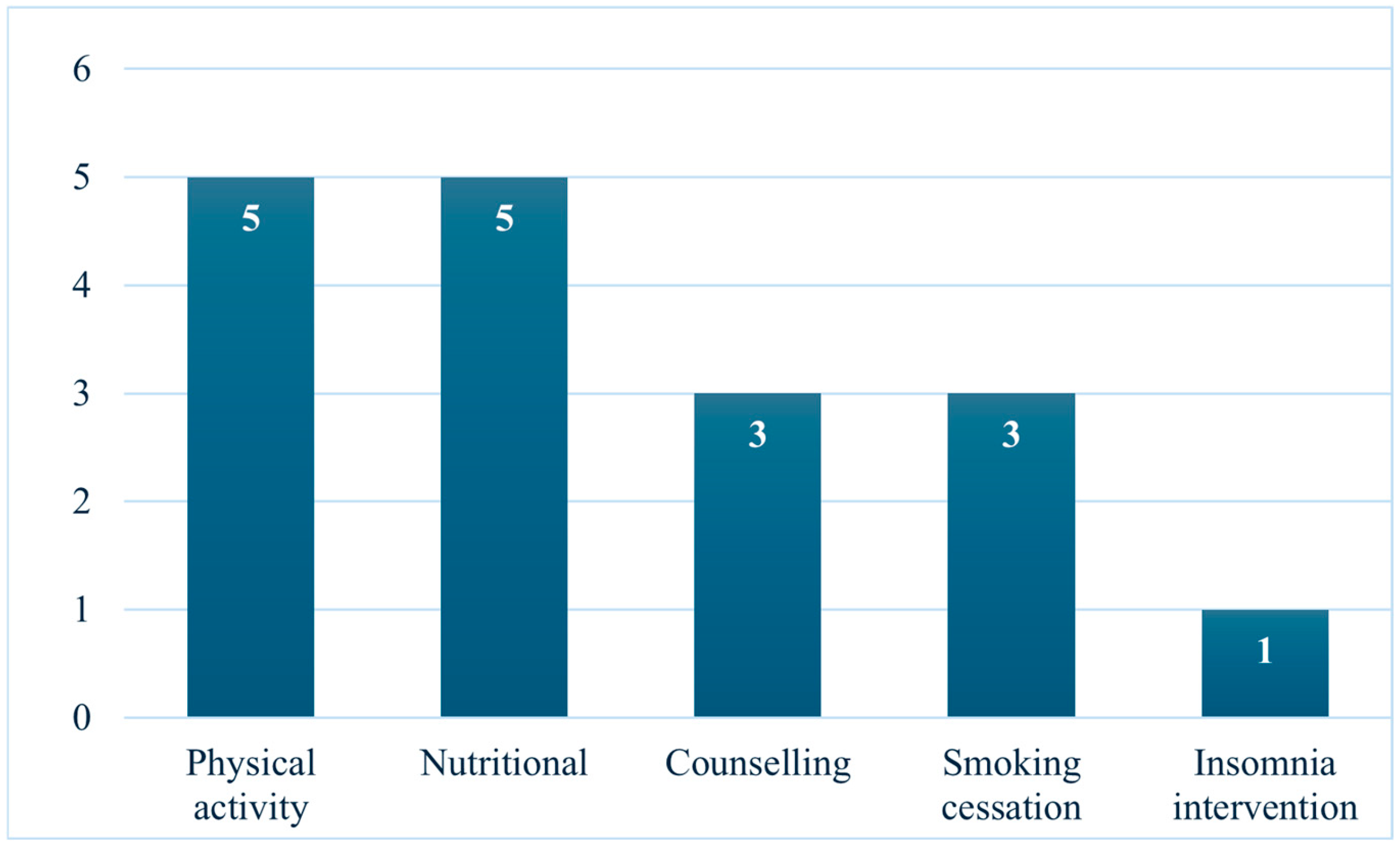
3. Results
3.1. Nutritional Interventions
3.2. Physical Activity Interventions
3.3. Counselling Interventions
3.4. Smoking Cessation
3.5. Other Interventions
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| QOL | Quality of life |
| TDT | Time from treatment to diagnosis |
| TTI | Time to treatment initiation |
| ERAS | Enhanced recovery after surgery |
| VO2max | Maximal oxygen uptake |
| VO2peak | Peak oxygen uptake |
| MEDLINE/Pubmed | Medical Literature Analysis and Retrieval System Online/ National Library of Medicine |
| ERP | Enhanced recovery therapy |
| TRACERx | Tracking Non-Small-Cell Lung Cancer Evolution through Therapy |
| BC | Breast Cancer |
| WCRF | World Cancer Research Fund |
| ATENTO | Adjusting the Dose of Therapeutic Exercise to Prevent Neurotoxicity Due to Anticancer Treatment |
| VATs | Vagal activation techniques |
| TE | Therapeutic exercise |
| NCCN | National Comprehensive Cancer Network |
| GSES | General Self-Efficacy Scale |
| DT | Distress thermometer |
| HADS | Hospital Anxiety Depression Scale |
| SRT | Sleep restriction therapy |
| RCT | Randomized clinical trial |
| EPA | Eicosapentaenoic acid |
| DHA | Docosahexaenoic acid |
| FITT | Frequency, intensity, time, and type |
| ESPEN | European Society for Clinical Nutrition and Metabolism |
| MPS | Muscle protein synthesis |
| CIPN | Chemotherapy-induced peripheral neuropathy |
| TPE | Therapeutic physical exercise |
| CRF | Cardiorespiratory fitness |
| UNCCRI | Northern Colorado Cancer Rehabilitation Institute |
| PTSD | Post-traumatic stress disorder |
| TRE | Therapeutic physical exercise |
| CPR | Cardiorespiratory fitness |
References
- Anderson, E.; Durstine, J.L. Physical activity, exercise, and chronic diseases: A brief review. Sports Med. Health Sci. 2019, 1, 3–10. [Google Scholar] [CrossRef]
- Katta, B.; Vijayakumar, C.; Dutta, S.; Dubashi, B.; Nelamangala Ramakrishnaiah, V.P. The Incidence and Severity of Patient-Reported Side Effects of Chemotherapy in Routine Clinical Care: A Prospective Observational Study. Cureus 2023, 15, e38301. [Google Scholar] [CrossRef]
- Pai, V.B.; Nahata, M.C. Cardiotoxicity of chemotherapeutic agents: Incidence, treatment and prevention. Drug Saf. 2000, 22, 263–302. [Google Scholar] [CrossRef]
- Tchen, N.; Juffs, H.G.; Downie, F.P.; Yi, Q.L.; Hu, H.; Chemerynsky, I.; Clemons, M.; Crump, M.; Goss, P.E.; Warr, D.; et al. Cognitive function, fatigue, and menopausal symptoms in women receiving adjuvant chemotherapy for breast cancer. J. Clin. Oncol. 2003, 21, 4175–4183. [Google Scholar] [CrossRef] [PubMed]
- Del Mastro, L.; Boni, L.; Michelotti, A.; Gamucci, T.; Olmeo, N.; Gori, S.; Giordano, M.; Garrone, O.; Pronzato, P.; Bighin, C.; et al. Effect of the gonadotropin-releasing hormone analogue triptorelin on the occurrence of chemotherapy-induced early menopause in premenopausal women with breast cancer: A randomized trial. JAMA 2011, 306, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Ahles, T.A.; Saykin, A.J.; Furstenberg, C.T.; Cole, B.; Mott, L.A.; Titus-Ernstoff, L.; Skalla, K.; Bakitas, M.; Silberfarb, P.M. Quality of life of long-term survivors of breast cancer and lymphoma treated with standard-dose chemotherapy or local therapy. J. Clin. Oncol. 2005, 23, 4399–4405. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, A.; Morita, T.; Miyashita, M.; Kimura, F. Symptom prevalence and longitudinal follow-up in cancer outpatients receiving chemotherapy. J. Pain. Symptom Manag. 2009, 37, 823–830. [Google Scholar] [CrossRef]
- Roman Souza, G.; Nooruddin, Z.; Lee, S.; Boyle, L.; Lucero, K.T.; Ananth, S.; Franklin, K.; Mader, M.; Toro Velez, E.; Naqvi, A.; et al. The Impact of Time from Diagnosis to Initiation of Chemotherapy on Survival of Patients with Newly Diagnosed Diffuse Large B-Cell Lymphoma in the Veterans Health Administration. Clin. Lymphoma Myeloma Leuk. 2024, 24, e67–e77. [Google Scholar] [CrossRef]
- Cone, E.B.; Marchese, M.; Paciotti, M.; Nguyen, D.D.; Nabi, J.; Cole, A.P.; Molina, G.; Molina, R.L.; Minami, C.A.; Mucci, L.A.; et al. Assessment of Time-to-Treatment Initiation and Survival in a Cohort of Patients with Common Cancers. JAMA Netw. Open 2020, 3, e2030072. [Google Scholar] [CrossRef]
- Silver, J.K.; Baima, J.; Newman, R.; Galantino, M.L.; Shockney, L.D. Cancer rehabilitation may improve function in survivors and decrease the economic burden of cancer to individuals and society. Work 2013, 46, 455–472. [Google Scholar] [CrossRef]
- Shapiro, C.L.; Recht, A. Side effects of adjuvant treatment of breast cancer. N. Engl. J. Med. 2001, 344, 1997–2008. [Google Scholar] [CrossRef]
- Silver, J.K.; Baima, J. Cancer prehabilitation: An opportunity to decrease treatment-related morbidity, increase cancer treatment options, and improve physical and psychological health outcomes. Am. J. Phys. Med. Rehabil. 2013, 92, 715–727. [Google Scholar] [CrossRef]
- Mina, D.S.; Brahmbhatt, P.; Lopez, C.; Baima, J.; Gillis, C.; Trachtenberg, L.; Silver, J.K. The Case for Prehabilitation Prior to Breast Cancer Treatment. Phys. Med. Rehabil. 2017, 9, S305–S316. [Google Scholar] [CrossRef]
- Society, E. Guidelines. Available online: https://erassociety.org/guidelines/ (accessed on 20 December 2024).
- Whittemore, R.; Knafl, K. The integrative review: Updated methodology. J. Adv. Nurs. 2005, 52, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Torraco, R.J. Writing Integrative Literature Reviews. Hum. Resour. Dev. Rev. 2016, 15, 404–428. [Google Scholar] [CrossRef]
- Watson, A.; Hand, S. Relevant Pharmacology and Interventions. In A Prehabilitation Guide for All Providers; Springer Nature Switzerland AG: Cham, Switzerland, 2024; pp. 39–71. [Google Scholar]
- Brown, C.; Orada, R.; Henderson, M. Cancer Prehabilitation. In A Prehabilitation Guide for All Providers; Springer Nature Switzerland AG: Cham, Switzerland, 2024; pp. 119–183. [Google Scholar]
- Di Leone, A.; Terribile, D.; Magno, S.; Sanchez, A.M.; Scardina, L.; Mason, E.J.; D’Archi, S.; Maggiore, C.; Rossi, C.; Di Micco, A.; et al. Neoadjuvant Chemotherapy in Breast Cancer: An Advanced Personalized Multidisciplinary Prehabilitation Model (APMP-M) to Optimize Outcomes. J. Pers. Med. 2021, 11, 324. [Google Scholar] [CrossRef] [PubMed]
- González-Santos, A.; Postigo-Martin, P.; Gallart-Aragón, T.; Esteban-Cornejo, I.; Lopez-Garzon, M.; Galiano-Castillo, N.; Arroyo-Morales, M.; Illescas-Montes, R.; Artacho-Cordón, F.; Martín-Martín, L.; et al. Neurotoxicity prevention with a multimodal program (ATENTO) prior to cancer treatment versus throughout cancer treatment in women newly diagnosed for breast cancer: Protocol for a randomized clinical trial. Res. Nurs. Health 2021, 44, 598–607. [Google Scholar] [CrossRef]
- Shaw, C. Management of diet in gastrointestinal cancer. Proc. Nutr. Soc. 2021, 80, 65–72. [Google Scholar] [CrossRef]
- Wagoner, C.W.; Capozzi, L.C.; Culos-Reed, S.N. Tailoring the Evidence for Exercise Oncology within Breast Cancer Care. Curr. Oncol. 2022, 29, 4827–4841. [Google Scholar] [CrossRef]
- Bertrand, N.; Bridoux, M.; Gaxatte, C.; Abi Rached, H.; Turpin, A.; Letarouilly, J.G.; Vieillard, M.H. Preserving bone in cancers of the elderly: A necessity. Jt. Bone Spine 2023, 90, 105549. [Google Scholar] [CrossRef]
- Jang, M.K.; Park, S.; Raszewski, R.; Park, C.G.; Doorenbos, A.Z.; Kim, S. Prevalence and clinical implications of sarcopenia in breast cancer: A systematic review and meta-analysis. Support. Care Cancer 2024, 32, 328. [Google Scholar] [CrossRef]
- Cedzyńska, M.; Przepiórka, I. Integrating smoking cessation counseling into oncology practice—Benefits and barriers. Nowotw. J. Oncol. 2024, 74, 314–316. [Google Scholar] [CrossRef]
- Fleming, L.; Zibaite, S.; Kyle, S.D.; Boyd, K.; Green, V.; Mansell, J.; Elsberger, B.; Young, D. Insomnia prehabilitation in newly diagnosed breast cancer patients: Protocol for a pilot, multicentre, randomised controlled trial comparing nurse delivered sleep restriction therapy to sleep hygiene education (INVEST trial). PLoS ONE 2024, 19, e0305304. [Google Scholar] [CrossRef]
- Phillips, I.; Nottelmann, L.; Stares, M. A traffic light approach for treatment and supportive care stratification in lung cancer. Curr. Opin. Support. Palliat. Care 2024, 18, 154–160. [Google Scholar] [CrossRef]
- Al-Sawaf, O.; Weiss, J.; Skrzypski, M.; Lam, J.M.; Karasaki, T.; Zambrana, F.; Kidd, A.C.; Frankell, A.M.; Watkins, T.B.K.; Martínez-Ruiz, C.; et al. Body composition and lung cancer-associated cachexia in TRACERx. Nat. Med. 2023, 29, 846–858. [Google Scholar] [CrossRef] [PubMed]
- Phillips, I.; Kestenbaum, S. Optimising patient fitness: Strategies to reduce the effects of cancer cachexia in patients with advanced lung cancer. Curr. Opin. Support. Palliat. Care 2020, 14, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef] [PubMed]
- Guise, T.A. Bone loss and fracture risk associated with cancer therapy. Oncologist 2006, 11, 1121–1131. [Google Scholar] [CrossRef]
- Stava, C.J.; Jimenez, C.; Hu, M.I.; Vassilopoulou-Sellin, R. Skeletal sequelae of cancer and cancer treatment. J. Cancer Surviv. 2009, 3, 75–88. [Google Scholar] [CrossRef]
- D’Oronzo, S.; Stucci, S.; Tucci, M.; Silvestris, F. Cancer treatment-induced bone loss (CTIBL): Pathogenesis and clinical implications. Cancer Treat. Rev. 2015, 41, 798–808. [Google Scholar] [CrossRef]
- Souberbielle, J.C.; Cormier, C.; Cavalier, E.; Breuil, V.; Debiais, F.; Fardellone, P.; Guggenbuhl, P.; Javier, R.M.; Legrand, E.; Lespessailles, E.; et al. Vitamin D Supplementation in France in patients with or at risk for osteoporosis: Recent data and new practices. Jt. Bone Spine 2020, 87, 25–29. [Google Scholar] [CrossRef]
- Coleman, R.; Hadji, P.; Body, J.J.; Santini, D.; Chow, E.; Terpos, E.; Oudard, S.; Bruland, Ø.; Flamen, P.; Kurth, A.; et al. Bone health in cancer: ESMO Clinical Practice Guidelines. Ann. Oncol. 2020, 31, 1650–1663. [Google Scholar] [CrossRef]
- World Cancer Research Fund/American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Cancer: Continuous Update Project Expert Report 2018; World Cancer Research Fund: London, UK, 2018. [Google Scholar]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Senesse, P.; Gioulbasanis, I.; Antoun, S.; Bozzetti, F.; Deans, C.; Strasser, F.; Thoresen, L.; Jagoe, R.T.; Chasen, M.; et al. Diagnostic criteria for the classification of cancer-associated weight loss. J. Clin. Oncol. 2015, 33, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, A.A.; Shave, R.E.; Bland, K.A.; Bovard, J.M.; Eves, N.D.; Gelmon, K.A.; McKenzie, D.C.; Virani, S.A.; Stöhr, E.J.; Warburton, D.E.R.; et al. Protective effects of acute exercise prior to doxorubicin on cardiac function of breast cancer patients: A proof-of-concept RCT. Int. J. Cardiol. 2017, 245, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Winters-Stone, K.M.; Horak, F.; Jacobs, P.G.; Trubowitz, P.; Dieckmann, N.F.; Stoyles, S.; Faithfull, S. Falls, Functioning, and Disability Among Women with Persistent Symptoms of Chemotherapy-Induced Peripheral Neuropathy. J. Clin. Oncol. 2017, 35, 2604–2612. [Google Scholar] [CrossRef]
- Janelsins, M.C.; Kesler, S.R.; Ahles, T.A.; Morrow, G.R. Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int. Rev. Psychiatry 2014, 26, 102–113. [Google Scholar] [CrossRef]
- Tudor-Locke, C.; Bassett, D.R. How Many Steps/Day Are Enough? Sports Med. 2004, 34, 1–8. [Google Scholar] [CrossRef]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Wechsler, D. Wechsler Adult Intelligence Scale—Fourth Edition (WAIS-IV); APA Pearson: San Antonio, TX, USA, 2008. [Google Scholar]
- Postma, T.J.; Aaronson, N.K.; Heimans, J.J.; Muller, M.J.; Hildebrand, J.G.; Delattre, J.Y.; Hoang-Xuan, K.; Lantéri-Minet, M.; Grant, R.; Huddart, R.; et al. The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: The QLQ-CIPN20. Eur. J. Cancer 2005, 41, 1135–1139. [Google Scholar] [CrossRef]
- Bell-Krotoski, J.A.; Fess, E.E.; Figarola, J.H.; Hiltz, D. Threshold detection and Semmes-Weinstein monofilaments. J. Hand Ther. 1995, 8, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Piper, B.F.; Dibble, S.L.; Dodd, M.J.; Weiss, M.C.; Slaughter, R.E.; Paul, S.M. The revised Piper Fatigue Scale: Psychometric evaluation in women with breast cancer. Oncol. Nurs. Forum 1998, 25, 677–684. [Google Scholar] [PubMed]
- Poquet, N.; Lin, C. The Brief Pain Inventory (BPI). J. Physiother. 2016, 62, 52. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Shackelford, D.Y.K.; Brown, J.M.; Peterson, B.M.; Schaffer, J.R.; Hayward, R. The University of Northern Colorado Cancer Rehabilitation Institute Treadmill Protocol Accurately Measures VO2peak in Cancer Survivors. Int. J. Phys. Med. Rehabil. 2017, 5, 437–443. [Google Scholar]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Campbell, K.L.; Winters-Stone, K.M.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.S.; Matthews, C.E.; Ligibel, J.A.; Gerber, L.H.; et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef]
- Schmitz, K.H.; Campbell, A.M.; Stuiver, M.M.; Pinto, B.M.; Schwartz, A.L.; Morris, G.S.; Ligibel, J.A.; Cheville, A.; Galvão, D.A.; Alfano, C.M.; et al. Exercise is medicine in oncology: Engaging clinicians to help patients move through cancer. CA Cancer J. Clin. 2019, 69, 468–484. [Google Scholar] [CrossRef]
- Gradishar, W.J.; Anderson, B.O.; Abraham, J.; Aft, R.; Agnese, D.; Allison, K.H.; Blair, S.L.; Burstein, H.J.; Dang, C.; Elias, A.D.; et al. Breast Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2020, 18, 452–478. [Google Scholar] [CrossRef]
- Carli, F.; Bousquet-Dion, G.; Awasthi, R.; Elsherbini, N.; Liberman, S.; Boutros, M.; Stein, B.; Charlebois, P.; Ghitulescu, G.; Morin, N.; et al. Effect of Multimodal Prehabilitation vs Postoperative Rehabilitation on 30-Day Postoperative Complications for Frail Patients Undergoing Resection of Colorectal Cancer: A Randomized Clinical Trial. JAMA Surg. 2020, 155, 233–242. [Google Scholar] [CrossRef]
- Pamoukdjian, F.; Bouillet, T.; Lévy, V.; Soussan, M.; Zelek, L.; Paillaud, E. Prevalence and predictive value of pre-therapeutic sarcopenia in cancer patients: A systematic review. Clin. Nutr. 2018, 37, 1101–1113. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef] [PubMed]
- Morton, R.W.; Murphy, K.T.; McKellar, S.R.; Schoenfeld, B.J.; Henselmans, M.; Helms, E.; Aragon, A.A.; Devries, M.C.; Banfield, L.; Krieger, J.W.; et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br. J. Sports Med. 2018, 52, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Capitão, C.; Coutinho, D.; Neves, P.M.; Capelas, M.L.; Pimenta, N.M.; Santos, T.; Mäkitie, A.; Ravasco, P. Protein intake and muscle mass maintenance in patients with cancer types with high prevalence of sarcopenia: A systematic review. Support. Care Cancer 2022, 30, 3007–3015. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M. Nutritional supplements in support of resistance exercise to counter age-related sarcopenia. Adv. Nutr. 2015, 6, 452–460. [Google Scholar] [CrossRef]
- Layman, D.K. Dietary Guidelines should reflect new understandings about adult protein needs. Nutr. Metab. 2009, 6, 12. [Google Scholar] [CrossRef]
- Baracos, V.E.; Mazurak, V.C.; Bhullar, A.S. Cancer cachexia is defined by an ongoing loss of skeletal muscle mass. Ann. Palliat. Med. 2019, 8, 3–12. [Google Scholar] [CrossRef]
- Szlendak, M.; Kapała, A. Does the ratio of eicosapentaenoic acid to docosahexaenoic acid matter in cancer treatment? A systematic review of their effects on cachexia-related inflammation. Nutrition 2024, 124, 112466. [Google Scholar] [CrossRef]
- Alcorta, A.; López-Gómez, L.; Capasso, R.; Abalo, R. Vitamins and fatty acids against chemotherapy-induced intestinal mucositis. Pharmacol. Ther. 2024, 261, 108689. [Google Scholar] [CrossRef]
- Artale, S.; Grillo, N.; Lepori, S.; Butti, C.; Bovio, A.; Barzaghi, S.; Colombo, A.; Castiglioni, E.; Barbarini, L.; Zanlorenzi, L.; et al. A Nutritional Approach for the Management of Chemotherapy-Induced Diarrhea in Patients with Colorectal Cancer. Nutrients 2022, 14, 1801. [Google Scholar] [CrossRef]
- Shu, C.; Yang, Q.; Huang, J.; Xie, X.; Li, H.; Wu, H.; Wang, X.; Chen, X.; Xie, Y.; Zhou, Y.; et al. Pretreatment plasma vitamin D and response to neoadjuvant chemotherapy in breast cancer: Evidence from pooled analysis of cohort studies. Int. J. Surg. 2024, 110, 8126–8135. [Google Scholar] [CrossRef]
- Chen, C.S.; Zirpoli, G.; Barlow, W.E.; Budd, G.T.; McKiver, B.; Pusztai, L.; Hortobagyi, G.N.; Albain, K.S.; Damaj, M.I.; Godwin, A.K.; et al. Vitamin D Insufficiency as a Risk Factor for Paclitaxel-Induced Peripheral Neuropathy in SWOG S0221. J. Natl. Compr. Canc. Netw. 2023, 21, 1172–1180.e1173. [Google Scholar] [CrossRef] [PubMed]
- Seretny, M.; Currie, G.L.; Sena, E.S.; Ramnarine, S.; Grant, R.; MacLeod, M.R.; Colvin, L.A.; Fallon, M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain 2014, 155, 2461–2470. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lin, Q.; Lu, X.; Li, W. Post-Diagnosis use of Antioxidant Vitamin Supplements and Breast Cancer Prognosis: A Systematic Review and Meta-Analysis. Clin. Breast Cancer 2021, 21, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Retzlaff, T.; Dörfler, J.; Kutschan, S.; Freuding, M.; Hübner, J. The benefits of vitamin A as a complementary treatment for oncology patients: A systematic review. J. Cancer Res. Clin. Oncol. 2023, 149, 2157–2177. [Google Scholar] [CrossRef]
- Daher, I.N.; Daigle, T.R.; Bhatia, N.; Durand, J.B. The prevention of cardiovascular disease in cancer survivors. Tex. Heart Inst. J. 2012, 39, 190–198. [Google Scholar]
- Wonders, K.Y.; Hydock, D.S.; Schneider, C.M.; Hayward, R. Acute exercise protects against doxorubicin cardiotoxicity. Integr. Cancer Ther. 2008, 7, 147–154. [Google Scholar] [CrossRef]
- Chicco, A.J.; Schneider, C.M.; Hayward, R. Exercise training attenuates acute doxorubicin-induced cardiac dysfunction. J. Cardiovasc. Pharmacol. 2006, 47, 182–189. [Google Scholar] [CrossRef]
- Del-Rosal-Jurado, A.; González-Sánchez, M.; Cuesta-Vargas, A.I. Effect of therapeutic exercise on peak oxygen consumption in oncological population: A systematic review with meta-analysis. Support. Care Cancer 2024, 32, 786. [Google Scholar] [CrossRef]
- Yi, J.C.; Syrjala, K.L. Anxiety and Depression in Cancer Survivors. Med. Clin. North. Am. 2017, 101, 1099–1113. [Google Scholar] [CrossRef]
- Eyigor, S.; Karapolat, H.; Yesil, H.; Uslu, R.; Durmaz, B. Effects of pilates exercises on functional capacity, flexibility, fatigue, depression and quality of life in female breast cancer patients: A randomized controlled study. Eur. J. Phys. Rehabil. Med. 2010, 46, 481–487. [Google Scholar]
- Bekhet, A.H.; Abdallah, A.R.; Ismail, H.M.; Genena, D.M.; Osman, N.A.; El Khatib, A.; Abbas, R.L. Benefits of Aerobic Exercise for Breast Cancer Survivors: A Systematic Review of Randomized Controlled Trials. Asian Pac. J. Cancer Prev. 2019, 20, 3197–3209. [Google Scholar] [CrossRef] [PubMed]
- Grimmett, C.; Heneka, N.; Chambers, S. Psychological Interventions Prior to Cancer Surgery: A Review of Reviews. Curr. Anesthesiol. Rep. 2022, 12, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ahmad, M. Effectiveness of adjunct psychotherapy for cancer treatment: A review. Future Oncol. 2018, 14, 1487–1496. [Google Scholar] [CrossRef] [PubMed]
- van Rooijen, S.; Carli, F.; Dalton, S.; Thomas, G.; Bojesen, R.; Le Guen, M.; Barizien, N.; Awasthi, R.; Minnella, E.; Beijer, S.; et al. Multimodal prehabilitation in colorectal cancer patients to improve functional capacity and reduce postoperative complications: The first international randomized controlled trial for multimodal prehabilitation. BMC Cancer 2019, 19, 98. [Google Scholar] [CrossRef]
- Kyte, S.L.; Gewirtz, D.A. The Influence of Nicotine on Lung Tumor Growth, Cancer Chemotherapy, and Chemotherapy-Induced Peripheral Neuropathy. J. Pharmacol. Exp. Ther. 2018, 366, 303–313. [Google Scholar] [CrossRef]
- Gild, P.; Vetterlein, M.W.; Seiler, R.; Necchi, A.; Hendricksen, K.; Mertens, L.S.; Roghmann, F.; Landenberg, N.V.; Gontero, P.; Cumberbatch, M.; et al. The association of cigarette smoking and pathological response to neoadjuvant platinum-based chemotherapy in patients undergoing treatment for urinary bladder cancer—A prospective European multicenter observational study of the EAU Young Academic Urologists (YAU) urothelial carcinoma working group. Surg. Oncol. 2020, 34, 312–317. [Google Scholar] [CrossRef]
- Yuan, C.; Morales-Oyarvide, V.; Babic, A.; Clish, C.B.; Kraft, P.; Bao, Y.; Qian, Z.R.; Rubinson, D.A.; Ng, K.; Giovannucci, E.L.; et al. Cigarette Smoking and Pancreatic Cancer Survival. J. Clin. Oncol. 2017, 35, 1822–1828. [Google Scholar] [CrossRef]
- Thomsen, T.; Tønnesen, H.; Okholm, M.; Kroman, N.; Maibom, A.; Sauerberg, M.L.; Møller, A.M. Brief smoking cessation intervention in relation to breast cancer surgery: A randomized controlled trial. Nicotine Tob. Res. 2010, 12, 1118–1124. [Google Scholar] [CrossRef]
- Edinger, J.D.; Arnedt, J.T.; Bertisch, S.M.; Carney, C.E.; Harrington, J.J.; Lichstein, K.L.; Sateia, M.J.; Troxel, W.M.; Zhou, E.S.; Kazmi, U.; et al. Behavioral and psychological treatments for chronic insomnia disorder in adults: An American Academy of Sleep Medicine clinical practice guideline. J. Clin. Sleep Med. 2021, 17, 255–262. [Google Scholar] [CrossRef]
- Zachariae, R.; Amidi, A.; Damholdt, M.F.; Clausen, C.D.R.; Dahlgaard, J.; Lord, H.; Thorndike, F.P.; Ritterband, L.M. Internet-Delivered Cognitive-Behavioral Therapy for Insomnia in Breast Cancer Survivors: A Randomized Controlled Trial. J. Natl. Cancer Inst. 2018, 110, 880–887. [Google Scholar] [CrossRef]
- Bean, H.R.; Diggens, J.; Ftanou, M.; Alexander, M.; Stafford, L.; Bei, B.; Francis, P.A.; Wiley, J.F. Light enhanced cognitive behavioral therapy for insomnia and fatigue during chemotherapy for breast cancer: A randomized controlled trial. Sleep 2022, 45, zsab246. [Google Scholar] [CrossRef]
- Zhang, S. Chemotherapy-induced peripheral neuropathy and rehabilitation: A review. Semin. Oncol. 2021, 48, 193–207. [Google Scholar] [CrossRef]
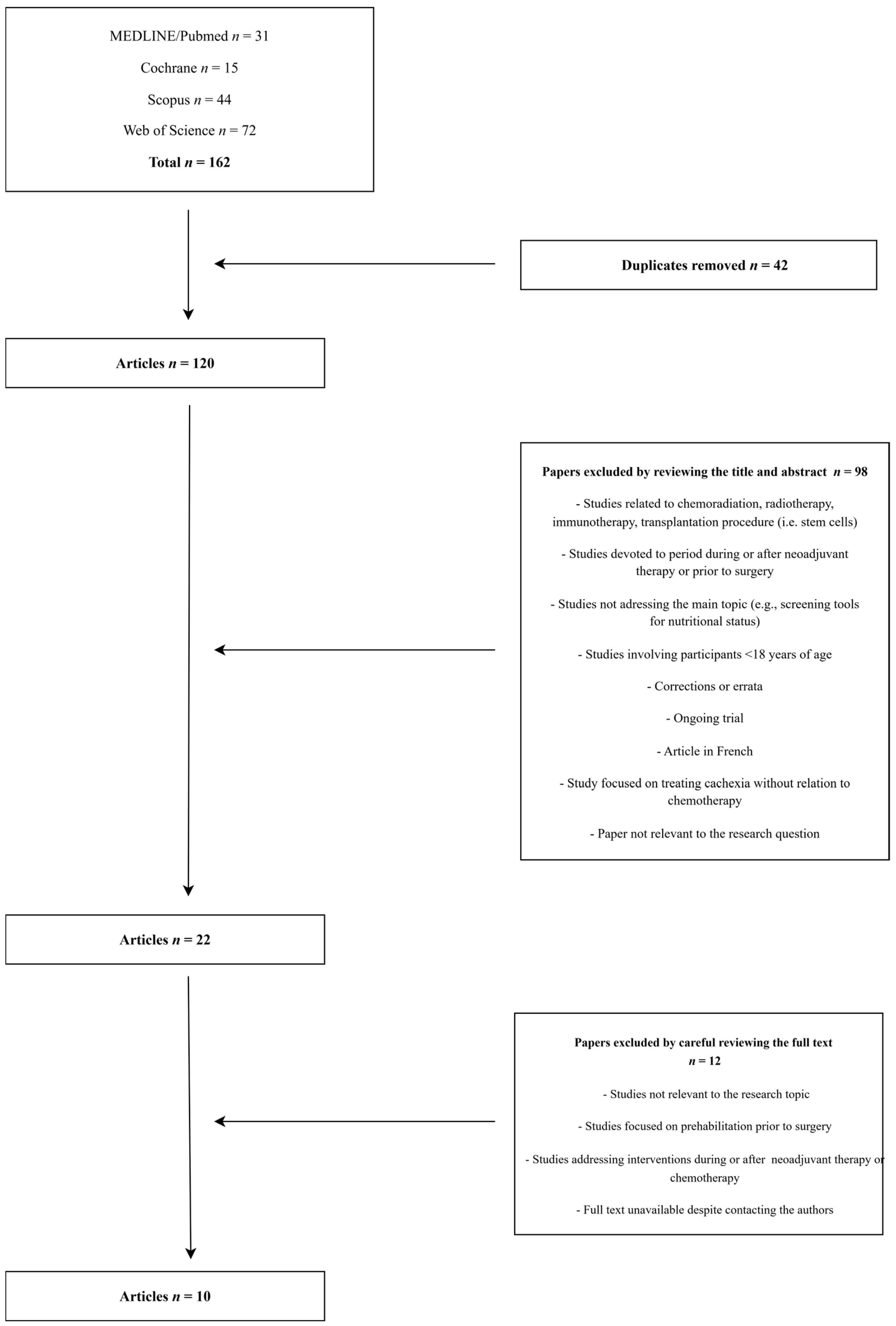
| Database Name (Filters Applied) | Search Query Used | Results (n) |
|---|---|---|
| MEDLINE/PubMed (Title/Abstract) | ((prehabilitation) AND (chemotherapy OR neoadjuvant OR “drug therapy”)) NOT (surg*) | 31 |
| Cochrane (Title Abstract Keywords) | ((prehabilitation) AND (chemotherapy OR neoadjuvant OR “drug therapy”)) NOT (surg*) | 15 |
| Scopus (Article Title, Abstract, Keywords) | ( TITLE-ABS-KEY ( prehabilitation ) AND TITLE-ABS-KEY ( chemotherapy OR “drug therapy: OR neoadjuvant ) AND NOT TITLE-ABS-KEY ( surgery ) ) AND PUBYEAR > 2009 AND PUBYEAR < 2026 AND ( LIMIT-TO ( LANGUAGE , “English” ) ) | 44 |
| Web of Science (Topic) | (TS=(Prehabilitation) AND TS=(chemotherapy OR “drug therapy” OR neoadjuvant)) NOT TS=(surgery) | 72 |
| Total | 162 | |
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Human subjects (≥18 years old) | Studies concerning radiotherapy, chemoradiotherapy, surgery, hormonotherapy, immunotherapy |
| Sampling = patients prior to chemotherapy | Studies concerning ERP and ERAS |
| Peer reviewed papers | Studies describing interventions during or after the treatment |
| Papers published in English | |
| Published between 2010 and January 13th 2025 |
| Reference | First Author’s Name, Journal, Year of Publication | Digital Object Identifier | Design of the Study | Type of Prehabilitation | Type of Cancer |
|---|---|---|---|---|---|
| [13] | Mina, PM&R, 2017 | 10.1016/j.pmrj.2017.08.402 | Narrative review | Physical activity | Breast |
| Nutrition | |||||
| Counselling | |||||
| Smoking cessation | |||||
| [19] | Di Leone, J. Pers. Med., 2021 | 10.3390/jpm11050324 | Narrative review | Physical activity | Breast |
| Nutrition | |||||
| Counselling | |||||
| [20] | González-Santos, Res Nurs Health, 2021 | 10.1002/nur.22136 | Study protocol for a randomized controlled trial | Physical activity | Breast |
| [21] | Shaw, Proc. Nutr. Soc., 2021 | 10.1017/S0029665120007041 | Narrative review | Nutrition | Gastrointestinal |
| [22] | Wagoner, Curr Oncol, 2022 | 10.3390/curroncol29070383 | Narrative review | Physical activity | Breast |
| [23] | Bertrand, Joint Bones Spine, 2023 | 10.1016/j.jbspin.2023.105549 | Narrative review | Nutrition | Non-specified |
| [24] | Jang, Support Care Cancer, 2024 | 10.1007/s00520-024-08532-0 | Systematic review and meta-analysis | Nutrition | Breast |
| [25] | Cedzyńska, Nowotwory J Oncol, 2024 | 10.5603/njo.101552 | Narrative review | Smoking cessation | Non-specified |
| [26] | Fleming, PLoS ONE, 2024 | 10.1371/journal.pone.0305304 | Pilot randomized controlled trial protocol | Insomnia intervention | Breast |
| [27] | Phillips, Curr Opin Support Palliat Care, 2024 | 10.1097/SPC.0000000000000716 | Narrative review | Physical activity | Lung |
| Session’s Parts (in Order) | Time of Duration | Sport Equipment Used |
|---|---|---|
| 8–10 min | Elliptical trainer, dumbbells, elastic bands, mat |
| Total of 60 min (cardiovascular 10–30 min, strength 30–50 min) | |
| 20 min |
| Parameters Assessed | Assessment Tool Used |
|---|---|
| Quality of life (QOL) | European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQC30) version 3.0 [43] |
| Mental flexibility, speed of processing, and executive function | The Trail Making Test (TMT) |
| Memory and cognitive processing speed | Wechsler Adult Intelligence Scale (Wechsler, 2008) [44] |
| Neuropathic symptoms | The EORTC QLQ-Chemotherapy-induced peripheral neuropathy (QLQCIPN20) (Postma, 2005) [45] |
| Peripheral sensory neuropathy | Semmes–Weinstein monofilaments (SWMs) (Bell-Krotoski, 1995) [46] |
| Anxiety and depression | The Hospital Anxiety and Depression Scale (HADS) (Zigmond & Snalth, 1983) [47] |
| Cancer-related fatigue | The Piper Fatigue Scale-Revised (PFS-R) (Piper, 1998) [48] |
| Pain severity and the interference of pain with daily activities | Brief Pain Inventory (BPI) (Poquet & Lin, 2016) [49] |
| Exploring the quadriceps, deltoid, trapezius, and cervical muscles bilaterally | Pressure pain thresholds and algometer |
| Sleep quality | The Pittsburgh Sleep Quality Index (PSQI) (Buysse, 1989) [50] |
| VO2peak | Cardiopulmonary exercise test (Medisoft, 870A treadmill) and Jaeger MasterScreen® CPX gas analyser (protocol of the University of Northern Colorado Cancer Rehabilitation Institute) [51] |
| Whole-body balance | Flamingo test |
| Physical activity or inactivity level | International Physical Activity Questionnaire (IPAQ) (Craig, 2003) [52] |
| Estimates lean body mass, fat mass, abdominal adipose tissue, and body max index (kg/m2) | InBody 720 impedance meter |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pietrakiewicz, K.; Stec, R.; Sobocki, J. Prehabilitation Prior to Chemotherapy in Humans: A Review of Current Evidence and Future Directions. Cancers 2025, 17, 2670. https://doi.org/10.3390/cancers17162670
Pietrakiewicz K, Stec R, Sobocki J. Prehabilitation Prior to Chemotherapy in Humans: A Review of Current Evidence and Future Directions. Cancers. 2025; 17(16):2670. https://doi.org/10.3390/cancers17162670
Chicago/Turabian StylePietrakiewicz, Karolina, Rafał Stec, and Jacek Sobocki. 2025. "Prehabilitation Prior to Chemotherapy in Humans: A Review of Current Evidence and Future Directions" Cancers 17, no. 16: 2670. https://doi.org/10.3390/cancers17162670
APA StylePietrakiewicz, K., Stec, R., & Sobocki, J. (2025). Prehabilitation Prior to Chemotherapy in Humans: A Review of Current Evidence and Future Directions. Cancers, 17(16), 2670. https://doi.org/10.3390/cancers17162670







