Survival Study: International Multicentric Pancreatic Left Resections (SIMPLR-2): Does Surgical Approach Matter for Recurrence-Free Survival and Overall Survival?
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
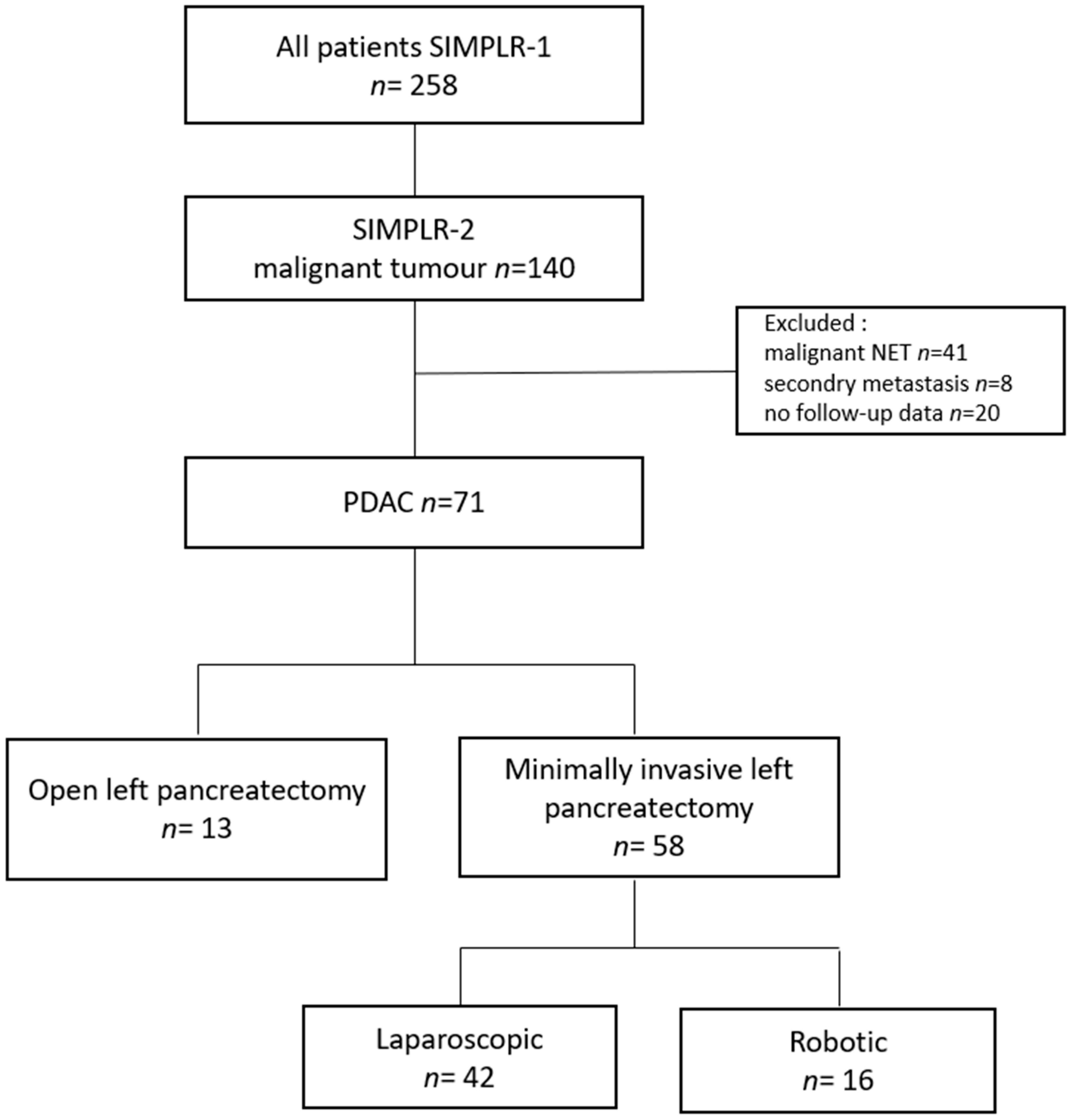
2.1. Study Design and Population
2.2. Institutional Review Board Statement
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
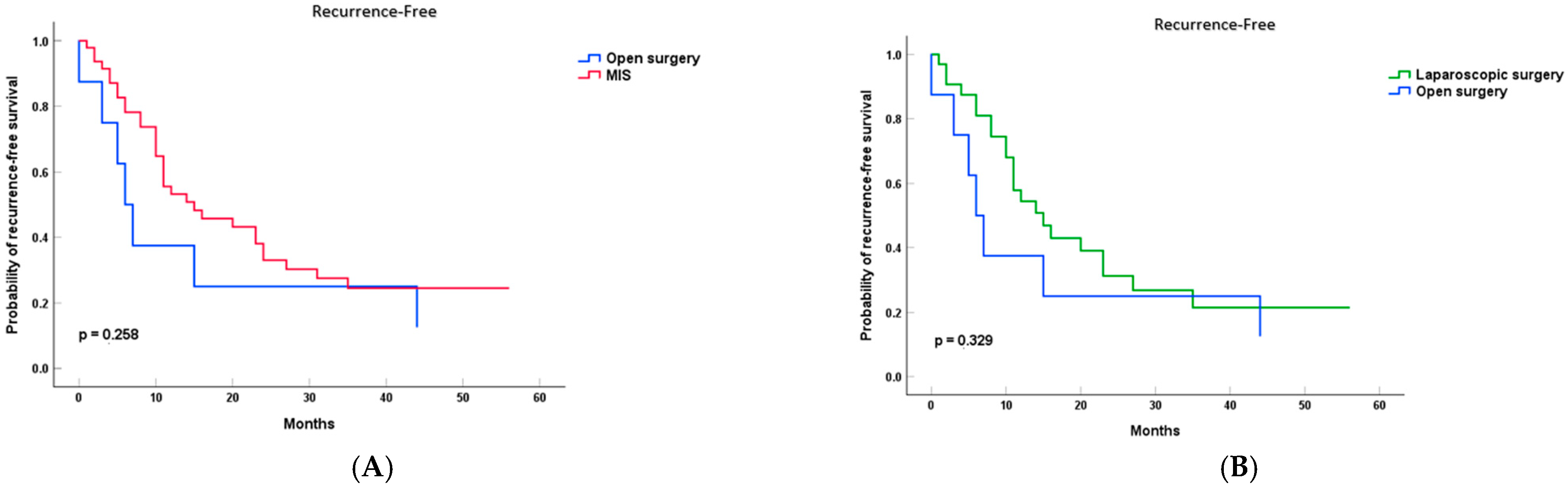
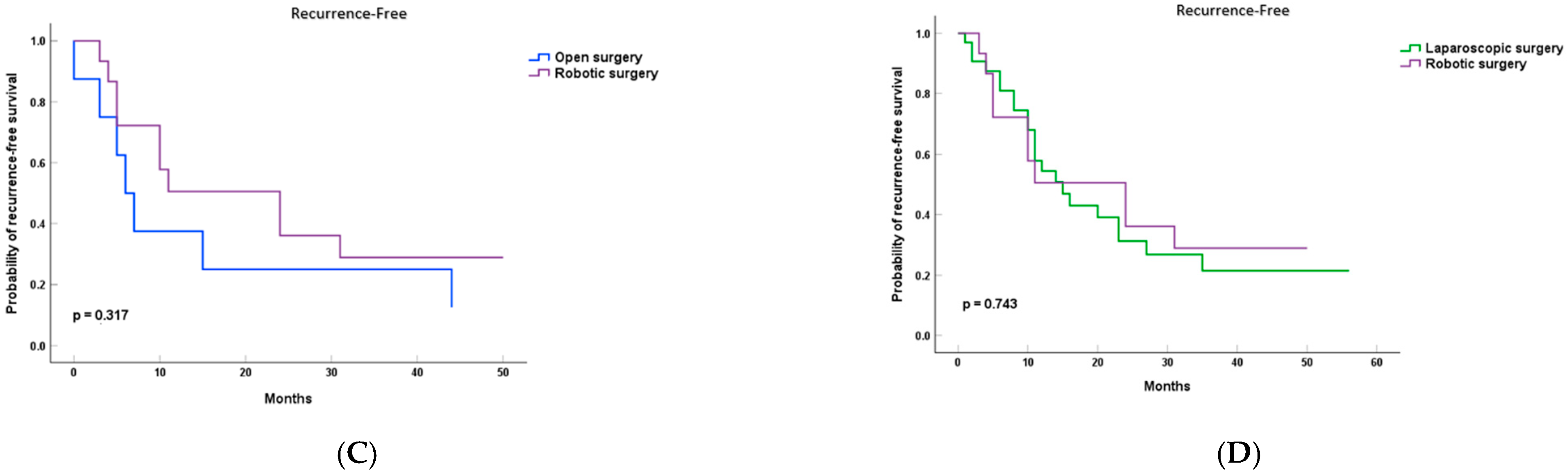
3.2. Survival Analysis
3.3. Univariable Covariant Balance and Propensity-Adjusted Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cuschieri, A. Laparoscopic surgery of the pancreas. J. R. Coll. Surg. Edinb. 1994, 39, 178–184. [Google Scholar] [PubMed]
- Melvin, W.S.; Needleman, B.J.; Krause, K.R.; Ellison, E.C. Robotic resection of pancreatic neuroendocrine tumor. J. Laparoendosc. Adv. Surg. Tech. A 2003, 13, 33–36. [Google Scholar] [CrossRef]
- Asbun, H.J.; Moekotte, A.L.; Vissers, F.L.; Kunzler, F.; Cipriani, F.; Alseidi, A.; D’Angelica, M.I.; Balduzzi, A.; Bassi, C.; Björnsson, B.; et al. The Miami International Evidence-based Guidelines on Minimally Invasive Pancreas Resection. Ann. Surg. 2020, 271, 1–14. [Google Scholar] [CrossRef]
- van Ramshorst, T.M.E.; van Hilst, J.; Bannone, E.; Pulvirenti, A.; Asbun, H.J.; Boggi, U.; Busch, O.R.; Dokmak, S.; Edwin, B.; Hogg, M.; et al. International survey on opinions and use of robot-assisted and laparoscopic minimally invasive pancreatic surgery: 5-year follow up. HPB 2024, 26, 63–72. [Google Scholar] [CrossRef]
- Luo, G.; Jin, K.; Cheng, H.; Guo, M.; Gong, Y.; Fan, Z.; Yang, C.; Huang, Q.; Ni, Q.; Liu, C.; et al. Prognosis of distal pancreatic cancers controlled by stage. Exp. Ther. Med. 2020, 20, 1091–1097. [Google Scholar] [CrossRef]
- Ryan, D.P.; Hong, T.S.; Bardeesy, N. Pancreatic adenocarcinoma. N. Engl. J. Med. 2014, 371, 1039–1049. [Google Scholar] [CrossRef]
- Ruess, D.A.; Makowiec, F.; Chikhladze, S.; Sick, O.; Riediger, H.; Hopt, U.T.; Wittel, U.A. The prognostic influence of intrapancreatic tumor location on survival after resection of pancreatic ductal adenocarcinoma. BMC Surg. 2015, 15, 123. [Google Scholar] [CrossRef]
- Birnbaum, D.J.; Bertucci, F.; Finetti, P.; Birnbaum, D.; Mamessier, E. Head and Body/Tail Pancreatic Carcinomas Are Not the Same Tumors. Cancers 2019, 11, 497. [Google Scholar] [CrossRef]
- Korrel, M.; Jones, L.R.; van Hilst, J.; Balzano, G.; Björnsson, B.; Boggi, U.; Bratlie, S.O.; Busch, O.R.; Butturini, G.; Capretti, G.; et al. Minimally invasive versus open distal pancreatectomy for resectable pancreatic cancer (DIPLOMA): An international randomised non-inferiority trial. Lancet Reg. Health Eur. 2023, 31, 100673. [Google Scholar] [CrossRef] [PubMed]
- Butturini, G.; Stocken, D.D.; Wente, M.N.; Jeekel, H.; Klinkenbijl, J.H.; Bakkevold, K.E.; Takada, T.; Amano, H.; Dervenis, C.; Bassi, C.; et al. Influence of resection margins and treatment on survival in patients with pancreatic cancer: Meta-analysis of randomized controlled trials. Arch. Surg. 2008, 143, 75–83; discussion 83. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Chen, H.; Lu, M.; Zhang, Y.; Lu, B.; You, L.; Zhang, T.; Dai, M.; Zhao, Y. Advances in the epidemiology of pancreatic cancer: Trends, risk factors, screening, and prognosis. Cancer Lett. 2021, 520, 1–11. [Google Scholar] [CrossRef]
- Acciuffi, S.; Hilal, M.A.; Ferrari, C.; Al-Madhi, S.; Chouillard, M.A.; Messaoudi, N.; Croner, R.S.; Gumbs, A.A. Study International Multicentric Pancreatic Left Resections (SIMPLR): Does Surgical Approach Matter? Cancers 2024, 16, 1051. [Google Scholar] [CrossRef]
- Kwon, J.; Lee, J.H.; Park, S.Y.; Park, Y.; Lee, W.; Song, K.B.; Hwang, D.W.; Kim, S.C. A comparison of robotic versus laparoscopic distal pancreatectomy: Propensity score matching analysis. Int. J. Med. Robot. Comput. Assist. Surg. 2022, 18, e2347. [Google Scholar] [CrossRef]
- van Veldhuisen, E.; Klompmaker, S.; Janssen, Q.P.; Hilal, M.A.; Alseidi, A.; Balduzzi, A.; Balzano, G.; Bassi, C.; Berrevoet, F.; Bonds, M.; et al. Surgical and Oncological Outcomes After Preoperative FOLFIRINOX Chemotherapy in Resected Pancreatic Cancer: An International Multicenter Cohort Study. Ann. Surg. Oncol. 2023, 30, 1463–1473. [Google Scholar] [CrossRef]
- Yang, D.J.; Xiong, J.J.; Lu, H.M.; Wei, Y.; Zhang, L.; Lu, S.; Hu, W.M. The oncological safety in minimally invasive versus open distal pancreatectomy for pancreatic ductal adenocarcinoma: A systematic review and meta-analysis. Sci. Rep. 2019, 9, 1159. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.W.; van Ramshorst, T.M.E.; Lof, S.; Al-Sarireh, B.; Bjornsson, B.; Boggi, U.; Burdio, F.; Butturini, G.; Casadei, R.; Coratti, A.; et al. Robot-Assisted Versus Laparoscopic Distal Pancreatectomy in Patients with Resectable Pancreatic Cancer: An International, Retrospective, Cohort Study. Ann. Surg. Oncol. 2023, 30, 3023–3032. [Google Scholar] [CrossRef] [PubMed]
- Björnsson, B.; Larsson, A.L.; Hjalmarsson, C.; Gasslander, T.; Sandström, P. Comparison of the duration of hospital stay after laparoscopic or open distal pancreatectomy: Randomized controlled trial. Br. J. Surg. 2020, 107, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- de Rooij, T.; Besselink, M.G.; Shamali, A.; Butturini, G.; Busch, O.R.; Edwin, B.; Troisi, R.; Fernández-Cruz, L.; Dagher, I.; Bassi, C.; et al. Pan-European survey on the implementation of minimally invasive pancreatic surgery with emphasis on cancer. HPB 2016, 18, 170–176. [Google Scholar] [CrossRef]
- Gavriilidis, P.; Roberts, K.J.; Sutcliffe, R.P. Comparison of robotic vs laparoscopic vs open distal pancreatectomy. A systematic review and network meta-analysis. HPB 2019, 21, 1268–1276. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, M.; Caruso, R.; D’Ovidio, A.; Núñez-Alfonsel, J.; Burdió Pinilla, F.; Quijano Collazo, Y.; Vicente, E.; Ielpo, B. Robotic versus laparoscopic distal pancreatectomies: A systematic review and meta-analysis on costs and perioperative outcome. Int. J. Med. Robot. Comput. Assist. Surg. 2021, 17, e2295. [Google Scholar] [CrossRef]
- van Hilst, J.; Korrel, M.; de Rooij, T.; Lof, S.; Busch, O.R.; Groot Koerkamp, B.; Kooby, D.A.; van Dieren, S.; Abu Hilal, M.; Besselink, M.G. Oncologic outcomes of minimally invasive versus open distal pancreatectomy for pancreatic ductal adenocarcinoma: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2019, 45, 719–727. [Google Scholar] [CrossRef]
- van Ramshorst, T.M.E.; Chen, J.W.; Hilal, M.A.; Besselink, M.G. Robot-Assisted versus Laparoscopic Distal Pancreatectomy in Patients with Resectable Pancreatic Cancer: An International Retrospective Cohort Study-Authors’ Reply. Ann. Surg. Oncol. 2023, 30, 5117–5118. [Google Scholar] [CrossRef]
- Gumbs, A.A.; Croner, R.; Rodriguez, A.; Zuker, N.; Perrakis, A.; Gayet, B. 200 consecutive laparoscopic pancreatic resections performed with a robotically controlled laparoscope holder. Surg. Endosc. 2013, 27, 3781–3791. [Google Scholar] [CrossRef]
- Gumbs, A.A.; Grès, P.; Madureira, F.; Gayet, B. Laparoscopic vs open resection of pancreatic endocrine neoplasms: Single institution’s experience over 14 years. Langenbecks Arch. Surg. 2008, 393, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Abu Hilal, M.; van Ramshorst, T.M.E.; Boggi, U.; Dokmak, S.; Edwin, B.; Keck, T.; Khatkov, I.; Ahmad, J.; Al Saati, H.; Alseidi, A.; et al. The Brescia Internationally Validated European Guidelines on Minimally Invasive Pancreatic Surgery (EGUMIPS). Ann. Surg. 2024, 279, 45–57. [Google Scholar] [CrossRef] [PubMed]
- de Rooij, T.; van Hilst, J.; van Santvoort, H.; Boerma, D.; van den Boezem, P.; Daams, F.; van Dam, R.; Dejong, C.; van Duyn, E.; Dijkgraaf, M.; et al. Minimally Invasive Versus Open Distal Pancreatectomy (LEOPARD): A Multicenter Patient-blinded Randomized Controlled Trial. Ann. Surg. 2019, 269, 2–9. [Google Scholar] [CrossRef]
- Kwon, J.; Park, S.Y.; Park, Y.; Jun, E.; Lee, W.; Song, K.B.; Lee, J.H.; Hwang, D.W.; Kim, S.C. A comparison of minimally invasive vs open distal pancreatectomy for resectable pancreatic ductal adenocarcinoma: Propensity score matching analysis. J. Hepato-Biliary-Pancreat. Sci. 2021, 28, 967–982. [Google Scholar] [CrossRef] [PubMed]
- Nassour, I.; Winters, S.B.; Hoehn, R.; Tohme, S.; Adam, M.A.; Bartlett, D.L.; Lee, K.K.; Paniccia, A.; Zureikat, A.H. Long-term oncologic outcomes of robotic and open pancreatectomy in a national cohort of pancreatic adenocarcinoma. J. Surg. Oncol. 2020, 122, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Gumbs, A.A.; Chouillard, E.; Abu Hilal, M.; Croner, R.; Gayet, B.; Gagner, M. The experience of the minimally invasive (MI) fellowship-trained (FT) hepatic-pancreatic and biliary (HPB) surgeon: Could the outcome of MI pancreatoduodenectomy for peri-ampullary tumors be better than open? Surg. Endosc. 2021, 35, 5256–5267. [Google Scholar] [CrossRef]
- Hong, S.; Song, K.B.; Madkhali, A.A.; Hwang, K.; Yoo, D.; Lee, J.W.; Youn, W.Y.; Alshammary, S.; Park, Y.; Lee, W.; et al. Robotic versus laparoscopic distal pancreatectomy for left-sided pancreatic tumors: A single surgeon’s experience of 228 consecutive cases. Surg. Endosc. 2020, 34, 2465–2473. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Zhiming, Z.; Xianglong, T.; Yuanxing, G.; Yong, X.U.; Rong, L.; Yee, L.W. Short- and mid-term outcomes of robotic versus laparoscopic distal pancreatosplenectomy for pancreatic ductal adenocarcinoma: A retrospective propensity score-matched study. Int. J. Surg. 2018, 55, 81–86. [Google Scholar] [CrossRef]
- Joseph, N.; Varghese, C.; Lucocq, J.; McGuinness, M.J.; Tingle, S.; Marchegiani, G.; Soreide, K.; Abu-Hilal, M.; Samra, J.; Besselink, M.; et al. Network Meta-Analysis and Trial Sequential Analysis of Randomised Controlled Trials Comparing Robotic, Laparoscopic, and Open Pancreatoduodenectomy. Ann. Surg. Open 2024, 5, e507. [Google Scholar] [CrossRef] [PubMed]

| Total | Open | Laparoscopic | Robotic | p-Value | |
|---|---|---|---|---|---|
| (n = 71) | (n = 13) | (n = 42) | (n = 16) | ||
| Gender | |||||
| Female | 39 (54.9%) | 6 (46.2%) | 23 (54.8%) | 10 (62.5%) | 0.713 |
| Male | 32 (45.1%) | 7 (53.8%) | 19 (45.2%) | 6 (37.5%) | |
| Age | |||||
| Mean (SD) | 68.92 (9.47) | 63.62 (11.77) | 70.52 (7.39) | 69 (11.29) | 0.025 |
| <65 | 19(26.8%) | 7 (53.8%) | 7 (16.7%) | 5 (31.3%) | |
| ≥65 | 52 (73.2%) | 6 (46.2%) | 35 (83.3%) | 11 (68.8%) | |
| BMI | |||||
| Mean (SD) | 26.45 (4.14) | 27.37 (3.89) | 27.29 (3.8) | 23.65 (4.13) | 0.006 |
| ASA Score | |||||
| 1 | 0 | 0 | 0 | 0.382 | |
| 2 | 45 (63.4%) | 8 (61.5%) | 29 (69%) | 8 (50%) | |
| 3 | 26 (36.6%) | 5 (38.5%) | 13 (31%) | 8 (50%) | |
| 4 | 0 | 1 (1.3%) | 0 | ||
| Neoadjuvant Chemotherapy | |||||
| No | 68 (97.1%) | 12 (92.3%) | 40 (97.6%) | 16 (100%) | 0.389 |
| Yes | 2 (2.9%) | 1 (7.7%) | 1 (2.4%) | 0 (0%) | |
| Diameter of Largest Resected Tumor (mm) | |||||
| Mean (SD) | 35.61 (22.23) | 57.46 (23.6) | 39.9 (15.9) | 25.64 (11.41) | <0.001 |
| Stage | |||||
| 0 | 1 (1.4%) | 1 (2.4%) | 0 | <0.001 | |
| 1 | 8 (11.6%) | 2 (16.7%) | 1 (2.4%) | 5 (31.3%) | |
| 2 | 42 (60.9%) | 9 (75.0%) | 29 (70.7%) | 4 (25%) | |
| 3 | 12 (17.4%) | 1 (8.3%) | 4 (9.08%) | 7 (43.8%) | |
| 4 | 6 (8.7%) | 6 (14.6%) | 0 | ||
| # LNN Retrieved | |||||
| Mean (SD) | 16.1 (11.93) | 23.08 (14.48) | 14.51 (8.32) | 27.81 (10.63) | <0.001 |
| # Pathologic.LNN | |||||
| Mean (SD) | 2.53 (9.5) | 2.08 (2.63) | 1.56 (1.42) | 2.94 (3.09) | 0.474 |
| Resection margin | |||||
| R0 | 49 (69%) | 9 (69.2%) | 25 (59.5%) | 15 (93.8%) | 0.034 |
| R1 | 22 (31%) | 4 (30.8%) | 17 (40.5%) | 1 (6.3%) |
| HR (95% CI) | p-Value | ||
|---|---|---|---|
| Gender | 0.848 | (0.446–1.609) | 0.613 |
| Age (<65 vs. ≥65) | 0.767 | (0.392–1.502) | 0.439 |
| BMI | 1.113 | (1.025–1.209) | 0.011 |
| ASA-Score (2 or 3) | 1.395 | (0.724–2.688) | 0.319 |
| Stage (<3 vs. ≥3) | 3.250 | (1.501–7.037) | 0.003 |
| Resection margin (R0 vs. R1) | 1.031 | (0.500–2.126) | 0.934 |
| Surgical approach (OS vs. MIS) | 0.608 | (0.295–1.254) | 0.178 |
| Diameter of largest resected tumor (mm) | 1.002 | (0.987–1.017) | 0.809 |
| Neoadjuvant chemotherapy | 0.858 | (0.117–6.289) | 0.880 |
| # LNN Retrieved | 1.003 | (0.976–1.031) | 0.823 |
| # Pathologic LNN | 1.154 | (1.022–1.303) | 0.021 |
| Resection margin | 1.031 | (0.500–2.126) | 0.934 |
| HR (95% CI) | p-Value | ||
|---|---|---|---|
| OS vs. MIS | 0.759 | (0.274–2.102) | 0.595 |
| Propensity score | 0.884 | (0.179–4.365) | 0.880 |
| Covariate | Open (Mean (±SD) or n (%)) | MIS (Mean (±SD) or n (%)) | p-Value (Bevor PS Adjustment) | p-Value (After PS Adjustment) |
|---|---|---|---|---|
| Gender (male) | 7 (21.9%) | 25 (78.1%) | 0.255 | 0.634 |
| Age ≥ 65 | 6 (11.5%) | 46 (88.5%) | 0.015 | 0.263 |
| BMI | 27.37 (±3.89) | 26.23 (±4.21) | 0.509 | 0.228 |
| ASA (1 or 2) | 8 (17.8%) | 37 (82.2%) | 0.886 | 0.184 |
| Neoadjuvant Chemotherapy (no) | 12 (17.6%) | 56 (82.4%) | 0.307 | 0.718 |
| Diameter of Largest Resected Tumor (mm) | 57.46 (±23.6) | 36.27 (±16.06) | <0.001 | 0.19 |
| Stage (<3) | 11 (21.6%) | 40 (78.4%) | 0.067 | 0.077 |
| # LNN Retrieved | 23.08 (±14.48) | 18.69 (±10.95) | 0.267 | 0.163 |
| # Pathologic.LNN | 2.08 (±2.63) | 2 ± 2.17 | 0.583 | 0.844 |
| Resection Margin (R0) | 9 (18.4%) | 40 (81.6%) | 0.765 | 0.847 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Madhi, S.; Abu Hilal, M.; Acciuffi, S.; Rahimli, M.; Jeong, S.; Rawicz-Pruszyński, K.; Chouillard, M.-A.; Messaoudi, N.; Chouillard, E.; Dagher, I.; et al. Survival Study: International Multicentric Pancreatic Left Resections (SIMPLR-2): Does Surgical Approach Matter for Recurrence-Free Survival and Overall Survival? Cancers 2025, 17, 2659. https://doi.org/10.3390/cancers17162659
Al-Madhi S, Abu Hilal M, Acciuffi S, Rahimli M, Jeong S, Rawicz-Pruszyński K, Chouillard M-A, Messaoudi N, Chouillard E, Dagher I, et al. Survival Study: International Multicentric Pancreatic Left Resections (SIMPLR-2): Does Surgical Approach Matter for Recurrence-Free Survival and Overall Survival? Cancers. 2025; 17(16):2659. https://doi.org/10.3390/cancers17162659
Chicago/Turabian StyleAl-Madhi, Sara, Mohammad Abu Hilal, Sara Acciuffi, Mirhasan Rahimli, Seong Jeong, Karol Rawicz-Pruszyński, Marc-Anthony Chouillard, Nouredin Messaoudi, Elie Chouillard, Ibrahim Dagher, and et al. 2025. "Survival Study: International Multicentric Pancreatic Left Resections (SIMPLR-2): Does Surgical Approach Matter for Recurrence-Free Survival and Overall Survival?" Cancers 17, no. 16: 2659. https://doi.org/10.3390/cancers17162659
APA StyleAl-Madhi, S., Abu Hilal, M., Acciuffi, S., Rahimli, M., Jeong, S., Rawicz-Pruszyński, K., Chouillard, M.-A., Messaoudi, N., Chouillard, E., Dagher, I., Croner, R. S., & Gumbs, A. A. (2025). Survival Study: International Multicentric Pancreatic Left Resections (SIMPLR-2): Does Surgical Approach Matter for Recurrence-Free Survival and Overall Survival? Cancers, 17(16), 2659. https://doi.org/10.3390/cancers17162659









