Nanoparticle-Based Delivery Strategies for Combating Drug Resistance in Cancer Therapeutics
Simple Summary
Abstract
1. Introduction
2. Mechanisms of Drug Resistance in Cancer
2.1. ABC Transporters and Multidrug Resistance
2.1.1. ABCB1(P-Glycoprotein/P-gp)
2.1.2. ABCG2 (Breast Cancer Resistance Protein)
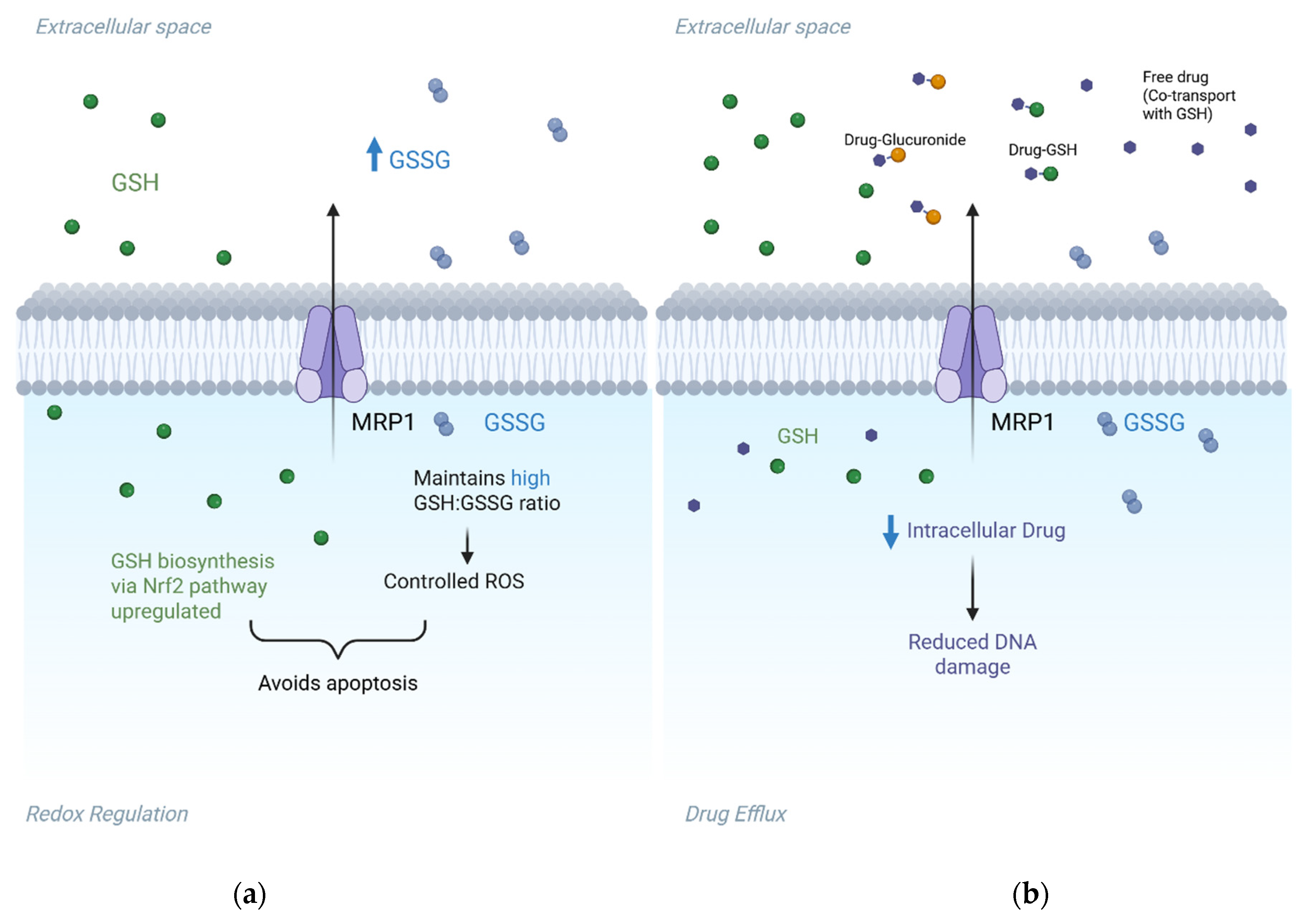
2.1.3. ABCC Family Members (Multidrug Resistance-Associated Proteins)
2.2. Beyond Drug Efflux: Mechanistic Roles of ABC Transporters in Cancer Resistance
2.3. Other Mechanisms
2.3.1. Alterations in Drug Targets
2.3.2. Enhanced DNA Repair Mechanisms
2.3.3. Evasion of Apoptosis
2.3.4. Tumour Microenvironment Barriers
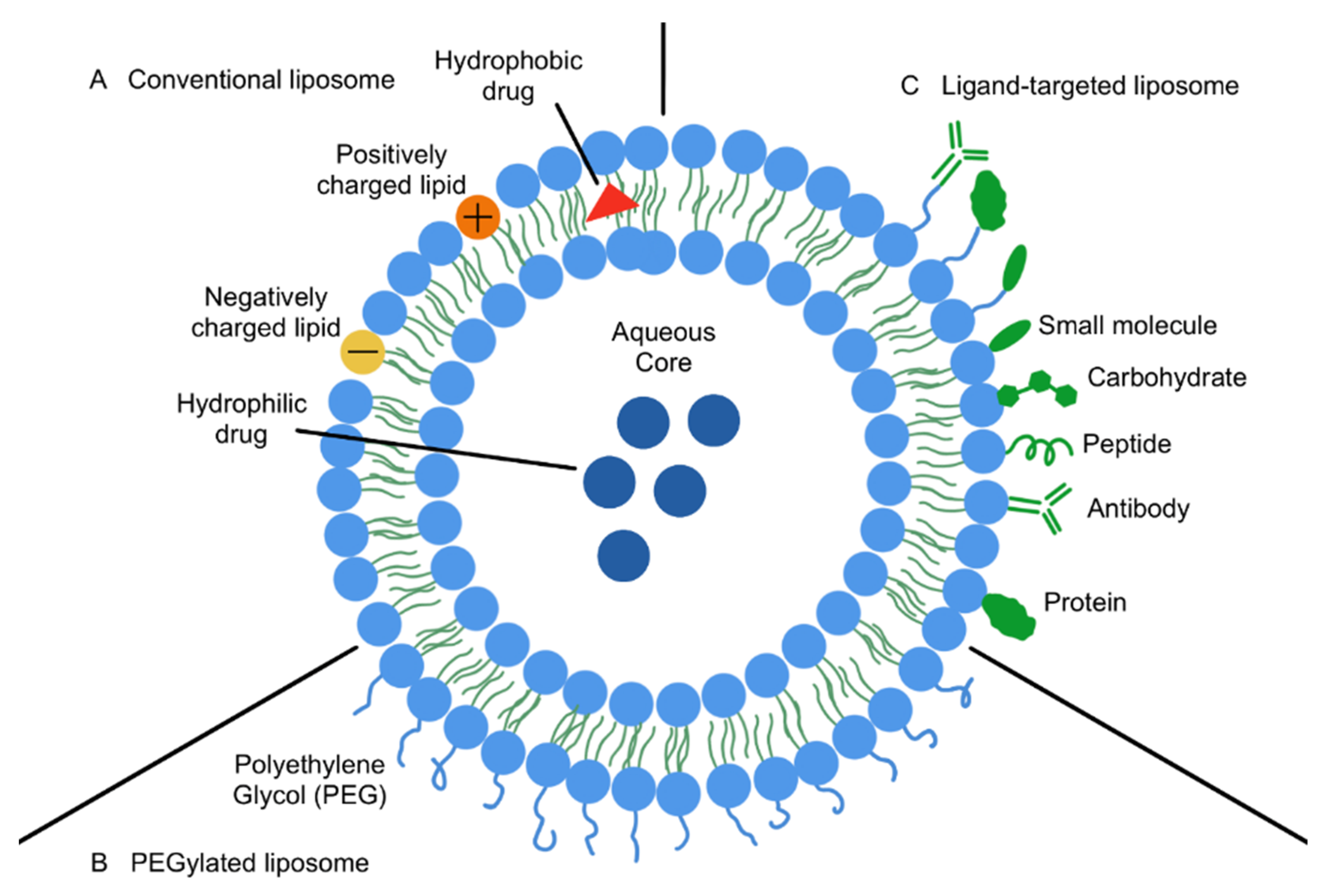
3. Nanoparticle-Based Drug Delivery Systems: An Overview
3.1. Nanoparticles in Cancer Therapy
3.2. Strategies to Minimise Immune Clearance
4. Nanoparticle Strategies to Overcome Multidrug Resistance
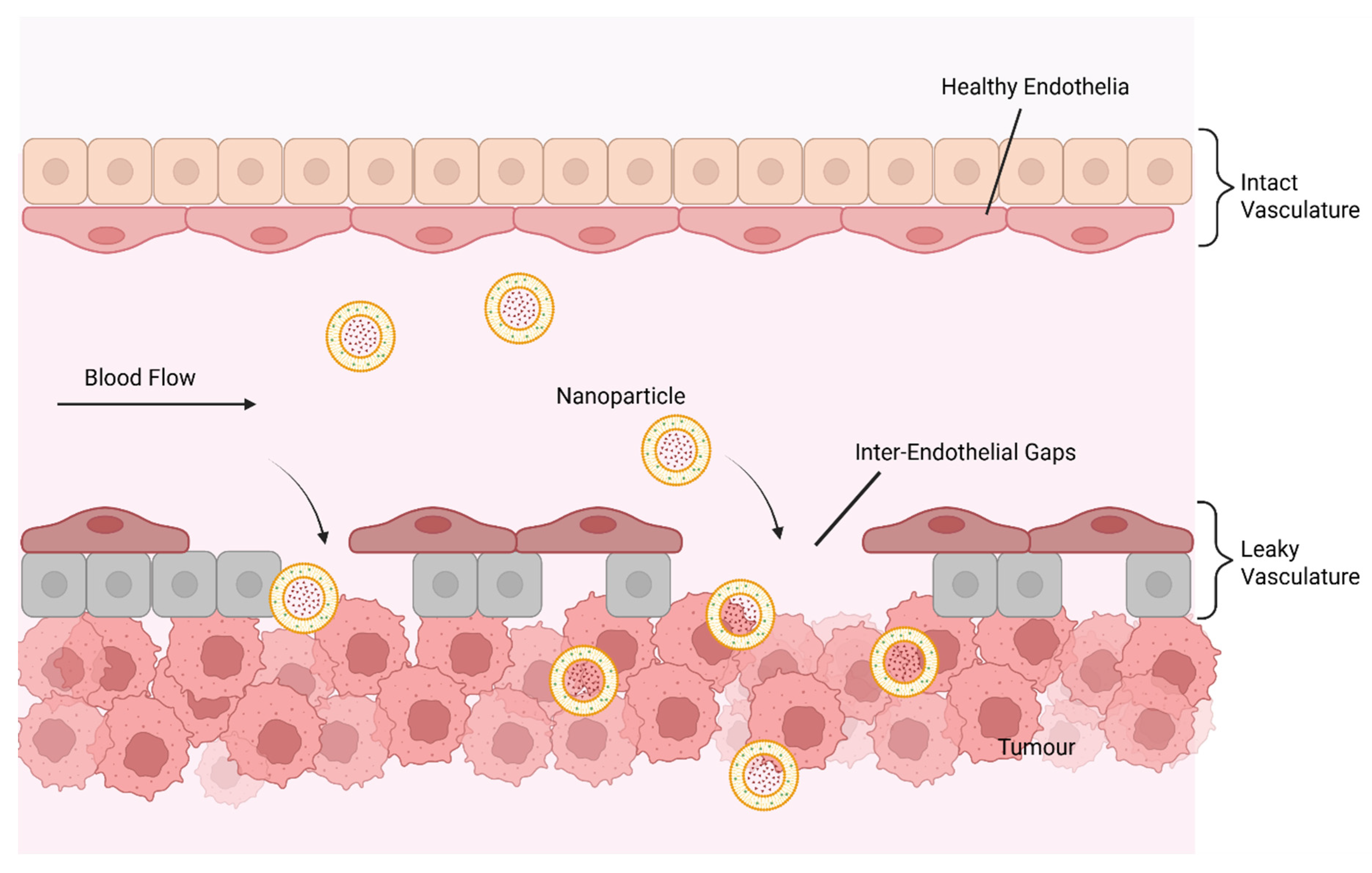
4.1. Enhanced Tumour Accumulation (The EPR Effect)
4.2. Active Targeting via Surface Conjugation
4.2.1. Folate Conjugation
4.2.2. RGD Peptide Conjugation
4.2.3. Aptamer-Functionalised NPs
4.2.4. Transferrin-Receptor-Targeted NPs
4.2.5. CD44-Targeted NPs
4.2.6. Monoclonal Antibody-Conjugated NPs
4.2.7. Dynamic Surface Engineering for Tumour Heterogeneity
4.3. Stimuli-Responsive Nanoparticles
4.3.1. Metabolic Targeting via Lactate-Responsive NPs for Tumour-Specific Drug Release
4.3.2. Tumour-Responsive Nanocarriers in Chemoimmunotherapy
4.3.3. pH Sensitivity
4.3.4. Enzyme Responsiveness
4.3.5. Redox Responsiveness
4.3.6. Hypoxia Responsiveness
4.4. Stability and Reproducibility Challenges
4.5. Combination and Co-Delivery Approaches
4.6. Cancer Types Showing Promise with NP-Based Approaches
5. Advanced Nanoparticle Strategies
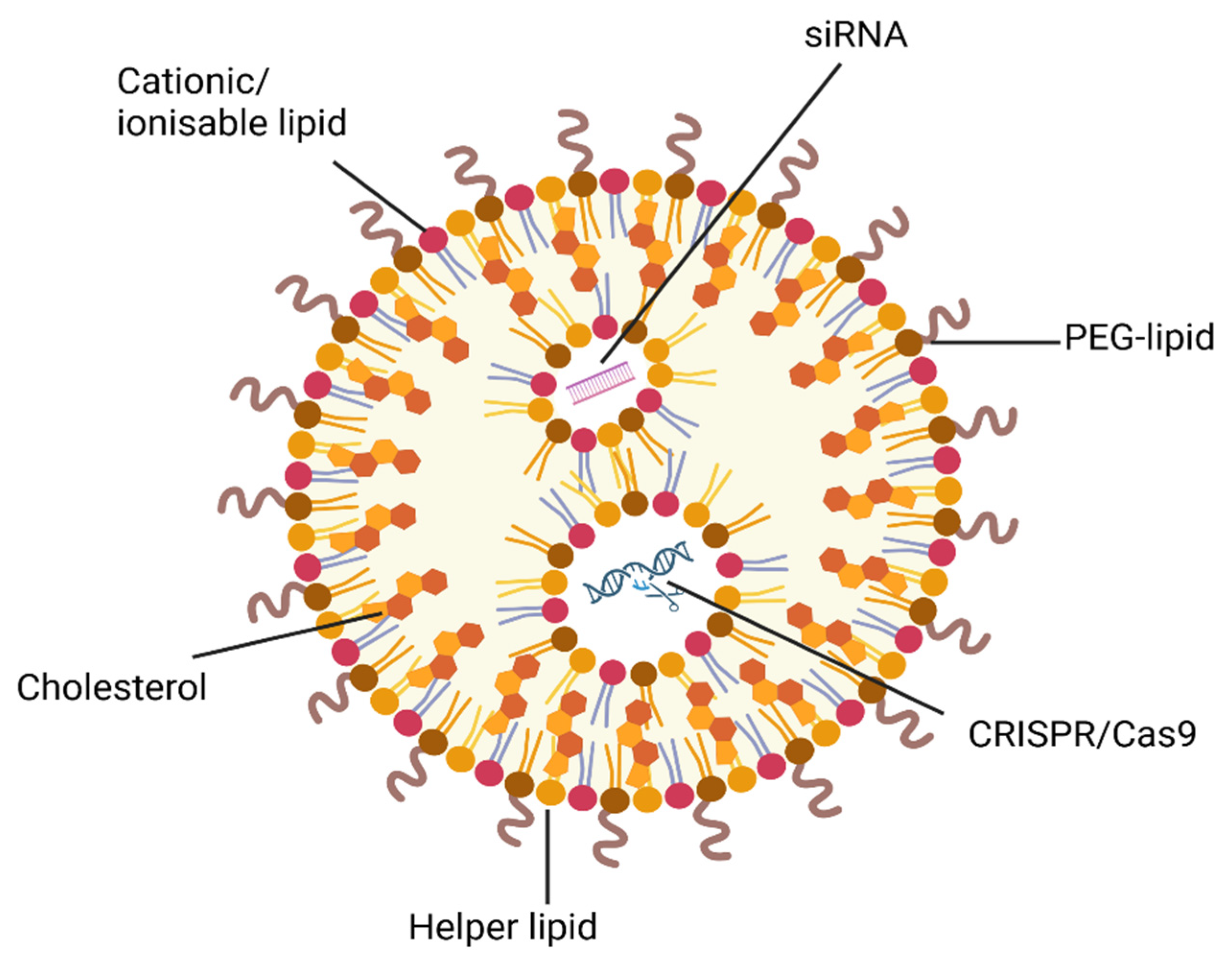
5.1. Gene-Directed NP Strategies to Overcome MDR
5.2. Safety and Ethical Considerations for Gene Editing Nanomedicines
5.2.1. Off-Target Gene Editing
5.2.2. Immunogenicity of CRISPR Components
5.2.3. Ethical and Societal Implications
5.3. Extracellular Vesicles: Opportunities and Limitations
6. Translational and Clinical Considerations
6.1. Manufacturing Scalability and Reproducibility
6.2. Regulatory Pathways and Quality by Design
6.3. Patient Heterogeneity and Companion Diagnostics
6.4. Clinical Evaluation and Key Translational Barriers
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MDR | multidrug resistance |
| NP | nanoparticle |
| siRNA | small interfering RNA |
| CRISPR | clustered regularly interspaced short palindromic repeat |
| TME | tumour microenvironment |
| ABC | ATP-binding cassette |
| P-gp | P-glycoprotein |
| MRP | multidrug resistance-associated protein |
| BCRP | breast cancer resistance protein |
| GI | gastrointestinal |
| CRC | colorectal cancer |
| GSH | glutathione |
| ROS | reactive oxygen species |
| S1P | sphingosine-1-phosphate |
| EGFR | epidermal growth factor receptor |
| HIF-1 | hypoxia-inducible factor-1 |
| EPR | enhance permeability and retention |
| SLN | solid lipid nanoparticles |
| LNP | lipid nanoparticles |
| PEG | polyethylene glycol |
| ATR | active transport and retention |
| ECM | extracellular matrix |
| RGD | arginine-glycine-aspartic |
| PSMA | prostate-specific membrane antigen |
| Tf | transferrin |
| MAbs | monoclonal antibodies |
| PTXNR-TTZ | trastuzumab-conjugated paclitaxel-loaded nanorods |
| PD-1 | programmed death-1 |
| ICD | immunogenic cell death |
| PSL | pH-sensitive liposome |
| MMP | matrix metalloproteinases |
| FSCNO | fucoidan−silica−carbon nano-onion NPs |
| PTX | unencapsulated paclitaxel |
| PTX-SL | paclitaxel liposome without trastuzumab |
| ssPalm | pH- and redox-responsive LNP |
| RNP | ribonucleoprotein |
| TKI | tyrosine kinase inhibitor |
| PDAC | pancreatic ductal adenocarcinoma |
References
- Siegel, R.L.; Kratzer, T.B.; Giaquinto, A.N.; Sung, H.; Jemal, A. Cancer statistics, 2025. CA A Cancer J. Clin. 2025, 75, 10–45. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 2 May 2025).
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Khatami, M. Analyses of repeated failures in cancer therapy for solid tumors: Poor tumor-selective drug delivery, low therapeutic efficacy and unsustainable costs. Clin. Transl. Med. 2018, 7, 11. [Google Scholar] [CrossRef]
- Davodabadi, F.; Sajjadi, S.F.; Sarhadi, M.; Mirghasemi, S.; Nadali Hezaveh, M.; Khosravi, S.; Kamali Andani, M.; Cordani, M.; Basiri, M.; Ghavami, S. Cancer chemotherapy resistance: Mechanisms and recent breakthrough in targeted drug delivery. Eur. J. Pharmacol. 2023, 958, 176013. [Google Scholar] [CrossRef]
- Emran, T.B.; Shahriar, A.; Mahmud, A.R.; Rahman, T.; Abir, M.H.; Siddiquee, M.F.-R.; Ahmed, H.; Rahman, N.; Nainu, F.; Wahyudin, E.; et al. Multidrug Resistance in Cancer: Understanding Molecular Mechanisms, Immunoprevention and Therapeutic Approaches. Front. Oncol. 2022, 12, 891652. [Google Scholar] [CrossRef]
- Manjón, A.G.; Manzo, S.G.; Prekovic, S.; Potgeter, L.; van Schaik, T.; Liu, N.Q.; Flach, K.; Peric-Hupkes, D.; Joosten, S.; Teunissen, H.; et al. Perturbations in 3D genome organization can promote acquired drug resistance. Cell Rep. 2023, 42, 113124. [Google Scholar] [CrossRef]
- Cao, J.Y.; Poddar, A.; Magtanong, L.; Lumb, J.H.; Mileur, T.R.; Reid, M.A.; Dovey, C.M.; Wang, J.; Locasale, J.W.; Stone, E.; et al. A Genome-wide Haploid Genetic Screen Identifies Regulators of Glutathione Abundance and Ferroptosis Sensitivity. Cell Rep. 2019, 26, 1544–1556.e1548. [Google Scholar] [CrossRef]
- Boehnke, N.; Straehla, J.P.; Safford, H.C.; Kocak, M.; Rees, M.G.; Ronan, M.; Rosenberg, D.; Adelmann, C.H.; Chivukula, R.R.; Nabar, N.; et al. Massively parallel pooled screening reveals genomic determinants of nanoparticle delivery. Science 2022, 377, eabm5551. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Peng, X.; Zoulikha, M.; Boafo, G.F.; Magar, K.T.; Ju, Y.; He, W. Multifunctional nanoparticle-mediated combining therapy for human diseases. Signal Transduct. Target. Ther. 2024, 9, 1. [Google Scholar] [CrossRef]
- Tran, S.; DeGiovanni, P.-J.; Piel, B.; Rai, P. Cancer nanomedicine: A review of recent success in drug delivery. Clin. Transl. Med. 2017, 6, e44. [Google Scholar] [CrossRef]
- Sun, X.; Zhao, P.; Lin, J.; Chen, K.; Shen, J. Recent advances in access to overcome cancer drug resistance by nanocarrier drug delivery system. Cancer Drug Resist. 2023, 6, 390–415. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, X.; Wei, Y.; Song, C.; Wang, Y. Recent advances in targeted gene silencing and cancer therapy by nanoparticle-based delivery systems. Biomed. Pharmacother. 2023, 157, 114065. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.; Kim, Y.; Kim, J.; Cho, H.; Kim, K. Overcoming Cancer Drug Resistance with Nanoparticle Strategies for Key Protein Inhibition. Molecules 2024, 29, 3994. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Agrawal, P.; Singh, S.K.; Chhonker, Y.S.; Sun, J.; Murry, D.J. Polymer-Based Drug Delivery Systems for Cancer Therapeutics. Polymers 2024, 16, 843. [Google Scholar] [CrossRef] [PubMed]
- Vaghari-Tabari, M.; Hassanpour, P.; Sadeghsoltani, F.; Malakoti, F.; Alemi, F.; Qujeq, D.; Asemi, Z.; Yousefi, B. CRISPR/Cas9 gene editing: A new approach for overcoming drug resistance in cancer. Cell. Mol. Biol. Lett. 2022, 27, 49. [Google Scholar] [CrossRef]
- Sun, L.; Liu, H.; Ye, Y.; Lei, Y.; Islam, R.; Tan, S.; Tong, R.; Miao, Y.-B.; Cai, L. Smart nanoparticles for cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 418. [Google Scholar] [CrossRef]
- Benderski, K.; Lammers, T.; Sofias, A.M. Analysis of multi-drug cancer nanomedicine. Nat. Nanotechnol. 2025; Online ahead of print. [Google Scholar] [CrossRef]
- Gottesman, M.M. Mechanisms of Cancer Drug Resistance. Annu. Rev. Med. 2002, 53, 615–627. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Wang, X.; Yao, P.; Dai, Q.; Qi, X.; Yang, M.; Zhang, X.; Huang, R.; Yang, J.; et al. Docetaxel-loaded pH/ROS dual-responsive nanoparticles with self-supplied ROS for inhibiting metastasis and enhancing immunotherapy of breast cancer. J. Nanobiotechnol. 2023, 21, 286. [Google Scholar] [CrossRef]
- Zhang, R.; Jagessar, K.L.; Brownd, M.; Polasa, A.; Stein, R.A.; Moradi, M.; Karakas, E.; McHaourab, H.S. Conformational cycle of a protease-containing ABC transporter in lipid nanodiscs reveals the mechanism of cargo-protein coupling. Nat. Commun. 2024, 15, 9055. [Google Scholar] [CrossRef]
- Holohan, C.; Van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer drug resistance: An evolving paradigm. Nat. Rev. Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Longley, D.; Johnston, P. Molecular mechanisms of drug resistance. J. Pathol. 2005, 205, 275–292. [Google Scholar] [CrossRef]
- Wang, Q.; Atluri, K.; Tiwari, A.K.; Babu, R.J. Exploring the Application of Micellar Drug Delivery Systems in Cancer Nanomedicine. Pharmaceuticals 2023, 16, 433. [Google Scholar] [CrossRef]
- Randic, T.; Magni, S.; Philippidou, D.; Margue, C.; Grzyb, K.; Preis, J.R.; Wroblewska, J.P.; Nazarov, P.V.; Mittelbronn, M.; Frauenknecht, K.B.M.; et al. Single-cell transcriptomics of NRAS-mutated melanoma transitioning to drug resistance reveals P2RX7 as an indicator of early drug response. Cell Rep. 2023, 42, 112696. [Google Scholar] [CrossRef]
- Sajid, A.; Rahman, H.; Ambudkar, S.V. Advances in the structure, mechanism and targeting of chemoresistance-linked ABC transporters. Nat. Rev. Cancer 2023, 23, 762–779. [Google Scholar] [CrossRef]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef]
- Balasubramanian, A.; Sundrud, M.S. ATP-dependent transporters: Emerging players at the crossroads of immunity and metabolism. Front. Immunol. 2023, 14, 1286696. [Google Scholar] [CrossRef]
- Fletcher, J.I.; Haber, M.; Henderson, M.J.; Norris, M.D. ABC transporters in cancer: More than just drug efflux pumps. Nat. Rev. Cancer 2010, 10, 147–156. [Google Scholar] [CrossRef]
- Pote, M.S.; Gacche, R.N. ATP-binding cassette efflux transporters and MDR in cancer. Drug Discov. Today 2023, 28, 103537. [Google Scholar] [CrossRef]
- Saxena, M.; Stephens, M.A.; Pathak, H.; Rangarajan, A. Transcription factors that mediate epithelial–mesenchymal transition lead to multidrug resistance by upregulating ABC transporters. Cell Death Dis. 2011, 2, e179. [Google Scholar] [CrossRef]
- Galetin, A.; Brouwer, K.L.R.; Tweedie, D.; Yoshida, K.; Sjöstedt, N.; Aleksunes, L.; Chu, X.; Evers, R.; Hafey, M.J.; Lai, Y.; et al. Membrane transporters in drug development and as determinants of precision medicine. Nat. Rev. Drug Discov. 2024, 23, 255–280. [Google Scholar] [CrossRef]
- Seelig, A. P-Glycoprotein: One Mechanism, Many Tasks and the Consequences for Pharmacotherapy of Cancers. Front. Oncol. 2020, 10, 576559. [Google Scholar] [CrossRef]
- Bugde, P.; Biswas, R.; Merien, F.; Lu, J.; Liu, D.X.; Chen, M.; Zhou, S.; Li, Y. The therapeutic potential of targeting ABC transporters to combat multi-drug resistance. Expert Opin. Ther. Targets 2017, 21, 511–530. [Google Scholar] [CrossRef] [PubMed]
- Mello, F.V.C.; de Moraes, G.N.; Maia, R.C.; Kyeremateng, J.; Iram, S.H.; Santos-Oliveira, R. The Effect of Nanosystems on ATP-Binding Cassette Transporters: Understanding the Influence of Nanosystems on Multidrug Resistance Protein-1 and P-glycoprotein. Int. J. Mol. Sci. 2020, 21, 2630. [Google Scholar] [CrossRef] [PubMed]
- Nanayakkara, A.K.; Follit, C.A.; Chen, G.; Williams, N.S.; Vogel, P.D.; Wise, J.G. Targeted inhibitors of P-glycoprotein increase chemotherapeutic-induced mortality of multidrug resistant tumor cells. Sci. Rep. 2018, 8, 967. [Google Scholar] [CrossRef]
- Chen, X.-Y.; Wu, Z.-X.; Wang, J.-Q.; Teng, Q.-X.; Tang, H.; Liu, Q.; Chen, Z.-S.; Chen, W. Multidrug resistance transporters P-gp and BCRP limit the efficacy of ATR inhibitor ceralasertib in cancer cells. Front. Pharmacol. 2024, 15, 1400699. [Google Scholar] [CrossRef] [PubMed]
- Ambjørner, S.E.B.; Wiese, M.; Köhler, S.C.; Svindt, J.; Lund, X.L.; Gajhede, M.; Saaby, L.; Brodin, B.; Rump, S.; Weigt, H.; et al. The Pyrazolo[3,4-d]pyrimidine Derivative, SCO-201, Reverses Multidrug Resistance Mediated by ABCG2/BCRP. Cells 2020, 9, 613. [Google Scholar] [CrossRef]
- Anish Ruban, S.; Raj, F.J.; Thangaraj, P. Phytochemical intervention in BCRP-driven cancer drug resistance: A comprehensive review. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2025, 1880, 189349. [Google Scholar] [CrossRef]
- Gambacorta, N.; Gasperi, V.; Guzzo, T.; Di Leva, F.S.; Ciriaco, F.; Sánchez, C.; Tullio, V.; Rozzi, D.; Marinelli, L.; Topai, A.; et al. Exploring the 1,3-benzoxazine chemotype for cannabinoid receptor 2 as a promising anti-cancer therapeutic. Eur. J. Med. Chem. 2023, 259, 115647. [Google Scholar] [CrossRef]
- Myint, K.; Biswas, R.; Li, Y.; Jong, N.; Jamieson, S.; Liu, J.; Han, C.; Squire, C.; Merien, F.; Lu, J.; et al. Identification of MRP2 as a targetable factor limiting oxaliplatin accumulation and response in gastrointestinal cancer. Sci. Rep. 2019, 9, 2245. [Google Scholar] [CrossRef]
- Rottenberg, S.; Disler, C.; Perego, P. The rediscovery of platinum-based cancer therapy. Nat. Rev. Cancer 2021, 21, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Myint, K.; Li, Y.; Paxton, J.; McKeage, M. Multidrug Resistance-Associated Protein 2 (MRP2) Mediated Transport of Oxaliplatin-Derived Platinum in Membrane Vesicles. PLoS ONE 2015, 10, e0130727. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.; Cui, D.; Sun, L.; Lu, Y.; Han, M.; Liu, J. Inhibition of IGF-IR increases chemosensitivity in human colorectal cancer cells through MRP-2 promoter suppression. J. Cell. Biochem. 2012, 113, 2086–2097. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, X.; Feng, Y.; Liu, X.; Zhou, L.; Sui, H.; Ji, Q.; E, Q.; Chen, J.; Wu, L.; et al. Dihydromyricetin reverses MRP2-mediated MDR and enhances anticancer activity induced by oxaliplatin in colorectal cancer cells. Anticancer Drugs 2017, 28, 281–288. [Google Scholar] [CrossRef]
- Cecchin, E.; D’Andrea, M.; Lonardi, S.; Zanusso, C.; Pella, N.; Errante, D.; De Mattia, E.; Polesel, J.; Innocenti, F.; Toffoli, G. A prospective validation pharmacogenomic study in the adjuvant setting of colorectal cancer patients treated with the 5-fluorouracil/leucovorin/oxaliplatin (FOLFOX4) regimen. Pharmacogenom. J. 2013, 13, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Custodio, A.; Moreno-Rubio, J.; Aparicio, J.; Gallego-Plazas, J.; Yaya, R.; Maurel, J.; Higuera, O.; Burgos, E.; Ramos, D.; Calatrava, A.; et al. Pharmacogenetic predictors of severe peripheral neuropathy in colon cancer patients treated with oxaliplatin-based adjuvant chemotherapy: A GEMCAD group study. Ann. Oncol. 2014, 25, 398–403. [Google Scholar] [CrossRef]
- Mirakhorli, M.; Rahman, S.A.; Abdullah, S.; Vakili, M.; Rozafzon, R.; Khoshzaban, A. Multidrug resistance protein 2 genetic polymorphism and colorectal cancer recurrence in patients receiving adjuvant FOLFOX-4 chemotherapy. Mol. Med. Rep. 2013, 7, 613–617. [Google Scholar] [CrossRef]
- He, J.; Bugde, P.; Li, J.; Biswas, R.; Li, S.; Yang, X.; Tian, F.; Wu, Z.; Li, Y. Multidrug resistance protein 5 affects cell proliferation, migration and gemcitabine sensitivity in pancreatic cancer MIA Paca-2 and PANC-1 cells. Oncol. Rep. 2024, 51, 7. [Google Scholar] [CrossRef]
- Huang, Y.; Xue, C.; Bu, R.; Wu, C.; Li, J.; Zhang, J.; Chen, J.; Shi, Z.; Chen, Y.; Wang, Y.; et al. Inhibition and transport mechanisms of the ABC transporter hMRP5. Nat. Commun. 2024, 15, 4811. [Google Scholar] [CrossRef]
- Duvivier, L.; Gerard, L.; Diaz, A.; Gillet, J.-P. Linking ABC transporters to the hallmarks of cancer. Trends Cancer 2024, 10, 124–134. [Google Scholar] [CrossRef]
- Ichihara, G.; Katsumata, Y.; Sugiura, Y.; Matsuoka, Y.; Maeda, R.; Endo, J.; Anzai, A.; Shirakawa, K.; Moriyama, H.; Kitakata, H.; et al. MRP1-Dependent Extracellular Release of Glutathione Induces Cardiomyocyte Ferroptosis After Ischemia-Reperfusion. Circ. Res. 2023, 133, 861–876. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.P. Multidrug resistance protein 1 (MRP1, ABCC1), a “multitasking” ATP-binding cassette (ABC) transporter. J. Biol. Chem. 2014, 289, 30880–30888. [Google Scholar] [CrossRef]
- Lv, H.; Zhen, C.; Liu, J.; Yang, P.; Hu, L.; Shang, P. Unraveling the Potential Role of Glutathione in Multiple Forms of Cell Death in Cancer Therapy. Oxidative Med. Cell. Longev. 2019, 2019, 3150145. [Google Scholar] [CrossRef]
- Nasr, R.; Lorendeau, D.; Khonkarn, R.; Dury, L.; Pérès, B.; Boumendjel, A.; Cortay, J.-C.; Falson, P.; Chaptal, V.; Baubichon-Cortay, H. Molecular analysis of the massive GSH transport mechanism mediated by the human Multidrug Resistant Protein 1/ABCC1. Sci. Rep. 2020, 10, 7616. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, J.-K.; Kim, H. ABCB7 simultaneously regulates apoptotic and non-apoptotic cell death by modulating mitochondrial ROS and HIF1α-driven NFκB signaling. Oncogene 2020, 39, 1969–1982. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, C.; Ozvegy-Laczka, C.; Szakacs, G.; Sarkadi, B.; Di Pietro, A. ABCG2 is not able to catalyze glutathione efflux and does not contribute to GSH-dependent collateral sensitivity. Front. Pharmacol. 2013, 4, 138. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, X.; Xu, Y.; La, X.; Tian, J.; Li, A.; Li, H.; Wu, C.; Xi, Y.; Song, G.; et al. Tumor-associated macrophages confer colorectal cancer 5-fluorouracil resistance by promoting MRP1 membrane translocation via an intercellular CXCL17/CXCL22–CCR4–ATF6–GRP78 axis. Cell Death Dis. 2023, 14, 582. [Google Scholar] [CrossRef]
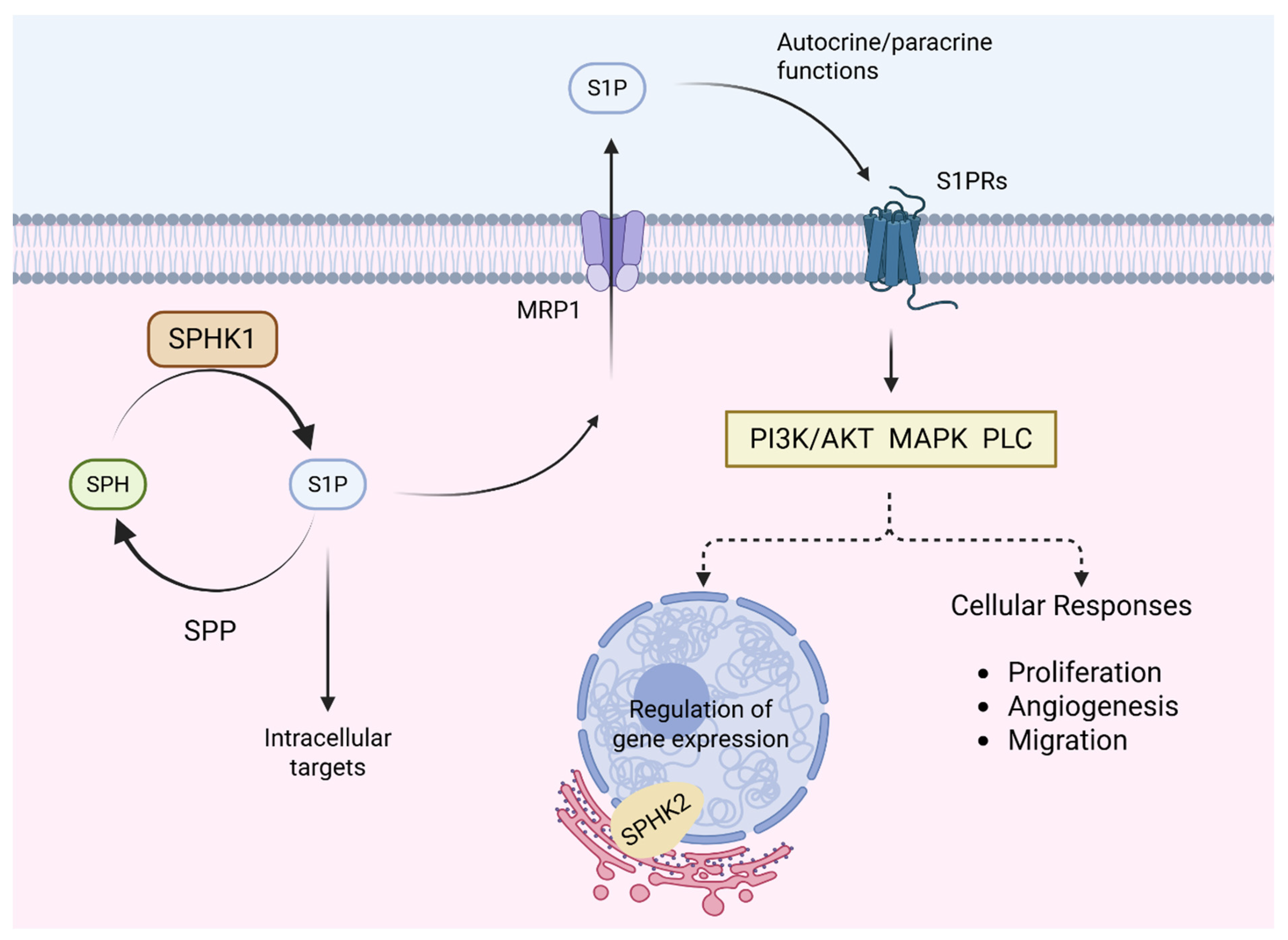
- Yamada, A.; Nagahashi, M.; Aoyagi, T.; Huang, W.C.; Lima, S.; Hait, N.C.; Maiti, A.; Kida, K.; Terracina, K.P.; Miyazaki, H.; et al. ABCC1-Exported Sphingosine-1-phosphate, Produced by Sphingosine Kinase 1, Shortens Survival of Mice and Patients with Breast Cancer. Mol. Cancer Res. 2018, 16, 1059–1070. [Google Scholar] [CrossRef]
- Alkafaas, S.S.; Elsalahaty, M.I.; Ismail, D.F.; Radwan, M.A.; Elkafas, S.S.; Loutfy, S.A.; Elshazli, R.M.; Baazaoui, N.; Ahmed, A.E.; Hafez, W.; et al. The emerging roles of sphingosine 1-phosphate and SphK1 in cancer resistance: A promising therapeutic target. Cancer Cell Int. 2024, 24, 89. [Google Scholar] [CrossRef]
- Hanouna, G.; Tang, E.; Perez, J.; Vandermeersch, S.; Haymann, J.-P.; Baud, L.; Letavernier, E. Preventing Calpain Externalization by Reducing ABCA1 Activity with Probenecid Limits Melanoma Angiogenesis and Development. J. Investig. Dermatol. 2020, 140, 445–454. [Google Scholar] [CrossRef]
- Liu, X.; Yao, D.; Liu, C.; Cao, Y.; Yang, Q.; Sun, Z.; Liu, D. Overexpression of ABCC3 promotes cell proliferation, drug resistance, and aerobic glycolysis and is associated with poor prognosis in urinary bladder cancer patients. Tumor Biol. 2016, 37, 8367–8374. [Google Scholar] [CrossRef] [PubMed]
- Dhatchinamoorthy, K.; Colbert, J.D.; Rock, K.L. Cancer Immune Evasion Through Loss of MHC Class I Antigen Presentation. Front. Immunol. 2021, 12, 636568. [Google Scholar] [CrossRef]
- Mravec, B. Nonmutational Epigenetic Reprogramming. In Neurobiology of Cancer: Role of the Nervous System in Cancer Etiopathogenesis, Treatment, and Prevention; Mravec, B., Ed.; Springer Nature: Cham, Switzerland, 2024; pp. 317–319. [Google Scholar]
- Xie, C.; Jiang, X.H.; Zhang, J.T.; Sun, T.T.; Dong, J.D.; Sanders, A.J.; Diao, R.Y.; Wang, Y.; Fok, K.L.; Tsang, L.L.; et al. CFTR suppresses tumor progression through miR-193b targeting urokinase plasminogen activator (uPA) in prostate cancer. Oncogene 2013, 32, 2282–2291. [Google Scholar] [CrossRef]
- El-Ashmawy, N.E.; Al-Ashmawy, G.M.; Hamada, O.B.; Khedr, N.F. The role of ABCG2 in health and disease: Linking cancer therapy resistance and other disorders. Life Sci. 2025, 360, 123245. [Google Scholar] [CrossRef]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug Resistance in Cancer: An Overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef] [PubMed]
- Gibson, E.G.; Deweese, J.E. Structural and Biochemical Basis of Etoposide-Resistant Mutations in Topoisomerase IIα. Symmetry 2022, 14, 1309. [Google Scholar] [CrossRef]
- Kara, A.; Özgür, A.; Nalbantoğlu, S.; Karadağ, A. DNA repair pathways and their roles in drug resistance for lung adenocarcinoma. Mol. Biol. Rep. 2021, 48, 3813–3825. [Google Scholar] [CrossRef]
- O’Leary, B.; Skinner, H.; Schoenfeld, J.D.; Licitra, L.; Le Tourneau, C.; Esdar, C.; Schroeder, A.; Salmio, S.; Psyrri, A. Evasion of apoptosis and treatment resistance in squamous cell carcinoma of the head and neck. Cancer Treat. Rev. 2024, 129, 102773. [Google Scholar] [CrossRef]
- Garcia, G.G.; Schmidt, C.J.; Hajal, C. The tumor microenvironment in therapy resistance. Front. Lab. Chip Technol. 2024, 3, 1420233. [Google Scholar] [CrossRef]
- Tiwari, A.; Trivedi, R.; Lin, S.Y. Tumor microenvironment: Barrier or opportunity towards effective cancer therapy. J. Biomed. Sci. 2022, 29, 83. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Jain, R.K.; Langer, R. Engineering and physical sciences in oncology: Challenges and opportunities. Nat. Rev. Cancer 2017, 17, 659–675. [Google Scholar] [CrossRef] [PubMed]
- Karimi, S.; Bakhshali, R.; Bolandi, S.; Zahed, Z.; Mojtaba Zadeh, S.S.; Kaveh Zenjanab, M.; Jahanban Esfahlan, R. For and against tumor microenvironment: Nanoparticle-based strategies for active cancer therapy. Mater. Today Bio 2025, 31, 101626. [Google Scholar] [CrossRef]
- Wang, S.; Hou, Y. New Types of Magnetic Nanoparticles for Stimuli-Responsive Theranostic Nanoplatforms. Adv. Sci. 2024, 11, 2305459. [Google Scholar] [CrossRef]
- Maimaitijiang, A.; He, D.; Li, D.; Li, W.; Su, Z.; Fan, Z.; Li, J. Progress in Research of Nanotherapeutics for Overcoming Multidrug Resistance in Cancer. Int. J. Mol. Sci. 2024, 25, 9973. [Google Scholar] [CrossRef]
- Lammers, T. Nanomedicine Tumor Targeting. Adv. Mater. 2024, 36, 2312169. [Google Scholar] [CrossRef]
- Nirmala, M.J.; Kizhuveetil, U.; Johnson, A.; G, B.; Nagarajan, R.; Muthuvijayan, V. Cancer nanomedicine: A review of nano-therapeutics and challenges ahead. RSC Adv. 2023, 13, 8606–8629. [Google Scholar] [CrossRef]
- Beltrán-Gracia, E.; López-Camacho, A.; Higuera-Ciapara, I.; Velázquez-Fernández, J.B.; Vallejo-Cardona, A.A. Nanomedicine review: Clinical developments in liposomal applications. Cancer Nanotechnol. 2019, 10, 11. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, W.; Wang, Z.; Lu, J. Lipid-Based Nanotechnology: Liposome. Pharmaceutics 2024, 16, 34. [Google Scholar] [CrossRef] [PubMed]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles─From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef]
- Bhagchandani, S.; Johnson, J.A.; Irvine, D.J. Evolution of Toll-like receptor 7/8 agonist therapeutics and their delivery approaches: From antiviral formulations to vaccine adjuvants. Adv. Drug Deliv. Rev. 2021, 175, 113803. [Google Scholar] [CrossRef]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Gatto, M.S.; Johnson, M.P.; Najahi-Missaoui, W. Targeted Liposomal Drug Delivery: Overview of the Current Applications and Challenges. Life 2024, 14, 672. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, A.; Alhakamy, N.A.; Md, S.; Kesharwani, P. Recent Progress of RGD Modified Liposomes as Multistage Rocket Against Cancer. Front. Pharmacol. 2022, 12, 803304. [Google Scholar] [CrossRef]
- Barenholz, Y. Doxil®--the first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Batist, G.; Barton, J.; Chaikin, P.; Swenson, C.; Welles, L. Myocet (liposome-encapsulated doxorubicin citrate): A new approach in breast cancer therapy. Expert Opin. Pharmacother. 2002, 3, 1739–1751. [Google Scholar] [CrossRef]
- Glantz, M.J.; Jaeckle, K.A.; Chamberlain, M.C.; Phuphanich, S.; Recht, L.; Swinnen, L.J.; Maria, B.; LaFollette, S.; Schumann, G.B.; Cole, B.F.; et al. A randomized controlled trial comparing intrathecal sustained-release cytarabine (DepoCyt) to intrathecal methotrexate in patients with neoplastic meningitis from solid tumors. Clin. Cancer Res. 1999, 5, 3394–3402. [Google Scholar]
- Lancet, J.E.; Uy, G.L.; Cortes, J.E.; Newell, L.F.; Lin, T.L.; Ritchie, E.K.; Stuart, R.K.; Strickland, S.A.; Hogge, D.; Solomon, S.R.; et al. CPX-351 (cytarabine and daunorubicin) Liposome for Injection Versus Conventional Cytarabine Plus Daunorubicin in Older Patients With Newly Diagnosed Secondary Acute Myeloid Leukemia. J. Clin. Oncol. 2018, 36, 2684–2692. [Google Scholar] [CrossRef]
- Alphandéry, E.; Pierre, G.-D.; Raphael, L.; Chalani, M.; Durand-Dubief, M. Cancer therapy using nanoformulated substances: Scientific, regulatory and financial aspects. Expert Rev. Anticancer Ther. 2015, 15, 1233–1255. [Google Scholar] [CrossRef]
- Drummond, D.C.; Noble, C.O.; Guo, Z.; Hong, K.; Park, J.W.; Kirpotin, D.B. Development of a highly active nanoliposomal irinotecan using a novel intraliposomal stabilization strategy. Cancer Res. 2006, 66, 3271–3277. [Google Scholar] [CrossRef]
- Forssen, E.A. The design and development of DaunoXome® for solid tumor targeting in vivo. Adv. Drug Deliv. Rev. 1997, 24, 133–150. [Google Scholar] [CrossRef]
- Webb, M.S.; Harasym, T.O.; Masin, D.; Bally, M.B.; Mayer, L.D. Sphingomyelin-cholesterol liposomes significantly enhance the pharmacokinetic and therapeutic properties of vincristine in murine and human tumour models. Br. J. Cancer 1995, 72, 896–904. [Google Scholar] [CrossRef]
- Jassim, Z.; Ayash, N. An Insight into Polymeric Micelles Preparation Methods and Applications as Drug Delivery Approach: A Review. Int. J. Pharm. Investig. 2025, 15, 849–857. [Google Scholar] [CrossRef]
- Zenze, M.; Daniels, A.; Singh, M. Dendrimers as Modifiers of Inorganic Nanoparticles for Therapeutic Delivery in Cancer. Pharmaceutics 2023, 15, 398. [Google Scholar] [CrossRef] [PubMed]
- Rastinehad, A.R.; Anastos, H.; Wajswol, E.; Winoker, J.S.; Sfakianos, J.P.; Doppalapudi, S.K.; Carrick, M.R.; Knauer, C.J.; Taouli, B.; Lewis, S.C.; et al. Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. Proc. Natl. Acad. Sci. USA 2019, 116, 18590–18596. [Google Scholar] [CrossRef]
- Thomas, R.; Park, I.-K.; Jeong, Y.Y. Magnetic Iron Oxide Nanoparticles for Multimodal Imaging and Therapy of Cancer. Int. J. Mol. Sci. 2013, 14, 15910–15930. [Google Scholar] [CrossRef]
- Kesharwani, P.; Ma, R.; Sang, L.; Fatima, M.; Sheikh, A.; Abourehab, M.A.S.; Gupta, N.; Chen, Z.-S.; Zhou, Y. Gold nanoparticles and gold nanorods in the landscape of cancer therapy. Mol. Cancer 2023, 22, 98. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Li, M.; Jia, L.; Xie, Y.; Ma, W.; Yan, Z.; Liu, F.; Deng, J.; Zhu, A.; Siwei, X.; Su, W.; et al. Lyophilization process optimization and molecular dynamics simulation of mRNA-LNPs for SARS-CoV-2 vaccine. NPJ Vaccines 2023, 8, 153. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, M.M.; Pandey, N.; Winkles, J.A.; Woodworth, G.F.; Kim, A.J. Toward the scale-up production of polymeric nanotherapeutics for cancer clinical trials. Nano Today 2024, 56, 102314. [Google Scholar] [CrossRef]
- Eltaib, L. Polymeric Nanoparticles in Targeted Drug Delivery: Unveiling the Impact of Polymer Characterization and Fabrication. Polymers 2025, 17, 833. [Google Scholar] [CrossRef]
- Gajbhiye, K.R.; Salve, R.; Narwade, M.; Sheikh, A.; Kesharwani, P.; Gajbhiye, V. Lipid polymer hybrid nanoparticles: A custom-tailored next-generation approach for cancer therapeutics. Mol. Cancer 2023, 22, 160. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Jalouli, M.; Bhajan, S.K.; Al-Zharani, M.; Harrath, A.H. A Comprehensive Review of Nanoparticle-Based Drug Delivery for Modulating PI3K/AKT/mTOR-Mediated Autophagy in Cancer. Int. J. Mol. Sci. 2025, 26, 1868. [Google Scholar] [CrossRef]
- Fatima, R.; Katiyar, P.; Kushwaha, K. Recent advances in mesoporous silica nanoparticle: Synthesis, drug loading, release mechanisms, and diverse applications. Front. Nanotechnol. 2025, 7, 1564188. [Google Scholar] [CrossRef]
- Janaszewska, A.; Lazniewska, J.; Trzepiński, P.; Marcinkowska, M.; Klajnert-Maculewicz, B. Cytotoxicity of Dendrimers. Biomolecules 2019, 9, 330. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Qiu, L.; Qiao, X.; Yang, H. Dendrimer-based drug delivery systems: History, challenges, and latest developments. J. Biol. Eng. 2022, 16, 18. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hou, S. Recent progress in the effect of magnetic iron oxide nanoparticles on cells and extracellular vesicles. Cell Death Discov. 2023, 9, 195. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, H.; Zhu, X.; Liu, C.; Wu, M.; Li, C.; Li, X.; Gao, L.; Ding, Y. Tolerance, Variability and Pharmacokinetics of Albumin-Bound Paclitaxel in Chinese Breast Cancer Patients. Front. Pharmacol. 2018, 9, 1372. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, Y.; Lu, S.; Zeng, M.; Liu, L.; Dai, Q.; Yin, R. Adverse event profile of albumin-bound paclitaxel: A real-world pharmacovigilance analysis. Front. Pharmacol. 2024, 15, 1448144. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, X.; Li, J.; Nie, Y.; Liao, G.; Yu, Y.; Li, C. Overcoming the reticuloendothelial system barrier to drug delivery with a “Don’t-Eat-Us” strategy. ACS Nano 2019, 13, 13015–13026. [Google Scholar] [CrossRef]
- Luozhong, S.; Liu, P.; Li, R.; Yuan, Z.; Debley, E.; Chen, Y.; Hu, Y.; Cao, Z.; Cui, M.; McIlhenny, K.; et al. Poly(carboxybetaine) lipids enhance mRNA therapeutics efficacy and reduce their immunogenicity. Nat. Mater. 2025; Online ahead of print. [Google Scholar] [CrossRef]
- Tilden, S.G.; Ricco, M.H.; Hemann, E.A.; Anchordoquy, T.J. Reducing off-target drug accumulation by exploiting a type-III interferon response. J. Control. Release 2023, 358, 729–738. [Google Scholar] [CrossRef] [PubMed]
- La-Beck, N.M.; Gabizon, A.A. Nanoparticle Interactions with the Immune System: Clinical Implications for Liposome-Based Cancer Chemotherapy. Front. Immunol. 2017, 8, 416. [Google Scholar] [CrossRef]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef] [PubMed]
- Rajan, R.; Sabnani, M.K.; Mavinkurve, V.; Shmeeda, H.; Mansouri, H.; Bonkoungou, S.; Le, A.D.; Wood, L.M.; Gabizon, A.A.; La-Beck, N.M. Liposome-induced immunosuppression and tumor growth is mediated by macrophages and mitigated by liposome-encapsulated alendronate. J. Control. Release 2018, 271, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Patel, J.; Back, P.I.; Shmeeda, H.; Adamsky, K.; Yang, H.; Alvarez, C.; Gabizon, A.A.; La-Beck, N.M. Comparative effects of free doxorubicin, liposome encapsulated doxorubicin and liposome co-encapsulated alendronate and doxorubicin (PLAD) on the tumor immunologic milieu in a mouse fibrosarcoma model. Nanotheranostics 2022, 6, 451–464. [Google Scholar] [CrossRef]
- Betker, J.L.; Anchordoquy, T.J. The Effect of Repeat Administration of Lipoplexes on Gene Delivery, Biodistribution, and Cytokine Response in Immunocompetent Tumor-Bearing Mice. J. Pharm. Sci. 2022, 111, 1926–1936. [Google Scholar] [CrossRef]
- Nakamura, T.; Sato, T.; Endo, R.; Sasaki, S.; Takahashi, N.; Sato, Y.; Hyodo, M.; Hayakawa, Y.; Harashima, H. STING agonist loaded lipid nanoparticles overcome anti-PD-1 resistance in melanoma lung metastasis via NK cell activation. J. Immunother. Cancer 2021, 9, e002852. [Google Scholar] [CrossRef]
- Morton, J.J.; Bird, G.; Refaeli, Y.; Jimeno, A. Humanized Mouse Xenograft Models: Narrowing the Tumor–Microenvironment Gap. Cancer Res. 2016, 76, 6153–6158. [Google Scholar] [CrossRef]
- Dasgupta, A.; Sofias, A.M.; Kiessling, F.; Lammers, T. Nanoparticle delivery to tumours: From EPR and ATR mechanisms to clinical impact. Nat. Rev. Bioeng. 2024, 2, 714–716. [Google Scholar] [CrossRef]
- Linderman, S.W.; DeRidder, L.; Sanjurjo, L.; Foote, M.B.; Alonso, M.J.; Kirtane, A.R.; Langer, R.; Traverso, G. Enhancing immunotherapy with tumour-responsive nanomaterials. Nat. Rev. Clin. Oncol. 2025, 22, 262–282. [Google Scholar] [CrossRef]
- Deng, M.; Rao, J.-D.; Guo, R.; Li, M.; He, Q. Size-Adjustable Nano-Drug Delivery Systems for Enhanced Tumor Retention and Penetration. Pharm. Front. 2021, 3, e98–e112. [Google Scholar] [CrossRef]
- Subhan, M.A.; Yalamarty, S.S.K.; Filipczak, N.; Parveen, F.; Torchilin, V.P. Recent Advances in Tumor Targeting via EPR Effect for Cancer Treatment. J. Pers. Med. 2021, 11, 571. [Google Scholar] [CrossRef] [PubMed]
- Bazak, R.; Houri, M.; Achy, S.E.; Hussein, W.; Refaat, T. Passive targeting of nanoparticles to cancer: A comprehensive review of the literature. Mol. Clin. Oncol. 2014, 2, 904–908. [Google Scholar] [CrossRef] [PubMed]
- Toy, R.; Peiris, P.M.; Ghaghada, K.B.; Karathanasis, E. Shaping cancer nanomedicine: The effect of particle shape on the in vivo journey of nanoparticles. Nanomedicine 2014, 9, 121–134. [Google Scholar] [CrossRef]
- Nguyen, L.N.M.; Lin, Z.P.; Sindhwani, S.; MacMillan, P.; Mladjenovic, S.M.; Stordy, B.; Ngo, W.; Chan, W.C.W. The exit of nanoparticles from solid tumours. Nat. Mater. 2023, 22, 1261–1272. [Google Scholar] [CrossRef]
- Sindhwani, S.; Syed, A.M.; Ngai, J.; Kingston, B.R.; Maiorino, L.; Rothschild, J.; MacMillan, P.; Zhang, Y.; Rajesh, N.U.; Hoang, T.; et al. The entry of nanoparticles into solid tumours. Nat. Mater. 2020, 19, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef]
- Yu, M.K.; Park, J.; Jon, S. Targeting strategies for multifunctional nanoparticles in cancer imaging and therapy. Theranostics 2012, 2, 3–44. [Google Scholar] [CrossRef]
- Bazak, R.; Houri, M.; El Achy, S.; Kamel, S.; Refaat, T. Cancer active targeting by nanoparticles: A comprehensive review of literature. J. Cancer Res. Clin. Oncol. 2015, 141, 769–784. [Google Scholar] [CrossRef]
- Elamir, A.; Ajith, S.; Sawaftah, N.A.; Abuwatfa, W.; Mukhopadhyay, D.; Paul, V.; Al-Sayah, M.H.; Awad, N.; Husseini, G.A. Ultrasound-triggered herceptin liposomes for breast cancer therapy. Sci. Rep. 2021, 11, 7545. [Google Scholar] [CrossRef]
- Mal, S.; Chakraborty, S.; Mahapatra, M.; Pakeeraiah, K.; Das, S.; Paidesetty, S.K.; Roy, P. Tackling breast cancer with gold nanoparticles: Twinning synthesis and particle engineering with efficacy. Nanoscale Adv. 2024, 6, 2766–2812. [Google Scholar] [CrossRef] [PubMed]
- Soe, Z.C.; Kwon, J.B.; Thapa, R.K.; Ou, W.; Nguyen, H.T.; Gautam, M.; Oh, K.T.; Choi, H.G.; Ku, S.K.; Yong, C.S.; et al. Transferrin-Conjugated Polymeric Nanoparticle for Receptor-Mediated Delivery of Doxorubicin in Doxorubicin-Resistant Breast Cancer Cells. Pharmaceutics 2019, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, M.J.; Loureiro, J.A.; Coelho, M.A.N.; Pereira, M.C. Transferrin Receptor-Targeted Nanocarriers: Overcoming Barriers to Treat Glioblastoma. Pharmaceutics 2022, 14, 279. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Silva, J.; Fernandes, R.S.; Ramos Oda, C.M.; Ferreira, T.H.; Machado Botelho, A.F.; Martins Melo, M.; de Miranda, M.C.; Assis Gomes, D.; Dantas Cassali, G.; Townsend, D.M.; et al. Folate-coated, long-circulating and pH-sensitive liposomes enhance doxorubicin antitumor effect in a breast cancer animal model. Biomed. Pharmacother. 2019, 118, 109323. [Google Scholar] [CrossRef] [PubMed]
- Chitgupi, U.; Qin, Y.; Ghosh, S.; Quinn, B.; Carter, K.; He, X.; Sunar, U.; Lovell, J.F. Folate-Targeted Nanoliposomal Chemophototherapy. Pharmaceutics 2023, 15, 2385. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Q.; Zhu, Q.; Gao, J.; Zhu, X.; Yu, H.; Li, Y.; Zhang, C. Copackaging photosensitizer and PD-L1 siRNA in a nucleic acid nanogel for synergistic cancer photoimmunotherapy. Sci. Adv. 2022, 8, eabn2941. [Google Scholar] [CrossRef]
- Feng, X.; Zhu, Y.; Wang, F.; Guo, T.; Dou, X.; Lin, M.; Tian, W. The Aptamer Functionalized Nanocomposite Used for Prostate Cancer Diagnosis and Therapy. J. Nanomater. 2022, 2022, 9946357. [Google Scholar] [CrossRef]
- Yin, X.; He, Z.; Ge, W.; Zhao, Z. Application of aptamer functionalized nanomaterials in targeting therapeutics of typical tumors. Front. Bioeng. Biotechnol. 2023, 11, 1092901. [Google Scholar] [CrossRef]
- Cesarini, V.; Scopa, C.; Silvestris, D.A.; Scafidi, A.; Petrera, V.; Del Baldo, G.; Gallo, A. Aptamer-Based In Vivo Therapeutic Targeting of Glioblastoma. Molecules 2020, 25, 4267. [Google Scholar] [CrossRef] [PubMed]
- Qi, N.; Zhou, X.; Ma, N.; Zhang, J.; Wang, Z.; Zhang, X.; Li, A. Integrin αvβ3 and LHRH Receptor Double Directed Nano-Analogue Effective Against Ovarian Cancer in Mice Model. Int. J. Nanomed. 2024, 19, 3071–3086. [Google Scholar] [CrossRef] [PubMed]
- Farhoudi, L.; Hosseinikhah, S.M.; Kazemi-Beydokhti, A.; Arabi, L.; Alavizadeh, S.H.; Moosavian, S.A.; Jaafari, M.R. pH-sensitive polymeric micelles enhance the co-delivery of doxorubicin and docetaxel: An emerging modality for treating breast cancer. Cancer Nanotechnol. 2024, 15, 37. [Google Scholar] [CrossRef]
- Zheng, G.; Zheng, M.; Yang, B.; Fu, H.; Li, Y. Improving breast cancer therapy using doxorubicin loaded solid lipid nanoparticles: Synthesis of a novel arginine-glycine-aspartic tripeptide conjugated, pH sensitive lipid and evaluation of the nanomedicine in vitro and in vivo. Biomed. Pharmacother. 2019, 116, 109006. [Google Scholar] [CrossRef]
- Caraway, C.A.; Gaitsch, H.; Wicks, E.E.; Kalluri, A.; Kunadi, N.; Tyler, B.M. Polymeric Nanoparticles in Brain Cancer Therapy: A Review of Current Approaches. Polymers 2022, 14, 2963. [Google Scholar] [CrossRef]
- Adamo, F.M.; De Falco, F.; Dorillo, E.; Sorcini, D.; Stella, A.; Esposito, A.; Arcaleni, R.; Rosati, E.; Sportoletti, P. Nanotechnology Advances in the Detection and Treatment of Lymphoid Malignancies. Int. J. Mol. Sci. 2024, 25, 9253. [Google Scholar] [CrossRef]
- Madej, M.; Kurowska, N.; Strzalka-Mrozik, B. Polymeric Nanoparticles—Tools in a Drug Delivery System in Selected Cancer Therapies. Appl. Sci. 2022, 12, 9479. [Google Scholar] [CrossRef]
- Almomen, A.; Alhowyan, A. A Comprehensive Study on Folate-Targeted Mesoporous Silica Nanoparticles Loaded with 5-Fluorouracil for the Enhanced Treatment of Gynecological Cancers. J. Funct. Biomater. 2024, 15, 74. [Google Scholar] [CrossRef]
- Yin, H.; Yan, Q.; Liu, Y.; Yang, L.; Liu, Y.; Luo, Y.; Chen, T.; Li, N.; Wu, M. Co-encapsulation of paclitaxel and 5-fluorouracil in folic acid-modified, lipid-encapsulated hollow mesoporous silica nanoparticles for synergistic breast cancer treatment. RSC Adv. 2022, 12, 32534–32551. [Google Scholar] [CrossRef]
- Tonbul, H.; Sahin, A.; Tavukcuoglu, E.; Ultav, G.; Akbas, S.; Aktas, Y.; Esendaglı, G.; Capan, Y. Folic acid decoration of mesoporous silica nanoparticles to increase cellular uptake and cytotoxic activity of doxorubicin in human breast cancer cells. J. Drug Deliv. Sci. Technol. 2021, 63, 102535. [Google Scholar] [CrossRef]
- Mozafarinia, M.; Karimi, S.; Farrokhnia, M.; Esfandiari, J. In vitro breast cancer targeting using Trastuzumab-conjugated mesoporous silica nanoparticles: Towards the new strategy for decreasing size and high drug loading capacity for drug delivery purposes in MSN synthesis. Microporous Mesoporous Mater. 2021, 316, 110950. [Google Scholar] [CrossRef]
- Ngamcherdtrakul, W.; Morry, J.; Gu, S.; Castro, D.J.; Goodyear, S.M.; Sangvanich, T.; Reda, M.M.; Lee, R.; Mihelic, S.A.; Beckman, B.L.; et al. Cationic Polymer Modified Mesoporous Silica Nanoparticles for Targeted SiRNA Delivery to HER2+ Breast Cancer. Adv. Funct. Mater. 2015, 25, 2646–2659. [Google Scholar] [CrossRef]
- Mikled, P.; Chavasiri, W.; Khongkow, M. Dual folate/biotin-decorated liposomes mediated delivery of methylnaphthazarin for anti-cancer activity. Sci. Rep. 2024, 14, 21796. [Google Scholar] [CrossRef] [PubMed]
- Ruzycka-Ayoush, M.; Kowalik, P.; Kowalczyk, A.; Bujak, P.; Nowicka, A.M.; Wojewodzka, M.; Kruszewski, M.; Grudzinski, I.P. Quantum dots as targeted doxorubicin drug delivery nanosystems in human lung cancer cells. Cancer Nanotechnol. 2021, 12, 8. [Google Scholar] [CrossRef]
- Kunachowicz, D.; Kłosowska, K.; Sobczak, N.; Kepinska, M. Applicability of Quantum Dots in Breast Cancer Diagnostic and Therapeutic Modalities—A State-of-the-Art Review. Nanomaterials 2024, 14, 1424. [Google Scholar] [CrossRef]
- Graham, W.; Torbett-Dougherty, M.; Islam, A.; Soleimani, S.; Bruce-Tagoe, T.A.; Johnson, J.A. Magnetic Nanoparticles and Drug Delivery Systems for Anti-Cancer Applications: A Review. Nanomaterials 2025, 15, 285. [Google Scholar] [CrossRef]
- Nunes, S.S.; Miranda, S.E.M.; de Oliveira Silva, J.; Fernandes, R.S.; de Alcântara Lemos, J.; de Aguiar Ferreira, C.; Townsend, D.M.; Cassali, G.D.; Oliveira, M.C.; Branco de Barros, A.L. pH-responsive and folate-coated liposomes encapsulating irinotecan as an alternative to improve efficacy of colorectal cancer treatment. Biomed. Pharmacother. 2021, 144, 112317. [Google Scholar] [CrossRef]
- Driscoll, J.; Gondaliya, P.; Zinn, D.A.; Jain, R.; Yan, I.K.; Dong, H.; Patel, T. Using aptamers for targeted delivery of RNA therapies. Mol. Ther. 2025, 33, 1344–1367. [Google Scholar] [CrossRef]
- Zhou, G.; Wilson, G.; Hebbard, L.; Duan, W.; Liddle, C.; George, J.; Qiao, L. Aptamers: A promising chemical antibody for cancer therapy. Oncotarget 2016, 7, 13446–13463. [Google Scholar] [CrossRef]
- Kesharwani, P.; Chadar, R.; Sheikh, A.; Rizg, W.Y.; Safhi, A.Y. CD44-Targeted Nanocarrier for Cancer Therapy. Front. Pharmacol. 2021, 12, 800481. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Huang, Y.; Batra, N.; Chen, Y.; Huang, H.; Wang, Y.; Zhang, Z.; Li, S.; Chen, C.-Y.; Wang, Z.; et al. Inhibition of iRhom1 by CD44-targeting nanocarrier for improved cancer immunochemotherapy. Nat. Commun. 2024, 15, 255. [Google Scholar] [CrossRef]
- Chen, Y.; Qin, Z.; Wang, Y.; Gu, B.; Wang, J.; Zheng, Y.; Niu, Y.; Jia, L. CD44-targeted virus-mimicking nanomedicine eliminates cancer stem cells and mitigates chemoresistance in head and neck squamous cell carcinoma. Mater. Today Bio 2025, 32, 101721. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Acharya, A.; Krishnamurthy, P.T. Antibody-conjugated nanoparticles for target-specific drug delivery of chemotherapeutics. Beilstein J. Nanotechnol. 2023, 14, 912–926. [Google Scholar] [CrossRef] [PubMed]
- Abedin, M.R.; Powers, K.; Aiardo, R.; Barua, D.; Barua, S. Antibody–drug nanoparticle induces synergistic treatment efficacies in HER2 positive breast cancer cells. Sci. Rep. 2021, 11, 7347. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Cui, F.; Huang, D.; Zhang, D.; Zhu, A.; Sun, X.; Cao, Y.; Ding, S.; Wang, Y.; Gao, E.; et al. PD-L1 monoclonal antibody-conjugated nanoparticles enhance drug delivery level and chemotherapy efficacy in gastric cancer cells. Int. J. Nanomed. 2019, 14, 17–32. [Google Scholar] [CrossRef]
- Liang, Y.; Wu, J.; Yan, Y.; Wang, Y.; Zhao, H.; Wang, X.; Chang, S.; Li, S. Charge-Reversal Nano-Drug Delivery Systems in the Tumor Microenvironment: Mechanisms, Challenges, and Therapeutic Applications. Int. J. Mol. Sci. 2024, 25, 9779. [Google Scholar] [CrossRef]
- Luo, Z.; Chen, C.-Y.; Li, S. Improving Tumor Targeting and Penetration for Nanoparticle-Mediated Cancer Therapy. Small Methods, 2025; 2401860, Online ahead of print. [Google Scholar] [CrossRef]
- Ouyang, X.; Liu, Y.; Zheng, K.; Pang, Z.; Peng, S. Recent advances in zwitterionic nanoscale drug delivery systems to overcome biological barriers. Asian J. Pharm. Sci. 2024, 19, 100883. [Google Scholar] [CrossRef]
- Noreen, S.; Maqbool, I.; Saleem, A.; Mahmood, H.; Rai, N. Recent insights and applications of nanocarriers-based drug delivery systems for colonic drug delivery and cancer therapy: An updated review. Crit. Rev. Oncol. /Hematol. 2025, 208, 104646. [Google Scholar] [CrossRef]
- Fatima, M.; Almalki, W.H.; Khan, T.; Sahebkar, A.; Kesharwani, P. Harnessing the Power of Stimuli-Responsive Nanoparticles as an Effective Therapeutic Drug Delivery System. Adv. Mater. 2024, 36, 2312939. [Google Scholar] [CrossRef]
- Li, M.; Zhao, G.; Su, W.-K.; Shuai, Q. Enzyme-Responsive Nanoparticles for Anti-tumor Drug Delivery. Front. Chem. 2020, 8, 647. [Google Scholar] [CrossRef]
- Badparvar, F.; Marjani, A.P.; Salehi, R.; Ramezani, F. Dual pH/redox-responsive hyperbranched polymeric nanocarriers with TME-trigger size shrinkage and charge reversible ability for amplified chemotherapy of breast cancer. Sci. Rep. 2024, 14, 8567. [Google Scholar] [CrossRef]
- Guo, X.; Cheng, Y.; Zhao, X.; Luo, Y.; Chen, J.; Yuan, W.-E. Advances in redox-responsive drug delivery systems of tumor microenvironment. J. Nanobiotechnol. 2018, 16, 74. [Google Scholar] [CrossRef]
- Xia, Y.; Duan, S.; Han, C.; Jing, C.; Xiao, Z.; Li, C. Hypoxia-responsive nanomaterials for tumor imaging and therapy. Front. Oncol. 2022, 12, 1089446. [Google Scholar] [CrossRef]
- Xing, Y.; Zeng, B.; Yang, W. Light responsive hydrogels for controlled drug delivery. Front. Bioeng. Biotechnol. 2022, 10, 1075670. [Google Scholar] [CrossRef]
- Linsley, C.S.; Wu, B.M. Recent advances in light-responsive on-demand drug-delivery systems. Ther. Deliv. 2017, 8, 89–107. [Google Scholar] [CrossRef] [PubMed]
- Moradi Kashkooli, F.; Jakhmola, A.; Ferrier, G.A.; Hornsby, T.K.; Tavakkoli, J.; Kolios, M.C. Integrating Therapeutic Ultrasound With Nanosized Drug Delivery Systems in the Battle Against Cancer. Technol. Cancer Res. Treat. 2023, 22, 15330338231211472. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Cornel, E.J.; Du, J. Ultrasound-responsive polymer-based drug delivery systems. Drug Deliv. Transl. Res. 2021, 11, 1323–1339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Pan, T.; Lee, J.; Goldberg, S.; King, S.A.; Tang, E.; Hu, Y.; Chen, L.; Hoover, A.; Zhu, L.; et al. Enabling tumor-specific drug delivery by targeting the Warburg effect of cancer. Cell Rep. Med. 2025, 6, 101920. [Google Scholar] [CrossRef]
- Song, J.; Cheng, M.; Xie, Y.; Li, K.; Zang, X. Efficient tumor synergistic chemoimmunotherapy by self-augmented ROS-responsive immunomodulatory polymeric nanodrug. J. Nanobiotechnol. 2023, 21, 93. [Google Scholar] [CrossRef]
- Hosonuma, M.; Yoshimura, K. Association between pH regulation of the tumor microenvironment and immunological state. Front. Oncol. 2023, 13, 1175563. [Google Scholar] [CrossRef]
- Kanamala, M.; Wilson, W.R.; Yang, M.; Palmer, B.D.; Wu, Z. Mechanisms and biomaterials in pH-responsive tumour targeted drug delivery: A review. Biomaterials 2016, 85, 152–167. [Google Scholar] [CrossRef]
- Zhao, Y.; Ren, W.; Zhong, T.; Zhang, S.; Huang, D.; Guo, Y.; Yao, X.; Wang, C.; Zhang, W.Q.; Zhang, X.; et al. Tumor-specific pH-responsive peptide-modified pH-sensitive liposomes containing doxorubicin for enhancing glioma targeting and anti-tumor activity. J. Control. Release 2016, 222, 56–66. [Google Scholar] [CrossRef]
- Monteiro, L.O.F.; Malachias, Â.; Pound-Lana, G.; Magalhães-Paniago, R.; Mosqueira, V.C.F.; Oliveira, M.C.; de Barros, A.L.B.; Leite, E.A. Paclitaxel-Loaded pH-Sensitive Liposome: New Insights on Structural and Physicochemical Characterization. Langmuir 2018, 34, 5728–5737. [Google Scholar] [CrossRef]
- Alrbyawi, H.; Poudel, I.; Annaji, M.; Boddu, S.H.S.; Arnold, R.D.; Tiwari, A.K.; Babu, R.J. pH-Sensitive Liposomes for Enhanced Cellular Uptake and Cytotoxicity of Daunorubicin in Melanoma (B16-BL6) Cell Lines. Pharmaceutics 2022, 14, 1128. [Google Scholar] [CrossRef]
- de Oliveira Silva, J.; Miranda, S.E.M.; Leite, E.A.; de Paula Sabino, A.; Borges, K.B.G.; Cardoso, V.N.; Cassali, G.D.; Guimarães, A.G.; Oliveira, M.C.; de Barros, A.L.B. Toxicological study of a new doxorubicin-loaded pH-sensitive liposome: A preclinical approach. Toxicol. Appl. Pharmacol. 2018, 352, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Katti, P.S.; Gu, Z. Enzyme-responsive nanomaterials for controlled drug delivery. Nanoscale 2014, 6, 12273–12286. [Google Scholar] [CrossRef] [PubMed]
- Junnuthula, V.; Kolimi, P.; Nyavanandi, D.; Sampathi, S.; Vora, L.K.; Dyawanapelly, S. Polymeric Micelles for Breast Cancer Therapy: Recent Updates, Clinical Translation and Regulatory Considerations. Pharmaceutics 2022, 14, 1860. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, X.; Zhong, W.; Ren, X.; Sha, X.; Fang, X. Matrix metalloproteinases-2/9-sensitive peptide-conjugated polymer micelles for site-specific release of drugs and enhancing tumor accumulation: Preparation and in vitro and in vivo evaluation. Int. J. Nanomed. 2016, 11, 1643–1661. [Google Scholar] [CrossRef]
- Abed, H.F.; Abuwatfa, W.H.; Husseini, G.A. Redox-Responsive Drug Delivery Systems: A Chemical Perspective. Nanomaterials 2022, 12, 3183. [Google Scholar] [CrossRef]
- Gisbert-Garzarán, M.; Vallet-Regí, M. Redox-Responsive Mesoporous Silica Nanoparticles for Cancer Treatment: Recent Updates. Nanomaterials 2021, 11, 2222. [Google Scholar] [CrossRef]
- Debnath, S.K.; Debnath, M.; Ghosh, A.; Srivastava, R.; Omri, A. Targeting Tumor Hypoxia with Nanoparticle-Based Therapies: Challenges, Opportunities, and Clinical Implications. Pharmaceuticals 2024, 17, 1389. [Google Scholar] [CrossRef]
- Kumari, R.; Sunil, D.; Ningthoujam, R.S. Hypoxia-responsive nanoparticle based drug delivery systems in cancer therapy: An up-to-date review. J. Control. Release 2020, 319, 135–156. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yao, S.-Y.; Luo, J.; Ding, C.; Huang, Q.; Yang, Y.; Shi, Z.; Lin, J.; Pan, Y.-C.; Zeng, X.; et al. Engineered hypoxia-responsive albumin nanoparticles mediating mitophagy regulation for cancer therapy. Nat. Commun. 2025, 16, 596. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Chu, D.; Yang, F.; Li, Z.; Fan, B.; Jin, L.; Li, J. Hypoxia-Responsive Polymeric Micelles for Enhancing Cancer Treatment. Front. Chem. 2020, 8, 742. [Google Scholar] [CrossRef]
- Hao, D.; Meng, Q.; Jiang, B.; Lu, S.; Xiang, X.; Pei, Q.; Yu, H.; Jing, X.; Xie, Z. Hypoxia-Activated PEGylated Paclitaxel Prodrug Nanoparticles for Potentiated Chemotherapy. ACS Nano 2022, 16, 14693–14702. [Google Scholar] [CrossRef]
- Wang, Z.; Mu, X.; Yang, Q.; Luo, J.; Zhao, Y. Hypoxia-responsive nanocarriers for chemotherapy sensitization via dual-mode inhibition of hypoxia-inducible factor-1 alpha. J. Colloid Interface Sci. 2022, 628, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Hashiba, K.; Taguchi, M.; Sakamoto, S.; Otsu, A.; Maeda, Y.; Ebe, H.; Okazaki, A.; Harashima, H.; Sato, Y. Overcoming thermostability challenges in mRNA–lipid nanoparticle systems with piperidine-based ionizable lipids. Commun. Biol. 2024, 7, 556. [Google Scholar] [CrossRef]
- Tang, M.; Mahri, S.; Shiau, Y.-P.; Mukarrama, T.; Villa, R.; Zong, Q.; Racacho, K.J.; Li, Y.; Lee, Y.; Huang, Y.; et al. Multifunctional and Scalable Nanoparticles for Bimodal Image-Guided Phototherapy in Bladder Cancer Treatment. Nano-Micro Lett. 2025, 17, 222. [Google Scholar] [CrossRef]
- Srinivasan, L.V.; Rana, S.S. A critical review of various synthesis methods of nanoparticles and their applications in biomedical, regenerative medicine, food packaging, and environment. Discov. Appl. Sci. 2024, 6, 371. [Google Scholar] [CrossRef]
- Xu, H.; Li, Y.; Paxton, J.W.; Wu, Z. Co-Delivery Using pH-Sensitive Liposomes to Pancreatic Cancer Cells: The Effects of Curcumin on Cellular Concentration and Pharmacokinetics of Gemcitabine. Pharm. Res. 2021, 38, 1209–1219. [Google Scholar] [CrossRef]
- Yadav, S.; Vlerken, L.; Little, S.; Amiji, M. Evaluations of combination MDR-1 gene silencing and paclitaxel administration in biodegradable polymeric nanoparticle formulations to overcome multidrug resistance in cancer cells. Cancer Chemother. Pharmacol. 2008, 63, 711–722. [Google Scholar] [CrossRef]
- Vergara, D.; Bellomo, C.; Zhang, X.; Vergaro, V.; Tinelli, A.; Lorusso, V.; Rinaldi, R.; Lvov, Y.M.; Leporatti, S.; Maffia, M. Lapatinib/Paclitaxel polyelectrolyte nanocapsules for overcoming multidrug resistance in ovarian cancer. Nanomedicine 2012, 8, 891–899. [Google Scholar] [CrossRef]
- Wei, X.; Song, M.; Li, W.; Huang, J.; Yang, G.; Wang, Y. Multifunctional nanoplatforms co-delivering combinatorial dual-drug for eliminating cancer multidrug resistance. Theranostics 2021, 11, 6334–6354. [Google Scholar] [CrossRef]
- Wang, H.; Liang, Y.; Yin, Y.; Zhang, J.; Su, W.; White, A.M.; Bin, J.; Xu, J.; Zhang, Y.; Stewart, S.; et al. Carbon nano-onion-mediated dual targeting of P-selectin and P-glycoprotein to overcome cancer drug resistance. Nat. Commun. 2021, 12, 312. [Google Scholar] [CrossRef]
- Zhang, G.-N.; Gupta, P.; Wang, M.; Barbuti, A.M.; Ashby, C.R.; Zhang, Y.-K.; Zeng, L.; Xu, Q.; Fan, Y.-F.; Chen, Z.-S. Lipid–Saporin Nanoparticles for the Intracellular Delivery of Cytotoxic Protein to Overcome ABC Transporter-Mediated Multidrug Resistance In Vitro and In Vivo. Cancers 2020, 12, 498. [Google Scholar] [CrossRef]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Atukorale, P.U.; Moon, T.J.; Bokatch, A.R.; Lusi, C.F.; Routhier, J.T.; Deng, V.J.; Karathanasis, E. Dual agonist immunostimulatory nanoparticles combine with PD1 blockade for curative neoadjuvant immunotherapy of aggressive cancers. Nanoscale 2022, 14, 1144–1159. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Hong, J.; Lee, J.; Fakhraei Lahiji, S.; Kim, Y.-H. Recent advances in tumor microenvironment-targeted nanomedicine delivery approaches to overcome limitations of immune checkpoint blockade-based immunotherapy. J. Control. Release 2021, 332, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Patel, K.; Kumar, A. Targeting drug resistance in breast cancer: The potential of miRNA and nanotechnology-driven delivery systems. Nanoscale Adv. 2024, 6, 6079–6095. [Google Scholar] [CrossRef]
- Guan, C.; Han, Y.; Ling, Z.; Meng, X.; Zhang, B.; Dong, W.; Zhang, D.; Chen, K. Nanomaterials: Breaking the bottleneck of breast cancer drug resistance. Front. Immunol. 2024, 15, 1492546. [Google Scholar] [CrossRef]
- Liu, M.; Fu, M.; Yang, X.; Jia, G.; Shi, X.; Ji, J.; Liu, X.; Zhai, G. Paclitaxel and quercetin co-loaded functional mesoporous silica nanoparticles overcoming multidrug resistance in breast cancer. Colloids Surf. B Biointerfaces 2020, 196, 111284. [Google Scholar] [CrossRef]
- Xiong, X.; Arvizo, R.R.; Saha, S.; Robertson, D.J.; McMeekin, S.; Bhattacharya, R.; Mukherjee, P. Sensitization of ovarian cancer cells to cisplatin by gold nanoparticles. Oncotarget 2014, 5, 6453–6465. [Google Scholar] [CrossRef]
- Ganju, V.; Marx, G.; Pattison, S.; Amaro-Mugridge, N.B.; Zhao, J.T.; Williams, B.R.G.; MacDiarmid, J.A.; Brahmbhatt, H. Phase I/IIa Trial in Advanced Pancreatic Ductal Adenocarcinoma Treated with Cytotoxic Drug-Packaged, EGFR-Targeted Nanocells and Glycolipid-Packaged Nanocells. Clin. Cancer Res. 2024, 30, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.T.; Abhi, C.; Kamalasanan, K.; Pavithran, K.; Unni, A.R.; Sithara, M.S.; Sarma, M.; Mangalanandan, T.S. Pathophysiology-Driven Approaches for Overcoming Nanomedicine Resistance in Pancreatic Cancer. Mol. Pharm. 2024, 21, 5960–5988. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, M.M.; Robey, R.W.; Ambudkar, S.V. New mechanisms of multidrug resistance: An introduction to the Cancer Drug Resistance special collection. Cancer Drug Resist. 2023, 6, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Setrerrahmane, S.; Li, C.; Hu, J.; Xu, H. Nucleic acid drugs: Recent progress and future perspectives. Signal Transduct. Target. Ther. 2024, 9, 316. [Google Scholar] [CrossRef]
- Okuda, T.; Okazaki, M.; Hayano, A.; Okamoto, H. Stability of Naked Nucleic Acids under Physical Treatment and Powder Formation: Suitability for Development as Dry Powder Formulations for Inhalation. Pharmaceutics 2023, 15, 2786. [Google Scholar] [CrossRef]
- Kumar, G.; Mullick, P.; Andugulapati, S.B.; Eedara, A.C.; Kumar, N.; Mutalik, S.; Nandakumar, K.; Chamallamudi, M.R. Trastuzumab-conjugated liposomes for co-delivery of paclitaxel and anti-abcb1 siRNA in HER2-positive breast cancer: In vitro and in vivo evaluations. J. Drug Deliv. Sci. Technol. 2024, 95, 105614. [Google Scholar] [CrossRef]
- Suo, A.; Qian, J.; Xu, M.; Xu, W.; Zhang, Y.; Yao, Y. Folate-decorated PEGylated triblock copolymer as a pH/reduction dual-responsive nanovehicle for targeted intracellular co-delivery of doxorubicin and Bcl-2 siRNA. Mater. Sci. Eng. C 2017, 76, 659–672. [Google Scholar] [CrossRef]
- Sakurai, Y.; Watanabe, H.; Nishio, K.; Hashimoto, K.; Harada, A.; Gomi, M.; Suzuki, M.; Oyama, R.; Handa, T.; Sato, R.; et al. pH-Responsive Lipid Nanoparticles Achieve Efficient mRNA Transfection in Brain Capillary Endothelial Cells. Pharmaceutics 2022, 14, 1560. [Google Scholar] [CrossRef]
- Duan, L.; Ouyang, K.; Xu, X.; Xu, L.; Wen, C.; Zhou, X.; Qin, Z.; Xu, Z.; Sun, W.; Liang, Y. Nanoparticle Delivery of CRISPR/Cas9 for Genome Editing. Front. Genet. 2021, 12, 673286. [Google Scholar] [CrossRef]
- Rosenblum, D.; Gutkin, A.; Kedmi, R.; Ramishetti, S.; Veiga, N.; Jacobi, A.M.; Schubert, M.S.; Friedmann-Morvinski, D.; Cohen, Z.R.; Behlke, M.A.; et al. CRISPR-Cas9 genome editing using targeted lipid nanoparticles for cancer therapy. Sci. Adv. 2020, 6, abc9450. [Google Scholar] [CrossRef]
- Hołubowicz, R.; Du, S.W.; Felgner, J.; Smidak, R.; Choi, E.H.; Palczewska, G.; Menezes, C.R.; Dong, Z.; Gao, F.; Medani, O.; et al. Safer and efficient base editing and prime editing via ribonucleoproteins delivered through optimized lipid-nanoparticle formulations. Nat. Biomed. Eng. 2025, 9, 57–78. [Google Scholar] [CrossRef] [PubMed]
- Molinar, C.; Tannous, M.; Meloni, D.; Cavalli, R.; Scomparin, A. Current Status and Trends in Nucleic Acids for Cancer Therapy: A Focus on Polysaccharide-Based Nanomedicines. Macromol. Biosci. 2023, 23, 2300102. [Google Scholar] [CrossRef] [PubMed]
- Schultheis, B.; Strumberg, D.; Kuhlmann, J.; Wolf, M.; Link, K.; Seufferlein, T.; Kaufmann, J.; Feist, M.; Gebhardt, F.; Khan, M.; et al. Safety, Efficacy and Pharcacokinetics of Targeted Therapy with The Liposomal RNA Interference Therapeutic Atu027 Combined with Gemcitabine in Patients with Pancreatic Adenocarcinoma. A Randomized Phase Ib/IIa Study. Cancers 2020, 12, 3130. [Google Scholar] [CrossRef]
- Cuciniello, R.; Filosa, S.; Crispi, S. Novel approaches in cancer treatment: Preclinical and clinical development of small non-coding RNA therapeutics. J. Exp. Clin. Cancer Res. 2021, 40, 383. [Google Scholar] [CrossRef]
- Lu, Y.; Xue, J.; Deng, T.; Zhou, X.; Yu, K.; Deng, L.; Huang, M.; Yi, X.; Liang, M.; Wang, Y.; et al. Safety and feasibility of CRISPR-edited T cells in patients with refractory non-small-cell lung cancer. Nat. Med. 2020, 26, 732–740. [Google Scholar] [CrossRef]
- Benjamin, R.; Jain, N.; Maus, M.V.; Boissel, N.; Graham, C.; Jozwik, A.; Yallop, D.; Konopleva, M.; Frigault, M.J.; Teshima, T.; et al. UCART19, a first-in-class allogeneic anti-CD19 chimeric antigen receptor T-cell therapy for adults with relapsed or refractory B-cell acute lymphoblastic leukaemia (CALM): A phase 1, dose-escalation trial. Lancet Haematol. 2022, 9, e833–e843. [Google Scholar] [CrossRef]
- He, L.; Javid Anbardan, Z.; Habibovic, P.; van Rijt, S. Doxorubicin- and Selenium-Incorporated Mesoporous Silica Nanoparticles as a Combination Therapy for Osteosarcoma. ACS Appl. Nano Mater. 2024, 7, 25400–25411. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Wang, S.; Xu, R.; Tang, Y.; Xia, X. Cancer cell membrane-coated nanoparticles: A promising anti-tumor bionic platform. RSC Adv. 2024, 14, 10608–10637. [Google Scholar] [CrossRef]
- Guo, C.; Ma, X.; Gao, F.; Guo, Y. Off-target effects in CRISPR/Cas9 gene editing. Front. Bioeng. Biotechnol. 2023, 11, 1143157. [Google Scholar] [CrossRef]
- Ewaisha, R.; Anderson, K.S. Immunogenicity of CRISPR therapeutics—Critical considerations for clinical translation. Front. Bioeng. Biotechnol. 2023, 11, 1138596. [Google Scholar] [CrossRef]
- Chehelgerdi, M.; Chehelgerdi, M.; Khorramian-Ghahfarokhi, M.; Shafieizadeh, M.; Mahmoudi, E.; Eskandari, F.; Rashidi, M.; Arshi, A.; Mokhtari-Farsani, A. Comprehensive review of CRISPR-based gene editing: Mechanisms, challenges, and applications in cancer therapy. Mol. Cancer 2024, 23, 9. [Google Scholar] [CrossRef]
- Thangudu, S.; Mehta, S.; Dhowre, H.S.; Bojic, S.; Haghverdi, G.; Wu, A.Y.; Massoud, T.F.; Paulmurugan, R. Autologous extracellular vesicles derived from conjunctival squamous cell carcinoma deliver therapeutic microRNAs to induce apoptosis in originating cancer. J. Mater. Chem. B, 2025; Online ahead of print. [Google Scholar] [CrossRef]
- Kim, H.I.; Park, J.; Zhu, Y.; Wang, X.; Han, Y.; Zhang, D. Recent advances in extracellular vesicles for therapeutic cargo delivery. Exp. Mol. Med. 2024, 56, 836–849. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Xu, W.; Li, Y.; Lai, R.; Qiu, X.; Chen, X.; Chen, Z.; Mi, B.; Wu, M.; et al. Translational Challenges and Prospective Solutions in the Implementation of Biomimetic Delivery Systems. Pharmaceutics 2023, 15, 2623. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Gómez, F.D.; Monferrer, D.; Penon, O.; Rivera-Gil, P. Regulatory pathways and guidelines for nanotechnology-enabled health products: A comparative review of EU and US frameworks. Front. Med. 2025, 12, 1544393. [Google Scholar] [CrossRef]
- Peng, C. Editorial: Nanomedicine development and clinical translation. Front. Chem. 2024, 12, 1458690. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.W.; Bae, Y.H. EPR: Evidence and fallacy. J. Control. Release 2014, 190, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Zhou, M.; Wang, Y.; Lu, C.; Yin, B.; Zhang, Y.; Liu, H.; Yin, X.; Song, G. Emerging biomedical imaging-based companion diagnostics for precision medicine. iScience 2023, 26, 107277. [Google Scholar] [CrossRef]
- Rama, E.; May, J.-N.; Rix, A.; Lammers, T.; Kiessling, F. Image-guided strategies to improve drug delivery to tumors beyond using the enhanced permeability and retention (EPR) effect. Biochem. Biophys. Res. Commun. 2025, 778, 152346. [Google Scholar] [CrossRef]
- Milano, G.; Innocenti, F.; Minami, H. Liposomal irinotecan (Onivyde): Exemplifying the benefits of nanotherapeutic drugs. Cancer Sci. 2022, 113, 2224–2231. [Google Scholar] [CrossRef] [PubMed]
- Gradishar, W.J. Albumin-bound paclitaxel: A next-generation taxane. Expert Opin. Pharmacother. 2006, 7, 1041–1053. [Google Scholar] [CrossRef]
- Adams, D.; Gonzalez-Duarte, A.; O’Riordan, W.D.; Yang, C.-C.; Ueda, M.; Kristen, A.V.; Tournev, I.; Schmidt, H.H.; Coelho, T.; Berk, J.L.; et al. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N. Engl. J. Med. 2018, 379, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Akinc, A.; Maier, M.A.; Manoharan, M.; Fitzgerald, K.; Jayaraman, M.; Barros, S.; Ansell, S.; Du, X.; Hope, M.J.; Madden, T.D.; et al. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat. Nanotechnol. 2019, 14, 1084–1087. [Google Scholar] [CrossRef] [PubMed]
- Gillmore, J.D.; Gane, E.; Taubel, J.; Kao, J.; Fontana, M.; Maitland, M.L.; Seitzer, J.; O’Connell, D.; Walsh, K.R.; Wood, K.; et al. CRISPR-Cas9 In Vivo Gene Editing for Transthyretin Amyloidosis. N. Engl. J. Med. 2021, 385, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Finn, J.D.; Smith, A.R.; Patel, M.C.; Shaw, L.; Youniss, M.R.; van Heteren, J.; Dirstine, T.; Ciullo, C.; Lescarbeau, R.; Seitzer, J.; et al. A Single Administration of CRISPR/Cas9 Lipid Nanoparticles Achieves Robust and Persistent In Vivo Genome Editing. Cell Rep. 2018, 22, 2227–2235. [Google Scholar] [CrossRef]

| Product Name | Drug | Key Feature (s) | Indication (s) | Reference |
|---|---|---|---|---|
| Doxil®/Caelyx® | Doxorubicin | PEGylated, prolonged circulation and enhanced tumour accumulation. | Kaposi’s sarcoma, ovarian cancer and multiple myeloma | [89] |
| Myocet® | Doxorubicin | Non-PEGylated liposomes and reduced cardiotoxicity. | Metastatic breast cancer (with cyclophosphamide) | [90] |
| DepoCyt® | Cytarabine | Sustained-release liposomes for intrathecal administration, prolonged drug half-life. | Lymphomatous meningitis | [91] |
| Vyxeos® (CPX-351) | Daunorubicin +Cytarabine | Co-encapsulation of drugs in liposomes, shown synergistic effects and enhanced anti-leukemic activity. | Acute myeloid leukaemia (AML) | [92] |
| Mepact® | Mifamurtide | Less than 100 nm multi-lamellar liposome, postoperative combination with other anti-neoplastic agents has improved survival. | Non-metastatic osteosarcoma | [93] |
| OnivydeTM | Irinotecan | PEGylated, prolonged circulation, enhanced controlled release and enhanced tumour accumulation. | Metastatic pancreatic cancer | [94] |
| DaunoXome® | Daunorubicin | Small (~45–80 nm) unilamellar liposome, reduced cardiotoxicity and improved tolerability. | HIV-related Kaposi’s sarcoma | [95] |
| Marqibo® | Vincristine sulfate | Liposomal vincristine, prolonged circulation and reduced neurotoxicity. | Philadelphia chromosome-negative (Ph-) acute lymphoblastic leukaemia | [96] |
| Platform | Key Feature(s) | Advantages | Limitations | References |
|---|---|---|---|---|
| Gold NPs (AuNPs) | Tunable size/shape and strong optical/plasmonic properties | Greate for imaging, photothermal therapy and targeted delivery | Potential hepatic accumulation, size/ligand-dependent toxicity and/or limited clinical translation | [101] |
| LNPs | Ionizable phospholipids, scalable microfluidic formulation | Clinically validated for RNA delivery (e.g., Onpattro® and vaccines) and high encapsulation efficiency | Cold-chain required, hydrolytic instability and immunogenicity risk | [18,102,103] |
| Polymeric NPs | Biodegradable polymers (e.g., PLGA and micelles, dendrimers) | Controlled/sustained release, multifunctionality and tunable chemistry | Complex synthesis, potential degradation toxicity and scale-up challenges | [18,104,105] |
| Lipid−polymer hybrid NPs | Core–shell combining polymer and lipid components | Blended benefits: stability, payload versatility and prolonged circulation | Complex formulation, mostly preclinical | [106] |
| Mesoporous silica NPs | High surface area and easily functionalized, porous structure | High drug loading and stimuli-responsive kinetics | Poor biodegradability and risk of long-term accumulation | [107,108] |
| Dendrimers | Highly branched, monodisperse synthetic polymers | Precise size control, abundant functional groups and high payload capacity | Potential cytotoxicity at high generation and renal clearance limitations | [18,109,110] |
| Magnetic (SPIO) NPs | Superparamagnetic iron oxide core | MRI traceability and magnetically guided targeting | Limited penetration depth and aggregation or iron overload risks | [111] |
| Albumin-based NPs (e.g., nab-paclitaxel) | Protein-based carrier (e.g., albumin) | Clinically approved, endogenous uptake pathways, biocompatible | Hypersensitivity risk and variable manufacturing batch quality | [112,113] |
| NP Design Feature | Contribution Towards the EPR Effect |
|---|---|
| Size (typically 50–200 nm) | Small enough to pass through the leaky tumour vasculature and large enough to avoid renal clearance. |
| Surface charge | Neutral or slightly negative/positive surfaces reduce opsonisation and RES (reticuloendothelial system) clearance, improving circulation time and tumour accumulation. |
| Hydrophilic coatings (e.g., PEGylation) | Increases blood circulation half-life by preventing protein adsorption and immune recognition—more time to accumulate via EPR. |
| Shape (e.g., spherical) | Influences margination, internalisation and clearance rates—improving tumour delivery. |
| Type of NP | Targeting Ligand | Drug | Cancer Types | Reference |
|---|---|---|---|---|
| Liposomes (Active Targeting) | Anti-HER2 monoclonal antibodies (e.g., Trastuzumab) | Doxorubicin | HER2-positive breast cancer | [136] |
| Gold NPs (AuNPs) | Estrogen receptor ligands | Doxorubicin | Breast cancer | [137] |
| Transferrin-conjugated NPs | Transferrin | Doxorubicin | Various cancers | [138,139] |
| Folate-conjugated liposomes | Folate receptor | Doxorubicin and Methotrexate | Ovarian, colon cancer and breast cancer | [140,141] |
| Aptamer-conjugated NPs | PD-L1, PSMA and CD44 | Doxorubicin and siRNA | Prostate cancer, lung cancer and glioblastoma | [142,143,144,145] |
| Liposomes (pH-responsive, active targeting) | pH-sensitive ligands (e.g., RGD peptide) | Paclitaxel and Doxorubicin | Ovarian cancer and breast cancer | [146,147,148] |
| Polymeric NPs | Anti-EGFR and Anti-CD20 | Doxorubicin and Paclitaxel | Glioblastoma, lymphoma and colon cancer | [149,150,151] |
| Mesoporous silica NPs | Folate and HER2 antibodies | Paclitaxel and Doxorubicin | Ovarian cancer and breast cancer | [152,153,154,155,156] |
| Folate- and biotin- conjugated liposomes | Folate and biotin receptors | Methylnaphthazarin | Cervical cancer | [157] |
| Quantum dots | Anti-EGFR and Anti-HER2 | Doxorubicin and siRNA | Prostate, breast and lung cancer | [158,159] |
| Stimulus Type | Trigger Mechanism | Advantages | Role in MDR | Reference |
|---|---|---|---|---|
| Enzyme-responsive | Overexpressed tumour enzymes (e.g., MMPs, cathepsins) degrade linkers/coatings | High tumour specificity and improved penetration | Enhancing accumulation and penetration | [175] |
| Redox-responsive (GSH) | High intracellular GSH accelerates cleavage of disulfide bonds | Selective intracellular release and bypasses MDR efflux | Bypassing efflux via cytoplasmic-triggered release | [176,177] |
| Hypoxia-responsive | Hypoxia-induced enzymes reduce nitro/azo groups or activate prodrugs | Targets resistant hypoxic cores and enhanced selectivity | Delivering drugs into resistant hypoxic zones | [178] |
| Magnetic-responsive | External magnetic field guides particles and induces hyperthermia | Precise guidance of NPs and combinatorial thermal therapy | Inducing magnetic hyperthermia and improved delivery | [77,160] |
| Light-responsive (NIR) | NIR light cleaves photo-labile linkers or activates photothermal/photosensitizers | Precise spatial and temporal control | Disrupting tumour survival via local heat or ROS | [179,180] |
| Ultrasound-responsive | Ultrasound causes cavitation or vaporization triggering release | Non-invasive, site-specific burst release | Boosting penetration and microbubble-assisted uptake | [181,182] |
| System | Type of NP | Cargo | Indication | Status | Reference |
|---|---|---|---|---|---|
| CALAA-01 | Cyclodextrin-based polymer NP | siRNA targeting RRM2 | Solid tumours | Phase I completed | [229] |
| Atu027 | Liposomal NP | siRNA targeting PKN3 | Metastatic pancreatic cancer | Phase I completed, in combination with gemcitabine | [230] |
| iPsiRNA (NCT00672542) | Liposomal NP | siRNA targeting immunoproteasome subunits | Metastatic melanoma | Phase I completed | [231] |
| PD-1 knockout T Cells | Gene-edited T cells (ex vivo, non-viral) | CRISPR-Cas9 PD-1 knockout | Non-small cell lung cancer | Phase I completed (China) | [232] |
| UCART19 | CRISPR-edited allogeneic CAR-T cells | CRISPR-edited TCR/CD52 deletion | Acute lymphoblastic leukaemia | Phase I dose-escalation cohorts completed | [233] |
| Design Strategy | Key Feature (s) | Targeted MDR Mechanism | Example | Reference |
|---|---|---|---|---|
| Stimuli-responsive release | Dual pH/redox-sensitive linkers | Endosomal entrapment and premature drug efflux | Hyperbranched polymeric NPs that release docetaxel in the acidic/reductive TME, enhancing uptake (100% at 0.5 h) and efficacy | [176] |
| Active targeting via ligands | Surface conjugation with hyaluronic acid | Low receptor-mediated uptake in MDR cells | Doxorubicin- and selenium-incorporated mesoporous silica NPs coated with hyaluronic acid for Haase-triggered DOX release, demonstrating enhanced tumour cell uptake and efficacy in osteosarcoma models | [234] |
| Charge-reversal and zwitterion surfaces | pH/enzyme-triggered switch from neutral to cationic surface | Variable tumour uptake and protein corona formation | Acid-responsive charge-reversal DDS that remains “stealth” in blood but flips positive in the TME, improving penetration | [170] |
| Co-delivery of drug + siRNA/CRISPR | Co-encapsulation of chemotherapeutic + gene-silencing/editing | ABC transporter overexpression and anti-apoptosis | LNPs delivering prime-editor RNPs + chemotherapeutic, boosting in vivo editing >300-fold compared to unencapsulated RNPs | [11] |
| Biomimetic cell-membrane coatings | Cancer-cell membrane cloak for immune evasion and homotypic binding | Mononuclear phagocyte clearance and poor targeting | Cancer cell membrane-encapsulated NPs that escape macrophages and homotypically target tumours, enhancing circulation | [235] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Lu, G.-L.; Zheng, Y.-C.; Davison, E.K.; Li, Y. Nanoparticle-Based Delivery Strategies for Combating Drug Resistance in Cancer Therapeutics. Cancers 2025, 17, 2628. https://doi.org/10.3390/cancers17162628
Park S, Lu G-L, Zheng Y-C, Davison EK, Li Y. Nanoparticle-Based Delivery Strategies for Combating Drug Resistance in Cancer Therapeutics. Cancers. 2025; 17(16):2628. https://doi.org/10.3390/cancers17162628
Chicago/Turabian StylePark, Seohyun, Guo-Liang Lu, Yi-Chao Zheng, Emma K. Davison, and Yan Li. 2025. "Nanoparticle-Based Delivery Strategies for Combating Drug Resistance in Cancer Therapeutics" Cancers 17, no. 16: 2628. https://doi.org/10.3390/cancers17162628
APA StylePark, S., Lu, G.-L., Zheng, Y.-C., Davison, E. K., & Li, Y. (2025). Nanoparticle-Based Delivery Strategies for Combating Drug Resistance in Cancer Therapeutics. Cancers, 17(16), 2628. https://doi.org/10.3390/cancers17162628





