Preliminary Outcomes of a Digital Remote Care Solution for Colorectal Cancer Patients
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Analysis and Mapping of the Healthcare Process
2.2. Identification of Opportunities for Improvement
2.3. Redesign and Digitalization of the Process
2.4. Design and Validation of the Educational Program
2.5. Remote Monitoring
2.6. Definition of Indicators, Dashboard of Indicators, and Outcome Measures
- -
- Clinical outcomes (such as postoperative complications, length of hospital stay, mortality within 30 days).
- -
- Indicators of adherence to the program.
- -
- Usage levels of the digital solution.
- -
- PROMs and PREMs collected at various stages of the process.
- -
- Overall patient satisfaction.
2.7. Inclusion of Patients
- -
- Those who were aged eighteen years of age or older
- -
- Those suitable for CRC surgery.
- -
- Those with access to an email address, which is necessary for inclusion in the digital solution, either autonomously by the patient or with the assistance of a family member or caregiver who can help with the use and monitoring of the solution.
- -
- Those with access to a smartphone, either autonomously by the patient or with the assistance of a family member or caregiver who can help with the use and monitoring of the solution.
- -
- Those with the ability to follow the program through the digital solution, either autonomously by the patient or through the support of a family member or caregiver who can actively collaborate in the use and monitoring of the solution.
2.8. Exclusion of Patients
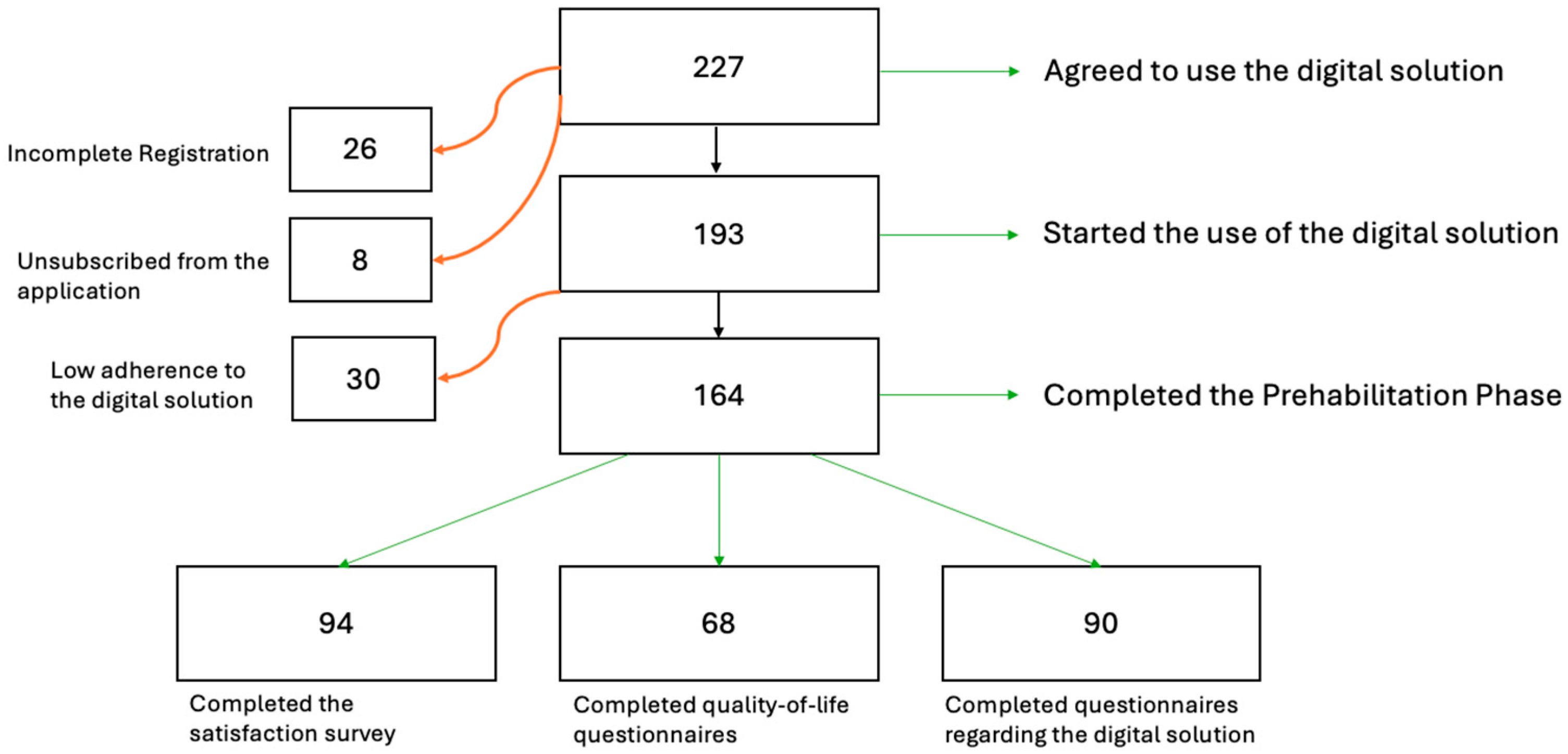
3. Results
3.1. Demographics
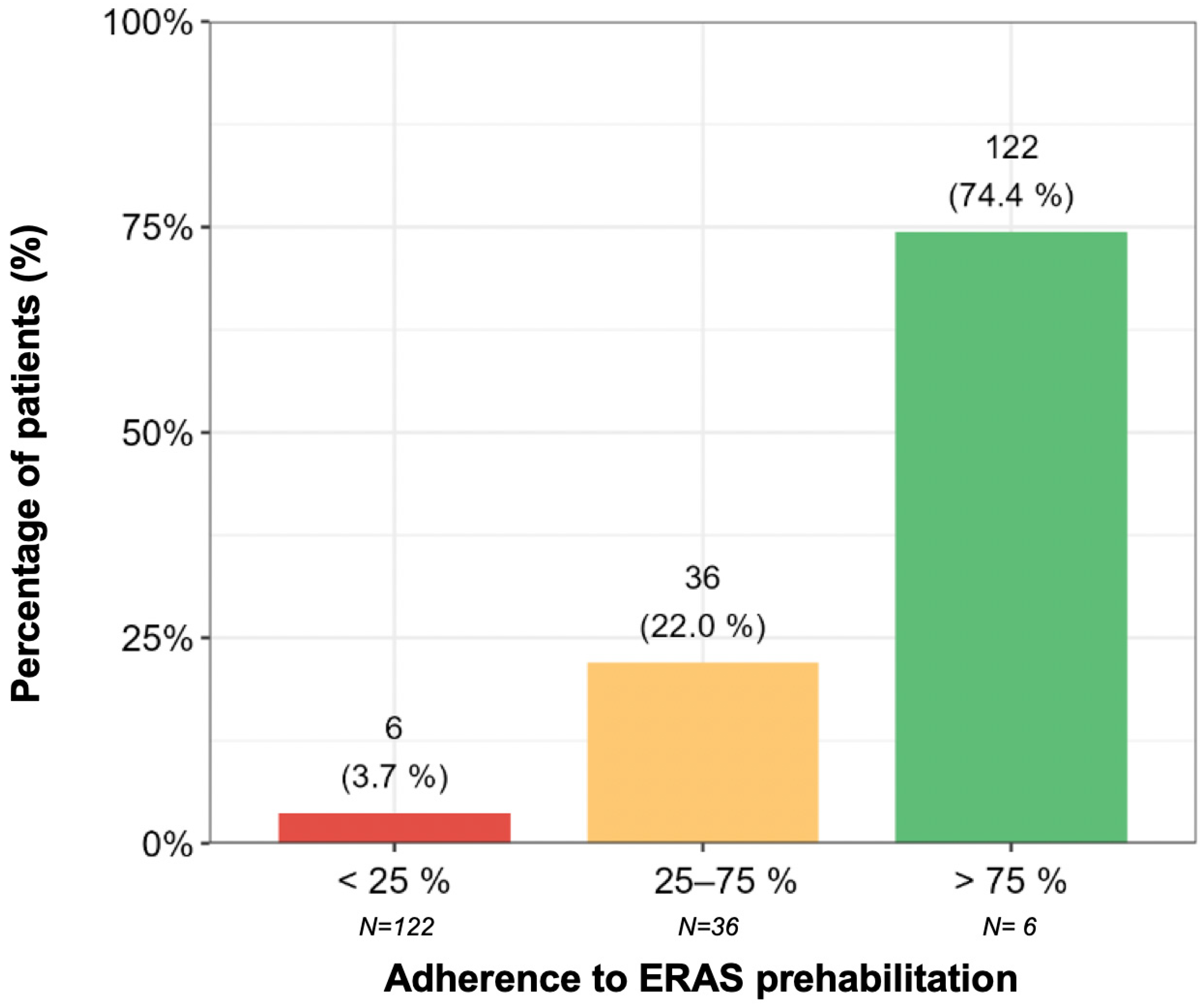
3.2. Adherence to the ERAS Protocol
3.3. Length of Hospital Stay (LoS)
- Low adherence (<25%): 10 days (95% CI: 4.45–22.48).
- Intermediate adherence (25–75%): 6.60 days (95% CI: 7.27–8.26).
- High adherence (>75%): 6.73 days (95% CI: 5.96–7.59) (Figure 3).
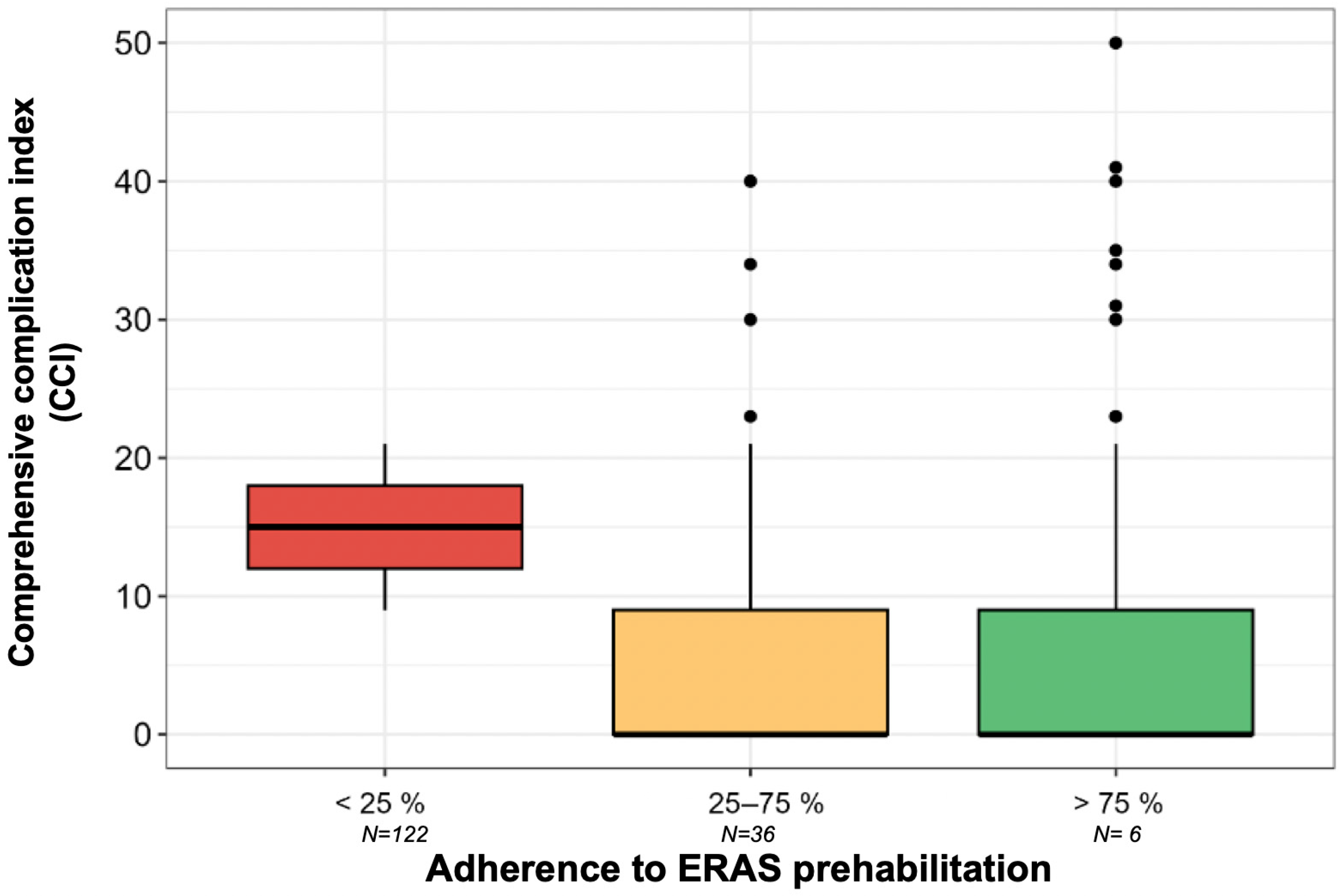
3.4. Comprehensive Complication Index (CCI)
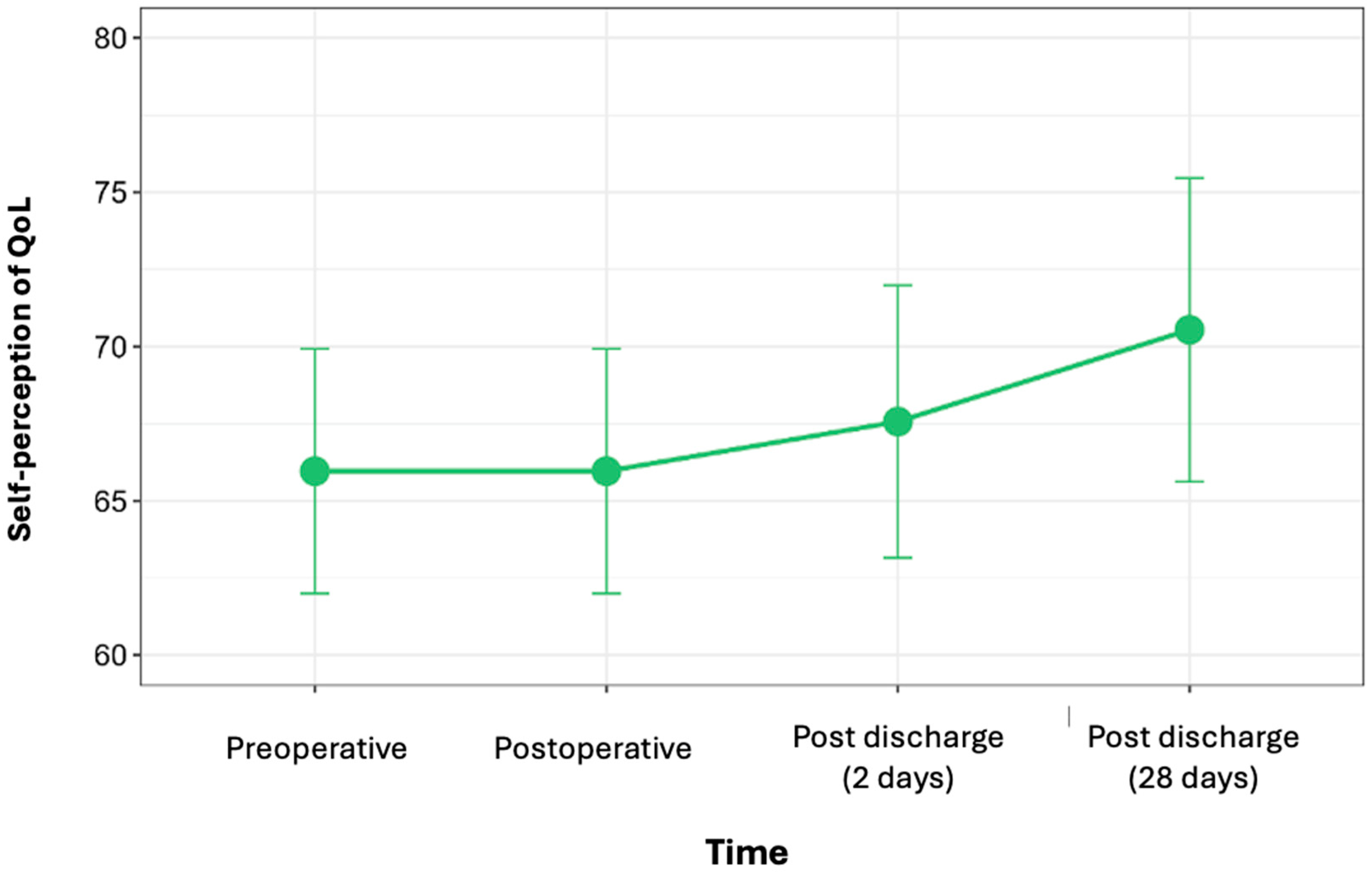
3.5. PROMs (Quality of Life)
3.6. PREMs (Satisfaction and Experience)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CCI | Comprehensive Complication Index |
| CRC | Colorectal cancer |
| ERAS | Enhanced Recovery After Surgery |
| FRAIL | Fatigue, Resistance, Ambulation, Illness, Loss of Weight |
| HADS | Hospital Anxiety and Depression Scale |
| LoS | Length of hospital stay |
| PROM | Patient-reported outcome |
| PREM | Patient-reported experience |
| TIRS | Total Individual Risk Score |
| VBHC | Value-based healthcare |
| VSM | Value Stream Mapping |
References
- Perera, S.K.; Jacob, S.; Wilson, E.B.; Ferlay, J.; Bray, F.; Sullivan, R.; Barton, M. Global demand for cancer surgery and an estimate of the optimal surgical and anaesthesia workforce between 2018 and 2040: A population-based modelling study. Lancet Oncol. 2021, 22, 182–189. [Google Scholar] [CrossRef]
- Lawrence, V.A.; Hazuda, H.P.; Cornell, J.E.; Pederson, T.; Bradshaw, P.T.; Mulrow, C.D.; Page, C.P. Functional independence after major abdominal surgery in the elderly. J. Am. Coll. Surg. 2004, 199, 762–772. [Google Scholar] [CrossRef]
- Stabenau, H.F.; Becher, R.D.; Gahbauer, E.A.; Leo-Summers, L.; Allore, H.G.; Gill, T.M. Functional Trajectories Before and After Major Surgery in Older Adults. Ann. Surg. 2018, 268, 911–917. [Google Scholar] [CrossRef]
- Sharp, S.P.; Malizia, R.; Skancke, M.; Arsoniadis, E.G.; Ata, A.; Stain, S.C.; Valerian, B.T.; Lee, E.C.; Wexner, S.D. A NSQIP analysis of trends in surgical outcomes for rectal cancer: What can we improve upon? Am. J. Surg. 2020, 220, 401–407. [Google Scholar] [CrossRef]
- Steffens, D.; Ismail, H.; Denehy, L.; Beckenkamp, P.R.; Solomon, M.; Koh, C.; Bartyn, J.; Pillinger, N. Preoperative Cardiopulmonary Exercise Test Associated with Postoperative Outcomes in Patients Undergoing Cancer Surgery: A Systematic Review and Meta-Analyses. Ann. Surg. Oncol. 2021, 28, 7120–7146. [Google Scholar] [CrossRef]
- Wischmeyer, P.E.; Carli, F.; Evans, D.C.; Guilbert, S.; Kozar, R.; Pryor, A.; Thiele, R.H.; Everett, S.; Grocott, M.; Gan, T.J.; et al. American Society for Enhanced Recovery and Perioperative Quality Initiative Joint Consensus Statement on Nutrition Screening and Therapy within a Surgical Enhanced Recovery Pathway. Anesth. Analg. 2018, 126, 1883–1895. [Google Scholar] [CrossRef] [PubMed]
- Molenaar, C.J.L.; Janssen, L.; van der Peet, D.L.; Winter, D.C.; Roumen, R.M.H.; Slooter, G.D. Conflicting Guidelines: A Systematic Review on the Proper Interval for Colorectal Cancer Treatment. World J Surg. 2021, 45, 2235–2250. [Google Scholar] [CrossRef] [PubMed]
- Barberan-Garcia, A.; Ubré, M.; Roca, J.; Lacy, A.M.; Burgos, F.; Risco, R.; Momblán, D.; Balust, J.; Blanco, I.; Martínez-Pallí, G. Personalised prehabilitation in high-risk patients undergoing elective major abdominal surgery: A randomized blinded controlled trial. Ann. Surg. 2018, 267, 50–56. [Google Scholar] [CrossRef]
- Barker, A.L.; Gray, C.; Wilson, L.; Thomson, B.N.J.; Shedda, S.; Crowe, T.C. Preoperative immunonutrition and its effect on postoperative outcomes in well-nourished and malnourished gastrointestinal surgery patients: A randomised controlled trial. Eur. J. Clin. Nutr. 2013, 67, 802–807. [Google Scholar] [CrossRef]
- Bousquet-Dion, G.; Awasthi, R.; Loiselle, S.; Minnella, E.M.; Agnihotram, R.V.; Bergdahl, A.; Carli, F.; Scheede-Bergdahl, C. Evaluation of supervised multimodal prehabilitation programme in cancer patients undergoing colorectal resection: A randomized control trial. Acta Oncol. 2018, 57, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Burden, S.T.; Gibson, D.J.; Lal, S.; Hill, J.; Pilling, M.; Soop, M.; Ramesh, A.; Todd, C. Pre-operative oral nutritional supplementation with dietary advice versus dietary advice alone in weight-losing patients with colorectal cancer: Single-blind randomized controlled trial. J. Cachexia Sarcopenia Muscle 2017, 8, 437–446. [Google Scholar] [CrossRef]
- Minnella, E.M.; Ferreira, V.; Awasthi, R.; Charlebois, P.; Stein, B.; Liberman, A.S.; Scheede-Bergdahl, C.; Morais, J.A.; Carli, F. Effect of two different pre-operative exercise training regimens before colorectal surgery on functional capacity. Eur. J. Anaesthesiol. 2020, 37, 969–978. [Google Scholar] [CrossRef]
- Polakowski, C.B.; Kato, M.; Preti, V.B.; Schieferdecker, M.E.M.; Campos, A.C.L. Impact of the preoperative use of synbiotics in colorectal cancer patients: A prospective, randomized, double-blind, placebo-controlled study. Nutrition 2019, 58, 40–46. [Google Scholar] [CrossRef]
- Onerup, A.; Andersson, J.; Angenete, E.; Bock, D.; Börjesson, M.; Ehrencrona, C.; Olsén, M.F.; Larsson, P.-A.; de la Croix, H.; Wedin, A.; et al. Effect of Short-term Homebased Pre- and Postoperative Exercise on Recovery After Colorectal Cancer Surgery (PHYSSURG-C). Ann. Surg. 2021, 275, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Molenaar, C.J.L.; Minnella, E.M.; Coca-Martinez, M.; Cate, D.W.G.T.; Regis, M.; Awasthi, R.; Martínez-Palli, G.; López-Baamonde, M.; Sebio-Garcia, R.; Feo, C.V.; et al. Effect of Multimodal Prehabilitation on Reducing Postoperative Complications and Enhancing Functional Capacity Following Colorectal Cancer Surgery. JAMA Surg. 2023, 158, 572–581. [Google Scholar] [CrossRef] [PubMed]
- van Rooijen, S.; Carli, F.; Dalton, S.; Thomas, G.; Bojesen, R.; Le Guen, M.; Barizien, N.; Awasthi, R.; Minnella, E.; Beijer, S.; et al. Multimodal prehabilitation in colorectal cancer patients to improve functional capacity and reduce postoperative complications: The first international randomized controlled trial for multimodal prehabilitation. BMC Cancer 2019, 19, 98. [Google Scholar] [CrossRef]
- Domenghino, A.; Walbert, C.; Birrer, D.L.; Puhan, M.A.; Clavien, P.-A. Consensus recommendations on how to assess the quality of surgical interventions. Nat. Med. 2023, 29, 811–822. [Google Scholar] [CrossRef]
- Fiore, J.F.; Figueiredo, S.; Balvardi, S.; Lee, L.; Nauche, B.; Landry, T.; Mayo, N.E.; Feldman, L.S. How Do We Value Postoperative Recovery? Ann. Surg. 2018, 267, 656–669. [Google Scholar] [CrossRef]
- Burch, J.; Taylor, C. Patients’ need for nursing telephone follow-up after enhanced recovery. Gastrointest. Nurs. 2012, 10, 51–58. [Google Scholar] [CrossRef]
- Anderson, M. Mobile Technology and Home Broadband 2019; Pew Research Center: Washington, DC, USA, 2019; Available online: https://www.pewresearch.org/internet/2019/06/13/mobile-technology-and-home-broadband-2019/ (accessed on 1 March 2025).
- Krebs, P.; Duncan, D.T. Health App Use Among US Mobile Phone Owners: A National Survey. JMIR mHealth uHealth 2015, 3, e101. [Google Scholar] [CrossRef]
- Kumar, D.; Arya, M. mHealth is an Innovative Approach to Address Health Literacy and Improve Patient-Physician Communication—An HIV Testing Exemplar. J. Mob. Technol. Med. 2015, 4, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Eustache, J.; El-Kefraoui, C.; Ekmekjian, T.; Latimer, E.; Lee, L. Do postoperative telemedicine interventions with a communication feature reduce emergency department visits and readmissions?—A systematic review and meta-analysis. Surg. Endosc. 2021, 35, 5889–5904. [Google Scholar] [CrossRef] [PubMed]
- van Staalduinen, D.J.; van den Bekerom, P.; Groeneveld, S.; Kidanemariam, M.; Stiggelbout, A.M.; van den Akker-van Marle, M.E. The implementation of value-based healthcare: A scoping review. BMC Health Serv. Res. 2022, 22, 270. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marin-Garcia, J.A.; Vidal-Carreras, P.I.; Garcia-Sabater, J.J. The Role of Value Stream Mapping in Healthcare Services: A Scoping Review. Int. J. Environ. Res. Public Health 2021, 18, 951. [Google Scholar] [CrossRef]
- Mason, S.; Nicolay, C.; Darzi, A. The use of Lean and Six Sigma methodologies in surgery: A systematic review. Surgeon 2015, 13, 91–100. [Google Scholar] [CrossRef]
- Goyal, S.; Law, E. An introduction to Kaizen in health care. Br. J. Hosp. Med. 2019, 80, 168–169. [Google Scholar] [CrossRef]
- Gustafsson, U.; Scott, M.; Schwenk, W.; Demartines, N.; Roulin, D.; Francis, N.; McNaught, C.; MacFie, J.; Liberman, A.; Soop, M.; et al. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Clin. Nutr. 2012, 31, 783–800. [Google Scholar] [CrossRef]
- Slankamenac, K.; Nederlof, N.; Pessaux, P.; de Jonge, J.; Wijnhoven, B.P.L.; Breitenstein, S.; Oberkofler, C.E.; Graf, R.; Puhan, M.A.; Clavien, P.-A. The Comprehensive Complication Index. Ann. Surg. 2014, 260, 757–763. [Google Scholar] [CrossRef]
- The EuroQol Group. EuroQol-a new facility for the measurement of health-related quality of life. Health Policy 1990, 16, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, P.; Clavien, P.-A.; Hahnloser, D. Complications in colorectal surgery: Risk factors and preventive strategies. Patient Saf. Surg. 2010, 4, 5. [Google Scholar] [CrossRef]
- McDermott, F.D.; Heeney, A.; Kelly, E.M.; Steele, R.J.; Carlson, G.L.; Winter, D.C. Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. Br. J. Surg. 2015, 102, 462–479. [Google Scholar] [CrossRef] [PubMed]
- Govaert, J.; Fiocco, M.; van Dijk, W.; Scheffer, A.; de Graaf, E.; Tollenaar, R.; Wouters, M.; Lamme, B.; Hess, D.; Belgers, H.; et al. Costs of complications after colorectal cancer surgery in the Netherlands: Building the business case for hospitals. Eur. J. Surg. Oncol. 2015, 41, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Popa, V.; Geissler, J.; Vermeulen, R.; Priest, E.; Capperella, K.; Susuzlu, G.; Terry, S.F.; Brooke, N. Delivering Digital Health Solutions that Patients Need: A Call to Action. Ther. Innov. Regul. Sci. 2023, 58, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Boletín Oficial del Estado. Real Decreto 311/2022, de 3 de mayo, por el que se Regula el Esquema Nacional de Seguridad; Boletín Oficial del Estado: Madrid, Spain, 2022; Volume 112, pp. 54321–54350. Available online: https://www.boe.es/buscar/act.php?id=BOE-A-2022-7191 (accessed on 10 March 2025).
- He, M.; Chen, M.; Ji, Y.; Lu, G. Effectiveness of smartphone app-based interventions after surgery on quality of recovery among cancer patients: A systematic review and meta-analysis. Ann. Med. 2024, 56, 2390167. [Google Scholar] [CrossRef]
- Carli, F.; Bousquet-Dion, G.; Awasthi, R.; Elsherbini, N.; Liberman, S.; Boutros, M.; Stein, B.; Charlebois, P.; Ghitulescu, G.; Morin, N.; et al. Effect of Multimodal Prehabilitation vs Postoperative Rehabilitation on 30-Day Postoperative Complications for Frail Patients Undergoing Resection of Colorectal Cancer. JAMA Surg. 2020, 155, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Gloor, S.; Misirlic, M.; Frei-Lanter, C.; Herzog, P.; Müller, P.; Schäfli-Thurnherr, J.; Lamdark, T.; Schregel, D.; Wyss, R.; Unger, I.; et al. Prehabilitation in patients undergoing colorectal surgery fails to confer reduction in overall morbidity: Results of a single-center, blinded, randomized controlled trial. Langenbeck’s Arch. Surg. 2022, 407, 897–907. [Google Scholar] [CrossRef]
- Forsmo, H.M.; Pfeffer, F.; Rasdal, A.; Østgaard, G.; Mohn, A.C.; Körner, H.; Erichsen, C. Compliance with enhanced recovery after surgery criteria and preoperative and postoperative counselling reduces length of hospital stay in colorectal surgery: Results of a randomized controlled trial. Color. Dis. 2016, 18, 603–611. [Google Scholar] [CrossRef]
- Lv, L.; Shao, Y.-F.; Zhou, Y.-B. The enhanced recovery after surgery (ERAS) pathway for patients undergoing colorectal surgery: An update of meta-analysis of randomized controlled trials. Int. J. Color. Dis. 2012, 27, 1549–1554. [Google Scholar] [CrossRef]
- Berian, J.R.; Ko, C.Y.; Ban, K.A. Does Implementation of Enhanced Recovery after Surgery (ERAS) Protocols in Colorectal Surgery Improve Patient Outcomes? Clin. Colon Rectal Surg. 2019, 32, 109–113. [Google Scholar] [CrossRef]
- van Waart, H.; Stuiver, M.M.; van Harten, W.H.; Sonke, G.S.; Aaronson, N.K. Design of the Physical exercise during Adjuvant Chemotherapy Effectiveness Study (PACES): A randomized controlled trial to evaluate effectiveness and cost-effectiveness of physical exercise in improving physical fitness and reducing fatigue. BMC Cancer 2010, 10, 673. [Google Scholar] [CrossRef]
- van Rooijen, S.J.; Engelen, M.A.; Scheede-Bergdahl, C.; Carli, F.; Roumen, R.M.H.; Slooter, G.D.; Schep, G. Systematic review of exercise training in colorectal cancer patients during treatment. Scand. J. Med. Sci. Sports 2017, 28, 360–370. [Google Scholar] [CrossRef] [PubMed]
- De Backer, I.C.; Van Breda, E.; Vreugdenhil, A.; Nijziel, M.R.; Kester, A.D.; Schep, G. High-intensity strength training improves quality of life in cancer survivors. Acta Oncol. 2007, 46, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Porter, M.E. Value-Based Health Care Delivery. Ann. Surg. 2008, 248, 503–509. [Google Scholar] [CrossRef]
- Feldman, L.S.; Lee, L.; Fiore, J. What outcomes are important in the assessment of Enhanced Recovery After Surgery (ERAS) pathways? Can. J. Anaesth. 2014, 62, 120–130. [Google Scholar] [CrossRef]
- Krogsgaard, M.R.; Brodersen, J.; Christensen, K.B.; Siersma, V.; Kreiner, S.; Jensen, J.; Hansen, C.F.; Comins, J.D. What is a PROM and why do we need it? Scand. J. Med. Sci. Sports 2020, 31, 967–971. [Google Scholar] [CrossRef]
- Lau, J.; Ng, J.S.; Lee, D.; Tan, J.K.; Tan, L.L.Y.; Pang, N.Q.; Tham, S.Y.; Ng, C.K.; Tan, K.K. Use of patient-reported experience and outcome measures within the colorectal cancer care continuum: A scoping review. J. Cancer Surviv. 2024. [Google Scholar] [CrossRef] [PubMed]
- Iroz, C.B.; Johnson, J.K.; Ager, M.S.; Joung, R.H.-S.; Brajcich, B.C.; Cella, D.; Franklin, P.D.; Holl, J.L.; Bilimoria, K.Y.; Merkow, R.P. Barriers and Facilitators to Implementing Patient-Reported Outcome Monitoring in Gastrointestinal Surgery. J. Surg. Res. 2023, 288, 341–349. [Google Scholar] [CrossRef] [PubMed]

| Men | Women | |||
|---|---|---|---|---|
| N | % | N | % | |
| Demographics | ||||
| N | 142 | 62.56% | 85 | 37.44% |
| Age (mean) years | 67 | 70 | ||
| <50 | 9 | 3.96% | 5 | 2.20% |
| 50–75 | 95 | 41.85% | 42 | 18.50% |
| >75 | 38 | 16.74% | 38 | 16.74% |
| Diagnosis | ||||
| Right colon cancer | 36 | 18.75% | 19 | 9.90% |
| Rectal cancer | 35 | 18.23% | 21 | 10.94% |
| Sigmoid colon cancer | 33 | 17.19% | 17 | 8.85% |
| Left colon cancer | 12 | 6.25% | 10 | 5.21% |
| Cecum cancer | 2 | 1.04% | 4 | 2.08% |
| Transverse colon cancer | 3 | 1.56% | - | - |
| Procedure | ||||
| Right hemicolectomy | 41 | 20.60% | 23 | 11.56% |
| Sigmoidectomy | 33 | 16.58% | 15 | 7.54% |
| Anterior rectal resection | 25 | 12.56% | 20 | 10.05% |
| Left hemicolectomy | 16 | 8.04% | 8 | 4.02% |
| Local resection | 3 | 1.51% | 5 | 2.51% |
| Abdominoperineal resection | 3 | 1.51% | 2 | 1.01% |
| Exploratory Laparotomy | 1 | 0.50% | 1 | 0.50% |
| Pancolectomy | 1 | 0.50% | 1 | 0.50% |
| Subtotal colectomy | 1 | 0.50% | - | - |
| Medical history and risk factors | ||||
| Smoker | 12 | 6.86% | 5 | 2.86% |
| Former smoker | 54 | 30.86% | 21 | 12% |
| Alcohol consumption (>14 consumptions/week) | 49 | 28% | 13 | 7.43% |
| Diabetes | 19 | 11.66% | 10 | 6.13% |
| Hypertension | 49 | 30.06% | 31 | 19.02% |
| Overweight (BMI: 25–30) | 47 | 26.55% | 28 | 15.82% |
| Obesity (BMI: >30) | 34 | 19.21% | 14 | 7.91% |
| Cardiovascular disease | ||||
| Heart disease | 20 | 12.27% | 9 | 5.52% |
| Arrhythmia | 11 | 37.93% | 2 | 6.9% |
| Myocardial ischemia history | 7 | 24.14% | 2 | 6.9% |
| Chronic kidney disease | 12 | 7.36% | 6 | 3.68% |
| Lung disease | 11 | 6.75% | 11 | 6.75% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaparro-Mirete, M.; Callejas, C.G.; García-Martínez, M.d.l.Á.; Ramos-Sanfiel, J.; Zurita-Saavedra, M.S.; De Castro-Monedero, P.; Gómez-Sánchez, J.; Argote-Camacho, Á.; Ubiña-Martínez, A.; González-Puga, C.; et al. Preliminary Outcomes of a Digital Remote Care Solution for Colorectal Cancer Patients. Cancers 2025, 17, 2622. https://doi.org/10.3390/cancers17162622
Chaparro-Mirete M, Callejas CG, García-Martínez MdlÁ, Ramos-Sanfiel J, Zurita-Saavedra MS, De Castro-Monedero P, Gómez-Sánchez J, Argote-Camacho Á, Ubiña-Martínez A, González-Puga C, et al. Preliminary Outcomes of a Digital Remote Care Solution for Colorectal Cancer Patients. Cancers. 2025; 17(16):2622. https://doi.org/10.3390/cancers17162622
Chicago/Turabian StyleChaparro-Mirete, Marta, Cristina González Callejas, María de los Ángeles García-Martínez, Jorge Ramos-Sanfiel, Maria Sol Zurita-Saavedra, Paola De Castro-Monedero, Javier Gómez-Sánchez, Ángela Argote-Camacho, Alfredo Ubiña-Martínez, Cristina González-Puga, and et al. 2025. "Preliminary Outcomes of a Digital Remote Care Solution for Colorectal Cancer Patients" Cancers 17, no. 16: 2622. https://doi.org/10.3390/cancers17162622
APA StyleChaparro-Mirete, M., Callejas, C. G., García-Martínez, M. d. l. Á., Ramos-Sanfiel, J., Zurita-Saavedra, M. S., De Castro-Monedero, P., Gómez-Sánchez, J., Argote-Camacho, Á., Ubiña-Martínez, A., González-Puga, C., Garde-Lecumberri, C., Nestares, T., & Mirón-Pozo, B. (2025). Preliminary Outcomes of a Digital Remote Care Solution for Colorectal Cancer Patients. Cancers, 17(16), 2622. https://doi.org/10.3390/cancers17162622





