YAP/TAZ Promote GLUT1 Expression and Are Associated with Prognosis in Endometrial Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Small Interfering RNA (siRNA) Knockdown and Adenovirus Infection
2.3. RNA Isolation and RT-qPCR Analysis
2.4. Patients
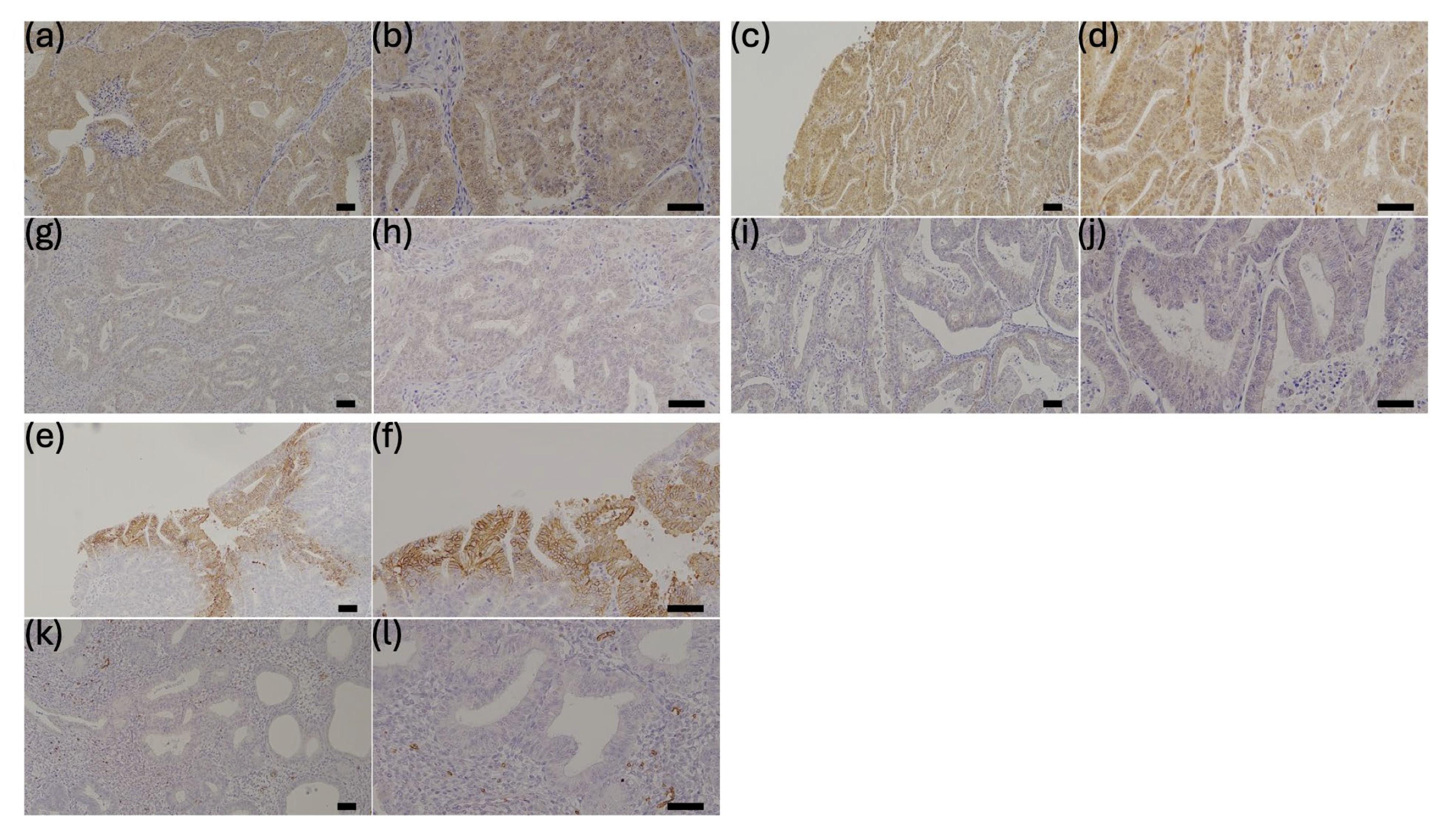
2.5. Tissue Samples and Immunohistochemistry
2.6. Statistical Analysis
3. Results
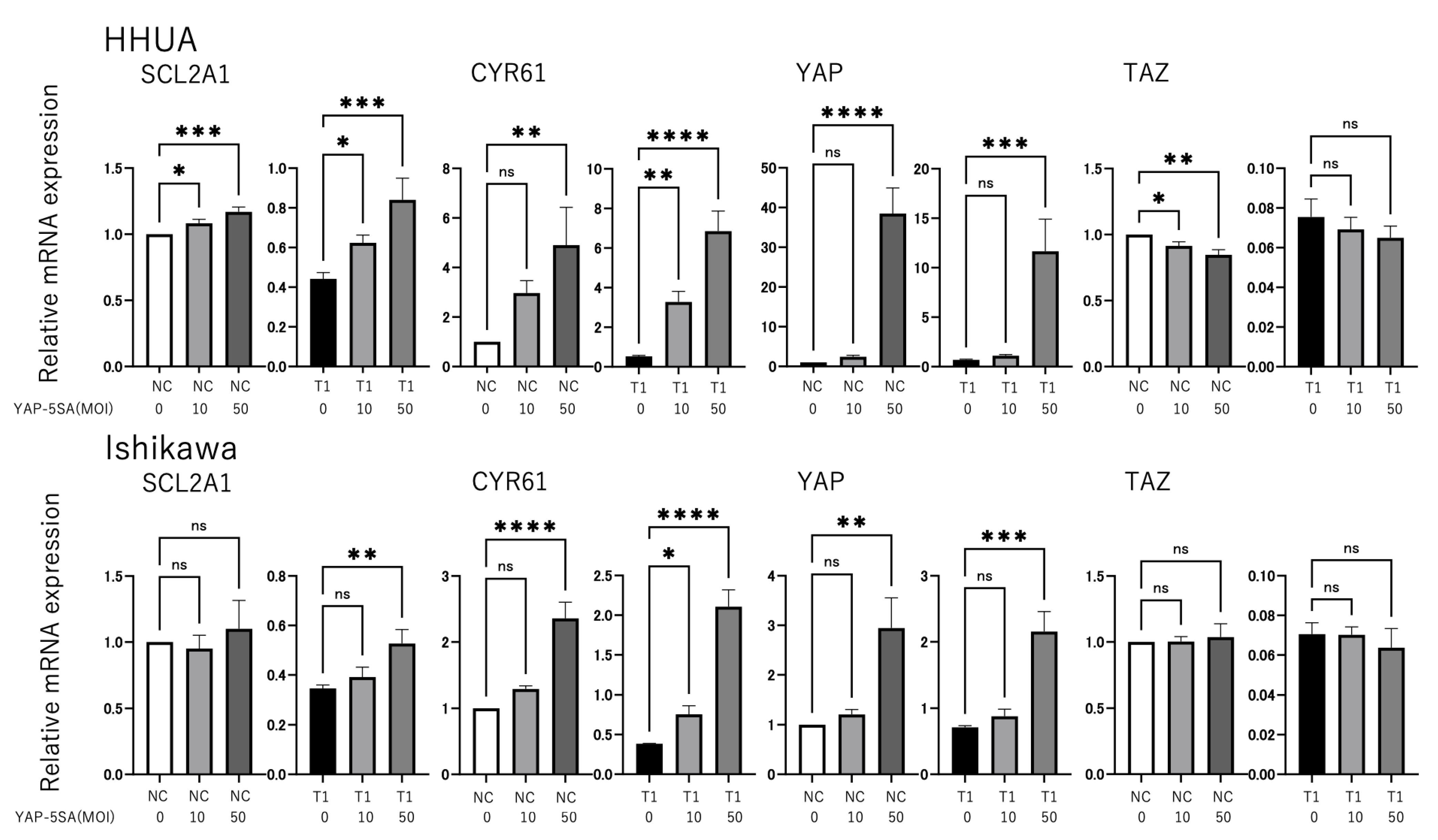
3.1. YAP and TAZ Positively Regulate GLUT1 Expression in Endometrial Cancer Cells
3.2. Patient Characteristics
3.3. Correlation of YAP, TAZ, and GLUT1 Expression and Association of Each Protein with Clinical Parameters
3.4. Correlation Between YAP and TAZ Expression and Clinicopathologic Parameters
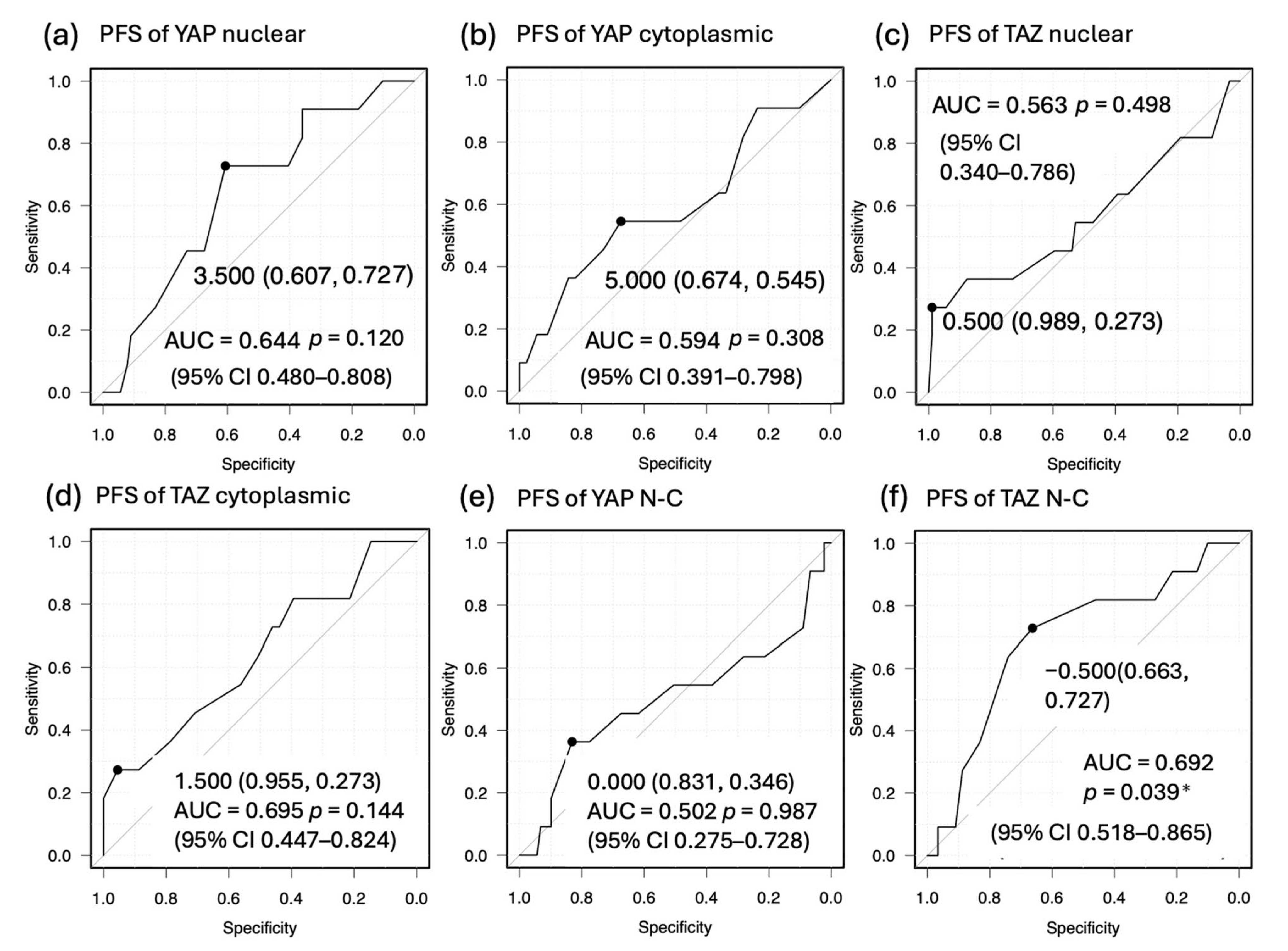
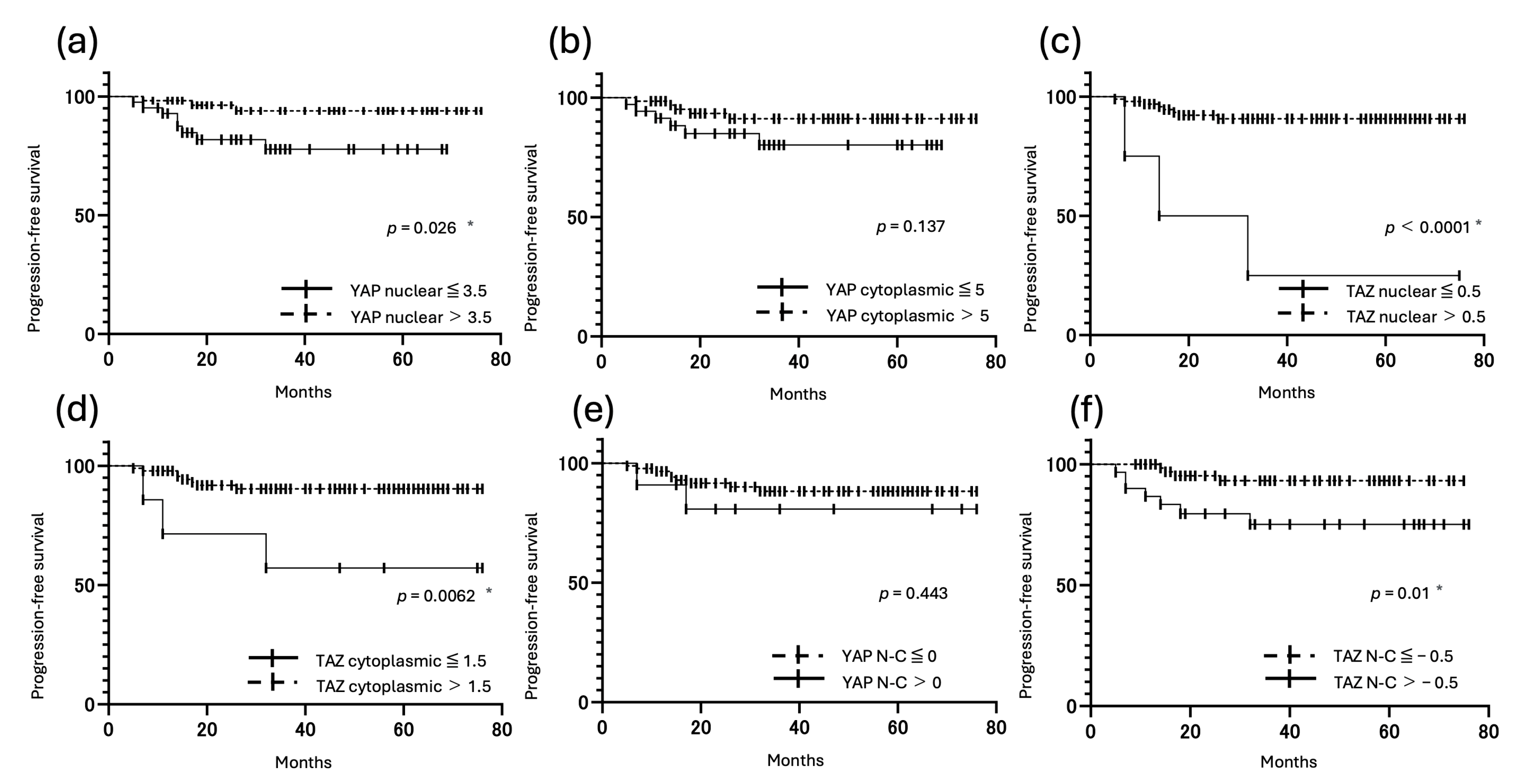
3.5. Correlation Between YAP and TAZ Expression and Prognosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | area under the curve |
| CI | confidence interval |
| CYR61 | cysteine-rich angiogenic inducer 61 |
| GLUT | glucose transporter |
| IRB | institutional review board |
| IRS | immunoreactivity score |
| LATS1/2 | large tumor suppressor kinase 1/2 |
| MOB1A/B | MOB kinase activator 1A/B |
| MST1/2 | mammalian STE20-like kinase 1/2 |
| OS | overall survival |
| PFS | progression-free survival |
| PP | percentage of positive cells |
| ROC | receiver operating characteristic |
| SAV1 | protein Salvador homologue 1 |
| SI | signal intensity |
| SLC2A1 | solute carrier family 2 member 1 |
| TAZ | transcriptional coactivator with PDZ-binding motif |
| TEAD | TEA domain |
| YAP | yes-associated protein |
| 5SA | 5 serine-to-alanine |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Makker, V.; MacKay, H.; Ray-Coquard, I.; Levine, D.A.; Westin, S.N.; Aoki, D.; Oaknin, A. Endometrial Cancer. Nat. Rev. Dis. Primers 2021, 7, 88. [Google Scholar] [CrossRef] [PubMed]
- Szablewski, L. Expression of Glucose Transporters in Cancers. Biochim. Biophys. Acta 2013, 1835, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Yongzhi, H.; Chen, S.; Luo, X.; Lin, Y.; Zhou, Y.; Jin, H.; Hou, B.; Deng, Y.; Tu, L.; et al. The Prognostic Value of GLUT1 in Cancers: A Systematic Review and Meta-Analysis. Oncotarget 2017, 8, 43356–43367. [Google Scholar] [CrossRef]
- Fu, M.; Hu, Y.; Lan, T.; Guan, K.-L.; Luo, T.; Luo, M. The Hippo Signalling Pathway and Its Implications in Human Health and Diseases. Signal Transduct. Target. Ther. 2022, 7, 376. [Google Scholar] [CrossRef]
- Shen, X.; Li, Q.; Sun, Y.; Chen, L.; Xue, F.; Tian, W.; Wang, Y. The Hippo Pathway in Endometrial Cancer: A Potential Therapeutic Target? Front. Oncol. 2023, 13, 1273345. [Google Scholar] [CrossRef]
- Tsujiura, M.; Mazack, V.; Sudol, M.; Kaspar, H.G.; Nash, J.; Carey, D.J.; Gogoi, R. Yes-Associated Protein (YAP) Modulates Oncogenic Features and Radiation Sensitivity in Endometrial Cancer. PLoS ONE 2014, 9, e100974. [Google Scholar] [CrossRef]
- Kuo, C.-C.; Ling, H.-H.; Chiang, M.-C.; Chung, C.-H.; Lee, W.-Y.; Chu, C.-Y.; Wu, Y.-C.; Chen, C.-H.; Lai, Y.-W.; Tsai, I.-L.; et al. Metastatic Colorectal Cancer Rewrites Metabolic Program through a Glut3-YAP-Dependent Signaling Circuit. Theranostics 2019, 9, 2526–2540. [Google Scholar] [CrossRef]
- Cox, A.G.; Tsomides, A.; Yimlamai, D.; Hwang, K.L.; Miesfeld, J.; Galli, G.G.; Fowl, B.H.; Fort, M.; Ma, K.Y.; Sullivan, M.R.; et al. Yap Regulates Glucose Utilization and Sustains Nucleotide Synthesis to Enable Organ Growth. EMBO J. 2018, 37, e100294. [Google Scholar] [CrossRef]
- Lin, C.; Xu, X. YAP1-TEAD1-Glut1 Axis Dictates the Oncogenic Phenotypes of Breast Cancer Cells by Modulating Glycolysis. Biomed. Pharmacother. 2017, 95, 789–794. [Google Scholar] [CrossRef]
- Peng, C.; Zhu, Y.; Zhang, W.; Liao, Q.; Chen, Y.; Zhao, X.; Guo, Q.; Shen, P.; Zhen, B.; Qian, X.; et al. Regulation of the Hippo-YAP Pathway by Glucose Sensor O-GlcNAcylation. Mol. Cell 2017, 68, 591–604.e5. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Parsels, J.D.; Lohse, I.; Lawrence, T.S.; Pasca di Magliano, M.; Sun, Y.; Morgan, M.A. Fbxw7 Deletion Accelerates KrasG12D-Driven Pancreatic Tumorigenesis via Yap Accumulation. Neoplasia 2016, 18, 666–673. [Google Scholar] [CrossRef]
- Rodríguez-Núñez, P.; Romero-Pérez, L.; Amaral, A.T.; Puerto-Camacho, P.; Jordán, C.; Marcilla, D.; Grünewald, T.G.; Alonso, J.; de Alava, E.; Díaz-Martín, J. Hippo Pathway Effectors YAP1/TAZ Induce an EWS-FLI1-Opposing Gene Signature and Associate with Disease Progression in Ewing Sarcoma. J. Pathol. 2020, 250, 374–386. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-H.; Ding, C.-K.C.; Sun, T.; Rupprecht, G.; Lin, C.-C.; Hsu, D.; Chi, J.-T. The Hippo Pathway Effector TAZ Regulates Ferroptosis in Renal Cell Carcinoma. Cell Rep. 2019, 28, 2501–2508.e4. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Wei, X.; Li, W.; Udan, R.S.; Yang, Q.; Kim, J.; Xie, J.; Ikenoue, T.; Yu, J.; Li, L.; et al. Inactivation of YAP Oncoprotein by the Hippo Pathway Is Involved in Cell Contact Inhibition and Tissue Growth Control. Genes Dev. 2007, 21, 2747–2761. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, T.; Orisaka, M.; Kawabe, S.; Morichika, R.; Uesaka, M.; Yoshida, Y. YAP/TAZ-TEAD Is a Novel Transcriptional Regulator of Genes Encoding Steroidogenic Enzymes in Rat Granulosa Cells and KGN Cells. Mol. Cell. Endocrinol. 2023, 559, 111808. [Google Scholar] [CrossRef]
- Mizutani, T.; Orisaka, M.; Miyazaki, Y.; Morichika, R.; Uesaka, M.; Miyamoto, K.; Yoshida, Y. Inhibition of YAP/TAZ-TEAD Activity Induces Cytotrophoblast Differentiation into Syncytiotrophoblast in Human Trophoblast. Mol. Hum. Reprod. 2022, 28, gaac032. [Google Scholar] [CrossRef]
- Lv, X.; He, C.; Huang, C.; Wang, H.; Hua, G.; Wang, Z.; Zhou, J.; Chen, X.; Ma, B.; Timm, B.K.; et al. Timely Expression and Activation of YAP1 in Granulosa Cells is Essential for Ovarian Follicle Development. FASEB J. 2019, 33, 10049–10064. [Google Scholar] [CrossRef]
- Kaumeyer, B.A.; Fidai, S.S.; Thakral, B.; Wang, S.A.; Arber, D.A.; Cheng, J.X.; Gurbuxani, S.; Venkataraman, G. GLUT1 Immunohistochemistry is a Highly Sensitive and Relatively Specific Marker for Erythroid Lineage in Benign and Malignant Hematopoietic Tissues. Am. J. Clin. Pathol. 2022, 158, 228–234. [Google Scholar] [CrossRef]
- Yoshida, Y.; Kurokawa, T.; Horiuchi, Y.; Sawamura, Y.; Shinagawa, A.; Kotsuji, F. Localisation of Phosphorylated MTOR Expression is Critical to Tumour Progression and Outcomes in Patients with Endometrial Cancer. Eur. J. Cancer 2010, 46, 3445–3452. [Google Scholar] [CrossRef]
- Vermij, L.; Jobsen, J.J.; León-Castillo, A.; Brinkhuis, M.; Roothaan, S.; Powell, M.E.; de Boer, S.M.; Khaw, P.; Mileshkin, L.R.; Fyles, A.; et al. Prognostic Refinement of NSMP High-Risk Endometrial Cancers Using Oestrogen Receptor Immunohistochemistry. Br. J. Cancer 2023, 128, 1360–1368. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the Freely Available Easy-to-Use Software “EZR” for Medical Statistics. Bone Marrow Transpl. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Japan Society of Gynecologic Oncology. Guidelines for Treatment of Uterine Body Neoplasms 2018 Edition; Kanehara & Co., Ltd.: Tokyo, Japan, 2018. [Google Scholar]
- Callus, B.A.; Finch-Edmondson, M.L.; Fletcher, S.; Wilton, S.D. YAPping about and Not Forgetting TAZ. FEBS Lett. 2019, 593, 253–276. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Vega, F.; Mina, M.; Armenia, J.; Chatila, W.K.; Luna, A.; La, K.C.; Dimitriadoy, S.; Liu, D.L.; Kantheti, H.S.; Saghafinia, S.; et al. Oncogenic Signaling Pathways in The Cancer Genome Atlas. Cell 2018, 173, 321–337.e10. [Google Scholar] [CrossRef]
- Ma, S.; Meng, Z.; Chen, R.; Guan, K.-L. The Hippo Pathway: Biology and Pathophysiology. Annu. Rev. Biochem. 2019, 88, 577–604. [Google Scholar] [CrossRef]
- Zanconato, F.; Cordenonsi, M.; Piccolo, S. YAP/TAZ at the Roots of Cancer. Cancer Cell 2016, 29, 783–803. [Google Scholar] [CrossRef]
- Gong, W.; Han, Z.; Fang, F.; Chen, L. Yap Expression is Closely Related to Tumor Angiogenesis and Poor Prognosis in Hepatoblastoma. Fetal Pediatr. Pathol. 2022, 41, 929–939. [Google Scholar] [CrossRef]
- Soyama, H.; Nishio, M.; Otani, J.; Sakuma, T.; Takao, S.; Hara, S.; Masuda, T.; Mimori, K.; Toyokuni, S.; Lydon, J.P.; et al. Hippo-TAZ Signaling Is the Master Regulator of the Onset of Triple-Negative Basal-like Breast Cancers. Proc. Natl. Acad. Sci. USA 2022, 119, e2123134119. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Banno, K.; Kunitomi, H.; Takahashi, T.; Takeda, T.; Nakamura, K.; Tsuji, K.; Tominaga, E.; Aoki, D. Warburg Effect in Gynecologic Cancers: Warburg Effect in Gynecologic Cancers. J. Obstet. Gynaecol. Res. 2019, 45, 542–548. [Google Scholar] [CrossRef]
- Goldman, N.A.; Katz, E.B.; Glenn, A.S.; Weldon, R.H.; Jones, J.G.; Lynch, U.; Fezzari, M.J.; Runowicz, C.D.; Goldberg, G.L.; Charron, M.J. GLUT1 and GLUT8 in Endometrium and Endometrial Adenocarcinoma. Mod. Pathol. 2006, 19, 1429–1436. [Google Scholar] [CrossRef]
- Song, D.H.; Jo, J.Y.; Kim, C.H.; Kim, M.H.; Cho, I.A.; Shin, J.K.; Choi, W.J.; Baek, J.C. Hypoxia-Regulated Proteins: Expression in Endometrial Cancer and Their Association with Clinicopathologic Features. Diagnostics 2024, 14, 1735. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Rago, C.; Cheong, I.; Pagliarini, R.; Angenendt, P.; Rajagopalan, H.; Schmidt, K.; Willson, J.K.V.; Markowitz, S.; Zhou, S.; et al. Glucose Deprivation Contributes to the Development of KRAS Pathway Mutations in Tumor Cells. Science 2009, 325, 1555–1559. [Google Scholar] [CrossRef] [PubMed]
- Misra, J.R.; Irvine, K.D. The Hippo Signaling Network and Its Biological Functions. Annu. Rev. Genet. 2018, 52, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.G.; Moroishi, T.; Guan, K.-L. YAP and TAZ: A Nexus for Hippo Signaling and Beyond. Trends Cell Biol. 2015, 25, 499–513. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, Z.; Shi, Y.; Sun, F. AMOT is Required for YAP Function in High Glucose Induced Liver Malignancy. Biochem. Biophys. Res. Commun. 2018, 495, 1555–1561. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Huang, H.-Y.; Lin, Z.; Ranieri, M.; Li, S.; Sahu, S.; Liu, Y.; Ban, Y.; Guidry, K.; Hu, H.; et al. Genome-Wide CRISPR Screens Identify Multiple Synthetic Lethal Targets That Enhance KRASG12C Inhibitor Efficacy. Cancer Res. 2023, 83, 4095–4111. [Google Scholar] [CrossRef]
- Edwards, A.C.; Stalnecker, C.A.; Jean Morales, A.; Taylor, K.E.; Klomp, J.E.; Klomp, J.A.; Waters, A.M.; Sudhakar, N.; Hallin, J.; Tang, T.T.; et al. TEAD Inhibition Overcomes YAP1/TAZ-Driven Primary and Acquired Resistance to KRASG12C Inhibitors. Cancer Res. 2023, 83, 4112–4129. [Google Scholar] [CrossRef]
- Byron, S.A.; Gartside, M.; Powell, M.A.; Wellens, C.L.; Gao, F.; Mutch, D.G.; Goodfellow, P.J.; Pollock, P.M. FGFR2 Point Mutations in 466 Endometrioid Endometrial Tumors: Relationship with MSI, KRAS, PIK3CA, CTNNB1 Mutations and Clinicopathological Features. PLoS ONE 2012, 7, e30801. [Google Scholar] [CrossRef]





| Patient and Tumor Characteristics | |
|---|---|
| Characteristic | n |
| Total number of patients | 100 |
| Histology | |
| Endometrioid | |
| G1 | 65 |
| G2 | 12 |
| G3 | 13 |
| Nonendometrioid | |
| Mixed | 1 |
| Serous | 4 |
| Carcinosarcoma | 1 |
| Clear | 2 |
| Mesonephric | 1 |
| Small cell | 1 |
| Stage (FIGO 2008) | |
| IA | 58 |
| IB | 19 |
| II | 8 |
| III | 9 |
| IV | 6 |
| Treatment | |
| Surgery | 61 |
| Surgery + chemotherapy | 38 |
| Lymphadenectomy | 50 |
| Hormone therapy | 1 |
| Pathological features | |
| Myometrial invasion ≥ 1/2 | 37 |
| Tumor size ≥ 2 cm | 75 |
| Presence of LVSI | 29 |
| Presence of lymph-node metastasis | 10 |
| Patient outcomes | |
| Tumor progression | 11 |
| Death | 5 |
| Variable | Patients (n) | Nuclear YAP | Cytoplasmic YAP | YAP N-C | |||
|---|---|---|---|---|---|---|---|
| Mean ± SE | p | Mean ± SE | p | Mean ± SE | p | ||
| Age (y) | |||||||
| <50 | 12 | 4.792 ± 2.518 | 0.4756 | 6.667 ± 2.716 | 0.8315 | −1.875 ± 2.001 | 0.6875 |
| ≥50 | 88 | 4.165 ± 2.615 | 6.5 ± 3.071 | −2.335 ± 2.363 | |||
| FIGO | |||||||
| I–II | 85 | 4.418 ± 2.652 | 0.1156 | 6.818 ± 2.896 | 0.0197 * | −2.4 ± 2.22 | 0.1638 |
| III–IV | 15 | 3.233 ± 2.069 | 4.833 ± 3.244 | −1.6 ± 2.804 | |||
| Histology | |||||||
| Endometrioid G1 and G2 | 77 | 4.558 ± 2.565 | 0.0201 * | 6.734 ± 2.84 | 0.1963 | −2.175 ± 2.38 | 0.3211 |
| G3 and others | 23 | 3.174 ± 2.475 | 5.804 ± 3.528 | −2.63 ± 2.112 | |||
| Myometrial invasion | |||||||
| <1/2 | 63 | 4.595 ± 2.707 | 0.0934 | 7.095 ± 2.95 | 0.0179 * | −2.5 ± 2.584 | 0.2953 |
| ≥1/2 | 37 | 3.635 ± 2.314 | 5.541 ± 2.916 | −1.936 ± 1.777 | |||
| Tumor size | |||||||
| <2 cm | 25 | 4.96 ± 3.119 | 0.1801 | 7.68 ± 2.802 | 0.0238 * | −2.72 ± 2.517 | 0.075 |
| ≥2 cm | 75 | 4 ± 2.377 | 6.133 ± 3.006 | −2.133 ± 2.247 | |||
| LVSI | |||||||
| Absent | 71 | 4.57 ± 2.631 | 0.0448 * | 6.859 ± 3.026 | 0.0754 | −2.289 ± 2.42 | 0.8157 |
| Present | 29 | 3.431 ± 2.371 | 5.69 ± 2.883 | −2.259 ± 2.09 | |||
| Lymph-node metastasis | |||||||
| Absent | 90 | 4.406 ± 2.593 | 0.0479 * | 6.722 ± 2.883 | 0.0493 * | −2.317 ± 2.255 | 0.5001 |
| Present | 10 | 2.75 ± 2.252 | 4.7 ± 1.181 | −1.95 ± 2.948 | |||
| Nuclear TAZ | Cytoplasmic TAZ | TAZ N-C | GLUT1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Patients (n) | Mean ± SE | p | Mean ± SE | p | Mean ± SE | p | Mean ± SE | p |
| Age (y) | |||||||||
| <50 | 12 | 3.125 ± 1.932 | 0.0825 | 3.708 ± 1.751 | 0.009 * | −0.5833 ± 2.224 | 0.43 | 2.583 ± 1.222 | 0.0248 * |
| ≥50 | 88 | 4.483 ± 2.76 | 5.705 ± 2.842 | −1.222 ± 2.125 | 3.96 ± 2.172 | ||||
| FIGO | |||||||||
| I–II | 85 | 4.371 ± 2.629 | 0.6154 | 5.629 ± 2.798 | 0.2738 | −1.259 ± 2.162 | 0.0537 | 3.665 ± 1.983 | 0.1648 |
| III–IV | 15 | 4.033 ± 3.176 | 4.533 ± 2.748 | −0.5 ± 1.918 | 4.533 ± 2.768 | ||||
| Histology | |||||||||
| Endometrioid G1 and G2 | 77 | 4.182 ± 2.6 | 0.428 | 5.461 ± 2.821 | 0.8432 | −1.279 ± 2.21 | 0.0951 | 3.682 ± 2.042 | 0.3716 |
| G3 and others | 23 | 4.783 ± 3.037 | 5.478 ± 2.81 | −0.696 ± 1.839 | 4.174 ± 2.391 | ||||
| Myometrial invasion | |||||||||
| <1/2 | 63 | 4.068 ± 2.614 | 0.5881 | 5.754 ± 2.922 | 0.3203 | −1.286 ± 2.28 | 0.0833 | 3.492 ± 1.883 | 0.1123 |
| ≥1/2 | 37 | 4.468 ± 2.763 | 4.973 ± 2.555 | −0.905 ± 1.87 | 4.311 ± 2.425 | ||||
| Tumor size | |||||||||
| <2 cm | 25 | 4.44 ± 3.289 | 0.8658 | 4.94 ± 2.682 | 0.2579 | −0.5 ± 2.638 | 0.438 | 3.12 ± 2.078 | 0.094 |
| ≥2 cm | 75 | 4.28 ± 2.502 | 5.64 ± 2.84 | −1.36 ± 1.913 | 4.02 ± 2.106 | ||||
| LVSI | |||||||||
| Absent | 71 | 4.197 ± 2.671 | 0.3974 | 5.345 ± 2.767 | 0.548 | −1.148 ± 2.164 | 0.6691 | 3.507 ± 2.019 | 0.0421 * |
| Present | 29 | 4.621 ± 2.805 | 5.759 ± 2.92 | −1.138 ± 2.104 | 4.5 ± 2.248 | ||||
| Lymph-node metastasis | |||||||||
| Absent | 90 | 4.367 ± 2.651 | 0.6182 | 5.606 ± 2.767 | 0.2223 | −1.239 ± 2.174 | 0.0218 * | 3.8 ± 2.109 | 0.7996 |
| Present | 10 | 3.9 ± 3.264 | 4.2 ± 2.974 | −0.3 ± 1.602 | 3.75 ± 2.383 | ||||
| Variable | Univariate Analysis | p | Multivariate Analysis | p | Hazard Ratio (95% CI) | p |
|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | Hazard Ratio (95% CI) | |||||
| Age (≥50) | 1.14 × 10−11 | >0.9999 | ||||
| FIGO stage (III–IV) | 4.804 (11.36–302.7) | <0.0001 * | 20.5 (4.29–152.9) | 0.0006 * | 21.63 (4.192–167.6) | 0.0007 * |
| Histopathologic type (G3 and others) | 20.91 (5.347–137.7) | 0.0001 * | 5.691 (1.169–42.67) | 0.0484 * | 6.738 (1.328–51.35) | 0.0334 * |
| Myometrial invasion (≥1/2) | 8.958 (2.306–58.79) | 0.0051 * | ||||
| Tumor size (≥2 cm) | 9.47 × 10−5 | >0.9999 | ||||
| LVSI (present) | 7.499 (2.15–34.05) | 0.0031 * | ||||
| Lymph-node metastasis (present) | 31.01 (8.8–126) | <0.0001 * | ||||
| YAP nuclear (≤3.5) | 3.97 (1.147–18.13) | 0.0418 * | 1.793 (0.4546–9.031) | 0.43 | ||
| YAP cytoplasmic (≤5) | 2.142 (0.6172–7.116) | 0.2084 | ||||
| YAP N-C (>0) | 1.808 (0.2756–7.019) | 0.1504 | ||||
| TAZ nuclear (≤0.5) | 3.296 (0.8614–10.95) | 0.0576 | ||||
| TAZ cytoplasmic (≤1.5) | 0.3833 (0.05831–1.494) | 0.221 | ||||
| TAZ N-C (>−0.5) | 4.338 (1.309–16.57) | 0.0193 * | 0.8589 (0.2123–3.862) | 0.8335 |
| Variable | Univariate Analysis | p | Multivariate Analysis | p | Hazard Ratio (95% CI) | p | Hazard Ratio (95% CI) | p |
|---|---|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | Hazard Ratio (95% CI) | |||||||
| Age (≥50) | 88,465,320,564 | >0.9999 | ||||||
| FIGO stage (III–IV) | 37.09 (5.164–749.2) | 0.0016 * | 26.6 (2.361–606.2) | 0.0099 * | 22.77 (2.106–503.1) | 0.0122 * | 36.88 (4.907–767) | 0.0021 * |
| Histopathologic type (G3 and others) | 3.59966 × 1012 | >0.9999 | ||||||
| Myometrial invasion (≥1/2) | 1.53315 × 1012 | >0.9999 | ||||||
| Tumor size (≥2 cm) | 33,601,241,091 | >0.9999 | ||||||
| LVSI (present) | 3.982 (0.6506–29.83) | 0.1341 | ||||||
| Lymph-node metastasis (present) | 10.57 (1.255–89.1) | 0.0192 * | 3.76 × 10−5 | >0.9999 | ||||
| YAP nuclear (≤3.5) | 0.164 (0.008348–1.12) | 0.1074 | ||||||
| YAP cytoplasmic (≤4) | 0.2285 (0.2295–1.407) | 0.1106 | ||||||
| YAP N-C (>−3) | 0.4315 (0.05682–2.605) | 0.3573 | ||||||
| TAZ nuclear (≤1.5) | 0.127 (0.00167–0.7687) | 0.0024 * | 0.2493 (0.001093–2.843) | 0.2758 | 0.1964 (0.00864–2.165) | 0.1936 | ||
| TAZ cytoplasmic (≤6) | 2.43 × 10−12 | >0.9999 | ||||||
| TAZ N-C (>0) | 3.099 (0.4080–18.71) | 0.2155 | 2.486 (0.3137–16.37) | 0.3361 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujita, M.; Orisaka, M.; Mizutani, T.; Fujita, Y.; Onuma, T.; Tsuyoshi, H.; Yoshida, Y. YAP/TAZ Promote GLUT1 Expression and Are Associated with Prognosis in Endometrial Cancer. Cancers 2025, 17, 2554. https://doi.org/10.3390/cancers17152554
Fujita M, Orisaka M, Mizutani T, Fujita Y, Onuma T, Tsuyoshi H, Yoshida Y. YAP/TAZ Promote GLUT1 Expression and Are Associated with Prognosis in Endometrial Cancer. Cancers. 2025; 17(15):2554. https://doi.org/10.3390/cancers17152554
Chicago/Turabian StyleFujita, Masayuki, Makoto Orisaka, Tetsuya Mizutani, Yuko Fujita, Toshimichi Onuma, Hideaki Tsuyoshi, and Yoshio Yoshida. 2025. "YAP/TAZ Promote GLUT1 Expression and Are Associated with Prognosis in Endometrial Cancer" Cancers 17, no. 15: 2554. https://doi.org/10.3390/cancers17152554
APA StyleFujita, M., Orisaka, M., Mizutani, T., Fujita, Y., Onuma, T., Tsuyoshi, H., & Yoshida, Y. (2025). YAP/TAZ Promote GLUT1 Expression and Are Associated with Prognosis in Endometrial Cancer. Cancers, 17(15), 2554. https://doi.org/10.3390/cancers17152554






