Oncolytic Therapies for Glioblastoma: Advances, Challenges, and Future Perspectives
Simple Summary
Abstract
1. Introduction
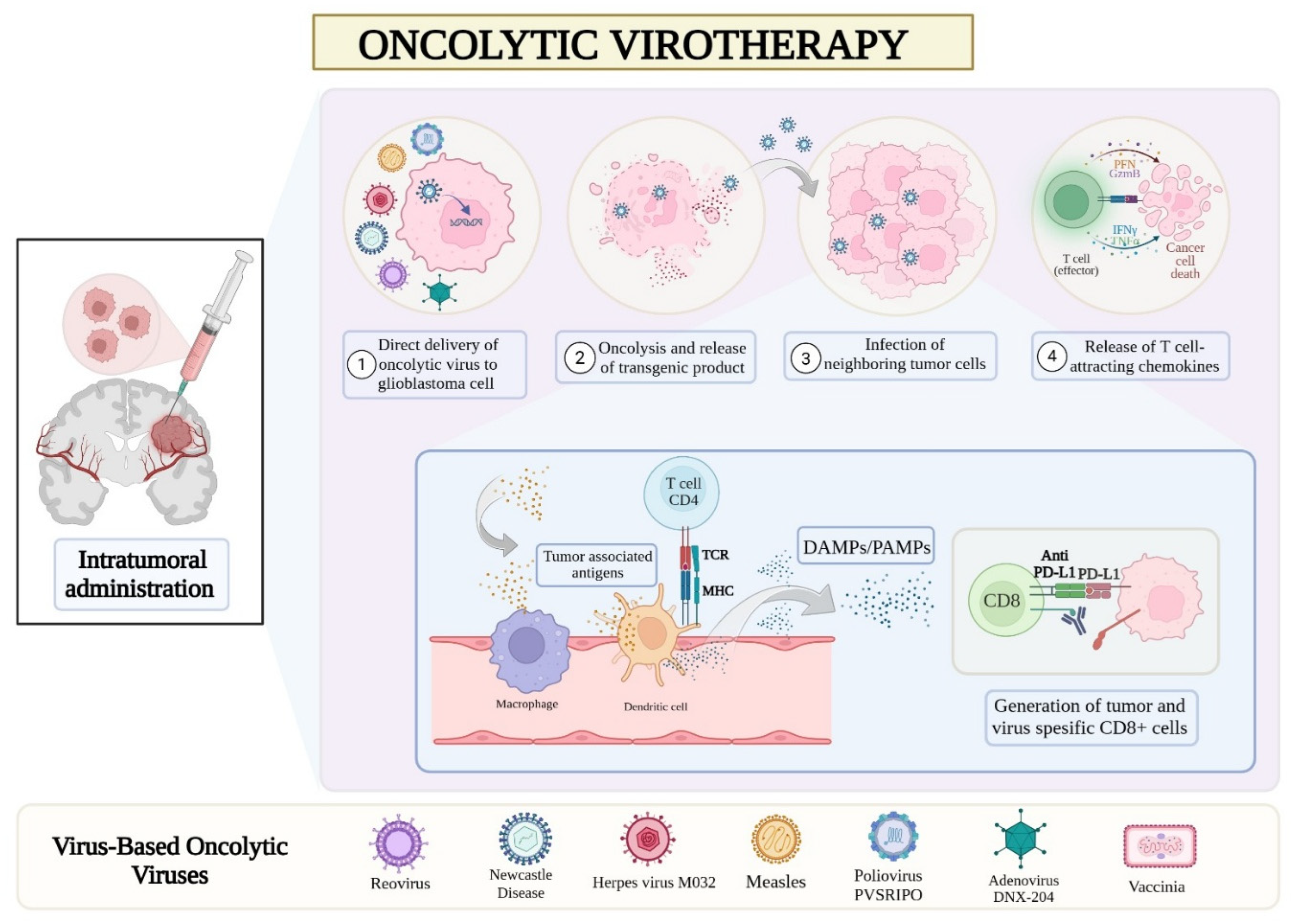
2. Virus-Based Oncolytic Therapies
2.1. Herpes Simplex Virus
2.1.1. Herpes Simplex Virus Oncolysis in Lab and Animal Studies
2.1.2. Herpes Simplex Virus Oncolysis in Clinical Studies
2.2. Adenovirus
2.2.1. Adenovirus Oncolysis in Lab and Animal Studies
2.2.2. Adenovirus Oncolysis in Clinical Studies
2.3. Measles Viruses
2.4. Newcastle Disease Virus
2.5. Reovirus
2.6. Retroviruses
2.7. Vaccinia Virus
2.8. Parvovirus H-1
2.9. Poliovirus
2.10. Zika Virus
3. Combination Oncolytic Therapies
3.1. Combination of OVs with Surgical Resection Strategies
3.2. Combination of OVs with Chemotherapeutic Agents
3.3. Combination of OVs with Radiotherapy
3.4. Combination of OVs with Immune Checkpoint Inhibitors
3.5. Arming OVs with Therapeutic Transgenes
4. Non-Viral Oncolytic Strategies
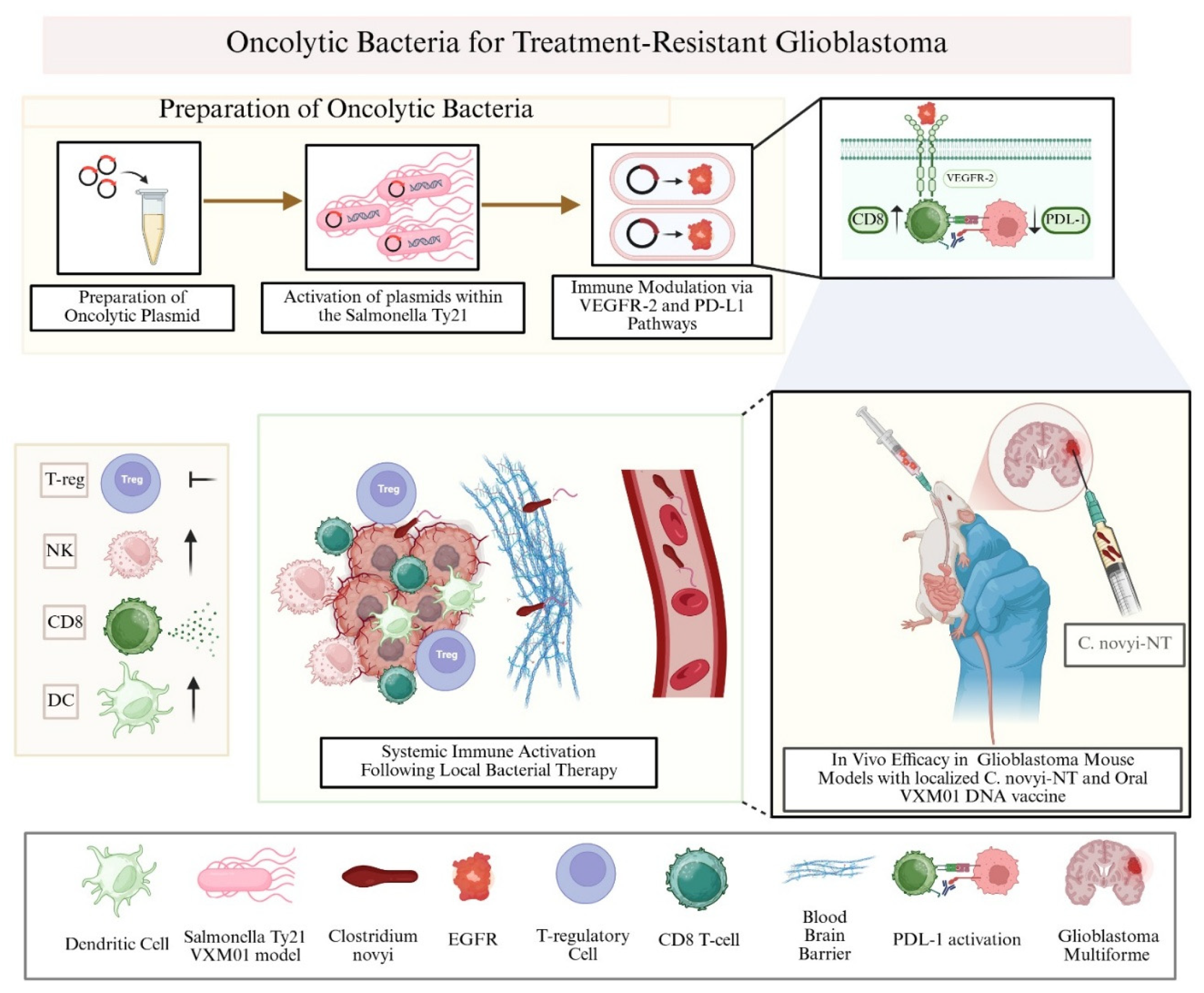
4.1. Oncolytic Bacteria
4.2. Oncolytic Peptides
5. Concerns and Future Directions in Oncolytic Therapies
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
References
- Miller, K.D.; Ostrom, Q.T.; Kruchko, C.; Patil, N.; Tihan, T.; Cioffi, G.; Brat, D.J.; Barnholtz-Sloan, J.S. Brain and other central nervous system tumor statistics, 2021. CA Cancer J. Clin. 2021, 71, 381–406. [Google Scholar] [CrossRef] [PubMed]
- Ohgaki, H.; Kleihues, P. The definition of primary and secondary glioblastoma. Clin. Cancer Res. 2013, 19, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee, S.U. Glioblastoma multiforme: A review of its epidemiology and pathogenesis through clinical presentation and treatment. Asian Pac. J. Cancer Prev. 2017, 18, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Angom, R.S.; Nakka, N.M.R.; Bhattacharya, S. Advances in glioblastoma therapy: An update on current approaches. Brain Sci. 2023, 13, 1536. [Google Scholar] [CrossRef] [PubMed]
- Ohgaki, H.; Dessen, P.; Jourde, B.; Horstmann, S.; Nishikawa, T.; Di Patre, P.L.; Burkhard, C.; Schuler, D.; Probst-Hensch, N.M.; Maiorka, P.C.; et al. Genetic pathways to glioblastoma: A population-based study. Cancer Res. 2004, 64, 6892–6899. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Klockow, J.L.; Zhang, M.; Lafortune, F.; Chang, E.; Jin, L.; Wu, Y.; Daldrup-Link, H.E. Glioblastoma multiforme (GBM): An overview of current therapies and mechanisms of resistance. Front. Oncol. 2021, 11, 769872. [Google Scholar] [CrossRef]
- Kreisl, T.N.; Kim, L.; Moore, K.; Duic, P.; Royce, C.; Stroud, I.; Garren, N.; Mackey, M.; Butman, J.A.; Camphausen, K.; et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J. Clin. Oncol. 2009, 27, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Gramatzki, D.; Roth, P.; Rushing, E.J.; Weller, J.; Andratschke, N.; Hofer, S.; Korol, D.; Regli, L.; Pangalu, A.; Pless, M.; et al. Bevacizumab may improve quality of life, but not overall survival in glioblastoma: An epidemiological study. Ann. Oncol. 2018, 29, 1431–1436. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; Le Rhun, E. How did lomustine become standard of care in recurrent glioblastoma? Cancer Treat. Rev. 2020, 87, 102029. [Google Scholar] [CrossRef] [PubMed]
- Salvato, I.; Marchini, A. Immunotherapeutic strategies for the treatment of glioblastoma: Current challenges and future perspectives. Cancers 2024, 16, 1276. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, F.; Ali, H.; Lathia, J.D.; Chen, P. Immunotherapy for glioblastoma: Current state, challenges, and future perspectives. Cell Mol. Immunol. 2024, 21, 1354–1375. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Y.; Zhang, A.; Yu, W.; Lei, Q.; Xiao, B.; Luo, Z. Investigational microbiological therapy for glioma. Cancers 2022, 14, 5977. [Google Scholar] [CrossRef] [PubMed]
- Hamad, A.; Yusubalieva, G.M.; Lipatova, A.V. Recent developments in glioblastoma therapy: Oncolytic viruses and emerging future strategies. Viruses 2023, 15, 547. [Google Scholar] [CrossRef] [PubMed]
- Asija, S.; Chatterjee, A.; Goda, J.S.; Yadav, S.; Chekuri, G.; Purwar, R. Oncolytic immunovirotherapy for high-grade gliomas: A novel and an evolving therapeutic option. Front. Immunol. 2023, 14, 1118246. [Google Scholar] [CrossRef] [PubMed]
- Todo, T.; Ito, H.; Ino, Y.; Ohtsu, H.; Ota, Y.; Shibahara, J.; Tanaka, M. Intratumoral oncolytic herpes virus G47∆ for residual or recurrent glioblastoma: A phase 2 trial. Nat. Med. 2022, 28, 1630–1639. [Google Scholar] [CrossRef] [PubMed]
- Shoaf, M.L.; Desjardins, A. Oncolytic viral therapy for malignant glioma and their application in clinical practice. Neurotherapeutics 2022, 19, 1732–1750. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, H.L.; Kohlhapp, F.J.; Zloza, A. Oncolytic viruses: A new class of immunotherapy drugs. Nat. Rev. Drug Discov. 2015, 14, 642–662. [Google Scholar] [CrossRef]
- Mihelson, N.; McGavern, D.B. Viral Control of Glioblastoma. Viruses 2021, 13, 1264. [Google Scholar] [CrossRef]
- Lin, D.; Shen, Y.; Liang, T. Oncolytic virotherapy: Basic principles, recent advances and future directions. Signal Transduct. Target. Ther. 2023, 8, 156. [Google Scholar] [CrossRef]
- Suzich, J.B.; Cliffe, A.R. Strength in diversity: Understanding the pathways to herpes simplex virus reactivation. Virology 2018, 522, 81–91. [Google Scholar] [CrossRef]
- Zhu, S.; Viejo-Borbolla, A. Pathogenesis and virulence of herpes simplex virus. Virulence 2021, 12, 2670–2702. [Google Scholar] [CrossRef]
- Ma, W.; He, H.; Wang, H. Oncolytic herpes simplex virus and immunotherapy. BMC Immunol. 2018, 19, 40. [Google Scholar] [CrossRef]
- Kardani, K.; Sanchez Gil, J.; Rabkin, S.D. Oncolytic herpes simplex viruses for the treatment of glioma and targeting glioblastoma stem-like cells. Front. Cell. Infect. Microbiol. 2023, 13, 1206111. [Google Scholar] [CrossRef]
- Miest, T.S.; Cattaneo, R. New viruses for cancer therapy: Meeting clinical needs. Nat. Rev. Microbiol. 2014, 12, 23–34. [Google Scholar] [CrossRef]
- Zhang, S.; Rabkin, S.D. The discovery and development of oncolytic viruses: Are they the future of cancer immunotherapy? Expert Opin. Drug Discov. 2021, 16, 391–410. [Google Scholar] [CrossRef]
- Conry, R.M.; Westbrook, B.; McKee, S.; Norwood, T.G. Talimogene laherparepvec: First in class oncolytic virotherapy. Hum. Vaccines Immunother. 2018, 14, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Kohlhapp, F.J.; Kaufman, H.L. Molecular Pathways: Mechanism of Action for Talimogene Laherparepvec, a New Oncolytic Virus Immunotherapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.J.; Peng, K.W. Oncolytic Virotherapy: A Contest between Apples and Oranges. Mol. Ther. J. Am. Soc. Gene Ther. 2017, 25, 1107–1116. [Google Scholar] [CrossRef]
- MartuzaR, L.; Malick, A.; Markert, J.M.; Ruffner, K.L.; Coen, D.M. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science 1991, 252, 854–856. [Google Scholar] [CrossRef]
- Sahu, U.; Mullarkey, M.P.; Pei, G.; Zhao, Z.; Hong, B.; Kaur, B. oHSV-P10 reduces glioma stem cell enrichment after oncolytic HSV therapy. Mol. Ther. Oncolytics 2023, 29, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Russell, L.; Swanner, J.; Jaime-Ramirez, A.C.; Wang, Y.; Sprague, A.; Banasavadi-Siddegowda, Y.; Yoo, J.Y.; Sizemore, G.M.; Kladney, R.; Zhang, J.; et al. PTEN expression by an oncolytic herpesvirus directs T-cell mediated tumor clearance. Nat. Commun. 2018, 9, 5006. [Google Scholar] [CrossRef]
- Rivas, T.; Goodrich, J.A.; Kugel, J.F. The Herpes Simplex Virus 1 Protein ICP4 Acts as both an Activator and a Repressor of Host Genome Transcription during Infection. Mol. Cell. Biol. 2021, 41, e0017121. [Google Scholar] [CrossRef] [PubMed]
- Reale, A.; Gatta, A.; Shaik, A.K.B.; Shallak, M.; Chiaravalli, A.M.; Cerati, M.; Zaccaria, M.; La Rosa, S.; Calistri, A.; Accolla, R.S.; et al. An oncolytic HSV-1 vector induces a therapeutic adaptive immune response against glioblastoma. J. Transl. Med. 2024, 22, 862. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, X.; Chen, X.; Liu, Y.; Huang, Y.; Cheng, Y.; Ren, P.; Zhao, J.; Zhou, G.G. Enhanced therapeutic efficacy for glioblastoma immunotherapy with an oncolytic herpes simplex virus armed with anti-PD-1 antibody and IL-12. Mol. Ther. Oncol. 2024, 32, 200799. [Google Scholar] [CrossRef]
- Bommareddy, P.K.; Wakimoto, H.; Martuza, R.L.; Kaufman, H.L.; Rabkin, S.D.; Saha, D. Oncolytic herpes simplex virus expressing IL-2 controls glioblastoma growth and improves survival. J. Immunother. Cancer 2024, 12, e008880. [Google Scholar] [CrossRef]
- Saha, D.; Martuza, R.L.; Rabkin, S.D. Macrophage Polarization Contributes to Glioblastoma Eradication by Combination Immunovirotherapy and Immune Checkpoint Blockade. Cancer Cell 2017, 32, 253–267.e5. [Google Scholar] [CrossRef]
- Alessandrini, F.; Menotti, L.; Avitabile, E.; Appolloni, I.; Ceresa, D.; Marubbi, D.; Campadelli-Fiume, G.; Malatesta, P. Eradication of glioblastoma by immuno-virotherapy with a retargeted oncolytic HSV in a preclinical model. Oncogene 2019, 38, 4467–4479. [Google Scholar] [CrossRef] [PubMed]
- Appolloni, I.; Alessandrini, F.; Menotti, L.; Avitabile, E.; Marubbi, D.; Piga, N.; Ceresa, D.; Piaggio, F.; Campadelli-Fiume, G.; Malatesta, P. Specificity, Safety, Efficacy of EGFRvIII-Retargeted Oncolytic HSV for Xenotransplanted Human Glioblastoma. Viruses 2021, 13, 1677. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, X.; Ji, Q.; Fang, A.; Song, L.; Xu, X.; Lin, Y.; Peng, Y.; Yu, J.; Xie, L.; et al. OH2 oncolytic virus: A novel approach to glioblastoma intervention through direct targeting of tumor cells and augmentation of anti-tumor immune responses. Cancer Lett. 2024, 589, 216834. [Google Scholar] [CrossRef]
- Nakashima, H.; Nguyen, T.; Kasai, K.; Passaro, C.; Ito, H.; Goins, W.F.; Shaikh, I.; Erdelyi, R.; Nishihara, R.; Nakano, I.; et al. Toxicity and Efficacy of a Novel GADD34-expressing Oncolytic HSV-1 for the Treatment of Experimental Glioblastoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 2574–2584. [Google Scholar] [CrossRef]
- Nehama, D.; Di Ianni, N.; Musio, S.; Du, H.; Patané, M.; Pollo, B.; Finocchiaro, G.; Park, J.J.H.; Dunn, D.E.; Edwards, D.S.; et al. B7-H3-redirected chimeric antigen receptor T cells target glioblastoma and neurospheres. EBioMedicine 2019, 47, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Chalise, L.; Kato, A.; Ohno, M.; Maeda, S.; Yamamichi, A.; Kuramitsu, S.; Shiina, S.; Takahashi, H.; Ozone, S.; Yamaguchi, J.; et al. Efficacy of cancer-specific anti-podoplanin CAR-T cells and oncolytic herpes virus G47Δ combination therapy against glioblastoma. Mol. Ther. Oncolytics 2022, 26, 265–274. [Google Scholar] [CrossRef]
- Meisen, W.H.; Wohleb, E.S.; Jaime-Ramirez, A.C.; Bolyard, C.; Yoo, J.Y.; Russell, L.; Hardcastle, J.; Dubin, S.; Muili, K.; Yu, J.; et al. The Impact of Macrophage- and Microglia-Secreted TNFα on Oncolytic HSV-1 Therapy in the Glioblastoma Tumor Microenvironment. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 3274–3285. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Swanner, J.; Otani, Y.; Nair, M.; Park, F.; Banasavadi-Siddegowda, Y.; Liu, J.; Jaime-Ramirez, A.C.; Hong, B.; Geng, F.; et al. Oncolytic HSV therapy increases trametinib access to brain tumors and sensitizes them in vivo. Neuro-Oncol. 2019, 21, 1131–1140. [Google Scholar] [CrossRef]
- Otani, Y.; Yoo, J.Y.; Chao, S.; Liu, J.; Jaime-Ramirez, A.C.; Lee, T.J.; Hurwitz, B.; Yan, Y.; Dai, H.; Glorioso, J.C.; et al. Oncolytic HSV-Infected Glioma Cells Activate NOTCH in Adjacent Tumor Cells Sensitizing Tumors to Gamma Secretase Inhibition. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 2381–2392. [Google Scholar] [CrossRef]
- Esaki, S.; Rabkin, S.D.; Martuza, R.L.; Wakimoto, H. Transient fasting enhances replication of oncolytic herpes simplex virus in glioblastoma. Am. J. Cancer Res. 2016, 6, 300–311. [Google Scholar]
- Peters, C.; Paget, M.; Tshilenge, K.T.; Saha, D.; Antoszczyk, S.; Baars, A.; Frost, T.; Martuza, R.L.; Wakimoto, H.; Rabkin, S.D. Restriction of Replication of Oncolytic Herpes Simplex Virus with a Deletion of γ34.5 in Glioblastoma Stem-Like Cells. J. Virol. 2018, 92, e00246-18. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Jaime-Ramirez, A.C.; Bolyard, C.; Dai, H.; Nallanagulagari, T.; Wojton, J.; Hurwitz, B.S.; Relation, T.; Lee, T.J.; Lotze, M.T.; et al. Bortezomib Treatment Sensitizes Oncolytic HSV-1-Treated Tumors to NK Cell Immunotherapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 5265–5276. [Google Scholar] [CrossRef]
- Monie, D.D.; Correia, C.; Zhang, C.; Ung, C.Y.; Vile, R.G.; Li, H. Modular network mechanism of CCN1-associated resistance to HSV-1-derived oncolytic immunovirotherapies for glioblastomas. Sci. Rep. 2021, 11, 11198. [Google Scholar] [CrossRef] [PubMed]
- Esaki, S.; Nigim, F.; Moon, E.; Luk, S.; Kiyokawa, J.; Curry, W., Jr.; Cahill, D.P.; Chi, A.S.; Iafrate, A.J.; Martuza, R.L.; et al. Blockade of transforming growth factor-β signaling enhances oncolytic herpes simplex virus efficacy in patient-derived recurrent glioblastoma models. Int. J. Cancer 2017, 141, 2348–2358. [Google Scholar] [CrossRef] [PubMed]
- Delwar, Z.M.; Kuo, Y.; Wen, Y.H.; Rennie, P.S.; Jia, W. Oncolytic Virotherapy Blockade by Microglia and Macrophages Requires STAT1/3. Cancer Res. 2018, 78, 718–730. [Google Scholar] [CrossRef] [PubMed]
- Jaime-Ramirez, A.C.; Dmitrieva, N.; Yoo, J.Y.; Banasavadi-Siddegowda, Y.; Zhang, J.; Relation, T.; Bolyard, C.; Wojton, J.; Kaur, B. Humanized chondroitinase ABC sensitizes glioblastoma cells to temozolomide. J. Gene Med. 2017, 19, e2942. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.; Muili, K.; Bolyard, C.; Russell, L.; Lee, T.J.; Banasavadi-Siddegowda, Y.; Yoo, J.Y.; Yan, Y.; Ballester, L.Y.; Bockhorst, K.H.; et al. Suppression of HMGB1 Released in the Glioblastoma Tumor Microenvironment Reduces Tumoral Edema. Mol. Ther. Oncolytics 2018, 12, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.W.; Hall, B.L.; Marzulli, M.; Shah, V.K.; Bailey, L.; Chiocca, E.A.; Goins, W.F.; Kohanbash, G.; Cohen, J.B.; Glorioso, J.C. Treatment of glioblastoma with current oHSV variants reveals differences in efficacy and immune cell recruitment. Mol. Ther. Oncolytics 2021, 22, 444–453. [Google Scholar] [CrossRef]
- Sanchez Gil, J.; Dubois, M.; Neirinckx, V.; Lombard, A.; Coppieters, N.; D’Arrigo, P.; Isci, D.; Aldenhoff, T.; Brouwers, B.; Lassence, C.; et al. Nanobody-based retargeting of an oncolytic herpesvirus for eliminating CXCR4+ GBM cells: A proof of principle. Mol. Ther. Oncolytics 2022, 26, 35–48. [Google Scholar] [CrossRef]
- Alayo, Q.A.; Ito, H.; Passaro, C.; Zdioruk, M.; Mahmoud, A.B.; Grauwet, K.; Zhang, X.; Lawler, S.E.; Reardon, D.A.; Goins, W.F.; et al. Glioblastoma infiltration of both tumor- and virus-antigen specific cytotoxic T cells correlates with experimental virotherapy responses. Sci. Rep. 2020, 10, 5095. [Google Scholar] [CrossRef]
- Markert, J.M.; Razdan, S.N.; Kuo, H.C.; Cantor, A.; Knoll, A.; Karrasch, M.; Nabors, L.B.; Markiewicz, M.; Agee, B.S.; Coleman, J.M.; et al. A phase 1 trial of oncolytic HSV-1, G207, given in combination with radiation for recurrent GBM demonstrates safety and radiographic responses. Mol. Ther. J. Am. Soc. Gene Ther. 2014, 22, 1048–1055. [Google Scholar] [CrossRef]
- Friedman, G.K.; Johnston, J.M.; Bag, A.K.; Bernstock, J.D.; Li, R.; Aban, I.; Kachurak, K.; Nan, L.; Kang, K.D.; Totsch, S.; et al. Oncolytic HSV-1 G207 Immunovirotherapy for Pediatric High-Grade Gliomas. N. Engl. J. Med. 2021, 384, 1613–1622. [Google Scholar] [CrossRef]
- Todo, T.; Ino, Y.; Ohtsu, H.; Shibahara, J.; Tanaka, M. A phase I/II study of triple-mutated oncolytic herpes virus G47∆ in patients with progressive glioblastoma. Nat. Commun. 2022, 13, 4119. [Google Scholar] [CrossRef]
- Estevez-Ordonez, D.; Stein, J.; Maleknia, P.; Gallegos, C.; Atchley, T.; Laskay, N.; Clements, J.; Lobbous, M.; Leavenworth, J.; Riley, K.; et al. CTIM-13. Phase I Clinical Trial of Oncolytic Hsv-1 M032, A Second-Generation Virus Armed to Expressed Il-12, for the Treatment of Adult Patients with Recurrent or Progressive Malignant Glioma. Neuro-Oncol. 2023, 25 (Suppl. S5), v64. [Google Scholar] [CrossRef]
- Ling, A.L.; Solomon, I.H.; Landivar, A.M.; Nakashima, H.; Woods, J.K.; Santos, A.; Masud, N.; Fell, G.; Mo, X.; Yilmaz, A.S.; et al. Clinical trial links oncolytic immunoactivation to survival in glioblastoma. Nature 2023, 623, 157–166. [Google Scholar] [CrossRef]
- Usman, N.; Suarez, M. Adenoviruses. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Binder, A.M.; Biggs, H.M.; Haynes, A.K.; Chommanard, C.; Lu, X.; Erdman, D.D.; Watson, J.T.; Gerber, S.I. Human Adenovirus Surveillance—United States, 2003–2016. MMWR. Morb. Mortal. Wkly. Rep. 2017, 66, 1039–1042. [Google Scholar] [CrossRef]
- Lynch, J.P., 3rd; Kajon, A.E. Adenovirus: Epidemiology, Global Spread of Novel Serotypes, and Advances in Treatment and Prevention. Semin. Respir. Crit. Care Med. 2016, 37, 586–602. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Bishop, E.S.; Zhang, R.; Yu, X.; Farina, E.M.; Yan, S.; Zhao, C.; Zheng, Z.; Shu, Y.; Wu, X.; et al. Adenovirus-Mediated Gene Delivery: Potential Applications for Gene and Cell-Based Therapies in the New Era of Personalized Medicine. Genes Dis. 2017, 4, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Westergren Jakobsson, A.; Segerman, B.; Wallerman, O.; Lind, S.B.; Zhao, H.; Rubin, C.J.; Pettersson, U.; Akusjärvi, G. The Human Adenovirus Type 2 Transcriptome: An Amazing Complexity of Alternatively Spliced mRNAs. J. Virol. 2021, 95, e01869-20. [Google Scholar] [CrossRef]
- Matsunaga, W.; Gotoh, A. Adenovirus as a Vector and Oncolytic Virus. Curr. Issues Mol. Biol. 2023, 45, 4826–4840. [Google Scholar] [CrossRef]
- Tian, Y.; Xie, D.; Yang, L. Engineering strategies to enhance oncolytic viruses in cancer immunotherapy. Signal Transduct. Target. Ther. 2022, 7, 117. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Miska, J.; Young, J.S.; Rashidi, A.; Kane, J.R.; Panek, W.K.; Kanojia, D.; Han, Y.; Balyasnikova, I.V.; Lesniak, M.S. A Comparative Study of Replication-Incompetent and -Competent Adenoviral Therapy-Mediated Immune Response in a Murine Glioma Model. Mol. Ther. Oncolytics 2017, 5, 97–104. [Google Scholar] [CrossRef]
- Wang, L.; Liu, W.; Li, Z.; Wang, X.; Feng, X.; Wang, Z.; Wu, J.; Zhang, H.; Wu, H.; Kong, W.; et al. A tropism-transformed Oncolytic Adenovirus with Dual Capsid Modifications for enhanced Glioblastoma Therapy. J. Cancer 2020, 11, 5713–5726. [Google Scholar] [CrossRef]
- Wang, S.; Yan, W.; Kong, L.; Zuo, S.; Wu, J.; Zhu, C.; Huang, H.; He, B.; Dong, J.; Wei, J. Oncolytic viruses engineered to enforce cholesterol efflux restore tumor-associated macrophage phagocytosis and anti-tumor immunity in glioblastoma. Nat. Commun. 2023, 14, 4367. [Google Scholar] [CrossRef]
- Kiyokawa, J.; Kawamura, Y.; Ghouse, S.M.; Acar, S.; Barçın, E.; Martínez-Quintanilla, J.; Martuza, R.L.; Alemany, R.; Rabkin, S.D.; Shah, K.; et al. Modification of Extracellular Matrix Enhances Oncolytic Adenovirus Immunotherapy in Glioblastoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, G.; Kiyokawa, J.; Tirmizi, Z.; Richardson, L.G.; Ning, J.; Das, S.; Martuza, R.L.; Stemmer-Rachamimov, A.; Rabkin, S.D.; et al. Characterization and oncolytic virus targeting of FAP-expressing tumor-associated pericytes in glioblastoma. Acta Neuropathol. Commun. 2020, 8, 221. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, X.; Bao, W.; Liu, G.; Wei, W.; Ping, Y. An oncolytic virus-T cell chimera for cancer immunotherapy. Nat. Biotechnol. 2024, 42, 1876–1887. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, J.; Tian, Y.; Wang, J.; Jin, G.; Liu, F. Ki67-targeted oncolytic adenovirus expressing IL-15 improves intratumoral T cell infiltration and PD-L1 expression in glioblastoma. Virology 2023, 587, 109885. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, J.; Zhang, Q.; Liu, F. Mesenchymal stem cells loaded with Ad5-Ki67/IL-15 enhance oncolytic adenovirotherapy in experimental glioblastoma. Biomed. Pharmacother. 2023, 157, 114035. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Zhang, Q.; Zhang, W.; Zhang, Q.; Jin, G.; Liu, F. TS-2021, a third-generation oncolytic adenovirus that carried Ki67 promoter, TGF-β2 5’UTR, and IL-15 against experimental glioblastoma. J. Med. Virol. 2024, 96, e29335. [Google Scholar] [CrossRef] [PubMed]
- El-Ayoubi, A.; Klawitter, M.; Rüttinger, J.; Wellhäusser, G.; Holm, P.S.; Danielyan, L.; Naumann, U. Intranasal Delivery of Oncolytic Adenovirus XVir-N-31 via Optimized Shuttle Cells Significantly Extends Survival of Glioblastoma-Bearing Mice. Cancers 2023, 15, 4912. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, S.; Wang, P.; Zhang, J.; Liu, F. Olaparib Enhances the Efficacy of Third-Generation Oncolytic Adenoviruses Against Glioblastoma by Modulating DNA Damage Response and p66shc-Induced Apoptosis. CNS Neurosci. Ther. 2024, 30, e70124. [Google Scholar] [CrossRef]
- Klawitter, M.; El-Ayoubi, A.; Buch, J.; Rüttinger, J.; Ehrenfeld, M.; Lichtenegger, E.; Krüger, M.A.; Mantwill, K.; Koll, F.J.; Kowarik, M.C.; et al. The Oncolytic Adenovirus XVir-N-31, in Combination with the Blockade of the PD-1/PD-L1 Axis, Conveys Abscopal Effects in a Humanized Glioblastoma Mouse Model. Int. J. Mol. Sci. 2022, 23, 9965. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Chai, H.H.; Fang, X.L.; Xu, H.S.; Li, T.W.; Tang, Q.S.; Gu, J.F.; Zhang, K.J.; Liu, X.Y.; Shi, Z.F.; et al. Double-modified oncolytic adenovirus armed with a recombinant interferon-like gene enhanced abscopal effects against malignant glioma. Neuro-Oncol. Adv. 2023, 5, vdad117. [Google Scholar] [CrossRef]
- Oh, E.; Hong, J.; Kwon, O.J.; Yun, C.O. A hypoxia- and telomerase-responsive oncolytic adenovirus expressing secretable trimeric TRAIL triggers tumor-specific apoptosis and promotes viral dispersion in TRAIL-resistant glioblastoma. Sci. Rep. 2018, 8, 1420. [Google Scholar] [CrossRef] [PubMed]
- Romanenko, M.V.; Dolgova, E.V.; Osipov, I.D.; Ritter, G.S.; Sizova, M.S.; Proskurina, A.S.; Efremov, Y.R.; Bayborodin, S.I.; Potter, E.A.; Taranov, O.S.; et al. Oncolytic Effect of Adenoviruses Serotypes 5 and 6 Against U87 Glioblastoma Cancer Stem Cells. Anticancer Res. 2019, 39, 6073–6086. [Google Scholar] [CrossRef]
- Bates, E.A.; Lovatt, C.; Plein, A.R.; Davies, J.A.; Siebzehnrubl, F.A.; Parker, A.L. Engineering Adenoviral Vectors with Improved GBM Selectivity. Viruses 2023, 15, 1086. [Google Scholar] [CrossRef]
- González-Morales, A.; Zabaleta, A.; García-Moure, M.; Alonso, M.M.; Fernández-Irigoyen, J.; Santamaría, E. Oncolytic adenovirus Delta-24-RGD induces a widespread glioma proteotype remodeling during autophagy. J. Proteom. 2019, 194, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Bhoopathi, P.; Mannangatti, P.; Pradhan, A.K.; Kumar, A.; Maji, S.; Lang, F.F.; Klibanov, A.L.; Madan, E.; Cavenee, W.K.; Keoprasert, T.; et al. Noninvasive therapy of brain cancer using a unique systemic delivery methodology with a cancer terminator virus. J. Cell. Physiol. 2024, 239, e31302. [Google Scholar] [CrossRef]
- Shimizu, Y.; Gumin, J.; Gao, F.; Hossain, A.; Shpall, E.J.; Kondo, A.; Parker Kerrigan, B.C.; Yang, J.; Ledbetter, D.; Fueyo, J.; et al. Characterization of patient-derived bone marrow human mesenchymal stem cells as oncolytic virus carriers for the treatment of glioblastoma. J. Neurosurg. 2021, 136, 757–767. [Google Scholar] [CrossRef]
- Meléndez-Vázquez, N.M.; Nguyen, T.T.; Fan, X.; López-Rivas, A.R.; Fueyo, J.; Gomez-Manzano, C.; Godoy-Vitorino, F. Gut microbiota composition is associated with the efficacy of Delta-24-RGDOX in malignant gliomas. Molecular therapy. Oncology 2024, 32, 200787. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Molina, Y.; Jiang, H.; Fueyo, J.; Nguyen, T.; Shin, D.H.; Youssef, G.; Fan, X.; Gumin, J.; Alonso, M.M.; Phadnis, S.; et al. GITRL-armed Delta-24-RGD oncolytic adenovirus prolongs survival and induces anti-glioma immune memory. Neuro-Oncol. Adv. 2019, 1, vdz009. [Google Scholar] [CrossRef]
- Berghauser Pont, L.M.; Kleijn, A.; Kloezeman, J.J.; van den Bossche, W.; Kaufmann, J.K.; de Vrij, J.; Leenstra, S.; Dirven, C.M.; Lamfers, M.L. The HDAC Inhibitors Scriptaid and LBH589 Combined with the Oncolytic Virus Delta24-RGD Exert Enhanced Anti-Tumor Efficacy in Patient-Derived Glioblastoma Cells. PLoS ONE 2015, 10, e0127058. [Google Scholar] [CrossRef]
- Qiao, J.; Dey, M.; Chang, A.L.; Kim, J.W.; Miska, J.; Ling, A.M.; Nettlebeck, D.; Han, Y.; Zhang, L.; Lesniak, M.S.; et al. Intratumoral oncolytic adenoviral treatment modulates the glioma microenvironment and facilitates systemic tumor-antigen-specific T cell therapy. Oncoimmunology 2015, 4, e1022302. [Google Scholar] [CrossRef]
- Srinivasan, V.M.; Gumin, J.; Camstra, K.M.; Collins, D.E.; Chen, M.M.; Shpall, E.J.; Parker Kerrigan, B.C.; Johnson, J.N.; Chen, S.R.; Fueyo, J.; et al. Endovascular Selective Intra-Arterial Infusion of Mesenchymal Stem Cells Loaded with Delta-24 in a Canine Model. Neurosurgery 2020, 88, E102–E113. [Google Scholar] [CrossRef] [PubMed]
- Stepanenko, A.A.; Sosnovtseva, A.O.; Valikhov, M.P.; Chernysheva, A.A.; Cherepanov, S.A.; Yusubalieva, G.M.; Ruzsics, Z.; Lipatova, A.V.; Chekhonin, V.P. Superior infectivity of the fiber chimeric oncolytic adenoviruses Ad5/35 and Ad5/3 over Ad5-delta-24-RGD in primary glioma cultures. Mol. Ther. Oncolytics 2021, 24, 230–248. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.S.; Zdioruk, M.; Nowicki, M.O.; Griffith, A.M.; Aguilar, E.; Aguilar, L.K.; Guzik, B.W.; Barone, F.; Tak, P.P.; Tabatabai, G.; et al. Systemic high-dose dexamethasone treatment may modulate the efficacy of intratumoral viral oncolytic immunotherapy in glioblastoma models. J. Immunother. Cancer 2022, 10, e003368. [Google Scholar] [CrossRef]
- Belcaid, Z.; Berrevoets, C.; Choi, J.; van Beelen, E.; Stavrakaki, E.; Pierson, T.; Kloezeman, J.; Routkevitch, D.; van der Kaaij, M.; van der Ploeg, A.; et al. Low-dose oncolytic adenovirus therapy overcomes tumor-induced immune suppression and sensitizes intracranial gliomas to anti-PD-1 therapy. Neuro-Oncol. Adv. 2020, 2, vdaa011. [Google Scholar] [CrossRef]
- Qiao, C.; Xu, Y.; He, Y.; Cai, Z.; Wang, H. Oncolytic adenovirus H101 enhances the anti-tumor effects of PD-1 blockade via CD47 downregulation in tumor cells. Oncol. Res. 2025, 33, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.S.; Zdioruk, M.; Nowicki, M.O.; Griffith, A.M.; Aguilar-Cordova, E.; Aguilar, L.K.; Guzik, B.W.; Barone, F.; Tak, P.P.; Schregel, K.; et al. Perturbing DDR signaling enhances cytotoxic effects of local oncolytic virotherapy and modulates the immune environment in glioma. Mol. Ther. Oncolytics 2022, 26, 275–288. [Google Scholar] [CrossRef]
- Niittykoski, M.; von Und Zu Fraunberg, M.; Martikainen, M.; Rauramaa, T.; Immonen, A.; Koponen, S.; Leinonen, V.; Vähä-Koskela, M.; Zhang, Q.; Kühnel, F.; et al. Immunohistochemical Characterization and Sensitivity to Human Adenovirus Serotypes 3, 5, and 11p of New Cell Lines Derived from Human Diffuse Grade II to IV Gliomas. Transl. Oncol. 2017, 10, 772–779. [Google Scholar] [CrossRef]
- Kleijn, A.; van den Bossche, W.; Haefner, E.S.; Belcaid, Z.; Burghoorn-Maas, C.; Kloezeman, J.J.; Pas, S.D.; Leenstra, S.; Debets, R.; de Vrij, J.; et al. The Sequence of Delta24-RGD and TMZ Administration in Malignant Glioma Affects the Role of CD8+T Cell Anti-tumor Activity. Mol. Ther. Oncolytics 2017, 5, 11–19. [Google Scholar] [CrossRef]
- Stepanenko, A.A.; Sosnovtseva, A.O.; Valikhov, M.P.; Vasiukova, A.A.; Abramova, O.V.; Lipatova, A.V.; Yusubalieva, G.M.; Chekhonin, V.P. Comparison of the L3-23K and L5-Fiber Regions for Arming the Oncolytic Adenovirus Ad5-Delta-24-RGD with Reporter and Therapeutic Transgenes. Int. J. Mol. Sci. 2025, 26, 3700. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, Z.; Zhong, K.; Wang, Z.; Yang, N.; Tang, X.; Li, H.; Lu, Q.; Wu, Z.; Yuan, B.; et al. CXCL11-armed oncolytic adenoviruses enhance CAR-T cell therapeutic efficacy and reprogram tumor microenvironment in glioblastoma. Mol. Ther. J. Am. Soc. Gene Ther. 2023, 31, 134–153. [Google Scholar] [CrossRef]
- Huang, J.; Zheng, M.; Zhang, Z.; Tang, X.; Chen, Y.; Peng, A.; Peng, X.; Tong, A.; Zhou, L. Interleukin-7-loaded oncolytic adenovirus improves CAR-T cell therapy for glioblastoma. Cancer Immunol. Immunother. 2021, 70, 2453–2465. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Mu, M.; Zhang, Z.; Chen, Y.; Yang, N.; Zhong, K.; Li, Y.; Lu, F.; Guo, G.; Tong, A. Systemic delivery of tannic acid-ferric-masked oncolytic adenovirus reprograms tumor microenvironment for improved therapeutic efficacy in glioblastoma. Cancer Gene Ther. 2024, 31, 1804–1817. [Google Scholar] [CrossRef]
- Batalla-Covello, J.; Ngai, H.W.; Flores, L.; McDonald, M.; Hyde, C.; Gonzaga, J.; Hammad, M.; Gutova, M.; Portnow, J.; Synold, T.; et al. Multiple Treatment Cycles of Neural Stem Cell Delivered Oncolytic Adenovirus for the Treatment of Glioblastoma. Cancers 2021, 13, 6320. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.S.; Zdioruk, M.; Nowicki, M.O.; Hoetker, M.S.; Herbert, Z.T.; Barone, F.; Tak, P.P.; Chiocca, E.A.; Tabatabai, G.; Lawler, S.E. Uncovering transcriptomic landscape alterations of CAN-2409 in in vitro and in vivo glioma models. Front. Med. 2023, 10, 1140352. [Google Scholar] [CrossRef]
- Fares, J.; Ahmed, A.U.; Ulasov, I.V.; Sonabend, A.M.; Miska, J.; Lee-Chang, C.; Balyasnikova, I.V.; Chandler, J.P.; Portnow, J.; Tate, M.C.; et al. Neural stem cell delivery of an oncolytic adenovirus in newly diagnosed malignant glioma: A first-in-human, phase 1, dose-escalation trial. The Lancet. Oncology 2021, 22, 1103–1114. [Google Scholar] [CrossRef]
- van Putten, E.H.P.; Kleijn, A.; van Beusechem, V.W.; Noske, D.; Lamers, C.H.J.; de Goede, A.L.; Idema, S.; Hoefnagel, D.; Kloezeman, J.J.; Fueyo, J.; et al. Convection Enhanced Delivery of the Oncolytic Adenovirus Delta24-RGD in Patients with Recurrent GBM: A Phase I Clinical Trial Including Correlative Studies. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2022, 28, 1572–1585. [Google Scholar] [CrossRef]
- van der Meulen-Muileman, I.H.; Amado-Azevedo, J.; Lamfers, M.L.M.; Kleijn, A.; Idema, S.; Noske, D.P.; Dirven, C.M.F.; van Beusechem, V.W. Adenovirus-Neutralizing and Infection-Promoting Activities Measured in Serum of Human Brain Cancer Patients Treated with Oncolytic Adenovirus Ad5-∆24.RGD. Int. J. Mol. Sci. 2025, 26, 854. [Google Scholar] [CrossRef]
- Peña Pino, I.; Darrow, D.P.; Chen, C.C. Magnetic Resonance Imaging-Aided SmartFlow Convection Delivery of DNX-2401: A Pilot, Prospective Case Series. World Neurosurg. 2024, 181, e833–e840. [Google Scholar] [CrossRef]
- Nassiri, F.; Patil, V.; Yefet, L.S.; Singh, O.; Liu, J.; Dang, R.M.A.; Yamaguchi, T.N.; Daras, M.; Cloughesy, T.F.; Colman, H.; et al. Oncolytic DNX-2401 virotherapy plus pembrolizumab in recurrent glioblastoma: A phase 1/2 trial. Nat. Med. 2023, 29, 1370–1378. [Google Scholar] [CrossRef]
- Taylor, O.G.; Brzozowski, J.S.; Skelding, K.A. Glioblastoma multiforme: An overview of emerging therapeutic targets. Front. Oncol. 2019, 9, 963. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bhattacharjee, S.; Yadava, P.K. Measles virus: Background and oncolytic virotherapy. Biochem. Biophys. Rep. 2018, 13, 58–62. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Allen, C.; Paraskevakou, G.; Liu, C.; Iankov, I.D.; Msaouel, P.; Zollman, P.; Myers, R.; Peng, K.W.; Russell, S.J.; Galanis, E. Oncolytic measles virus strains in the treatment of gliomas. Expert Opin. Biol. Ther. 2008, 8, 213–220. [Google Scholar] [CrossRef]
- Anker, S.C.; Szczeponik, M.G.; Dessila, J.; Dittus, K.; Engeland, C.E.; Jäger, D.; Ungerechts, G.; Leber, M.F. Oncolytic measles virus encoding microRNA for targeted RNA interference. Viruses 2023, 15, 308. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Forsyth, P.A.; Abate-Daga, D. Viral therapy gets personal: A potential gene signature to predict susceptibility to measles virus oncolysis. J. Natl. Cancer Inst. 2018, 110, 1139–1140. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Alía, M.Á.; Nace, R.A.; Tischer, A.; Zhang, L.; Bah, E.S.; Auton, M.; Russell, S.J. MeV-Stealth: A CD46-specific oncolytic measles virus resistant to neutralization by measles-immune human serum. PLoS Pathog. 2021, 17, e1009283. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ammour, Y.; Susova, O.; Krasnov, G.; Nikolaeva, E.; Varachev, V.; Schetinina, Y.; Gavrilova, M.; Mitrofanov, A.; Poletaeva, A.; Bekyashev, A.; et al. Transcriptome analysis of human glioblastoma cells susceptible to infection with the Leningrad-16 vaccine strain of measles virus. Viruses 2022, 14, 2433. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Galanis, E.; Dooley, K.E.; Keith Anderson, S.; Kurokawa, C.B.; Carrero, X.W.; Uhm, J.H.; Federspiel, M.J.; Leontovich, A.A.; Aderca, I.; Viker, K.B.; et al. Carcinoembryonic antigen-expressing oncolytic measles virus derivative in recurrent glioblastoma: A phase 1 trial. Nat. Commun. 2024, 15, 493. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abdullah, J.M.; Mustafa, Z.; Ideris, A. Newcastle disease virus interaction in targeted therapy against proliferation and invasion pathways of glioblastoma multiforme. Biomed. Res. Int. 2014, 2014, 386470. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Samal, S.K. Innovation in Newcastle Disease Virus Vectored Avian Influenza Vaccines. Viruses 2019, 11, 300. [Google Scholar] [CrossRef]
- García-Romero, N.; Palacín-Aliana, I.; Esteban-Rubio, S.; Madurga, R.; Rius-Rocabert, S.; Carrión-Navarro, J.; Presa, J.; Cuadrado-Castano, S.; Sánchez-Gómez, P.; García-Sastre, A.; et al. Newcastle disease virus (NDV) oncolytic activity in human glioma tumors is dependent on CDKN2A-Type I IFN gene cluster codeletion. Cells 2020, 9, 1405. [Google Scholar] [CrossRef]
- Yang, H.; Tian, J.; Zhao, J.; Zhao, Y.; Zhang, G. The Application of Newcastle Disease Virus (NDV): Vaccine Vectors and Tumor Therapy. Viruses 2024, 16, 886. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Soliman, R.M.; Nishioka, K.; Daidoji, T.; Noyori, O.; Nakaya, T. Chimeric Newcastle Disease Virus Vectors Expressing Human IFN-γ Mediate Target Immune Responses and Enable Multifaceted Treatments. Biomedicines 2023, 11, 455. [Google Scholar] [CrossRef]
- Kadhim, Z.A.; Sulaiman, G.M.; Al-Shammari, A.M.; Khan, R.A.; Al Rugaie, O.; Mohammed, H.A. Oncolytic Newcastle Disease Virus Co-Delivered with Modified PLGA Nanoparticles Encapsulating Temozolomide against Glioblastoma Cells: Developing an Effective Treatment Strategy. Molecules 2022, 27, 5757. [Google Scholar] [CrossRef]
- Jang, S.H.; Jung, B.K.; An, Y.H.; Jang, H. The phosphatase and tensin homolog gene inserted between NP and P gene of recombinant Newcastle disease virus oncolytic effect test to glioblastoma cell and xenograft mouse model. Virol. J. 2022, 19, 21. [Google Scholar] [CrossRef] [PubMed]
- Freeman, A.I.; Zakay-Rones, Z.; Gomori, J.M.; Linetsky, E.; Rasooly, L.; Greenbaum, E.; Rozenman-Yair, S.; Panet, A.; Libson, E.; Irving, C.S.; et al. Phase I/II trial of intravenous NDV-HUJ oncolytic virus in recurrent glioblastoma multiforme. Mol. Ther. J. Am. Soc. Gene Ther. 2006, 13, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Csatary, L.K.; Gosztonyi, G.; Szeberenyi, J.; Fabian, Z.; Liszka, V.; Bodey, B.; Csatary, C.M. MTH-68/H oncolytic viral treatment in human high-grade gliomas. J. Neuro-Oncol. 2004, 67, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.; Gerhards, R.; Kirches, E.; Firsching, R. Preliminary results of active specific immunization with modified tumor cell vaccine in glioblastoma multiforme. J. Neuro-Oncol. 2001, 53, 39–46. [Google Scholar] [CrossRef]
- Steiner, H.H.; Bonsanto, M.M.; Beckhove, P.; Brysch, M.; Geletneky, K.; Ahmadi, R.; Schuele-Freyer, R.; Kremer, P.; Ranaie, G.; Matejic, D.; et al. Antitumor vaccination of patients with glioblastoma multiforme: A pilot study to assess feasibility, safety, and clinical benefit. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2004, 22, 4272–4281. [Google Scholar] [CrossRef]
- Keshavarz, M.; Nejad, A.S.M.; Esghaei, M.; Bokharaei-Salim, F.; Dianat-Moghadam, H.; Keyvani, H.; Ghaemi, A. Oncolytic Newcastle disease virus reduces growth of cervical cancer cell by inducing apoptosis. Saudi J. Biol. Sci. 2020, 27, 47–52. [Google Scholar] [CrossRef]
- Cassel, W.A.; Murray, D.R. A ten-year follow-up on stage II malignant melanoma patients treated postsurgically with Newcastle disease virus oncolysate. Med. Oncol. Tumor Pharmacother. 1992, 9, 169–171. [Google Scholar] [CrossRef]
- Pomer, S.; Schirrmacher, V.; Thiele, R.; Lohrke, H.; Brkovic, D.; Staehler, G. Tumor response and 4 year survival-data of patients with advanced renal-cell carcinoma treated with autologous tumor vaccine and subcutaneous R-IL-2 and IFN-alpha(2b). Int. J. Oncol. 1995, 6, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Möbus, V.; Horn, S.; Stöck, M.; Schirrmacher, V. Tumor cell vaccination for gynecological tumors. Hybridoma 1993, 12, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Berkeley, R.; Barr, T.; Ilett, E.; Errington-Mais, F. Past, Present and Future of Oncolytic Reovirus. Cancers 2020, 12, 3219. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kicielinski, K.P.; Chiocca, E.A.; Yu, J.S.; Gill, G.M.; Coffey, M.; Markert, J.M. Phase 1 clinical trial of intratumoral reovirus infusion for the treatment of recurrent malignant gliomas in adults. Mol. Ther. 2014, 22, 1056–1062. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kendall, J.; Chalmers, A.; McBain, C.; Melcher, A.; Samson, A.; Phillip, R.; Brown, S.; Short, S. CTIM-14. Pelareorep and Granulocyte-Macrophage Colony-Stimulating Factor (Gm-Csf) with Standard Chemoradiotherapy/Adjuvant Temozolomide for Glioblastoma Multiforme (Gbm) Patients: Reoglio Phase I Trial Results. Neuro Oncol. 2020, 22 (Suppl. S2), ii35–ii36. [Google Scholar] [CrossRef] [PubMed Central]
- Samson, A.; Scott, K.J.; Taggart, D.; West, E.J.; Wilson, E.; Nuovo, G.J.; Thomson, S.; Corns, R.; Mathew, R.K.; Fuller, M.J.; et al. Intravenous delivery of oncolytic reovirus to brain tumor patients immunologically primes for subsequent checkpoint blockade. Sci. Transl. Med. 2018, 10, eaam7577. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schuelke, M.R.; Gundelach, J.H.; Coffey, M.; West, E.; Scott, K.; Johnson, D.R.; Samson, A.; Melcher, A.; Vile, R.G.; Bram, R.J. Phase I trial of sargramostim/pelareorep therapy in pediatric patients with recurrent or refractory high-grade brain tumors. Neuro-Oncol. Adv. 2022, 4, vdac085. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tai, C.K.; Wang, W.J.; Chen, T.C.; Kasahara, N. Single-shot, multicycle suicide gene therapy by replication-competent retrovirus vectors achieves long-term survival benefit in experimental glioma. Mol. Ther. J. Am. Soc. Gene Ther. 2005, 12, 842–851. [Google Scholar] [CrossRef]
- Perez, O.D.; Logg, C.R.; Hiraoka, K.; Diago, O.; Burnett, R.; Inagaki, A.; Jolson, D.; Amundson, K.; Buckley, T.; Lohse, D.; et al. Design and selection of Toca 511 for clinical use: Modified retroviral replicating vector with improved stability and gene expression. Mol. Ther. J. Am. Soc. Gene Ther. 2012, 20, 1689–1698. [Google Scholar] [CrossRef]
- Budzik, K.M.; Nace, R.A.; Ikeda, Y.; Russell, S.J. Oncolytic Foamy Virus—Generation and properties of a nonpathogenic replicating retroviral vector system that targets chronically proliferating cancer cells. J. Virol. 2021, 95, e00015-21. [Google Scholar] [CrossRef]
- Budzik, K.M.; Nace, R.A.; Ikeda, Y.; Russell, S.J. Evaluation of the stability and intratumoral delivery of foreign transgenes encoded by an oncolytic Foamy Virus vector. Cancer Gene Ther. 2022, 29, 1240–1251. [Google Scholar] [CrossRef]
- Cloughesy, T.F.; Landolfi, J.; Hogan, D.J.; Bloomfield, S.; Carter, B.; Chen, C.C.; Elder, J.B.; Kalkanis, S.N.; Kesari, S.; Lai, A.; et al. Phase 1 trial of vocimagene amiretrorepvec and 5-fluorocytosine for recurrent high-grade glioma. Sci. Transl. Med. 2016, 8, 341ra75. [Google Scholar] [CrossRef]
- Cloughesy, T.F.; Landolfi, J.; Vogelbaum, M.A.; Ostertag, D.; Elder, J.B.; Bloomfield, S.; Carter, B.; Chen, C.C.; Kalkanis, S.N.; Kesari, S.; et al. Durable complete responses in some recurrent high-grade glioma patients treated with Toca 511 + Toca FC. Neuro-Oncol. 2018, 20, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Accomando, W.P.; Rao, A.R.; Hogan, D.J.; Newman, A.M.; Nakao, A.; Alizadeh, A.A.; Diehn, M.; Diago, O.R.; Gammon, D.; Haghighi, A.; et al. Molecular and Immunologic Signatures are Related to Clinical Benefit from Treatment with Vocimagene Amiretrorepvec (Toca 511) and 5-Fluorocytosine (Toca FC) in Patients with Glioma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 6176–6186. [Google Scholar] [CrossRef] [PubMed]
- Hogan, D.J.; Zhu, J.J.; Diago, O.R.; Gammon, D.; Haghighi, A.; Lu, G.; Das, A.; Gruber, H.E.; Jolly, D.J.; Ostertag, D. Molecular Analyses Support the Safety and Activity of Retroviral Replicating Vector Toca 511 in Patients. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 4680–4693. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, Z.; Zhang, C.; Zhang, N.; Wang, P.; Chu, Y.; Chard Dunmall, L.S.; Lemoine, N.R.; Wang, Y. An effective therapeutic regime for treatment of glioma using oncolytic vaccinia virus expressing IL-21 in combination with immune checkpoint inhibition. Mol. Ther. Oncolytics 2022, 26, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Storozynsky, Q.T.; Agopsowicz, K.C.; Noyce, R.S.; Bukhari, A.B.; Han, X.; Snyder, N.; Umer, B.A.; Gamper, A.M.; Godbout, R.; Evans, D.H.; et al. Radiation combined with oncolytic vaccinia virus provides pronounced antitumor efficacy and induces immune protection in an aggressive glioblastoma model. Cancer Lett. 2023, 562, 216169. [Google Scholar] [CrossRef]
- Vasileva, N.; Ageenko, A.; Dmitrieva, M.; Nushtaeva, A.; Mishinov, S.; Kochneva, G.; Richter, V.; Kuligina, E. Double Recombinant Vaccinia Virus: A Candidate Drug against Human Glioblastoma. Life 2021, 11, 1084. [Google Scholar] [CrossRef]
- Chen, L.; Wang, P.; Di Gioia, C.; Yuan, M.; Zhang, Z.; Miao, J.; Yan, W.; Zhao, G.; Jia, Y.; Wang, N.; et al. A novel oncolytic Vaccinia virus armed with IL-12 augments antitumor immune responses leading to durable regression in murine models of lung cancer. Front. Immunol. 2025, 15, 1492464. [Google Scholar] [CrossRef]
- Duggal, R.; Geissinger, U.; Zhang, Q.; Aguilar, J.; Chen, N.G.; Binda, E.; Vescovi, A.L.; Szalay, A.A. Vaccinia virus expressing bone morphogenetic protein-4 in novel glioblastoma orthotopic models facilitates enhanced tumor regression and long-term survival. J. Transl. Med. 2013, 11, 155. [Google Scholar] [CrossRef]
- Cotmore, S.F.; Agbandje-McKenna, M.; Chiorini, J.A.; Mukha, D.V.; Pintel, D.J.; Qiu, J.; Soderlund-Venermo, M. The family Parvoviridae. Arch. Virol. 2019, 164, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Raykov, Z.; Grekova, S.; Leuchs, B.; Aprahamian, M.; Rommelaere, J. Arming parvoviruses with CpG motifs to improve their oncosuppressive capacity. Int. J. Cancer 2008, 122, 2880–2884. [Google Scholar] [CrossRef] [PubMed]
- Grekova, S.P.; Aprahamian, M.; Daeffler, L.; Leuchs, B.; Angelova, A.L.; Giese, N.A.; Rommelaere, J.; Raykov, Z. Interferon γ improves the vaccination potential of oncolytic H-1 parvovirus by modulating the tumor microenvironment. Cancer Gene Ther. 2012, 19, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Rayet, B.; Lopez-Guerrero, J.A.; Rommelaere, J.; Dinsart, C. Induction of programmed cell death by parvovirus H-1 in U937 cells: Connection with the tumor necrosis factor alpha signalling pathway. J Virol. 1998, 72, 8893–8903. [Google Scholar] [CrossRef]
- Angelova, A.L.; Barf, M.; Geletneky, K.; Unterberg, A.; and Rommelaere, J. Immunotherapeutic Potential of Oncolytic H-1 Parvovirus: Hints of Glioblastoma Microenvironment Conversion towards Immunogenicity. Viruses 2017, 9, 382. [Google Scholar] [CrossRef]
- Paglino, J.C.; Ozduman, K.; van den Pol, A.N. LuIII parvovirus selectively and efficiently targets, replicates in, and kills human glioma cells. J. Virol. 2012, 86, 7280–7291. [Google Scholar] [CrossRef] [PubMed]
- Geletneky, K.; Hajda, J.; Angelova, A.L.; Leuchs, B.; Capper, D.; Bartsch, A.J.; Neumann, J.-O.; Schöning, T.; Hüsing, J.; Beelte, B.; et al. Oncolytic H-1 Parvovirus Shows Safety and Signs of Immunogenic Activity in a First Phase I/IIa Glioblastoma Trial. Mol. Ther. 2017, 25, 2620–2634. [Google Scholar] [CrossRef]
- Geletneky, K.; Huesing, J.; Rommelaere, J.; Schlehofer, J.R.; Leuchs, B.; Dahm, M.; Krebs, O.; Doeberitz, M.V.K.; Huber, B.; Hajda, J. Phase I/IIa study of intratumoral/intracerebral or intravenous/intracerebral administration of Parvovirus H-1 (ParvOryx) in patients with progressive primary or recurrent glioblastoma multiforme: ParvOryx01 protocol. BMC Cancer 2012, 12, 99. [Google Scholar] [CrossRef] [PubMed]
- Gromeier, M.; Alexander, L.; Wimmer, E. Internal ribosomal entry site substitution eliminates neurovirulence in intergeneric poliovirus recombinants. Proc. Natl. Acad. Sci. USA 1996, 93, 2370–2375. [Google Scholar] [CrossRef]
- Chandramohan, V.; Bryant, J.D.; Piao, H.; Keir, S.T.; Lipp, E.S.; Lefaivre, M.; Perkinson, K.; Bigner, D.D.; Gromeier, M.; McLendon, R.E. Validation of an Immunohistochemistry Assay for Detection of CD155, the Poliovirus Receptor, in Malignant Gliomas. Arch. Pathol. Lab. Med. 2017, 141, 1697–1704. [Google Scholar] [CrossRef]
- Liu, F.; Huang, J.; Xiong, Y.; Li, S.; Liu, Z. Large-scale analysis reveals the specific clinical and immune features of CD155 in glioma. Aging 2019, 11, 5463–5482. [Google Scholar] [CrossRef]
- Merrill, M.K.; Bernhardt, G.; Sampson, J.H.; Wikstrand, C.J.; Bigner, D.D.; Gromeier, M. Poliovirus receptor CD155-targeted oncolysis of glioma. Neuro-Oncology 2004, 6, 208–217. [Google Scholar] [CrossRef]
- Brown, M.C.; Holl, E.K.; Boczkowski, D.; Dobrikova, E.; Mosaheb, M.; Chandramohan, V.; Bigner, D.D.; Gromeier, M.; Nair, S.K. Cancer immunotherapy with recombinant poliovirus induces IFN-dominant activation of dendritic cells and tumor antigen–specific CTLs. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef]
- Desjardins, A.; Sampson, J.H.; Peters, K.B.; Ranjan, T.; Vlahovic, G.; Threatt, S.; Herndon, J.E.; Boulton, S.; Lally-Goss, D.; McSherry, F.; et al. Oncolytic Polio/Rhinovirus Recombinant (PVSRIPO) in Recurrent Glioblastoma (GBM): First Phase I Clinical Trial Evaluating the Intratumoral Administration. Neuro-Oncology 2014, 16, iii43. [Google Scholar] [CrossRef][Green Version]
- Desjardins, A.; Gromeier, M.; Herndon, J.E.; Beaubier, N.; Bolognesi, D.P.; Friedman, A.H.; Friedman, H.S.; McSherry, F.; Muscat, A.M.; Nair, S.; et al. Recurrent Glioblastoma Treated with Recombinant Poliovirus. N. Engl. J. Med. 2018, 379, 150–161. [Google Scholar] [CrossRef]
- Beasley, G.M.; Nair, S.K.; E Farrow, N.; Landa, K.; Selim, M.A.; Wiggs, C.A.; Jung, S.-H.; Bigner, D.D.; Kelly, A.T.; Gromeier, M.; et al. Phase I trial of intratumoral PVSRIPO in patients with unresectable, treatment-refractory melanoma. J. Immunother. Cancer 2021, 9, e002203. [Google Scholar] [CrossRef]
- Bhagat, R.; Kaur, G.; Seth, P. Molecular mechanisms of zika virus pathogenesis: An update. Indian J. Med. Res. 2021, 154, 433–445. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bhardwaj, U.; Pandey, N.; Rastogi, M.; Singh, S.K. Gist of Zika Virus pathogenesis. Virology 2021, 560, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Meertens, L.; Labeau, A.; Dejarnac, O.; Cipriani, S.; Sinigaglia, L.; Bonnet-Madin, L.; Le Charpentier, T.; Hafirassou, M.L.; Zamborlini, A.; Cao-Lormeau, V.M.; et al. Axl Mediates ZIKA Virus Entry in Human Glial Cells and Modulates Innate Immune Responses. Cell Rep. 2017, 18, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Gorman, M.J.; McKenzie, L.D.; Chai, J.N.; Hubert, C.G.; Prager, B.C.; Fernandez, E.; Richner, J.M.; Zhang, R.; Shan, C.; et al. Zika virus has oncolytic activity against glioblastoma stem cells. J. Exp. Med. 2017, 214, 2843–2857, Erratum in J. Exp. Med. 2017, 214, 3145. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, Z.; Mesci, P.; Bernatchez, J.A.; Gimple, R.C.; Wang, X.; Schafer, S.T.; Wettersten, H.I.; Beck, S.; Clark, A.E.; Wu, Q.; et al. Zika Virus Targets Glioblastoma Stem Cells through a SOX2-Integrin αvβ5 Axis. Cell Stem Cell. 2020, 26, 187–204.e10. [Google Scholar] [CrossRef] [PubMed]
- Lima, E.O.; Guerreiro, T.M.; Melo, C.F.O.; de Oliveira, D.N.; Machado, D.; Lancelloti, M.; Catharino, R.R. MALDI imaging detects endogenous digoxin in glioblastoma cells infected by Zika virus-Would it be the oncolytic key? J. Mass Spectrom. 2018, 53, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Dabaja, M.Z.; Lima, E.O.; de Oliveira, D.N.; Guerreiro, T.M.; Melo, C.F.O.R.; Morishita, K.N.; Lancellotti, M.; Gois Ruiz, A.L.T.; Goulart, G.; Duarte, D.A.; et al. Metabolic alterations induced by attenuated Zika virus in glioblastoma cells. Cell Biosci. 2018, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hu, Y.; Huang, J.; Feng, Y.; Zhang, Z.; Zhong, K.; Chen, Y.; Wang, Z.; Huang, C.; Yang, H.; et al. Zika virus NS5 protein inhibits cell growth and invasion of glioma. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118655. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Mazzoccoli, L.; Jash, A.; Govero, J.; Bais, S.S.; Hu, T.; Fontes-Garfias, C.R.; Shan, C.; Okada, H.; Shresta, S.; et al. Zika virus oncolytic activity requires CD8+ T cells and is boosted by immune checkpoint blockade. JCI Insight 2021, 6, e144619. [Google Scholar] [CrossRef]
- Crane, A.T.; Chrostek, M.R.; Krishna, V.D.; Shiao, M.; Toman, N.G.; Pearce, C.M.; Tran, S.K.; Sipe, C.J.; Guo, W.; Voth, J.P.; et al. Zika virus-based immunotherapy enhances long-term survival of rodents with brain tumors through upregulation of memory T-cells. PLoS ONE 2020, 15, e0232858. [Google Scholar] [CrossRef]
- Garcez, P.P.; Guasti, A.; Ventura, N.; Higa, L.M.; Andreiuolo, F.; de Freitas, G.P.A.; Ribeiro, L.J.; Maia, R.A.; de Lima, S.M.B.; de Souza Azevedo, A.; et al. Case report: Regression of Glioblastoma after flavivirus infection. Front. Med. 2023, 10, 1192070. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Webb, M.J.; Sener, U.; Vile, R.G. Current status and challenges of oncolytic virotherapy for the treatment of glioblastoma. Pharmaceuticals 2023, 16, 793. [Google Scholar] [CrossRef]
- Liu, J.; Piranlioglu, R.; Ye, F.; Shu, K.; Lei, T.; Nakashima, H. Immunosuppressive cells in oncolytic virotherapy for glioma: Challenges and solutions. Front Cell Infect Microbiol. 2023, 13, 1141034. [Google Scholar] [CrossRef]
- Khoonkari, M.; Liang, D.; Kamperman, M.; van Rijn, P. The unfolded protein response sensor PERK mediates stiffness adaptation in glioblastoma cells. Int. J. Mol. Sci. 2022, 23, 6520. [Google Scholar] [CrossRef] [PubMed]
- Sottoriva, A.; Spiteri, I.; Piccirillo, S.G.M.; Touloumis, A.; Collins, V.P.; Marioni, J.C.; Curtis, C.; Watts, C.; Tavaré, S. Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc. Natl. Acad. Sci. USA. 2013, 110, 4009–4014. [Google Scholar] [CrossRef]
- Tamura, K.; Wakimoto, H.; Agarwal, A.S.; Rabkin, S.D.; Bhere, D.; Martuza, R.L.; Kuroda, T.; Kasmieh, R.; Shah, K. Multimechanistic tumor-targeted oncolytic virus overcomes resistance in brain tumors. Mol. Ther. 2013, 21, 68–77. [Google Scholar] [CrossRef]
- Ohka, F.; Natsume, A.; Wakabayashi, T. Current trends in targeted therapies for glioblastoma multiforme. Neurol. Res. Int. 2012, 2012, 878425. [Google Scholar] [CrossRef]
- Kircher, M.F.; de la Zerda, A.; Jokerst, J.V.; Zavaleta, C.L.; Kempen, P.J.; Mittra, E.; Pitter, K.; Huang, R.; Campos, C.; Habte, F.; et al. A brain tumor molecular imaging strategy using a new triple-modality MRI-photoacoustic-Raman nanoparticle. Nat. Med. 2012, 18, 829–834. [Google Scholar] [CrossRef]
- Ageenko, A.; Vasileva, N.; Richter, V.; Kuligina, E. Combination of oncolytic virotherapy with different antitumor approaches against glioblastoma. Int. J. Mol. Sci. 2024, 25, 2042. [Google Scholar] [CrossRef]
- Han, X.; Abdallah, M.O.E.; Breuer, P.; Evert, B. Downregulation of MGMT expression by targeted editing of DNA methylation enhances temozolomide sensitivity in glioblastoma. Cell Oncol. 2023, 46, 697–710. [Google Scholar] [CrossRef]
- Yu, W.; Zhang, L.; Wei, Q.; Shao, A. O6-methylguanine-DNA methyltransferase (MGMT): Challenges and new opportunities in glioma chemotherapy. Front. Oncol. 2020, 9, 1547. [Google Scholar] [CrossRef]
- Kanai, R.; Rabkin, S.D.; Yip, S.; Martuza, R.L. Oncolytic virus-mediated manipulation of DNA damage responses: Synergy with chemotherapy in killing glioblastoma stem cells. J. Natl. Cancer Inst. 2012, 104, 42–55. [Google Scholar] [CrossRef]
- Awada, H.; Paris, F.; Pecqueur, C. Exploiting radiation immunostimulatory effects to improve glioblastoma outcome. Neuro Oncol. 2023, 25, 433–446. [Google Scholar] [CrossRef]
- Chédeville, A.; Madureira, P.A. The role of hypoxia in glioblastoma radiotherapy resistance. Cancers 2021, 13, 542. [Google Scholar] [CrossRef]
- Rodriguez, S.M.B.; Tataranu, L.G.; Kamel, A.; Turliuc, S.; Rizea, R.E.; Dricu, A. Glioblastoma and Immune Checkpoint Inhibitors: A Glance at Available Treatment Options and Future Directions. Int. J. Mol. Sci. 2024, 25, 10765. [Google Scholar] [CrossRef]
- Berghoff, A.S.; Kiesel, B.; Widhalm, G.; Rajky, O.; Ricken, G.; Wöhrer, A.; Dieckmann, K.; Filipits, M.; Brandstetter, A.; Weller, M.; et al. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro Oncol. 2015, 17, 1064–1075. [Google Scholar] [CrossRef]
- Bagchi, S.; Yuan, R.; Engleman, E.G. Immune checkpoint inhibitors for the treatment of cancer: Clinical impact and mechanisms of response and resistance. Annu. Rev. Pathol. 2021, 16, 223–249. [Google Scholar] [CrossRef]
- Reardon, D.A.; Brandes, A.A.; Omuro, A.; Mulholland, P.; Lim, M.; Wick, A.; Baehring, J.; Ahluwalia, M.S.; Roth, P.; Bähr, O.; et al. Effect of nivolumab vs bevacizumab in patients with recurrent glioblastoma: The CheckMate 143 phase 3 randomized clinical trial. JAMA Oncol. 2020, 6, 1003–1010. [Google Scholar] [CrossRef]
- Arrieta, V.A.; Dmello, C.; McGrail, D.J.; Brat, D.J.; Lee-Chang, C.; Heimberger, A.B.; Chand, D.; Stupp, R.; Sonabend, A.M. Immune checkpoint blockade in glioblastoma: From tumor heterogeneity to personalized treatment. J. Clin. Investig. 2023, 133, e163447. [Google Scholar] [CrossRef]
- Sugawara, K.; Iwai, M.; Yajima, S.; Tanaka, M.; Yanagihara, K.; Seto, Y.; Todo, T. Oncolytic herpes virus G47Δ works synergistically with CTLA-4 inhibition through dynamic intratumoral immune modulation. Mol. Ther. Oncolytics 2021, 21, 132–145. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, F. Advances and potential pitfalls of oncolytic viruses expressing immunomodulatory transgene therapy for malignant gliomas. Cell Death Dis. 2020, 11, 485. [Google Scholar] [CrossRef]
- Vasileva, N.; Ageenko, A.; Byvakina, A.; Sen’kova, A.; Kochneva, G.; Mishinov, S.; Richter, V.; Kuligina, E. The recombinant oncolytic virus VV-GMCSF-Lact and chemotherapy drugs against human glioma. Int. J. Mol. Sci. 2024, 25, 4244. [Google Scholar] [CrossRef]
- Cheema, T.A.; Wakimoto, H.; Fecci, P.E.; Ning, J.; Kuroda, T.; Jeyaretna, D.S.; Martuza, R.L.; Rabkin, S.D. Multifaceted oncolytic virus therapy for glioblastoma in an immunocompetent cancer stem cell model. Proc. Natl. Acad. Sci. USA. 2013, 110, 12006–12011. [Google Scholar] [CrossRef]
- Jiang, H.; Shin, D.H.; Nguyen, T.T.; Fueyo, J.; Fan, X.; Henry, V.; Carrillo, C.C.; Yi, Y.; Alonso, M.M.; Collier, T.L.; et al. Localized treatment with oncolytic adenovirus Delta-24-RGDOX induces systemic immunity against disseminated subcutaneous and intracranial melanomas. Clin. Cancer Res. 2019, 25, 6801–6814. [Google Scholar] [CrossRef]
- Roberts, N.J.; Zhang, L.; Janku, F.; Collins, A.; Bai, R.Y.; Staedtke, V.; Rusk, A.W.; Tung, D.; Miller, M.; Roix, J.; et al. Intratumoral injection of Clostridium novyi-NT spores induces antitumor responses. Sci. Transl. Med. 2014, 6, 249ra111. [Google Scholar] [CrossRef]
- Andtbacka, R.H.; Kaufman, H.L.; Collichio, F.; Amatruda, T.; Senzer, N.; Chesney, J.; Delman, K.A.; Spitler, L.E.; Puzanov, I.; Agarwala, S.S.; et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J. Clin. Oncol. 2015, 33, 2780–2788. [Google Scholar] [CrossRef]
- Wilson, W.R.; Hay, M.P. Targeting hypoxia in cancer therapy. Nature reviews. Cancer 2011, 11, 393–410. [Google Scholar] [CrossRef]
- Horsman, M.R.; Mortensen, L.S.; Petersen, J.B.; Busk, M.; Overgaard, J. Imaging hypoxia to improve radiotherapy outcome. Nat. Rev. Clin. Oncol. 2012, 9, 674–687. [Google Scholar] [CrossRef]
- Agrawal, N.; Bettegowda, C.; Cheong, I.; Geschwind, J.F.; Drake, C.G.; Hipkiss, E.L.; Tatsumi, M.; Dang, L.H.; Diaz, L.A., Jr.; Pomper, M.; et al. Bacteriolytic therapy can generate a potent immune response against experimental tumors. Proc. Natl. Acad. Sci. USA 2004, 101, 15172–15177. [Google Scholar] [CrossRef]
- Krick, E.L.; Sorenmo, K.U.; Rankin, S.C.; Cheong, I.; Kobrin, B.; Thornton, K.; Kinzler, K.W.; Vogelstein, B.; Zhou, S.; Diaz, L.A., Jr. Evaluation of Clostridium novyi-NT spores in dogs with naturally occurring tumors. Am. J. Vet. Res. 2012, 73, 112–118. [Google Scholar] [CrossRef]
- Diaz, L.A., Jr.; Cheong, I.; Foss, C.A.; Zhang, X.; Peters, B.A.; Agrawal, N.; Bettegowda, C.; Karim, B.; Liu, G.; Khan, K.; et al. Pharmacologic and toxicologic evaluation of C. novyi-NT spores. Toxicol. Sci. Off. J. Soc. Toxicol. 2005, 88, 562–575. [Google Scholar] [CrossRef]
- Paoloni, M.; Khanna, C. Translation of new cancer treatments from pet dogs to humans. Nat. Rev. Cancer 2008, 8, 147–156. [Google Scholar] [CrossRef]
- Wick, W.; Wick, A.; Sahm, F.; Riehl, D.; von Deimling, A.; Bendszus, M.; Kickingereder, P.; Beckhove, P.; Schmitz-Winnenthal, F.H.; Jungk, C.; et al. VXM01 phase I study in patients with progressive glioblastoma: Final results. JCO 2018, 36, 2017. [Google Scholar] [CrossRef]
- Sun, R.; Liu, M.; Lu, J.; Chu, B.; Yang, Y.; Song, B.; Wang, H.; He, Y. Bacteria loaded with glucose polymer and photosensitive ICG silicon-nanoparticles for glioblastoma photothermal immunotherapy. Nat. Commun. 2022, 13, 5127. [Google Scholar] [CrossRef]
- Zhou, M.; Tang, Y.; Xu, W.; Hao, X.; Li, Y.; Huang, S.; Xiang, D.; Wu, J. Bacteria-based immunotherapy for cancer: A systematic review of preclinical studies. Front. Immunol. 2023, 14, 1140463. [Google Scholar] [CrossRef]
- Zhang, Y.; Xi, K.; Fu, Z.; Zhang, Y.; Cheng, B.; Feng, F.; Dong, Y.; Fang, Z.; Zhang, Y.; Shen, J.; et al. Stimulation of tumoricidal immunity via bacteriotherapy inhibits glioblastoma relapse. Nat. Commun. 2024, 15, 4241. [Google Scholar] [CrossRef]
- Banerjee, A.; Kim, B.J.; Carmona, E.M.; Cutting, A.S.; Gurney, M.A.; Carlos, C.; Feuer, R.; Prasadarao, N.V.; Doran, K.S. Bacterial Pili exploit integrin machinery to promote immune activation and efficient blood-brain barrier penetration. Nat. Commun. 2011, 2, 462. [Google Scholar] [CrossRef]
- Naghavian, R.; Faigle, W.; Oldrati, P.; Wang, J.; Toussaint, N.C.; Qiu, Y.; Medici, G.; Wacker, M.; Freudenmann, L.K.; Bonté, P.E.; et al. Microbial peptides activate tumour-infiltrating lymphocytes in glioblastoma. Nature 2023, 617, 807–817. [Google Scholar] [CrossRef]
- De Bonis, P.; Albanese, A.; Lofrese, G.; de Waure, C.; Mangiola, A.; Pettorini, B.L.; Pompucci, A.; Balducci, M.; Fiorentino, A.; Lauriola, L.; et al. Postoperative infection may influence survival in patients with glioblastoma: Simply a myth? Neurosurgery 2011, 69, 864–869. [Google Scholar] [CrossRef]
- Bowles, A.P., Jr.; Perkins, E. Long-term remission of malignant brain tumors after intracranial infection: A report of four cases. Neurosurgery 1999, 44, 636–643. [Google Scholar] [CrossRef]
- Solár, P.; Mackerle, Z.; Hendrych, M.; Pospisil, P.; Lakomy, R.; Valekova, H.; Hermanova, M.; Jancalek, R. Prolonged survival in patients with local chronic infection after high-grade glioma treatment: Two case reports. Front. Oncol. 2022, 12, 1073036. [Google Scholar] [CrossRef]
- Sari-Ak, D.; Alomari, O.; Shomali, R.A.; Lim, J.; Thimiri Govinda Raj, D.B. Advances in CRISPR-Cas9 for the Baculovirus Vector System: A Systematic Review. Viruses 2023, 15, 54. [Google Scholar] [CrossRef]
- Mander, S.; Naffouje, S.A.; Gao, J.; Li, W.; Christov, K.; Green, A.; Bongarzone, E.R.; Das Gupta, T.K.; Yamada, T. Tumor-targeting cell-penetrating peptide, p28, for glioblastoma imaging and therapy. Front. Oncol. 2022, 12, 940001. [Google Scholar] [CrossRef]
- von Spreckelsen, N.; Fadzen, C.M.; Hartrampf, N.; Ghotmi, Y.; Wolfe, J.M.; Dubey, S.; Yang, B.Y.; Kijewski, M.F.; Wang, S.; Farquhar, C.; et al. Targeting glioblastoma using a novel peptide specific to a deglycosylated isoform of brevican. Adv. Ther. 2021, 4, 2000244. [Google Scholar] [CrossRef]
- Latzer, P.; Zelba, H.; Battke, F.; Reinhardt, A.; Shao, B.; Bartsch, O.; Rabsteyn, A.; Harter, J.; Schulze, M.; Okech, T.; et al. A real-world observation of patients with glioblastoma treated with a personalized peptide vaccine. Nat. Commun. 2024, 15, 6870. [Google Scholar] [CrossRef]
- Collins, S.A.; Shah, A.H.; Ostertag, D.; Kasahara, N.; Jolly, D.J. Clinical development of retroviral replicating vector Toca 511 for gene therapy of cancer. Expert Opin. Biol. Ther. 2021, 21, 1199–1214. [Google Scholar] [CrossRef]
- D’Amico, R.S.; Aghi, M.K.; Vogelbaum, M.A.; Bruce, J.N. Convection-enhanced drug delivery for glioblastoma: A review. J. Neuro-Oncol. 2021, 151, 415–427. [Google Scholar] [CrossRef]
- Friedmann-Morvinski, D. Glioblastoma heterogeneity and cancer cell plasticity. Crit. Rev. Oncog. 2014, 19, 327–336. [Google Scholar] [CrossRef]
- Becker, A.P.; Sells, B.E.; Haque, S.J.; Chakravarti, A. Tumor Heterogeneity in Glioblastomas: From Light Microscopy to Molecular Pathology. Cancers 2021, 13, 761. [Google Scholar] [CrossRef]
- Zhang, P.; Xia, Q.; Liu, L.; Li, S.; Dong, L. Current Opinion on Molecular Characterization for GBM Classification in Guiding Clinical Diagnosis, Prognosis, and Therapy. Front. Mol. Biosci. 2020, 7, 562798. [Google Scholar] [CrossRef]
- Chongsathidkiet, P.; Jackson, C.; Koyama, S.; Loebel, F.; Cui, X.; Farber, S.H.; Woroniecka, K.; Elsamadicy, A.A.; Dechant, C.A.; Kemeny, H.R.; et al. Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat. Med. 2018, 24, 1459–1468. [Google Scholar] [CrossRef]
- Campian, J.L.; Ghosh, S.; Kapoor, V.; Yan, R.; Thotala, S.; Jash, A.; Hu, T.; Mahadevan, A.; Rifai, K.; Page, L.; et al. Long-Acting Recombinant Human Interleukin-7, NT-I7, Increases Cytotoxic CD8 T Cells and Enhances Survival in Mouse Glioma Models. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2022, 28, 1229–1239. [Google Scholar] [CrossRef]

| oHSV Variant | Genetic Modifications/Additions | Study Model | Key Findings | Reference |
|---|---|---|---|---|
| z | Lacks thymidine kinase (TK); attenuated neurovirulence | U87 glioma cells (in vitro, mice) | Cytotoxic to human glioma cells; improved survival and reduced tumor burden in mice | [29] |
| oHSV-P10 | Expresses PTEN-L | Human GBM12 cells and mice | Eliminated GSCs, blocked IL6/JAK/STAT3 signaling; overcame radiotherapy resistance | [30] |
| HSV-P10 | Expresses PTEN-α | GBM cells, immune-competent mice | Suppressed PI3K/AKT pathway, reduced PD-L1, enhanced adaptive immune response | [31] |
| oHSV-1 (ICP4+) | Deleted γ34.5 and ICP47; retains ICP4 | Glioma cell lines and animal models | Reduced invasion via downregulation of Sp1; high anti-tumor activity with less immune activation | [32] |
| EGFP-oHSV-1 | Δγ34.5/ΔUS12; includes EGFP reporter | GL261 glioma mouse model | Improved survival and immune response; greater tumor control vs. other oHSV-1 variants | [33] |
| C5252 | Deleted γ34.5 and 15 kb repeat; expresses IL-12 and anti-PD-1 fragment | Human GBM cells and mice | Strong cytotoxicity despite low replication; induced apoptosis, immune activation (IFN-γ, TNF-α) | [34] |
| G47Δ-mIL2 | Expresses IL-2 | Orthotopic glioma mouse model | Enhanced CD4+/CD8+ T-cell infiltration; prolonged survival; no synergy with PD-1 blockade | [35] |
| G47Δ-mIL12 | Expresses IL-12; combined with anti-PD-1/CTLA-4 | Glioma mouse model | Improved survival; increased M1 macrophages and effector T cells; enhanced immune response | [36] |
| R-115 | Targets HER2; expresses IL-12 | HER2+ glioma in BALB/c mice | Long-term remission, antibody generation, HER2-independent protection; no CD4/CD8 increase noted | [37] |
| R-613 | Targets EGFRvIII | hGICs and EGFRvIII+ glioma in mice | Effective in early-stage tumors; less effective in advanced disease; suitable for combination therapy | [38] |
| OH2 (HSV-2 based) | HSV-2-derived oncolytic virus with tumor-selective replication | In vitro, xenograft mice | Selective tumor replication, DNA damage, suppressed tumor cells and M2 TAMs, enhanced macrophage and T cell infiltration, slowed GBM, prolonged survival | [39] |
| NG34 (HSV-1 with GADD34) | HSV-1 engineered to express GADD34 under nestin promoter | In vitro, mouse models | Similar efficacy to rQNestin34.5, reduced neurotoxicity and brain damage | [40] |
| HSV + CAR-T cells (B7-H3-directed) | CAR-T cells engineered to express B7-H3-targeted CAR and carry HSV | Orthotopic GBM mice | CAR-T cells deliver HSV to distant tumor sites, enhanced T cell infiltration, prolonged survival | [41] |
| CAR-T + HSV-1 G47Δ | HSV-1 G47Δ combined with CAR-T cells | Mouse models | Tumor regression, extended survival | [42] |
| oHSV-1 | Wild-type or engineered oHSV-1 | Murine models, in vitro | TNFα from M1 macrophages/microglia inhibits viral replication; blocking TNFα increases viral spread and survival | [43] |
| MEK inhibitor + oHSV-1 | Pharmacological MEK inhibitor combined with oHSV-1 | In vitro, murine glioma | Trametinib reduces TNFα production, enhances oHSV replication and survival | [44] |
| oHSV + Gamma Secretase Inhibitor (GSI) | Wild-type oHSV combined with NOTCH pathway inhibitor | In vitro, orthotopic mice | Inhibition of NOTCH signaling boosts therapy efficacy with maintained safety | [45] |
| oHSV G47Δ | HSV-1 G47Δ | Patient-derived cultures, mice | Fasting boosts viral replication and cytotoxicity by suppressing JNK pathway | [46] |
| γ34.5-deficient oHSV | Deletion of γ34.5 gene in oHSV; Us11 protein expression | GSCs, ScGCs culture | GSCs resist virus replication due to translational blockade; Us11 protein restores replication | [47] |
| Bortezomib + oHSV1 + NK cells | oHSV + proteasome inhibitor + NK cells | In vitro, mice | Necroptosis induction, increased NK activation, improved tumor suppression | [48] |
| CCN1 and HSV resistance | Wild-type HSV-1; CCN1 expression modulates resistance | GBM cell lines | CCN1 mediates early viral resistance by innate immune activation | [49] |
| oHSV + TGF-β receptor inhibitor | oHSV combined with TGF-β pathway inhibitor | GSCs, animal models | Blocking TGF-β boosts viral replication and survival via JNK-MAPK pathway | [50] |
| oHSV + C16 inhibitor | Pharmacologic STAT1/3 inhibitor combined with oHSV | U87 xenograft, cell cultures | Inhibiting STAT1/3 in microglia/macrophages promotes oHSV replication and tumor regression | [51] |
| oHSV releasing ChaseM enzyme + temozolomide | oHSV engineered to release ChaseM enzyme (degrades CSPGs) | Preclinical mouse models | Degrades CSPGs, improves tumor penetration and apoptotic death, extends survival | [52] |
| oHSV + anti-HMGB1 antibodies | Wild-type oHSV combined with HMGB1 neutralizing antibodies | In vitro, mouse brain tumors | Blocking HMGB1 reduces edema and improves survival with oHSV therapy | [53] |
| KG4:T124 | HSV-1 derivative (specific modifications not detailed) | GL261N4 and CT2A murine glioma models | Cleared quickly in CT2A; limited immune response and therapeutic effect. | [54] |
| rQNestin34.5v.1 | ICP34.5 under nestin promoter | GL261N4 and CT2A murine glioma models | Higher viral load; persisted longer in GL261N4, enhancing immune infiltration and survival. | [55] |
| CXCR4-targeted oHSV | Glycoprotein D modified with CXCR4-specific nanobody in attenuated HSV-1 | GSC xenograft mouse models | Targeted CXCR4+ GSCs; reduced tumor growth and improved survival. | [56] |
| Virus Used | Patient Population | Design and Intervention | Adverse Events | Median Survival | Notable Findings | Reference |
|---|---|---|---|---|---|---|
| G207 (γ134.5-deleted HSV-1) | 9 adults with recurrent malignant glioma | Single intratumoral G207 injection + 24 h later 5 Gy radiation; 2 patients had a second injection | Well tolerated; no severe side effects | 7.5 months | Safe combination with radiotherapy; 3 patients showed marked radiologic response | [57] |
| G207 | 12 pediatric patients (7–18 y/o), mostly GBM | Intratumoral G207 ± radiation | High rate of AEs (e.g., diarrhea, bradycardia, seizures), but manageable | 12.2 months | 11/12 had clinical or radiological improvement; increased lymphocyte infiltration; 4 survived > 18 months | [58] |
| G47∆ (triple-mutated oHSV) | 13 adults with recurrent/advanced GBM | Up to 6 intratumoral injections over 2 weeks | Common: nausea, fever, headache; manageable with corticosteroids | Not reported; 3 > 46 mo | CD4+/CD8+ T-cell infiltration; 1 patient survived > 11 years | [59] |
| M032 (IL-12 expressing HSV-1) | 21 adults with recurrent glioma | Single intratumoral injection | Grade 3–4 AEs in only 1 patient at high dose; no severe toxicity at max dose | 9.38 months | Generally well tolerated; individualized response suggests need for personalized dosing | [60] |
| CAN-3110 (ICP34.5 under nestin promoter) | 41 patients with recurrent GBM | Single intratumoral injection | No serious AEs at highest doses | Not stated clearly | HSV-seropositive patients had better survival; T-cell activation and immune gene upregulation observed | [61] |
| oADV Variant | Genetic Modifications/Additions | Study Model | Key Findings | Ref. |
|---|---|---|---|---|
| Replicating vs. Non-replicating oAdVs | Replication-competent vs. incompetent adenoviruses | Cell lines, mouse models | Replicating oAdVs enhanced immune cell infiltration and survival | [69] |
| Ad5-pIX-Ad37 | IX capsid protein with dimerization domain; fiber knob from Ad37 | In vitro, in vivo | Enhanced cell entry and oncolytic activity | [70] |
| oAdV-ApoA1 | Carries apolipoprotein A1 | Cell lines, mouse models | Reduced 7-KC, activated TNF signaling, improved immune response | [71] |
| ICOVIR17 | Carries hyaluronidase enzyme | GBM mouse models | Increased macrophages, CD8+ T cells, and survival with anti-PD-1 | [72] |
| ICOVIR15 | ∆24-E1A, RGD-modified fiber | GBM cells, mouse models | Targeted FAP+ pericytes and tumor cells; induced apoptosis | [73] |
| ONCOTECH | T cell–associated, PD-L1 targeting | Cancer mouse models | Reduced PD-L1, enhanced survival | [74] |
| Ad5-Ki67/IL-15 | Ki67 promoter; IL-15 expression | Glioma cells, mouse models | Reduced PD-L1, boosted T cell infiltration | [75] |
| MSC-Ad5-Ki67/IL-15 | MSC-carried virus with IL-15 and Ki67 promoter | In vitro, in vivo | Enhanced macrophage infiltration and efficacy | [76] |
| TS-2021 (Ad5 KT-E1A-IL-15) | Ki67 promoter, TGF-β2 5′UTR, IL-15 | In vitro, in vivo | Reduced tumor burden, improved survival | [77] |
| TS-2021 + Olaparib | Same as TS-2021; combined with PARP inhibitor | GBM cells, mouse models | Synergistic tumor apoptosis and survival benefit | [78] |
| XVir-N-31 (Intranasal) | Carrier-cell optimized oAdV | GBM-bearing mice | Non-invasive delivery reduced tumor burden, improved survival | [79] |
| XVir-N-31 + ICI | Combined with anti-PD-1/PD-L1 | In vitro, humanized mouse models | Enhanced immune cell infiltration, tumor regression | [80] |
| YSCH-01 | Recombinant interferon-like gene | Glioma cells, hamster models | Strong local and distant tumor suppression | [81] |
| H5CmTERT-Ad/TRAIL | hTERT promoter, secretable trimeric TRAIL | In vitro, in vivo | Effective in TRAIL-resistant tumors, induced death in hypoxia | [82] |
| Ad6 | Native Ad6 serotype | GBM cells, mouse models | Cytotoxicity, reduced GBM stem cells | [83] |
| Ad5 (hTERT/survivin promoters) | GBM-specific promoters (hTERT, survivin) | GBM cell lines | Selective cytotoxicity in GBM cells | [84] |
| Delta-24-RGD (Proteomic analysis) | ∆24-E1A, RGD-modified fiber | Phase I clinical trial samples | Altered kinase/cytokine profiles, immune activation | [85] |
| CTV (Ad3 fiber + Ad5 capsid) | Produces MDA-7/IL-24 | In vivo, GBM models | Extended survival, enhanced with Temozolomide | [86] |
| PD-BM-MSC-D24 | Delta-24-RGD loaded in chemo-treated BM-hMSC | In vitro, in vivo | Effective delivery and tumor suppression | [87] |
| Delta-24-RGDOX | Oncolytic adenovirus with RGD motif and OX40L | Mouse models | Prolonged survival; changes in gut microbiota with dominance of Bifidobacterium. Microbiota may influence therapeutic efficacy. | [88] |
| Delta-24-GREAT | Delta-24 modified with GITRL gene | Human and mouse glioma cell lines; mouse models | Enhanced immune response, increased memory T cells, tumor rejection after re-challenge. | [89] |
| Delta-24-RGD + HDAC inhibitors | Combined with scriptaid and LBH589 | Patient-derived glioblastoma lines | Synergistic antitumor effects; scriptaid ↑ caspase-3/7 and apoptosis; LBH589 ↑ LDH and phospho-p70S6K. | [90] |
| AdCMVdelta24 | Delta-24 under CMV promoter | Mouse GBM models | Reduced Tregs, increased IFNγ+ CD8+ T cells; reprogrammed Tregs into stimulatory phenotype. | [91] |
| hMSC-D24 | Delta-24-RGD loaded into human MSCs | Dog GBM model (large animal); intra-arterial delivery (ESIA) | ESIA safe in anterior cerebral circulation; stroke risk in posterior; proof-of-concept for large-animal delivery method. | [92] |
| Ad5/35-delta-24, Ad5/3-delta-24 | Fiber region modified | Human and rodent glioma lines; mouse models | Ad5/35-delta-24: strong immune-mediated tumor suppression; induced immune memory; superior to Ad5-delta-24-RGD. | [93] |
| CAN-2409 + dexamethasone | Simultaneous administration with corticosteroid | In vitro and in vivo experiments | decreased immune activation; decreased tumor response; decreased median survival; dexamethasone suppresses CAN-2409 efficacy. | [94] |
| Delta-24-RGD + anti-PD-1 | Combination with immune checkpoint blockade | In vivo and in vitro models | Synergistic effect; Increased CD8+ T cells and IFNγ production; improved survival over monotherapies. | [95] |
| H101 + anti-PD-1 | H101 suppresses CD47; anti-PD-1 immunotherapy | Human glioblastoma lines (U87-MG) | Increased T cell infiltration, macrophage phagocytosis, cytokines; enhanced anti-tumor effect. | [96] |
| CAN-2409 + ATR inhibitor (AZD6738) | Combination with DNA damage repair inhibitor | In vitro and in vivo GBM models | Increased γH2AX; decreased PD-L1; improved survival; enhanced DNA damage and immune response. | [97] |
| Oncolytic Ad (receptor sensitivity study) | N/A | Human glioma cell lines (Grade II–IV) | Receptor expression (CAR, CD46, DSG-2) not predictive of infectivity or efficacy. | [98] |
| Delta-24-RGD + TMZ | Combination with temozolomide (standard chemo) | Murine glioma lines; mouse models | Synergy when Delta-24-RGD precedes TMZ; reversed effects if sequence is changed; Increased CD8+ T cells. | [99] |
| Ad5-Delta-24-RGD with L3-23K vs. L5-Fiber gene addition | Gene insertion at different viral regions | GBM cell lines; mouse models | Gene expression higher at L3-23K; insertion site affected oncolytic activity; no major difference in cytotoxicity. | [100] |
| CXCL11-carrying oAd | oAd encoding CXCL11 chemokine | Orthotopic GBM mouse models; cell cultures | Increased CD8+ T cell activation; Increased Tregs; improved CAR-T therapy efficacy in GBM. | [101] |
| oAd-IL7 + CAR-T (B7H3) | Oncolytic adenovirus expressing IL-7 | In vivo and in vitro models | Increased intratumoral T cells; Increased median survival; synergistic effect with CAR-T therapy. | [102] |
| OA@TA-Fe3+-CXCL11 oAd | CXCL11 oAd coated with tannic acid & Fe3+ ions | In vivo and in vitro GBM models | Increased retention and oncolytic activity; Fe3+ reduced hypoxia via O2 generation; immune stimulation. | [103] |
| NSC.CRAd-S-pk7 | CRAd-Survivin-pk7 delivered via neural stem cells | Mice with competent immune system | Multiple doses at high levels effective; immune system did not hinder therapy; shown to be safe in Phase I trials. | [104] |
| CAN-2409 | Expresses HSV-TK (thymidine kinase) | Glioma stem-like cells; mouse models | Enriched p53/cell cycle pathways; regulated MYC, CCNB1, PLK1, CDC20; Increased IL-12, Increased T cell activation. | [105] |
| Virus Used | Patient Population | Design and Intervention | Adverse Events | Median Survival | Notable Findings | Ref. |
|---|---|---|---|---|---|---|
| NSC-CRAd-S-pk7 | 11 newly diagnosed glioblastoma patients | Phase 1; oAdv delivered via neural stem cells (NSC) after tumor resection; followed by standard chemo-radiotherapy | Decreased lymphocytes, headache, anemia, fatigue, nausea, hypoalbuminemia; NSC-CRAd-S-pk7-related meningitis (1 pt), subdural fluid (1 pt) | 18.4 months | Safe; did not delay standard therapy; promising survival outcomes; supports Phase 2 progression | [106] |
| Delta24-RGD (DNX-2401) | Patients with recurrent glioblastoma | Phase 1; oAdv administered via convection-enhanced delivery (CED) to tumor and peritumoral areas | Dose-limiting: increased intracranial pressure, temporary viral meningitis | 129 days | 1 complete responder (8-year survival), 1 partial response (2.5 years); ↑ NK, T cells, proinflammatory cytokines; immune activation observed | [107] |
| Ad5-Δ24.RGD | GBM patients with pre-existing adenovirus antibodies | Observational immune monitoring study | Not specified | Not specified | Despite neutralizing antibodies, RGD modification enabled efficacy by alternative cellular entry; supports oAdv utility in seropositive patients | [108] |
| DNX-2401 + pembrolizumab | 3 recurrent glioblastoma patients | Phase 2 pilot; DNX-2401 delivered via SmartFlow catheter under real-time MRI guidance; followed by pembrolizumab | None reported | Not reported | 2 partial responders with infusion area >1 cm; 1 non-responder with <1 cm infusion; safe and feasible approach for image-guided intratumoral administration | [109] |
| DNX-2401 + pembrolizumab | 49 patients with recurrent glioblastoma | Phase 1/2; DNX-2401 combined with anti-PD-1 (pembrolizumab) | No dose-limiting toxicities reported | 12.5 months | Combination was safe; MRI response associated with long-term survival; supports benefit over monotherapy | [110] |
| oMeV Variant | Genetic Modifications/Additions | Study Model | Key Findings | Ref. |
|---|---|---|---|---|
| Preclinical | ||||
| MV-CEA | Insertion of the soluble N-terminal extracellular domain of human CEA into MV-NSe backbone (MV-Edm lineage); CEA gene placed before N gene | Human glioma cell lines (primary and GBM-derived); orthotopic glioma mouse models (e.g., U87, GBM14, GBM39, GBM6) | Enabled noninvasive monitoring of viral gene expression via serum CEA levels; induced selective cytopathic effects in glioma cells; promoted apoptosis; spared normal astrocytes and fibroblasts; prolonged survival in glioma models | [113] |
| MV-miR-122 | Engineered to encode microRNA-122 (miR-122); tested with luciferase reporter system | In vitro glioma cell model | Demonstrated that MV may have the potential to deliver functional miRNA and leads to a reduced target protein expression by 40%; limited by Drosha-mediated miRNA processing inefficiency in cytoplasm | [114] |
| MeV + Ruxolitinib | Combination of wild-type Edmonston-lineage MeV with JAK1 pathway inhibitor ruxolitinib | Patient-derived xenograft (PDX) glioblastoma models; 22-gene expression analysis | JAK1 inhibition increased MeV replication and therapeutic sensitivity; highlighted potential for combination virotherapy and immune modulation | [115] |
| MeV-stealth | MeV glycoproteins replaced with CDV glycoproteins; CDV-H fused with CD46-targeting scFv; CDV-F signal peptide modified to redirect tropism | Multiple tumor xenografts in immunodeficient mice | Immune-evasive tumor targeting; specific lysis of CD46-overexpressing tumors; reduced off-target effects | [116] |
| MV-L16 (live attenuated measles virus strain) | GBM-derived primary cell lines (Gbl7n, Gbl11n, etc.) | Preclinical translational study (ex vivo analysis of patient-derived glioma lines) | MV-L16 induced significant caspase-3/7 activation, confirming apoptosis. mRNA-seq showed predictive immunologic gene expression changes in virus-sensitive GBM cells. | [117] |
| Clinical Studies | ||||
| Virus Used | Design and Intervention | Adverse Events | Notable Findings | Ref. |
| MV-CEA (Edmonston strain expressing carcinoembryonic antigen) | 22 patients with recurrent glioblastoma (GBM) Phase I, First-in-human trial (NCT00390299). Group A: MV-CEA injected into resection cavity. Group B: Intratumoral injection Day 1, followed by resection cavity injection Day 5. | No dose-limiting toxicity, even at max dose. Minor, manageable adverse events (not specified in detail). | Repeated intratumoral MV-CEA is safe. CEA levels correlate with viral replication. Dual-Labeling Diagnostic Assay (DLDA) may predict viral response. | [118] |
| oNDV Variant | Genetic Modifications/Additions | Study Model | Key Findings | Ref. |
|---|---|---|---|---|
| Preclinical Studies | ||||
| Wild-type NDV | None | Human GBM cell lines (GBM18, GBM27, etc.) in immunodeficient mice | IFN-I gene cluster deletion enhanced NDV replication and tumor lethality; NS1-expressing NDV overcame IFN-I resistance | [121] |
| NDV-2F/2HN-IFNγ | Incorporated F and HN genes from APMV-2; added human IFN-γ gene | Human PBMCs, chicken embryo fibroblasts (CEFs), Caco-2 (colon cancer) cell line | Enhanced IFN-γ expression and immune response; increased cancer cell death; evaded NDV-specific antibodies | [123] |
| NDV + TMZ-PLGA-NPs | Combination of wild-type NDV with Temozolomide-loaded PLGA nanoparticles | Human GBM cell lines (in vitro) | Combination therapy improved cytotoxicity over individual agents; TMZ-NPs enhanced drug stability and delivery | [124] |
| rNDV-PTEN (Pos. 1 vs. 2) | PTEN gene inserted between NP-P (Pos. 1) or P-M (Pos. 2) genes in NDV genome | T98G GBM cells in immunodeficient mice (in vitro and in vivo) | PTEN overexpression (especially in Pos. 1) suppressed cancer growth markers (P-Akt, hTERT); intratumoral injection more effective than intravenous delivery | [125] |
| Clinical Studies | ||||
| Virus Used | Design and Intervention | Adverse Events | Notable Findings | Ref. |
| NDV-HUJ | Phase I/II, open-label trial (n = 11); phase I: escalating weekly cycles (0.1 to 55 BIU); phase II: 3 weekly cycles of 5 days at 11 BIU, then maintenance 2x/week | No adverse events related to NDV-HUJ | NDV found in blood, urine, CSF, saliva, tumor; 1 pt had complete remission but relapsed in 3 months; anti-NDV antibodies plateaued at 8 weeks | [126] |
| MTH-68/H | Case series (n = 4); daily to twice daily dosing ranging from 2 × 107 to 2.5 × 108 PFU | Not reported | Tumor regression, neurological improvement, steroid withdrawal; long-term survival 5–9 years from diagnosis | [127] |
| Ulster | Nonrandomized controlled study (n = 22); ASI vaccine (NDV + cisplatin-inactivated autologous tumor) via cutaneous injection every 2 wks × 5; CG had chemo (nimustine + VM-26) | No significant adverse events with ASI | ASI induced immune response via DTH; no survival difference, but TG (ASI) had mean survival of 46 weeks vs. 48 weeks for CG | [128] |
| Ulster (ATV-NDV) | Nonrandomized controlled study (n = 111); NDV-inactivated autologous tumor cells injected q3–4 wks up to 8 doses post-RT | No significant adverse events with ATV-NDV | Median OS: TG 100 wks, CG-NS 49 wks, CG-S 88 wks; elevated CD8+ TILs, increased memory T cells in long-term survivors; durable immune memory | [129] |
| Virus Used | Patient Population | Design and Intervention | Adverse Events | Notable Findings | Ref. |
|---|---|---|---|---|---|
| Reovirus (Pelareorep) | Recurrent malignant gliomas | Phase I trial; intratumoral catheter-based infusion for 72 hrs | One transient grade 3 seizure; otherwise well tolerated | Virus reached tumor; histology showed cell death and immune activation; safe at high doses | [135] |
| Reovirus + GM-CSF | Newly diagnosed GBM | ReoGlio (2017–2020): IV pelareorep + GM-CSF during concurrent chemoradiation and adjuvant TMZ | Flu-like symptoms, reversible hypotension; no major safety concerns or treatment delays | Virus delivery to tumor confirmed; some T cell activity observed in resected tumors; early signals of immunological engagement | [136] |
| Reovirus | Newly diagnosed GBM | Window-of-Opportunity trial: single IV dose prior to surgery | Well tolerated | Viral RNA/proteins detected in tumor tissue; increased CD8+ T cell activity; proof of BBB crossing and immunogenic modulation | [137] |
| Reovirus + GM-CSF | Pediatric high-grade gliomas (incl. GBM) | Small trial (IV Pelareorep + GM-CSF) | Grade 3 hyponatremia (1 patient); overall well tolerated | All patients progressed within weeks; early antibody neutralization likely impacted efficacy; confirms tumor entry via IV but poor clinical outcome | [138] |
| Virus Variant | Genetic Modifications/Additions | Study Model | Key Findings | Ref. |
|---|---|---|---|---|
| Pre-clinical Studies | ||||
| MLV-based RCR | RCR vector expressing yeast cytosine deaminase gene with 5-FC prodrug | Cell lines, mouse models | Single injection led to widespread tumor infection and enhanced survival; tumor-selective expression of suicide gene. | [139] |
| Toca 511 | Amphotropic MLV encoding modified yeast cytosine deaminase 2 (yCD2) | In vitro, in vivo | Tumor-targeted delivery; converts 5-FC to 5-FU; safe replication; efficient and stable gene delivery in glioblastoma. | [140] |
| oFV-GFP | oFV vector with chimpanzee virus PAN1/PAN2 genes and green fluorescent protein (GFP) transgene | GBM cells, mouse models | Safe, stable, and tumor-persistent; spreads well even in slow-growing tumors; prolongs survival; can reactivate after dormancy. | [141] |
| oFV-GFP, oFV-TK, oFV-iCasp9 | GFP, thymidine kinase (TK), and inducible caspase 9 (iCasp9) inserted into oFV vectors | Human GBM cells, mouse models | Effective long-term gene expression; GFP expression persisted for 66 days; larger genes like iCasp9 lost over time during replication in tumors. | [142] |
| Clinical Studies | ||||
| Virus Used | Design and Intervention | Adverse Events | Notable Findings | Ref. |
| Toca 511 | 45 patients with glioblastoma | Phase 1 trial; virus injected after surgery followed by oral Toca FC and standard chemoradiotherapy | Grade 3 asthenia, hydrocephalus, thrombocytopenia, procedural pain | [143] |
| Toca 511 | 56 patients with recurrent high-grade glioma | Phase 1 dose-escalation; intratumoral virus injection post-surgery + Toca FC oral prodrug | Grade 2 skin rash, mucositis, facial swelling; Grade 3 hemorrhagic enteritis and colitis | [144] |
| Toca 511 | 56 high-grade glioma patients | Phase 1; integrated omics analysis (WES, RNA-seq, ELISA) on tumor and blood to assess response | Not disclosed | [145] |
| Toca 511 | 127 high-grade glioma patients | Large study using extended-release Toca FC and molecular monitoring (RNA/DNA tracking, integration site analysis, mutation profiling) | Hematologic adverse events including lymphoma | [146] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alomari, O.; Eyvazova, H.; Güney, B.; Al Juhmani, R.; Odabasi, H.; Al-Rawabdeh, L.; Mokresh, M.E.; Erginoglu, U.; Keles, A.; Baskaya, M.K. Oncolytic Therapies for Glioblastoma: Advances, Challenges, and Future Perspectives. Cancers 2025, 17, 2550. https://doi.org/10.3390/cancers17152550
Alomari O, Eyvazova H, Güney B, Al Juhmani R, Odabasi H, Al-Rawabdeh L, Mokresh ME, Erginoglu U, Keles A, Baskaya MK. Oncolytic Therapies for Glioblastoma: Advances, Challenges, and Future Perspectives. Cancers. 2025; 17(15):2550. https://doi.org/10.3390/cancers17152550
Chicago/Turabian StyleAlomari, Omar, Habiba Eyvazova, Beyzanur Güney, Rana Al Juhmani, Hatice Odabasi, Lubna Al-Rawabdeh, Muhammed Edib Mokresh, Ufuk Erginoglu, Abdullah Keles, and Mustafa K. Baskaya. 2025. "Oncolytic Therapies for Glioblastoma: Advances, Challenges, and Future Perspectives" Cancers 17, no. 15: 2550. https://doi.org/10.3390/cancers17152550
APA StyleAlomari, O., Eyvazova, H., Güney, B., Al Juhmani, R., Odabasi, H., Al-Rawabdeh, L., Mokresh, M. E., Erginoglu, U., Keles, A., & Baskaya, M. K. (2025). Oncolytic Therapies for Glioblastoma: Advances, Challenges, and Future Perspectives. Cancers, 17(15), 2550. https://doi.org/10.3390/cancers17152550






