Evaluating the Therapeutic Role of Lymph Node Dissection in Variant Subtype Bladder Cancer
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Data Source
2.2. Study Outcomes
2.3. Statistical Analysis
3. Results
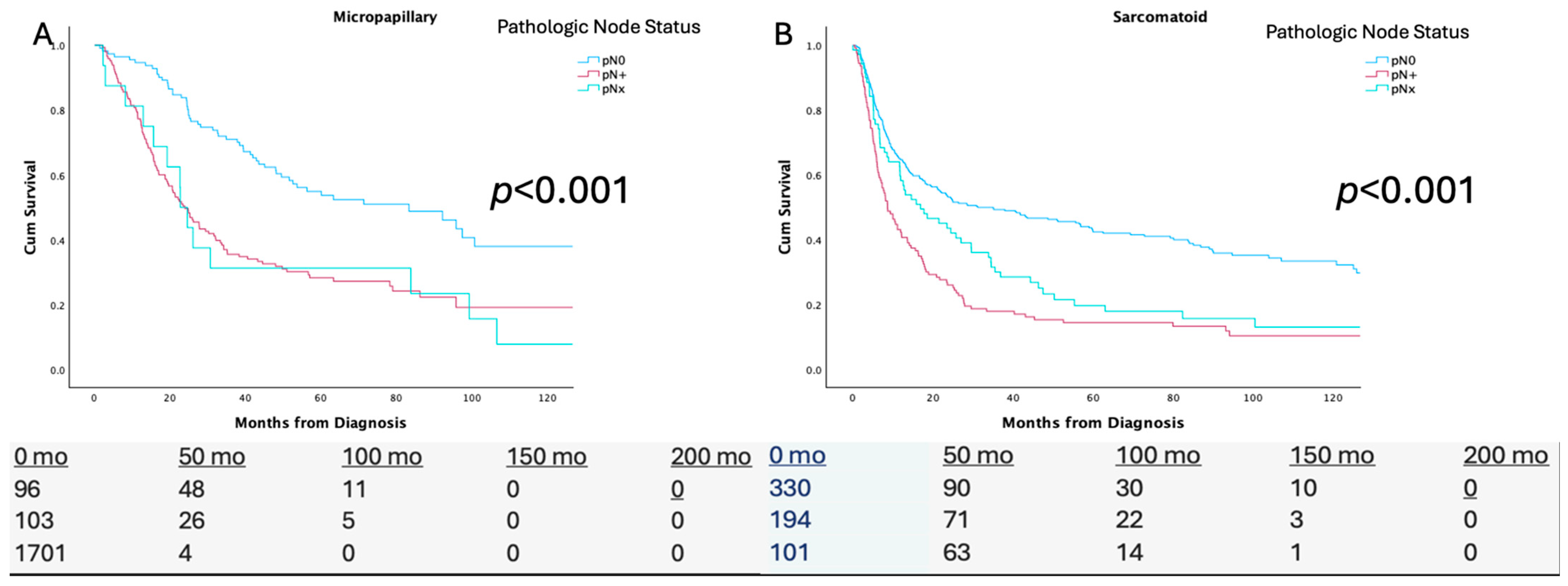
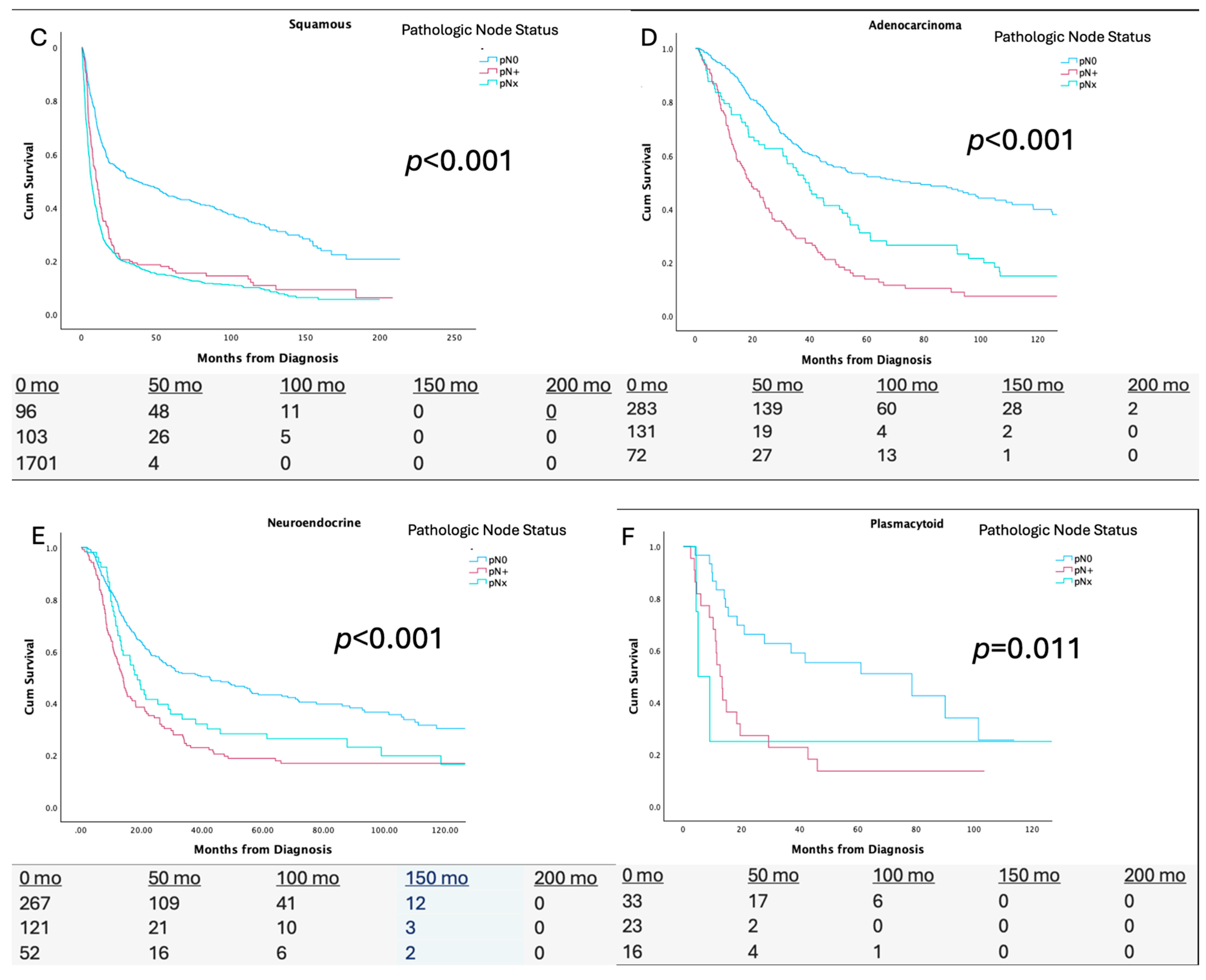
3.1. pN Status
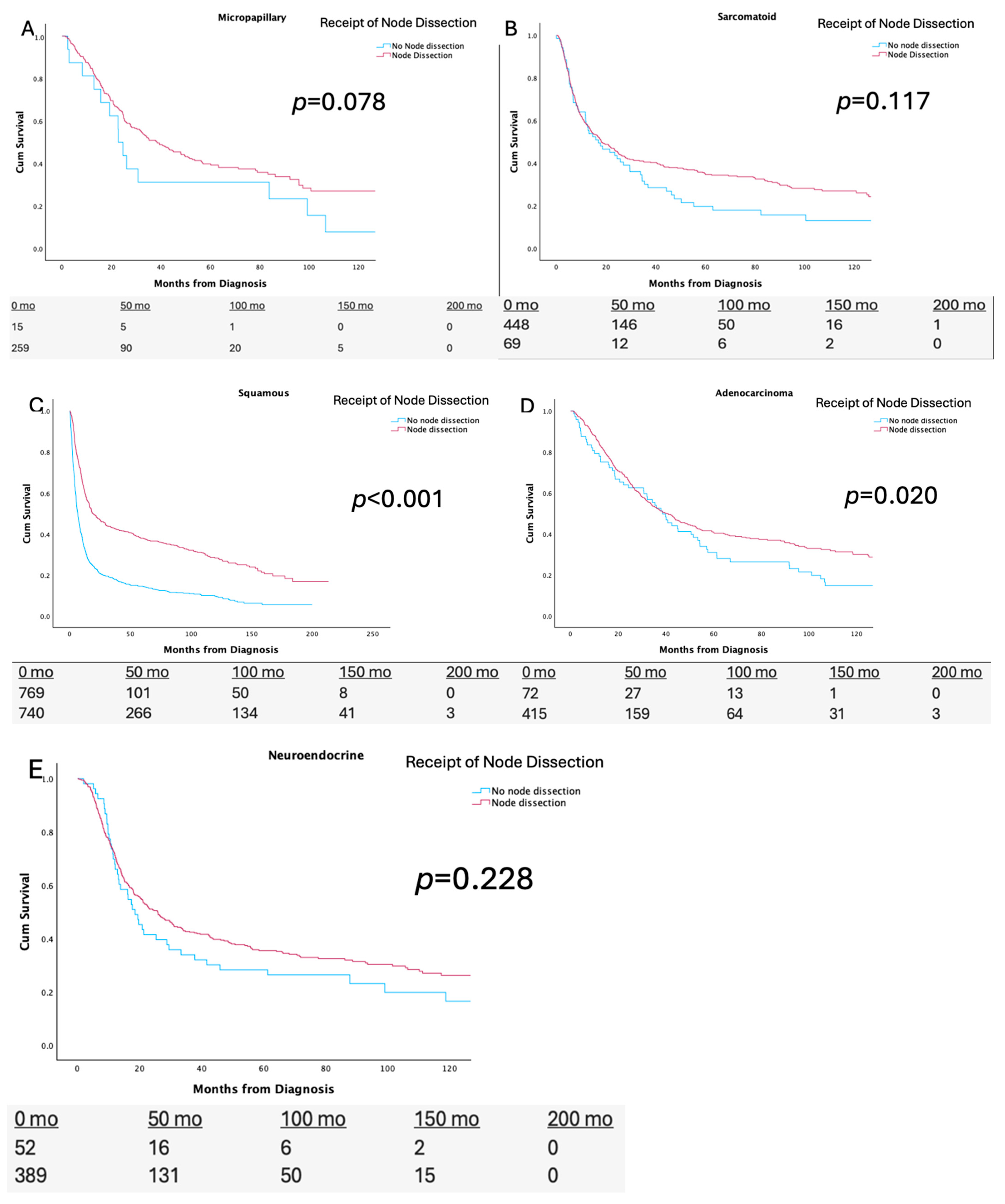
3.2. Node Dissection and Nodal Yield
3.3. Multivariate Cox Proportional Hazards Analysis
3.4. Receipt of Nodal Dissection Stratified by Receipt of NAC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Choi, S.Y.; You, D.; Hong, B.; Hong, J.H.; Ahn, H.; Kim, C.-S. Impact of lymph node dissection in radical cystectomy for bladder cancer: How many vs how far? Surg. Oncol. 2019, 30, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Perera, M.; McGrath, S.; Sengupta, S.; Crozier, J.; Bolton, D.; Lawrentschuk, N. Pelvic lymph node dissection during radical cystectomy for muscle-invasive bladder cancer. Nat. Rev. Urol. 2018, 15, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Lerner, S.P.; Tangen, C.; Svatek, R.S.; Daneshmand, S.; Pohar, K.S.; Skinner, E.; Schuckman, A.; Sagalowsky, A.I.; Smith, N.D.; Kamat, A.M.; et al. Standard or Extended Lymphadenectomy for Muscle-Invasive Bladder Cancer. N. Engl. J. Med. 2024, 391, 1206–1216. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Day, E.; Gavira, J.; Tapia, J.C.; Anguera, G.; Maroto, P. What About Variant Histologies in Bladder Cancer? Eur. Urol. Focus 2024, 10, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Abufaraj, M.; Mostafaei, H.; Quhal, F.; Karakiewicz, P.I.; Briganti, A.; Kimura, S.; Egawa, S.; Shariat, S.F. A Systematic Review and Meta-Analysis of Variant Histology in Urothelial Carcinoma of the Bladder Treated with Radical Cystectomy. J. Urol. 2020, 204, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.N.; Lokeshwar, S.D.; Syed, J.S.; Javier-Desloges, J.F.; Press, B.H.; Choksi, A.U.; Rajwa, P.; Ploussard, G.; Kim, J.W.; Monaghan, T.F.; et al. Oncologic outcomes of neoadjuvant chemotherapy in patients with micropapillary variant urothelial carcinoma of the bladder. Urol. Oncol. Semin. Orig. Investig. 2023, 41, 107.e1–107.e8. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Kong, V.; Jalfon, M.; Hesse, D.; Kim, J.; Wright, J.L.; Adeniran, A.; Humphrey, P.; Martin, D.T.; Ghali, F. Evaluating Treatment Patterns and the Role of Neoadjuvant Chemotherapy in Plasmacytoid Urothelial Carcinoma: Insights from a Combined National and Institutional Series. Cancers 2024, 16, 3050. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Loeb, S.; Partin, A.W.; Schaeffer, E.M. Complications of pelvic lymphadenectomy: Do the risks outweigh the benefits? Rev. Urol. 2010, 12, 20428290. [Google Scholar] [PubMed Central]
- May, M.; Herrmann, E.; Bolenz, C.; Brookman-May, S.; Tiemann, A.; Moritz, R.; Fritsche, H.-M.; Burger, M.; Trojan, L.; Michel, M.S.; et al. Association Between the Number of Dissected Lymph Nodes During Pelvic Lymphadenectomy and Cancer-Specific Survival in Patients with Lymph Node–Negative Urothelial Carcinoma of the Bladder Undergoing Radical Cystectomy. Ann. Surg. Oncol. 2011, 18, 2018–2025. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, F.; Motterle, G.; Zattoni, F.; Morlacco, A.; Moro, F.D. The Role of Lymph Node Dissection in the Treatment of Bladder Cancer. Front. Surg. 2018, 5, 62. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leissner, J.; Ghoneim, M.; Abol-Enein, H.; Thüroff, J.; Franzaring, L.; Fisch, M.; Schulze, H.; Managadze, G.; Allhoff, E.; El-Baz, M.; et al. Extended Radical Lymphadenectomy in Patients with Urothelial Bladder Cancer: Results of a Prospective Multicenter Study. J. Urol. 2004, 171, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Abdollah, F.; Sun, M.; Schmitges, J.; Djahangirian, O.; Tian, Z.; Jeldres, C.; Perrotte, P.; Shariat, S.F.; Montorsi, F.; Karakiewicz, P.I. Stage-specific impact of pelvic lymph node dissection on survival in patients with non-metastatic bladder cancer treated with radical cystectomy. BJU Int. 2012, 109, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Gofrit, O.N.; Zorn, K.C.; Steinberg, G.D.; Zagaja, G.P.; Shalhav, A.L. The Will Rogers Phenomenon in Urological Oncology. J. Urol. 2008, 179, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Sundi, D.; Svatek, R.S.; Nielsen, M.E.; Schoenberg, M.P.; Bivalacqua, T.J. Extent of pelvic lymph node dissection during radical cys-tectomy: Is bigger better? Rev. Urol. 2014, 16, 25548542. [Google Scholar] [PubMed Central]
- Nakagawa, T. Lymph node dissection for bladder cancer: Current standards and the latest evidence. Int. J. Urol. 2021, 28, 7–15. [Google Scholar] [CrossRef]
- Martini, A.; Afferi, L.; Zamboni, S.; Schultz, J.G.; Lonati, C.; Mattei, A.; Karnes, R.J.; Soligo, M.; Stabile, A.; Di Trapani, E.; et al. Oncologic Surveillance for Variant Histology Bladder Cancer after Radical Cystectomy. J. Urol. 2021, 206, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Rudzinski, J.K.; Labbate, C.V.; Hensley, P.J.; Bree, K.K.; Guo, C.C.; Alhalabi, O.; Campbell, M.T.; Siefker-Radtke, A.O.; Navai, N.; et al. Long-Term Oncological Outcomes in Patients Diagnosed with Nonmetastatic Plasmacytoid Variant of Bladder Cancer: A 20-Year University of Texas MD Anderson Cancer Center Experience. J. Urol. 2024, 211, 241–255. [Google Scholar] [CrossRef] [PubMed]
- McFadden, J.; Tachibana, I.; Adra, N.; Collins, K.; Cary, C.; Koch, M.; Kaimakliotis, H.; Masterson, T.; Rice, K. Impact of variant histology on upstaging and survival in patients with nonmuscle invasive bladder cancer undergoing radical cystectomy. Urol. Oncol. Semin. Orig. Investig. 2024, 42, 69.e11–69.e16. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T. Understanding the biology of urothelial cancer metastasis. Asian J. Urol. 2016, 3, 211–222. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Al-Ahmadie, H.A.; Iyer, G.; Lee, B.H.; Scott, S.N.; Mehra, R.; Bagrodia, A.; Jordan, E.J.; Gao, S.P.; Ramirez, R.; Cha, E.K.; et al. Frequent somatic CDH1 loss-of-function mutations in plasmacytoid variant bladder cancer. Nat. Genet. 2016, 48, 356–358. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ghali, F.; Vakar-Lopez, F.; Roudier, M.P.; Garcia, J.; Arora, S.; Cheng, H.H.; Schweizer, M.T.; Haffner, M.C.; Lee, J.K.; Yu, E.Y.; et al. Metastatic Bladder Cancer Expression and Subcellular Localization of Nectin-4 and Trop-2 in Variant Histology: A Rapid Autopsy Study. Clin. Genitourin. Cancer 2023, 21, 669–678. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Monaghan, T.F.; Flores, V.X.; Suss, N.R.; Robins, D.J.; Smith, M.T.; McNeil, B.K.; Hyacinthe, L.M.; Weiss, J.P.; Winer, A.G. Determinants of adequate lymph node dissection following neoadjuvant chemotherapy in patients with urothelial muscle-invasive bladder cancer: Results from the National Cancer Database. Int. Urol. Nephrol. 2021, 53, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Matulay, J.T.; Narayan, V.M.; Kamat, A.M. Clinical and Genomic Considerations for Variant Histology in Bladder Cancer. Curr. Oncol. Rep. 2019, 21, 23. [Google Scholar] [CrossRef] [PubMed]
- Meeks, J.J.; Sjödahl, G.; Lerner, S.P.; Das, A.; McConkey, D.J.; Black, P.C. Tumor Subtyping: Making Sense of Heterogeneity with a Goal Toward Treatment. Bl. Cancer 2021, 7, 1–11. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weiss, K.; Gessner, K.H.; Demzik, A.; Moreton, E.; Kim, W.Y.; Wobker, S.E.; Rose, T.L.; Milowsky, M.I.; Bjurlin, M.A. Molecular characterization of plasmacytoid urothelial carcinoma and the impact on treatment implications. Cancer Treat. Res. Commun. 2023, 37, 100779. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Narayan, V.M.; Gupta, S.; Davicioni, E.; Murugan, P.; Gibb, E.A.; Konety, B. Genomic Analysis and Treatment Response of a Bladder Urothelial Carcinoma with Sarcomatoid Variant Histology. Clin. Genitourin. Cancer 2019, 17, e888–e892. [Google Scholar] [CrossRef] [PubMed]
- Hoffman-Censits, J.; Choi, W.; Pal, S.; Trabulsi, E.; Kelly, W.K.; Hahn, N.M.; McConkey, D.; Comperat, E.; Matoso, A.; Cussenot, O.; et al. Urothelial Cancers with Small Cell Variant Histology Have Confirmed High Tumor Mutational Burden, Frequent TP53 and RB Mutations, and a Unique Gene Expression Profile. Eur. Urol. Oncol. 2021, 4, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Zennami, K.; Takahara, K.; Nukaya, T.; Takenaka, M.; Ichino, M.; Sasaki, H.; Kusaka, M.; Sumitomo, M.; Shiroki, R. The Role of Lymph Node Dissection in Patients with Muscle-Invasive Bladder Cancer Who Underwent Radical Cystectomy Following Neoadjuvant Chemotherapy. Clin. Genitourin. Cancer 2024, 22, 1–9. [Google Scholar] [CrossRef]
- Tochigi, K.; Nagayama, J.; Yuguchi, Y.; Hattori, K.; Hiroki, S.; Kanada, Y.; Matsui, H.; Akamatsu, S. MP53-17 association between the number of dissected lymph nodes and prognosis in patients undergoing radical cystectomy following neoadjuvant chemotherapy. J. Urol. 2024, 211, e870. [Google Scholar] [CrossRef]
- Marano, L.; Verre, L.; Carbone, L.; Poto, G.E.; Fusario, D.; Venezia, D.F.; Calomino, N.; Kaźmierczak-Siedlecka, K.; Polom, K.; Marrelli, D.; et al. Current Trends in Volume and Surgical Outcomes in Gastric Cancer. J. Clin. Med. 2023, 12, 2708. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Entire Cohort LND | Entire Cohort No LND | p-Value | Micropapillary (n = 426) | Sarcomatoid (n = 704) | Squamous (n = 28,491) | Adenocarcinoma (n = 601) | Neuroendocrine (n = 614) | Plasmacytoid (n = 75) | |
|---|---|---|---|---|---|---|---|---|---|
| Age | 70 | 71 | 0.73 | 70.9 | 72.9 | 71.64 | 67.83 | 72.61 | 68.98 |
| Sex | 0.69 | ||||||||
| Male | 74.8% | 73.2% | 77.0% | 66.4% | 75.4% | 63.4% | 77.7% | 73.2% | |
| Female | 25.2% | 26.8% | 23.0% | 33.6% | 24.6% | 36.6% | 22.3% | 26.8% | |
| cT | 0.34 | ||||||||
| T0 | 0.7% | 1.0% | 0.1% | 0.4% | 0.6% | 0.8% | 0.3% | 1.1% | |
| T1 | 35.6% | 34.6% | 35.4% | 21.6% | 52.8% | 29.7% | 15.7% | 21.1% | |
| T2 | 49.7% | 46.6% | 50.8% | 55.8% | 36.1% | 40.4% | 62.4% | 50.1% | |
| T3 | 8.5% | 9.7% | 7.4% | 11.9% | 4.7% | 12.4% | 10.3% | 12.2% | |
| T4 | 5.5% | 8.1% | 6.2% | 10.2% | 5.7% | 16.7% | 11.2% | 15.6% | |
| cN | <0.001 | ||||||||
| c0 | 78.4% | 61.9% | 79.3% | 77.5% | 75.3% | 70.5% | 70.3% | 85.2% | |
| c1 | 2.8% | 2.2% | 4.7% | 3.7% | 1.7% | 3.7% | 5.3% | 1.6% | |
| c2 | 2.4% | 1.9% | 3.6% | 2.0% | 1.6% | 2.7% | 4.8% | 1.6% | |
| c3 | 0.5% | 0.3% | 1.4% | 0.4% | 0.4% | 0.1% | 0.4% | 3.3% | |
| cX | 15.8% | 18.4% | 10.9% | 16.3% | 21.1% | 22.8% | 19.2% | 8.2% | |
| pT | <0.001 | ||||||||
| T0 | 6% | 7.1% | 4.6% | 5.2% | 3.4% | 2.5% | 6.8% | 11.2% | |
| T1 | 10.1% | 4.4% | 14.3% | 7.9% | 29.4% | 14.4% | 6.4% | 1.6% | |
| T2 | 28.3% | 31.5% | 25.6% | 31.2% | 25.4% | 28.9% | 41.6% | 12.5% | |
| T3 | 36.0% | 41.0% | 35.0% | 38.5% | 16.4% | 34.7% | 33.6% | 31.7% | |
| T4 | 19.6% | 16.0% | 19.2% | 17.2% | 8.4% | 19.5% | 11.6% | 43.1% | |
| NAC | 0.93 | ||||||||
| Yes | 20.4% | 18.3% | 18.9% | 13.3% | 21.4% | 13.8% | 36.5% | 13.3% | |
| No | 79.6% | 81.7% | 81.1% | 86.7% | 78.6% | 86.2% | 63.5% | 86.7% | |
| pN status | <0.001 | ||||||||
| pN0 | 73% | 0% | 31.5% | 43.0% | 27.4% | 39.8% | 32.1% | 45.3% | |
| pN+ | 27% | 0% | 35.4% | 14.8% | 4.2% | 11.3% | 11.7% | 32.0% | |
| pNx | 0% | 100% | 33.1% | 42.2% | 68.4% | 48.9% | 56.2% | 22.7% |
| Micropapillary | p | Sarcomatoid | p | Squamous | p | Adenocarcinoma | p | Neuroendocrine | p | |
|---|---|---|---|---|---|---|---|---|---|---|
| AGE | 1.05 (1.03–1.08) | <0.001 | 1.01 (1.00–1.03) | 0.04 | 1.02 (1.02–1.03) | <0.001 | 1.02 (1.01–1.03) | <0.001 | 1.03 (1.02–1.06) | <0.001 |
| CT STAGE | 1.23 (0.84–1.59) | 0.56 | 1.48 (1.19–2.69) | 0.77 | 1.21 (1.13–1.40) | <0.001 | 1.32 (0.54–2.71) | 0.93 | 1.56 (1.21–1.96) | 0.003 |
| NAC | 0.98 (0.63–1.5) | 0.91 | 1.38 (0.990–1.980) | 0.06 | 0.87 (0.59–1.29) | 0.31 | 1.18 (0.83–1.60) | 0.613 | 0.09 (0.07–0.94) | 0.002 |
| LN Y/N | 1.73 (0.88–3.43) | 0.17 | 1.06 (0.75–1.50) | 0.70 | 0.50 (0.44–0.58) | <0.001 | 0.065 (0.045–0.93) | 0.030 | 0.76 (0.04–1.50) | 0.120 |
| FACILITY TYPE Non-Academic (Reference) ACADEMIC | 1.13 (0.83–1.32) | 0.38 | 1.23 (0.67–1.63) | 0.53 | 1.04 (0.83–1.25) | 0.67 | 1.35 (0.93–1.74) | 0.25 | 0.98 (0.74–1.42) | 0.68 |
| CN+ STATUS | 1.21 (0.93–1.48) | 0.19 | 1.32 (0.84–1.69) | 0.39 | 1.66 (1.34–1.83) | 0.03 | 1.08 (0.73–1.43) | 0.41 | 1.34 (1.13–1.50) | 0.02 |
| INSURANCE STATUS PRIVATE (REFERENCE) MEDICARE/MEDICAID | 1.05 (0.67–1.54) | 0.67 | 0.95 (0.43–1.87) | 0.84 | 1.13 (0.84–1.28) | 0.19 | 1.04 (0.83–1.43) | 0.83 | 1.31 (0.65–1.95 | 0.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, S.N.; Martin, D.T.; Keervani, K.; James, S.; Humphrey, P.; Hesse, D.; Tan, W.S.; Patel, S.; Wright, J.; Ghali, F. Evaluating the Therapeutic Role of Lymph Node Dissection in Variant Subtype Bladder Cancer. Cancers 2025, 17, 2536. https://doi.org/10.3390/cancers17152536
Rahman SN, Martin DT, Keervani K, James S, Humphrey P, Hesse D, Tan WS, Patel S, Wright J, Ghali F. Evaluating the Therapeutic Role of Lymph Node Dissection in Variant Subtype Bladder Cancer. Cancers. 2025; 17(15):2536. https://doi.org/10.3390/cancers17152536
Chicago/Turabian StyleRahman, Syed Nahiyaan, Darryl T. Martin, Kandala Keervani, Spencer James, Peter Humphrey, David Hesse, Wei Shen Tan, Sunil Patel, Jonathan Wright, and Fady Ghali. 2025. "Evaluating the Therapeutic Role of Lymph Node Dissection in Variant Subtype Bladder Cancer" Cancers 17, no. 15: 2536. https://doi.org/10.3390/cancers17152536
APA StyleRahman, S. N., Martin, D. T., Keervani, K., James, S., Humphrey, P., Hesse, D., Tan, W. S., Patel, S., Wright, J., & Ghali, F. (2025). Evaluating the Therapeutic Role of Lymph Node Dissection in Variant Subtype Bladder Cancer. Cancers, 17(15), 2536. https://doi.org/10.3390/cancers17152536






