Advances and Challenges in the Management of Myelodysplastic Syndromes
Simple Summary
Abstract
1. Introduction
1.1. Challenges in Classification and Risk-Assessment: The Evolving System(s) for MDS
1.2. Risk-Assessment in the Era of Precision Medicine
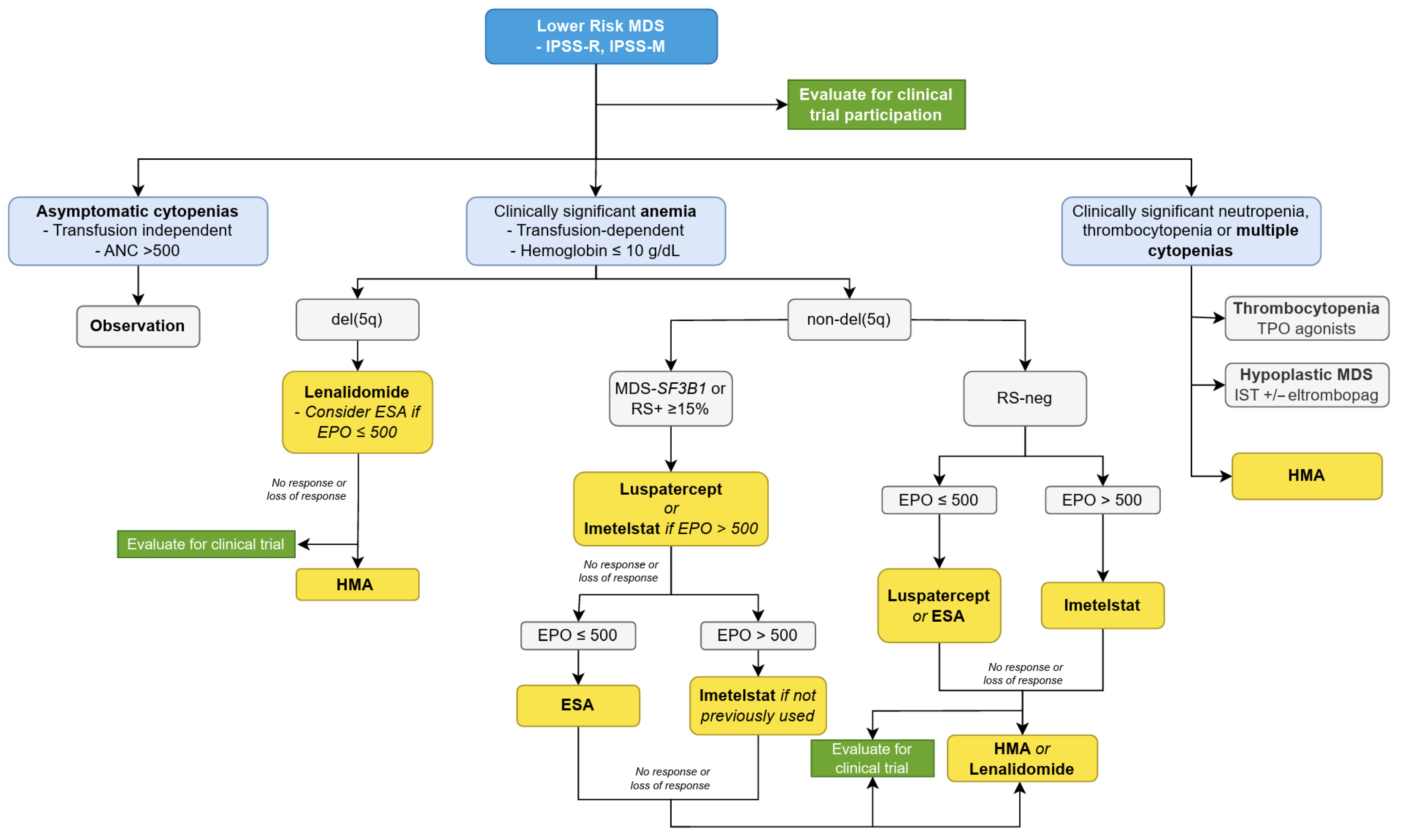
1.3. Treatment of Lower-Risk MDS
1.4. MDS with Chromosome 5 Deletion
1.5. Management of Anemia in LR-MDS
1.6. Luspatercept
1.7. Imetelstat
1.8. HMAs in LR-MDS
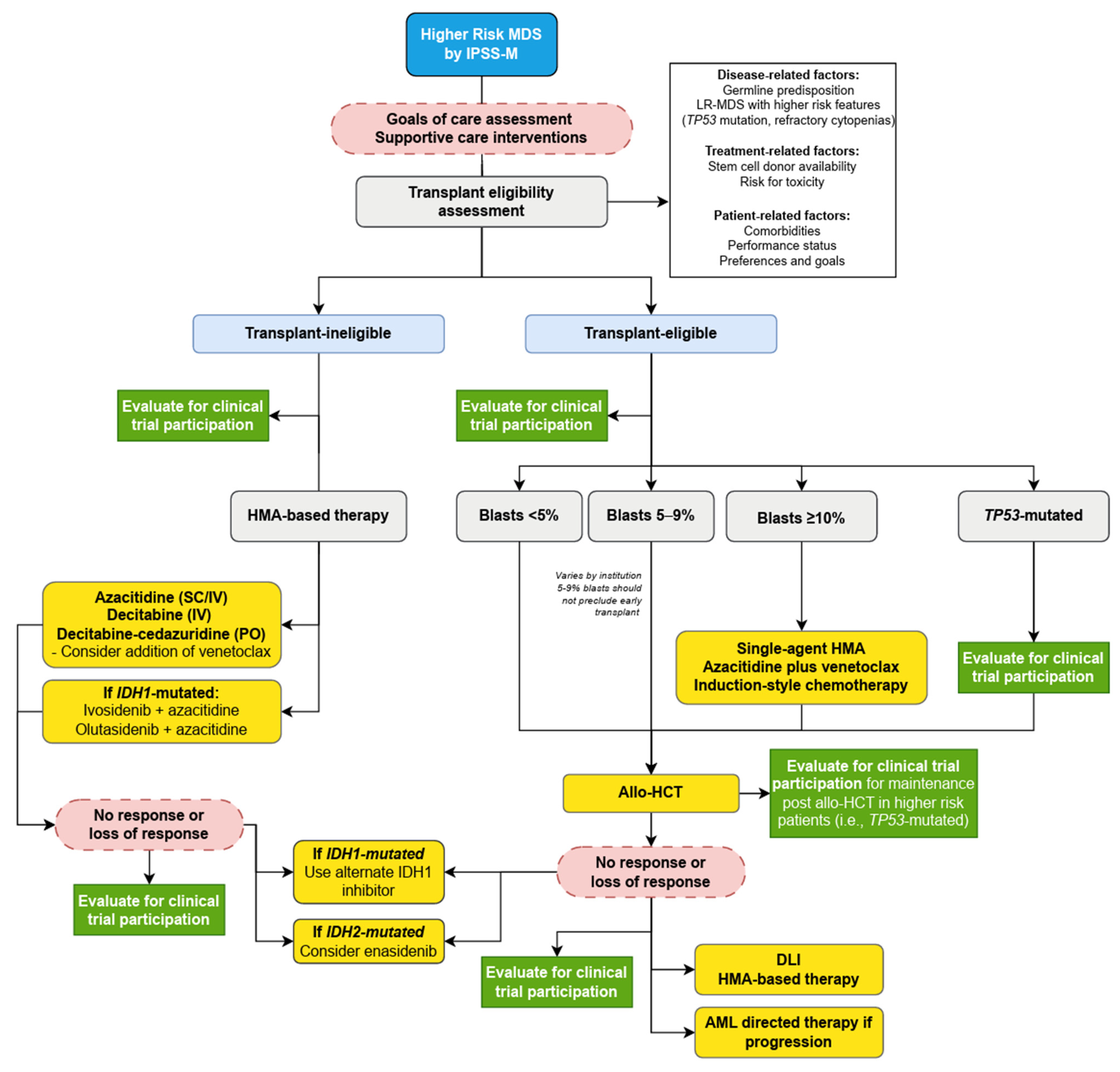
1.9. Treatment of Higher-Risk MDS
1.10. Transplant Eligible Patients
1.11. Transplant-Ineligible
1.12. Early Promise and Later Disappointments: HMA Combinations in HR-MDS
1.13. IDH1/2 Inhibitors
1.14. TP53-Mutated MDS
2. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- SEER*Explorer: An Interactive Website for SEER Cancer Statistics [Internet]. Surveillance Research Program, National Cancer Institute. 17 April 2024. [Updated: 5 November 2024]. Available online: https://seer.cancer.gov/statistics-network/explorer/ (accessed on 19 February 2025).
- Ma, X. Epidemiology of Myelodysplastic Syndromes. Am. J. Med. 2012, 125, S2–S5. [Google Scholar] [CrossRef] [PubMed]
- Kouroukli, O.; Symeonidis, A.; Foukas, P.; Maragkou, M.-K.; Kourea, E.P. Bone Marrow Immune Microenvironment in Myelodysplastic Syndromes. Cancers 2022, 14, 5656. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zou, C.; Xiang, X.; Zhao, L.; Chen, M.; Yang, C.; Wu, Y. Myelodysplastic Neoplasms (MDS): Pathogenesis and Therapeutic Prospects. Biomolecules 2025, 15, 761. [Google Scholar] [CrossRef] [PubMed]
- Arber, D.A.; Orazi, A.; Hasserjian, R.P.; Borowitz, M.J.; Calvo, K.R.; Kvasnicka, H.-M.; Wang, S.A.; Bagg, A.; Barbui, T.; Branford, S.; et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemias: Integrating morphologic, clinical, and genomic data. Blood 2022, 140, 1200–1228. [Google Scholar] [CrossRef] [PubMed]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.M.; Catovsky, D.; Daniel, M.T.; Flandrin, G.; Galton, D.A.; Gralnick, H.R.; Sultan, C. Proposals for the classification of the myelodysplastic syndromes. Br. J. Haematol. 1982, 51, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Bernard, E.; Hasserjian, R.P.; Greenberg, P.L.; Arango Ossa, J.E.; Creignou, M.; Tuechler, H.; Gutierrez-Abril, J.; Domenico, D.; Medina-Martinez, J.S.; Levine, M.; et al. Molecular taxonomy of myelodysplastic syndromes and its clinical implications. Blood 2024, 144, 1617–1632. [Google Scholar] [CrossRef] [PubMed]
- Kewan, T.; Durmaz, A.; Bahaj, W.; Gurnari, C.; Terkawi, L.; Awada, H.; Ogbue, O.D.; Ahmed, R.; Pagliuca, S.; Awada, H.; et al. Molecular patterns identify distinct subclasses of myeloid neoplasia. Nat. Commun. 2023, 14, 3136. [Google Scholar] [CrossRef] [PubMed]
- Komrokji, R.S.; Lanino, L.; Ball, S.; Bewersdorf, J.P.; Marchetti, M.; Maggioni, G.; Travaglino, E.; Al Ali, N.H.; Fenaux, P.; Platzbecker, U.; et al. Data-driven, harmonised classification system for myelodysplastic syndromes: A consensus paper from the International Consortium for Myelodysplastic Syndromes. Lancet Haematol. 2024, 11, e862–e872. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef] [PubMed]
- Dohner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef] [PubMed]
- Stahl, M.; Bewersdorf, J.P.; Xie, Z.; Porta, M.G.D.; Komrokji, R.; Xu, M.L.; Abdel-Wahab, O.; Taylor, J.; Steensma, D.P.; Starczynowski, D.T.; et al. Classification, risk stratification and response assessment in myelodysplastic syndromes/neoplasms (MDS): A state-of-the-art report on behalf of the International Consortium for MDS (icMDS). Blood Rev. 2023, 62, 101128. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, P.; Cox, C.; LeBeau, M.M.; Fenaux, P.; Morel, P.; Sanz, G.; Sanz, M.; Vallespi, T.; Hamblin, T.; Oscier, D.; et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 1997, 89, 2079–2088. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, P.L.; Tuechler, H.; Schanz, J.; Sanz, G.; Garcia-Manero, G.; Solé, F.; Bennett, J.M.; Bowen, D.; Fenaux, P.; Dreyfus, F.; et al. Revised International Prognostic Scoring System for Myelodysplastic Syndromes. Blood 2012, 120, 2454–2465. [Google Scholar] [CrossRef] [PubMed]
- Bernard, E.; Tuechler, H.; Greenberg, P.L.; Hasserjian, R.P.; Arango Ossa, J.E.; Nannya, Y.; Devlin, S.M.; Creignou, M.; Pinel, P.; Monnier, L.; et al. Molecular International Prognostic Scoring System for Myelodysplastic Syndromes. NEJM Evid. 2022, 1, EVIDoa2200008. [Google Scholar] [CrossRef] [PubMed]
- Kewan, T.; Bewersdorf, J.P.; Blaha, O.; Stahl, M.; Al Ali, N.H.; DeZern, A.E.; Sekeres, M.A.; Carraway, H.E. Validation of the Molecular International Prognostic Scoring System (IPSS-M) in Patients (Pts) with Myelodysplastic Syndromes/Neoplasms (MDS) Who Were Treated with Hypomethylating Agents (HMA). Blood 2023, 142 (Suppl. S1), 4980. [Google Scholar] [CrossRef]
- Gurnari, C.; Gagelmann, N.; Badbaran, A.; Awada, H.; Dima, D.; Pagliuca, S.; D’Aveni-Piney, M.; Attardi, E.; Voso, M.T.; Cerretti, R.; et al. Outcome prediction in myelodysplastic neoplasm undergoing hematopoietic cell transplant in the molecular era of IPSS-M. Leukemia 2023, 37, 717–719. [Google Scholar] [CrossRef] [PubMed]
- Stahl, M.; Deveaux, M.; De Witte, T.; Neukirchen, J.; Sekeres, M.A.; Brunner, A.M.; Roboz, G.J.; Steensma, D.P.; Bhatt, V.R.; Platzbecker, U.; et al. The use of immunosuppressive therapy in MDS: Clinical outcomes and their predictors in a large international patient cohort. Blood Adv. 2018, 2, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines. Myelodysplastic Syndromes (Version 1.2025). Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1446 (accessed on 10 January 2025).
- Oliva, E.N.; Riva, M.; Niscola, P.; Santini, V.; Breccia, M.; Giai, V.; Poloni, A.; Patriarca, A.; Crisà, E.; Capodanno, I.; et al. Eltrombopag for Low-Risk Myelodysplastic Syndromes with Thrombocytopenia: Interim Results of a Phase II, Randomized, Placebo-Controlled Clinical Trial (EQOL-MDS). J. Clin. Oncol. 2023, 41, 4486–4496. [Google Scholar] [CrossRef] [PubMed]
- Fenaux, P.; Muus, P.; Kantarjian, H.; Lyons, R.M.; Larson, R.A.; Sekeres, M.A.; Becker, P.S.; Orejudos, A.; Franklin, J. Romiplostim monotherapy in thrombocytopenic patients with myelodysplastic syndromes: Long-term safety and efficacy. Br. J. Haematol. 2017, 178, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Desmond, R.; Townsley, D.M.; Dumitriu, B.; Olnes, M.J.; Scheinberg, P.; Bevans, M.; Parikh, A.R.; Broder, K.; Calvo, K.R.; Wu, C.O.; et al. Eltrombopag restores trilineage hematopoiesis in refractory severe aplastic anemia that can be sustained on discontinuation of drug. Blood 2014, 123, 1818–1825. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, M.; Cherif, H.; Fenaux, P.; Mittelman, M.; Verma, A.; Portella, M.S.O.; Burgess, P.; Ramos, P.M.; Choi, J.; Platzbecker, U. Azacitidine with or without eltrombopag for first-line treatment of intermediate- or high-risk MDS with thrombocytopenia. Blood 2018, 132, 2629–2638. [Google Scholar] [CrossRef] [PubMed]
- Abel, G.A.; Hebert, D.; Lee, C.; Rollison, D.; Gillis, N.; Komrokji, R.; Foran, J.M.; Liu, J.J.; Al Baghdadi, T.; Deeg, J.; et al. Health-related quality of life and vulnerability among people with myelodysplastic syndromes: A US national study. Blood Adv. 2023, 7, 3506–3515. [Google Scholar] [CrossRef] [PubMed]
- De Swart, L.; Crouch, S.; Hoeks, M.; Smith, A.; Langemeijer, S.; Fenaux, P.; Symeonidis, A.; Cermâk, J.; Hellström-Lindberg, E.; Stauder, R.; et al. Impact of red blood cell transfusion dose density on progression-free survival in patients with lower-risk myelodysplastic syndromes. Haematologica 2020, 105, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Stempel, J.M.; Podoltsev, N.A.; Dosani, T. Supportive Care for Patients with Myelodysplastic Syndromes. Cancer J. 2023, 29, 168–178. [Google Scholar] [CrossRef] [PubMed]
- DeFilipp, Z.; Ciurea, S.O.; Cutler, C.; Robin, M.; Warlick, E.D.; Nakamura, R.; Brunner, A.M.; Dholaria, B.; Walker, A.R.; Kroger, N.; et al. Hematopoietic Cell Transplantation in the Management of Myelodysplastic Syndrome: An Evidence-Based Review from the American Society for Transplantation and Cellular Therapy Committee on Practice Guidelines. Transplant. Cell. Ther. 2023, 29, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Cutler, C.S. A decision analysis of allogeneic bone marrow transplantation for the myelodysplastic syndromes: Delayed transplantation for low-risk myelodysplasia is associated with improved outcome. Blood 2004, 104, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Gurnari, C.; Robin, M.; Godley, L.A.; Drozd-Sokolowska, J.; Wlodarski, M.W.; Raj, K.; Onida, F.; Worel, N.; Ciceri, F.; Carbacioglu, S.; et al. Germline predisposition traits in allogeneic hematopoietic stem-cell transplantation for myelodysplastic syndromes: A survey-based study and position paper on behalf of the Chronic Malignancies Working Party of the EBMT. Lancet Haematol. 2023, 10, e994–e1005. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Komrokji, R.; Al Ali, N.; Regelson, A.; Geyer, S.; Patel, A.; Saygin, C.; Zeidan, A.M.; Bewersdorf, J.P.; Mendez, L.; et al. Risk prediction for clonal cytopenia: Multicenter real-world evidence. Blood 2024, 144, 2033–2044. [Google Scholar] [CrossRef] [PubMed]
- Weeks, L.D.; Niroula, A.; Neuberg, D.; Wong, W.; Lindsley, R.C.; Luskin, M.R.; Berliner, N.; Stone, R.M.; Deangelo, D.J.; Soiffer, R.J.; et al. Prediction of Risk for Myeloid Malignancy in Clonal Hematopoiesis. NEJM Evid. 2023, 2, EVIDoa2200310. [Google Scholar] [CrossRef] [PubMed]
- List, A.; Dewald, G.; Bennett, J.; Giagounidis, A.; Raza, A.; Feldman, E.; Powell, B.; Greenberg, P.; Thomas, D.; Stone, R.; et al. Lenalidomide in the Myelodysplastic Syndrome with Chromosome 5q Deletion. N. Engl. J. Med. 2006, 355, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Cheson, B.D.; Bennett, J.M.; Kantarjian, H.; Pinto, A.; Schiffer, C.A.; Nimer, S.D.; Löwenberg, B.; Beran, M.; de Witte, T.M.; Stone, R.M. Report of an international working group to standardize response criteriafor myelodysplastic syndromes. Blood 2000, 96, 3671–3674. [Google Scholar] [CrossRef] [PubMed]
- Fenaux, P.; Giagounidis, A.; Selleslag, D.; Beyne-Rauzy, O.; Mufti, G.; Mittelman, M.; Muus, P.; Te Boekhorst, P.; Sanz, G.; Del Canizo, C.; et al. A randomized phase 3 study of lenalidomide versus placebo in RBC transfusion-dependent patients with Low-/Intermediate-1-risk myelodysplastic syndromes with del5q. Blood 2011, 118, 3765–3776. [Google Scholar] [CrossRef] [PubMed]
- Díez-Campelo, M.; López-Cadenas, F.; Xicoy, B.; Lumbreras, E.; González, T.; Del Rey González, M.; Sánchez-García, J.; Coll Jordà, R.; Slama, B.; Hernández-Rivas, J.-Á.; et al. Low dose lenalidomide versus placebo in non-transfusion dependent patients with low risk, del(5q) myelodysplastic syndromes (SintraREV): A randomised, double-blind, phase 3 trial. Lancet Haematol. 2024, 11, e659–e670. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kelaidi, C.; Meunier, M.; Casadevall, N.; Gerds, A.T.; Platzbecker, U. The prognostic value of serum erythropoietin in patients with lower-risk myelodysplastic syndromes: A review of the literature and expert opinion. Ann. Hematol. 2020, 99, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Hellström-Lindberg, E.; Negrin, R.; Stein, R.; Krantz, S.; Lindberg, G.; Vardiman, J.; Öst, Å.; Greenberg, P. Erythroid response to treatment with G-CSF plus erythropoietin for the anaemia of patients with myelodysplastic syndromes: Proposal for a predictive model. Br. J. Haematol. 1997, 99, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Grabar, S.; Kelaidi, C.; Beyne-Rauzy, O.; Picard, F.; Bardet, V.; Coiteux, V.; Leroux, G.; Lepelley, P.; Daniel, M.T.; et al. Predictive factors of response and survival in myelodysplastic syndrome treated with erythropoietin and G-CSF: The GFM experience. Blood 2008, 111, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Nguyen, A.N.; Sohal, D.; Ying Ma, J.; Pahanish, P.; Gundabolu, K.; Hayman, J.; Chubak, A.; Mo, Y.; Bhagat, T.D.; et al. Inhibition of the TGF-β receptor I kinase promotes hematopoiesis in MDS. Blood 2008, 112, 3434–3443. [Google Scholar] [CrossRef] [PubMed]
- Celgene Coorporation, Bristol-Myers Squibb Company. REBLOZYL® (Lusparercept-Aamt) for Injection, for Subcutaneous Use; Celgene Coorporation, Bristol-Myers Squibb Company: Summit, NY, USA, 2025. [Google Scholar]
- Platzbecker, U.; Germing, U.; Gotze, K.S.; Kiewe, P.; Mayer, K.; Chromik, J.; Radsak, M.; Wolff, T.; Zhang, X.; Laadem, A.; et al. Luspatercept for the treatment of anaemia in patients with lower-risk myelodysplastic syndromes (PACE-MDS): A multicentre, open-label phase 2 dose-finding study with long-term extension study. Lancet Oncol. 2017, 18, 1338–1347. [Google Scholar] [CrossRef] [PubMed]
- Platzbecker, U.; Gotze, K.S.; Kiewe, P.; Germing, U.; Mayer, K.; Radsak, M.; Wolff, T.; Chromik, J.; Sockel, K.; Oelschlagel, U.; et al. Long-Term Efficacy and Safety of Luspatercept for Anemia Treatment in Patients with Lower-Risk Myelodysplastic Syndromes: The Phase II PACE-MDS Study. J. Clin. Oncol. 2022, 40, 3800–3807. [Google Scholar] [CrossRef] [PubMed]
- Fenaux, P.; Platzbecker, U.; Mufti, G.J.; Garcia-Manero, G.; Buckstein, R.; Santini, V.; Díez-Campelo, M.; Finelli, C.; Cazzola, M.; Ilhan, O.; et al. Luspatercept in Patients with Lower-Risk Myelodysplastic Syndromes. N. Engl. J. Med. 2020, 382, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Platzbecker, U.; Della Porta, M.G.; Santini, V.; Zeidan, A.M.; Komrokji, R.S.; Shortt, J.; Valcarcel, D.; Jonasova, A.; Dimicoli-Salazar, S.; Tiong, I.S.; et al. Efficacy and safety of luspatercept versus epoetin alfa in erythropoiesis-stimulating agent-naive, transfusion-dependent, lower-risk myelodysplastic syndromes (COMMANDS): Interim analysis of a phase 3, open-label, randomised controlled trial. Lancet 2023, 402, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Oliva, E.N.; Platzbecker, U.; Della Porta, M.G.; Garcia-Manero, G.; Santini, V.; Fenaux, P.; Shortt, J.; Komrokji, R.S.; Pelligra, C.G.; Guo, S.; et al. Health-Related Quality of Life of Luspatercept Versus Epoetin Alfa in Red Blood Cell Transfusion-Dependent Lower-Risk Myelodysplastic Syndromes: Results from the Final Datacut of the Phase 3 COMMANDS Study. Blood 2024, 144, 3216. [Google Scholar] [CrossRef]
- Garcia-Manero, G.; Della Porta, M.G.; Zeidan, A.M.; Komrokji, R.; Pozharskaya, V.; Rose, S.; Keeperman, K.; Lai, Y.; Kalsekar, S.; Aggarwal, B.; et al. Overall survival (OS) and duration of response for transfusion independence (TI) in erythropoiesis stimulating agent (ESA)–naive patients (pts) with very low-, low-, or intermediate-risk myelodysplastic syndromes (MDS) treated with luspatercept (LUSPA) vs epoetin alfa (EA) in the COMMANDS trial. J. Clin. Oncol. 2025, 43, 6512. [Google Scholar] [CrossRef]
- Garcia-Manero, G.; Santini, V.; Zeidan, A.M.; Komrokji, R.S.; Pozharskaya, V.; Rose, S.; Keeperman, K.; Lai, Y.; Kalsekar, S.; Aggarwal, B.; et al. Long-Term Transfusion Independence with Luspatercept Versus Epoetin Alfa in Erythropoiesis-Stimulating Agent-Naive, Lower-Risk Myelodysplastic Syndromes in the COMMANDS Trial. Adv. Ther. 2025, 42, 3576–3589. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, A.M.; Komrokji, R.S.; Buckstein, R.; Santini, V.; Rose, S.; Malini, P.; Lew, G.; Aggarwal, D.; Keeperman, K.L.; Jiang, H.; et al. The ELEMENT-MDS Trial: A Phase 3 Randomized Study Evaluating Luspatercept Versus Epoetin Alfa in Erythropoiesis-Stimulating Agent-Naive, Non-Transfusion-Dependent, Lower-Risk Myelodysplastic Syndromes. Blood 2023, 142, 6503. [Google Scholar] [CrossRef]
- Mukherjee, S.; Brown-Bickerstaff, C.; Falkenstein, A.; Makinde, A.Y.; Bland, E.; Laney, J.; Garretson, M.; Huggar, D.; McBride, A. Treatment patterns and outcomes with luspatercept in patients with lower-risk myelodysplastic syndromes: A retrospective US cohort analysis. HemaSphere 2024, 8, e38. [Google Scholar] [CrossRef] [PubMed]
- Götze, K.S.S.; Hecht, A.; Kubasch, A.S.; Diez-Campelo, M.; Stüssi, G.; Valcarcel, D.; Font Lopez, P.; Mora Castera, E.; Xicoy, B.; Bernal Del Castillo, T.; et al. Preliminary Safety and Efficacy of Luspatercept Initiated at Maximum Approved Dose in Patients with Transfusion Dependent Anemia Due to Very Low, Low and Intermediate Risk MDS with Ring Sideroblasts: First Results of the Lusplus Trial. Blood 2024, 144, 3207. [Google Scholar] [CrossRef]
- Della Porta, M.G.; Diez-Campelo, M.; Santini, V.; Buckstein, R.; Ades, L.; Sahagun, L.; Das, G.; Bryant, T.; Zelinsky, T.; Lai, Y.; et al. Preliminary efficacy and safety analysis of the MAXILUS study of luspatercept initiated at the maximum approved dose in transfusion-dependent lower-risk myelodysplastic syndromes. HemaSphere 2025, 9, PF634. [Google Scholar]
- RYTELO® (Imetelstat) for Injection, for Intravenous Use; Geron Coorporation: Foster City, CA, USA, 2025.
- Platzbecker, U.; Santini, V.; Fenaux, P.; Sekeres, M.A.; Savona, M.R.; Madanat, Y.F.; Díez-Campelo, M.; Valcárcel, D.; Illmer, T.; Jonášová, A.; et al. Imetelstat in patients with lower-risk myelodysplastic syndromes who have relapsed or are refractory to erythropoiesis-stimulating agents (IMerge): A multinational, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2024, 403, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Platzbecker, U.; Santini, V.; Zeidan, A.M.; Sekeres, M.A.; Fenaux, P.; Raza, A.; Mittelman, M.; Thépot, S.; Buckstein, R.; Germing, U.; et al. Effect of Prior Treatments on the Clinical Activity of Imetelstat in Transfusion-Dependent Patients with Erythropoiesis-Stimulating Agent, Relapsed or Refractory/Ineligible Lower-Risk Myelodysplastic Syndromes. Blood 2024, 144, 352. [Google Scholar] [CrossRef]
- Malcovati, L.; Porta, M.G.D.; Pascutto, C.; Invernizzi, R.; Boni, M.; Travaglino, E.; Passamonti, F.; Arcaini, L.; Maffioli, M.; Bernasconi, P.; et al. Prognostic Factors and Life Expectancy in Myelodysplastic Syndromes Classified According to WHO Criteria: A Basis for Clinical Decision Making. J. Clin. Oncol. 2005, 23, 7594–7603. [Google Scholar] [CrossRef] [PubMed]
- Stauder, R.; Yu, G.; Koinig, K.A.; Bagguley, T.; Fenaux, P.; Symeonidis, A.; Sanz, G.; Cermak, J.; Mittelman, M.; Hellström-Lindberg, E.; et al. Health-related quality of life in lower-risk MDS patients compared with age- and sex-matched reference populations: A European LeukemiaNet study. Leukemia 2018, 32, 1380–1392. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tang, Z.; An, T.; Zhao, L. The impact of iron chelation therapy on patients with lower/intermediate IPSS MDS and the prognostic role of elevated serum ferritin in patients with MDS and AML: A meta-analysis. Medicine 2019, 98, e17406. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Jabbour, E.; Montalban-Bravo, G.; Darbaniyan, F.; Do, K.-A.; Class, C.; Short, N.J.; Kanagal-Shamana, R.; Kadia, T.; Borthakur, G.; et al. Low-Dose Decitabine versus Low-Dose Azacitidine in Lower-Risk MDS. NEJM Evid. 2022, 1, EVIDoa2200034. [Google Scholar] [CrossRef] [PubMed]
- Diez-Campelo, M.; Ross, D.M.; Giagounidis, A.; Tan, S.; Cluzeau, T.; Chee, L.C.Y.; Valcarcel, D.; Arnan, M.; Graham, C.; McGinty, A.; et al. Durable Clinical Benefit with Ker-050 Treatment: Findings from an Ongoing Phase 2 Study in Participants with Lower-Risk MDS. Blood 2023, 142, 196. [Google Scholar] [CrossRef]
- Tan, S.; Arnan, M.; Chee, L.C.Y.; Cluzeau, T.; Diez-Campelo, M.; Giagounidis, A.; Hiwase, D.; Ross, D.M.; Sekeres, M.A.; Valcarcel, D.; et al. Hematologic Improvement and Fatigue Reduction with Elritercept (KER-050) in Participants with Lower-Risk (LR) Myelodysplastic Neoplasms (MDS) with Non-Transfusion Dependent Anemia: New Analyses from an Ongoing Phase 2 Trial. Blood 2024, 144, 4591. [Google Scholar] [CrossRef]
- RVU120 for Treatment of Anemia in Patients with Lower-Risk Myelodysplastic Neoplasms (MDS), ClinicalTrials.gov ID NCT06243458. Available online: https://www.clinicaltrials.gov/study/NCT06243458?term=NCT06243458&rank=1 (accessed on 11 June 2025).
- Rodriguez Sevilla, J.J.; Adema, V.; Chien, K.S.; Ganan-Gomez, I.; Montalban-Bravo, G.; Urrutia, S.; Joseph, J.; Yang, H.; Borthakur, G.; Short, N.J.; et al. A Phase 2 Study of Canakinumab in Patients with Lower-Risk Myelodysplastic Syndromes or Chronic Myelomonocytic Leukemia. Blood 2023, 142, 1866. [Google Scholar] [CrossRef]
- Bouligny, I.; Carraway, H.E.; Sekeres, M.; Dezern, A.; Komrokji, R.; Stone, R.M.; Roboz, G.J.; Montalban-Bravo, G.; Bataller, A.; Sasaki, K.; et al. A randomized, multicenter phase II trial of 3-day decitabine, 3-day azacitidine, or 5-day azacitidine in lower-risk myelodysplastic syndrome. HemaSphere 2025, 9, PF629. [Google Scholar]
- Ades, L.; Cluzeau, T.; Comont, T.; Aguinaga, L.; Stamatoullas, A.; Meunier, M.; Gyan, E.; Garnier, A.; D’Aveni, M.; Thépot, S.; et al. Combining ESA and Luspatercept in Non-RS MDS Patients Having Failed ESA—Results of the Phase 1-2 Part a of the GFM Combola Study. Blood 2024, 144, 351. [Google Scholar] [CrossRef]
- Garcia-Manero, G.; Bachiashvili, K.; Griffiths, E.A.; Zeidan, A.M.; Traer, E.; Saini, L.; Amin, H.; Mohan, S.R.; Lubbert, M.; Maness-Harris, L.; et al. Randomized Phase 1-2 Study to Assess Safety and Efficacy of Low-Dose (LD) Oral Decitabine/Cedazuridine (ASTX727) in Lower-Risk Myelodysplastic Syndromes (LR-MDS) Patients: Interim Safety Analysis. Clin. Lymphoma Myeloma Leuk. 2023, 23 (Suppl. S1), S376–S377. [Google Scholar] [CrossRef]
- A Study of LB-100 in Patients with Low or Intermediate-1 Risk Myelodysplastic Syndromes (MDS), ClinicalTrials.gov ID NCT03886662. Available online: https://clinicaltrials.gov/study/NCT03886662?cond=NCT03886662&rank=1 (accessed on 11 June 2025).
- Garcia-Manero, G.; Madanat, Y.F.; Sekeres, M.A.; Carraway, H.E.; Cusnir, M.; McCloskey, J.; Naqvi, K.; Schiller, G.J.; Yan, L.; Gordi, T.; et al. R289, a Dual Irak 1/4 Inhibitor, in Patients with Relapsed/Refractory (R/R) Lower-Risk Myelodysplastic Syndrome (LR-MDS): Initial Results from a Phase 1b Study. Blood 2024, 144, 4595. [Google Scholar] [CrossRef]
- Garcia-Manero, G.; Abaza, Y.; Greenberg, P.L.; Haque, T.; Velázquez Kennedy, K.; Della Porta, M.G.; Ooi, M.G.; Stahl, M.; Xie, Z.; Mangaonkar, A.A.; et al. Preliminary Safety and Biomarker Results of the NLRP3 Inflammasome Inhibitor DFV890 in Adult Patients with Myeloid Diseases: A Phase 1b Study. Blood 2024, 144, 353. [Google Scholar] [CrossRef]
- Study of SX-682 Alone and in Combination with Oral or Intravenous Decitabine in Subjects with Myelodysplastic Syndrome, ClinicalTrials.gov ID NCT04245397. Available online: https://clinicaltrials.gov/study/NCT04245397?cond=NCT04245397&rank=1 (accessed on 11 June 2025).
- Gurnari, C.; Robin, M.; Ades, L.; Aljurf, M.; Almeida, A.M.; Duarte, F.B.; Bernard, E.; Cutler, C.S.; Della Porta, M.G.; de Witte, T.M.; et al. Clinical-genomic profiling of MDS to inform allo-HSCT:Recommendations from an international panel on behalf of the EBMT. Blood 2025, 145, 1987–2001. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, R.; Saber, W.; Martens, M.J.; Ramirez, A.; Scott, B.; Oran, B.; Leifer, E.; Tamari, R.; Mishra, A.; Maziarz, R.T.; et al. Biologic Assignment Trial of Reduced-Intensity Hematopoietic Cell Transplantation Based on Donor Availability in Patients 50-75 Years of Age with Advanced Myelodysplastic Syndrome. J. Clin. Oncol. 2021, 39, 3328–3339. [Google Scholar] [CrossRef] [PubMed]
- Kröger, N.; Sockel, K.; Wolschke, C.; Bethge, W.; Schlenk, R.F.; Wolf, D.; Stadler, M.; Kobbe, G.; Wulf, G.; Bug, G.; et al. Comparison Between 5-Azacytidine Treatment and Allogeneic Stem-Cell Transplantation in Elderly Patients with Advanced MDS According to Donor Availability (VidazaAllo Study). J. Clin. Oncol. 2021, 39, 3318–3327. [Google Scholar] [CrossRef] [PubMed]
- Robin, M.; Porcher, R.; Adès, L.; Raffoux, E.; Michallet, M.; François, S.; Cahn, J.Y.; Delmer, A.; Wattel, E.; Vigouroux, S.; et al. HLA-matched allogeneic stem cell transplantation improves outcome of higher risk myelodysplastic syndrome A prospective study on behalf of SFGM-TC and GFM. Leukemia 2015, 29, 1496–1501. [Google Scholar] [CrossRef] [PubMed]
- Bischof, L.; Ussmann, J.; Platzbecker, U.; Jentzsch, M.; Franke, G.N. Allogeneic stem cell transplantation for MDS-clinical issues, choosing preparative regimens and outcome. Leuk. Lymphoma 2025, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Shaw, B.E.; Jimenez-Jimenez, A.M.; Burns, L.J.; Logan, B.R.; Khimani, F.; Shaffer, B.C.; Shah, N.N.; Mussetter, A.; Tang, X.Y.; McCarty, J.M.; et al. National Marrow Donor Program-Sponsored Multicenter, Phase II Trial of HLA-Mismatched Unrelated Donor Bone Marrow Transplantation Using Post-Transplant Cyclophosphamide. J. Clin. Oncol. 2021, 39, 1971–1982. [Google Scholar] [CrossRef] [PubMed]
- Luznik, L.; Pasquini, M.C.; Logan, B.; Soiffer, R.J.; Wu, J.; Devine, S.M.; Geller, N.; Giralt, S.; Heslop, H.E.; Horowitz, M.M.; et al. Randomized Phase III BMT CTN Trial of Calcineurin Inhibitor-Free Chronic Graft-Versus-Host Disease Interventions in Myeloablative Hematopoietic Cell Transplantation for Hematologic Malignancies. J. Clin. Oncol. 2022, 40, 356–368. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, M.; Power, M.; Abou Mourad, Y.; Barnett, M.; Broady, R.; Forrest, D.; Gerrie, A.; Hogge, D.; Nantel, S.; Sanford, D.; et al. Improving Revised International Prognostic Scoring System Pre-Allogeneic Stem Cell Transplantation Does Not Translate Into Better Post-Transplantation Outcomes for Patients with Myelodysplastic Syndromes: A Single-Center Experience. Biol. Blood Marrow Transplant. 2018, 24, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Yahng, S.A.; Kim, M.; Kim, T.M.; Jeon, Y.W.; Yoon, J.H.; Shin, S.H.; Lee, S.E.; Eom, K.S.; Lee, S.; Min, C.K.; et al. Better transplant outcome with pre-transplant marrow response after hypomethylating treatment in higher-risk MDS with excess blasts. Oncotarget 2017, 8, 12342–12354. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, J.C.; Mix, L.; Faustmann, P.; Weller, J.F.; Fehn, A.; Phely, L.; Riedel, A.; Vogel, W.; Faul, C.; Lengerke, C.; et al. Superior outcome of upfront allogeneic hematopoietic cell transplantation versus hypomethylating agent induction in myelodysplastic syndrome. Bone Marrow Transplant. 2024, 59, 1332–1334. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, Y.; Zhou, W.; Wang, R.; Li, Y.; Yu, L. Pre-transplant therapy for patients with myelodysplastic syndromes: A systematic review and meta-analysis. Leuk. Res. 2021, 110, 106645. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, A.M.; Platzbecker, U.; Bewersdorf, J.P.; Stahl, M.; Ades, L.; Borate, U.; Bowen, D.T.; Buckstein, R.J.; Brunner, A.M.; Carraway, H.E.; et al. Consensus proposal for revised International Working Group response criteria for higher risk myelodysplastic syndromes. Blood 2023, 141, 2047–2061. [Google Scholar] [CrossRef] [PubMed]
- Rolles, B.; Bewersdorf, J.P.; Kewan, T.; Blaha, O.; Stempel, J.M.; Lanino, L.; Al Ali, N.H.; Dezern, A.E.; Sekeres, M.A.; Uy, G.L.; et al. Impact of Response to Hypomethylating Agent-Based Therapy on Survival Outcomes in the Context of Baseline Clinical-Molecular Risk and Transplant Status in Patients with Myelodysplastic Syndromes/Neoplasms (MDS): An Analysis from the International Consorti. Blood 2024, 144, 664. [Google Scholar] [CrossRef]
- Oran, B.; De Lima, M.; Garcia-Manero, G.; Thall, P.F.; Lin, R.; Popat, U.; Alousi, A.M.; Hosing, C.; Giralt, S.; Rondon, G.; et al. A phase 3 randomized study of 5-azacitidine maintenance vs observation after transplant in high-risk AML and MDS patients. Blood Adv. 2020, 4, 5580–5588. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhang, Y.; Wang, S.; Kong, P.; Su, Y.; Hu, J.; Jiang, M.; Bai, H.; Lang, T.; Wang, J.; et al. Effect of rhG-CSF Combined with Decitabine Prophylaxis on Relapse of Patients with High-Risk MRD-Negative AML After HSCT: An Open-Label, Multicenter, Randomized Controlled Trial. J. Clin. Oncol. 2020, 38, 4249–4259. [Google Scholar] [CrossRef] [PubMed]
- Randomised Study of Oral Azacitidine vs Placebo Maintenance in AML or MDS Patients After Allo-SCT (AMADEUS), ClinicalTrials.gov ID NCT04173533. Available online: https://clinicaltrials.gov/study/NCT04173533 (accessed on 21 February 2025).
- De Lima, M.; Oran, B.; Champlin, R.E.; Papadopoulos, E.B.; Giralt, S.A.; Scott, B.L.; William, B.M.; Hetzer, J.; Laille, E.; Hubbell, B.; et al. CC-486 Maintenance after Stem Cell Transplantation in Patients with Acute Myeloid Leukemia or Myelodysplastic Syndromes. Biol. Blood Marrow Transplant. 2018, 24, 2017–2024. [Google Scholar] [CrossRef] [PubMed]
- Preemptive CIML NK Cell Therapy After Hematopoietic Stem Cell Transplantation, ClinicalTrials.gov ID NCT06138587. Available online: https://clinicaltrials.gov/study/NCT06138587?cond=NCT06138587&rank=1 (accessed on 11 June 2025).
- Pre-Emptive Therapy with DEC-C to Improve Outcomes in MDS Patients with Measurable Residual Disease Post Allogeneic Hematopoietic Cell Transplant, ClinicalTrials.gov ID NCT04742634. Available online: https://clinicaltrials.gov/study/NCT04742634?cond=NCT04742634&rank=1 (accessed on 11 June 2025).
- Fathi, A.T.; Kim, H.T.; Soiffer, R.J.; Levis, M.J.; Li, S.; Kim, A.S.; Defilipp, Z.; El-Jawahri, A.; McAfee, S.L.; Brunner, A.M.; et al. Multicenter Phase I Trial of Ivosidenib as Maintenance Treatment Following Allogeneic Hematopoietic Cell Transplantation for IDH1-Mutated Acute Myeloid Leukemia. Clin. Cancer Res. 2023, 29, 2034–2042. [Google Scholar] [CrossRef] [PubMed]
- Fathi, A.T.; Kim, H.T.; Soiffer, R.J.; Levis, M.J.; Li, S.; Kim, A.S.; Mims, A.S.; Defilipp, Z.; El-Jawahri, A.; McAfee, S.L.; et al. Enasidenib as maintenance following allogeneic hematopoietic cell transplantation for IDH2-mutated myeloid malignancies. Blood Adv. 2022, 6, 5857–5865. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Tamari, R.; Dezern, A.E.; Byrne, M.T.; Gooptu, M.; Chen, Y.-B.; Deeg, H.J.; Sallman, D.; Gallacher, P.; Wennborg, A.; et al. Eprenetapopt Plus Azacitidine After Allogeneic Hematopoietic Stem-Cell Transplantation for TP53-Mutant Acute Myeloid Leukemia and Myelodysplastic Syndromes. J. Clin. Oncol. 2022, 40, 3985–3993. [Google Scholar] [CrossRef] [PubMed]
- Guillaume, T.; Thepot, S.; Peterlin, P.; Ceballos, P.; Bourgeois, A.L.; Garnier, A.; Orvain, C.; Giltat, A.; Francois, S.; Bris, Y.L.; et al. Prophylactic or Preemptive Low-Dose Azacitidine and Donor Lymphocyte Infusion to Prevent Disease Relapse following Allogeneic Transplantation in Patients with High-Risk Acute Myelogenous Leukemia or Myelodysplastic Syndrome. Transplant. Cell Ther. 2021, 27, 839.e1-839.e6. [Google Scholar] [CrossRef] [PubMed]
- Panobinostat Maintenance After HSCT fo High-Risk AML and MDS, ClinicalTrials.gov ID NCT04326764. Available online: https://clinicaltrials.gov/study/NCT04326764?cond=NCT04326764&rank=1 (accessed on 11 June 2025).
- Bug, G.; Burchert, A.; Wagner, E.M.; Kröger, N.; Berg, T.; Güller, S.; Metzelder, S.K.; Wolf, A.; Hünecke, S.; Bader, P.; et al. Phase I/II study of the deacetylase inhibitor panobinostat after allogeneic stem cell transplantation in patients with high-risk MDS or AML (PANOBEST trial). Leukemia 2017, 31, 2523–2525. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.Y.; Wei, Z.L.; Xu, Y.J.; Shi, J.M.; Yi, H.; Lai, Y.R.; Jiang, E.L.; Wang, S.B.; Wu, T.; Gao, L.; et al. Poor pretransplantation minimal residual disease clearance as an independent prognostic risk factor for survival in myelodysplastic syndrome with excess blasts: A multicenter, retrospective cohort study. Cancer 2023, 129, 2013–2022. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Jia, Y.; Yang, J.; Cai, Y.; Tong, Y.; Qiu, H.; Zhou, K.; Xia, X.; Zhang, Y.; Shen, C.; et al. Azacitidine combined with interferon-α for pre-emptive treatment of AML/MDS after allogeneic peripheral blood stem cell transplantation: A prospective phase II study. Br. J. Haematol. 2024, 205, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Sallman, D.; McLemore, A.F.; Komrokji, R.S.; Elmariah, H.; Kuykendall, A.T.; Bejanyan, N.; Chan, O.; Faramand, R.; Pidala, J.A.; Yoder, S.J.; et al. Measurable Residual Disease Monitoring By Duplex Sequencing for TP53 in the Post Allogeneic Stem Cell Transplantation Study with Eprenetapopt (APR-246) + Azacitidine Strongly Predicts Outcomes. Blood 2024, 144, 1046. [Google Scholar] [CrossRef]
- Schulz, E.; Aplan, P.D.; Freeman, S.D.; Pavletic, S.Z. Moving toward a conceptualization of measurable residual disease in myelodysplastic syndromes. Blood Adv. 2023, 7, 4381–4394. [Google Scholar] [CrossRef] [PubMed]
- Tobiasson, M.; Pandzic, T.; Illman, J.; Nilsson, L.; Westrom, S.; Ejerblad, E.; Olesen, G.; Bjorklund, A.; Olsnes Kittang, A.; Werlenius, O.; et al. Patient-Specific Measurable Residual Disease Markers Predict Outcome in Patients with Myelodysplastic Syndrome and Related Diseases After Hematopoietic Stem-Cell Transplantation. J. Clin. Oncol. 2024, 42, 1378–1390. [Google Scholar] [CrossRef] [PubMed]
- Kaminskas, E.; Farrell, A.T.; Wang, Y.-C.; Sridhara, R.; Pazdur, R. FDA Drug Approval Summary: Azacitidine (5-azacytidine, Vidaza™) for Injectable Suspension. Oncologist 2005, 10, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Abbvie Press Release, June 16, 2025. AbbVie Provides Update on VERONA Trial for Newly Diagnosed Higher-Risk Myelodysplastic Syndromes. Available online: https://news.abbvie.com/2025-06-16-AbbVie-Provides-Update-on-VERONA-Trial-for-Newly-Diagnosed-Higher-Risk-Myelodysplastic-Syndromes (accessed on 16 June 2025).
- APR-246 & Azacitidine for the Treatment of TP53 Mutant Myelodysplastic Syndromes (MDS), ClinicalTrials.gov ID NCT03745716. Available online: https://clinicaltrials.gov/study/NCT03745716?term=apr-246&cond=mds&rank=1&tab=results#more-information (accessed on 11 June 2025).
- Garcia-Manero, G.; Fenaux, P.; Al-Kali, A.; Baer, M.R.; Sekeres, M.A.; Roboz, G.J.; Gaidano, G.; Scott, B.L.; Greenberg, P.; Platzbecker, U.; et al. Rigosertib versus best supportive care for patients with high-risk myelodysplastic syndromes after failure of hypomethylating drugs (ONTIME): A randomised, controlled, phase 3 trial. Lancet Oncol. 2016, 17, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Manero, G.; McCloskey, J.; Scott, B.L.; Griffiths, E.A.; Kiner-Strachan, B.; Brunner, A.M.; Zeidan, A.M.; Traer, E.; Madanat, Y.F.; Meyer, J.; et al. Results from a Phase 1 Open-Label Dose Escalation and Expansion Trial of Oral Azacitidine + Cedazuridine (ASTX030) in Patients with Myelodysplastic Syndromes (MDS) and MDS/Myeloproliferative Neoplasms (MPN). Blood 2024, 144, 662. [Google Scholar] [CrossRef]
- Adès, L.; Girshova, L.; Doronin, V.A.; Díez-Campelo, M.; Valcárcel, D.; Kambhampati, S.; Viniou, N.-A.; Woszczyk, D.; De Paz Arias, R.; Symeonidis, A.; et al. Pevonedistat plus azacitidine vs azacitidine alone in higher-risk MDS/chronic myelomonocytic leukemia or low-blast-percentage AML. Blood Adv. 2022, 6, 5132–5145. [Google Scholar] [CrossRef] [PubMed]
- Magrolimab + Azacitidine Versus Azacitidine + Placebo in Untreated Participants with Myelodysplastic Syndrome (MDS) (ENHANCE), ClinicalTrials.gov ID NCT04313881. Available online: https://clinicaltrials.gov/study/NCT04313881 (accessed on 24 June 2025).
- Zeidan, A.M.; Ando, K.; Rauzy, O.; Turgut, M.; Wang, M.C.; Cairoli, R.; Hou, H.A.; Kwong, Y.L.; Arnan, M.; Meers, S.; et al. Sabatolimab plus hypomethylating agents in previously untreated patients with higher-risk myelodysplastic syndromes (STIMULUS-MDS1): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Haematol. 2024, 11, e38–e50. [Google Scholar] [CrossRef] [PubMed]
- Tamibarotene Plus Azacitidine in Participants with Newly Diagnosed RARA-Positive Higher-Risk Myelodysplastic Syndrome, ClinicalTrials.gov ID NCT04797780. Available online: https://clinicaltrials.gov/study/NCT04797780?tab=results (accessed on 24 June 2025).
- Lisaftoclax (APG-2575) Combined with Azacytidine (AZA) in the Treatment of Patients with Higher-Risk Myelodysplastic Syndrome (GLORA-4), ClinicalTrials.gov ID NCT06641414. Available online: https://clinicaltrials.gov/study/NCT06641414?term=lisaftoclax%20&rank=3 (accessed on 19 July 2025).
- A Trial of AK117 (Anti-CD47) in Patients with Myelodysplastic Syndrome, ClinicalTrials.gov ID NCT04900350. Available online: https://clinicaltrials.gov/study/NCT04900350 (accessed on 24 June 2025).
- A Study of BGB-11417 in Participants with Myeloid Malignancies, ClinicalTrials.gov ID NCT04771130. Available online: https://clinicaltrials.gov/study/NCT04771130?cond=mds&term=sonrotoclax%20&rank=1#study-record-dates (accessed on 19 July 2025).
- Dinardo, C.D.; Roboz, G.J.; Watts, J.M.; Madanat, Y.F.; Prince, G.T.; Baratam, P.; De Botton, S.; Stein, A.; Foran, J.M.; Arellano, M.L.; et al. Final phase 1 substudy results of ivosidenib for patients with mutant IDH1 relapsed/refractory myelodysplastic syndrome. Blood Adv. 2024, 8, 4209–4220. [Google Scholar] [CrossRef] [PubMed]
- Sebert, M.; Chevret, S.; Dimicoli-Salazar, S.; Cluzeau, T.; Rauzy, O.; Stamatoulas Bastard, A.; Laribi, K.; Fossard, G.; Thépot, S.; Gloaguen, S.; et al. Enasidenib (ENA) Monotherapy in Patients with IDH2 mutated Myelodysplastic Syndrome (MDS), the Ideal Phase 2 Study By the GFM and Emsco Groups. Blood 2024, 144, 1839. [Google Scholar] [CrossRef]
- Fenaux, P.; Mufti, G.J.; Hellstrom-Lindberg, E.; Santini, V.; Finelli, C.; Giagounidis, A.; Schoch, R.; Gattermann, N.; Sanz, G.; List, A.; et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: A randomised, open-label, phase III study. Lancet Oncol. 2009, 10, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, A.M.; Salimi, T.; Epstein, R.S. Real-world use and outcomes of hypomethylating agent therapy in higher-risk myelodysplastic syndromes: Why are we not achieving the promise of clinical trials? Future Oncol. 2021, 17, 5163–5175. [Google Scholar] [CrossRef] [PubMed]
- Rajakumaraswamy, N.; Gandhi, M.; Wei, A.H.; Sallman, D.A.; Daver, N.G.; Mo, S.; Iqbal, S.; Karalliyadda, R.; Chen, M.; Wang, Y.; et al. Real-world Effectiveness of Azacitidine in Treatment-Naive Patients with Higher-risk Myelodysplastic Syndromes. Clin. Lymphoma Myeloma Leuk. 2024, 24, 260-268.e2. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.; Issa, J.-P.J.; Rosenfeld, C.S.; Bennett, J.M.; Albitar, M.; Dipersio, J.; Klimek, V.; Slack, J.; De Castro, C.; Ravandi, F.; et al. Decitabine improves patient outcomes in myelodysplastic syndromes. Cancer 2006, 106, 1794–1803. [Google Scholar] [CrossRef] [PubMed]
- Nazha, A.; Sekeres, M.A.; Garcia-Manero, G.; Barnard, J.; Al Ali, N.H.; Roboz, G.J.; Steensma, D.P.; Dezern, A.E.; Zimmerman, C.; Jabbour, E.J.; et al. Outcomes of patients with myelodysplastic syndromes who achieve stable disease after treatment with hypomethylating agents. Leuk. Res. 2016, 41, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Manero, G.; Griffiths, E.A.; Steensma, D.P.; Roboz, G.J.; Wells, R.; McCloskey, J.; Odenike, O.; DeZern, A.E.; Yee, K.; Busque, L.; et al. Oral cedazuridine/decitabine for MDS and CMML: A phase 2 pharmacokinetic/pharmacodynamic randomized crossover study. Blood 2020, 136, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Manero, G.; McCloskey, J.; Griffiths, E.A.; Yee, K.W.L.; Zeidan, A.M.; Al-Kali, A.; Deeg, H.J.; Patel, P.A.; Sabloff, M.; Keating, M.M.; et al. Oral decitabine-cedazuridine versus intravenous decitabine for myelodysplastic syndromes and chronic myelomonocytic leukaemia (ASCERTAIN): A registrational, randomised, crossover, pharmacokinetics, phase 3 study. Lancet Haematol. 2024, 11, e15–e26. [Google Scholar] [CrossRef] [PubMed]
- Silverman, L.R.; Verma, A.; Odchimar-Reissig, R.; Feldman, E.J.; Navada, S.; Demakos, E.P.; Baer, M.R.; Najfeld, V.; Sparano, J.A.; Piekarz, R. A Phase II Trial Of Epigenetic Modulators Vorinostat In Combination with Azacitidine (azaC) In Patients with The Myelodysplastic Syndrome (MDS): Initial Results Of Study 6898 Of The New York Cancer Consortium. Blood 2013, 122, 383. [Google Scholar] [CrossRef]
- Sekeres, M.A.; Othus, M.; List, A.F.; Odenike, O.; Stone, R.M.; Gore, S.D.; Litzow, M.R.; Buckstein, R.; Fang, M.; Roulston, D.; et al. Randomized Phase II Study of Azacitidine Alone or in Combination with Lenalidomide or with Vorinostat in Higher-Risk Myelodysplastic Syndromes and Chronic Myelomonocytic Leukemia: North American Intergroup Study SWOG S1117. J. Clin. Oncol. 2017, 35, 2745–2753. [Google Scholar] [CrossRef] [PubMed]
- Sekeres, M.A.; Watts, J.; Radinoff, A.; Sangerman, M.A.; Cerrano, M.; Lopez, P.F.; Zeidner, J.F.; Campelo, M.D.; Graux, C.; Liesveld, J.; et al. Randomized phase 2 trial of pevonedistat plus azacitidine versus azacitidine for higher-risk MDS/CMML or low-blast AML. Leukemia 2021, 35, 2119–2124. [Google Scholar] [CrossRef] [PubMed]
- Sallman, D.A.; Al Malki, M.M.; Asch, A.S.; Wang, E.S.; Jurcic, J.G.; Bradley, T.J.; Flinn, I.W.; Pollyea, D.A.; Kambhampati, S.; Tanaka, T.N.; et al. Magrolimab in Combination with Azacitidine in Patients with Higher-Risk Myelodysplastic Syndromes: Final Results of a Phase Ib Study. J. Clin. Oncol. 2023, 41, 2815–2826. [Google Scholar] [CrossRef] [PubMed]
- Gilead Press Release, July 21, 2023. Gilead To Discontinue Phase 3 ENHANCE Study of Magrolimab Plus Azacitidine in Higher-Risk MDS. Available online: https://www.gilead.com/news/news-details/2023/gilead-to-discontinue-phase-3-enhance-study-of-magrolimab-plus-azacitidine-in-higher-risk-mds (accessed on 2 March 2025).
- Zeidan, A.M.; Xiao, Z.; Sanz, G.; Giagounidis, A.; Sekeres, M.; Lao, Z.; Deeren, D.; Gao, S.; Riva, M.; Lee, J.; et al. Primary results of the phase III STIMULUS-MDS2 study of sabatolimab + azacitidine vs placebo + azacitidine as frontline therapy for patients with higher-risk MDS or CMML-2. In Proceedings of the European Hematology Association Congress 2024, Madrid, Spain, 13–16 June 2024. Abstract S180. [Google Scholar]
- Brunner, A.M.; Esteve, J.; Porkka, K.; Knapper, S.; Traer, E.; Scholl, S.; Garcia-Manero, G.; Vey, N.; Wermke, M.; Janssen, J.J.W.M.; et al. Phase Ib study of sabatolimab (MBG453), a novel immunotherapy targeting TIM-3 antibody, in combination with decitabine or azacitidine in high- or very high-risk myelodysplastic syndromes. Am. J. Hematol. 2023, 99, E32–E36. [Google Scholar] [CrossRef] [PubMed]
- Dinardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Bogenberger, J.M.; Kornblau, S.M.; Pierceall, W.E.; Lena, R.; Chow, D.; Shi, C.X.; Mantei, J.; Ahmann, G.; Gonzales, I.M.; Choudhary, A.; et al. BCL-2 family proteins as 5-Azacytidine-sensitizing targets and determinants of response in myeloid malignancies. Leukemia 2014, 28, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Bazinet, A.; Darbaniyan, F.; Jabbour, E.; Montalban-Bravo, G.; Ohanian, M.; Chien, K.; Kadia, T.; Takahashi, K.; Masarova, L.; Short, N.; et al. Azacitidine plus venetoclax in patients with high-risk myelodysplastic syndromes or chronic myelomonocytic leukaemia: Phase 1 results of a single-centre, dose-escalation, dose-expansion, phase 1–2 study. Lancet Haematol. 2022, 9, e756–e765. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, A.M.; Borate, U.; Pollyea, D.A.; Brunner, A.M.; Roncolato, F.; Garcia, J.S.; Filshie, R.; Odenike, O.; Watson, A.M.; Krishnadasan, R.; et al. A phase 1b study of venetoclax and azacitidine combination in patients with relapsed or refractory myelodysplastic syndromes. Am. J. Hematol. 2023, 98, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Apodaca Chavez, E.I.; Crisp, R.; Varela Constantino, A.L.; Enrico, A.I.; Gomez-De Leon, A.; Ovilla-Martínez, R.; Wernicke, P.; Camargo Molano, C.; Boada, M.; Velloso, E.D.R.P.; et al. Azacitidine and Venetoclax in High-Risk Myelodysplastic Syndrome: A Real-World Perspective from the Glam Registry with Long-Term Follow-up. Blood 2024, 144, 6718. [Google Scholar] [CrossRef]
- Guru Murthy, G.S.; Ball, S.; Feld, J.; Abboud, R.; Haddadin, M.; Patel, S.A.; Kishtagari, A.; Hawkins, H.; Guerra, V.; Dworkin, E.; et al. Clinical Utilization and Outcomes of Hypomethylating Agents and Venetoclax in Patients with Myelodysplastic Syndrome—A Multicenter Retrospective Analysis. Blood 2024, 144, 3206. [Google Scholar] [CrossRef]
- Garcia, J.S.; Platzbecker, U.; Odenike, O.; Fleming, S.; Fong, C.Y.; Borate, U.; Jacoby, M.A.; Nowak, D.; Baer, M.R.; Peterlin, P.; et al. Efficacy and safety of venetoclax plus azacitidine for patients with treatment-naive high-risk myelodysplastic syndromes. Blood 2025, 145, 1126–1135. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, A.M.; Garcia, J.S.; Fenaux, P.; Platzbecker, U.; Miyazaki, Y.; Xiao, Z.; Zhou, Y.; Naqvi, K.; Kye, S.; Garcia-Manero, G. Phase 3 VERONA study of venetoclax with azacitidine to assess change in complete remission and overall survival in treatment-naïve higher-risk myelodysplastic syndromes. J. Clin. Oncol. 2021, 39, TPS7054. [Google Scholar] [CrossRef]
- Bataller, A.; Montalban-Bravo, G.; Bazinet, A.; Alvarado, Y.; Chien, K.; Venugopal, S.; Ishizawa, J.; Hammond, D.; Swaminathan, M.; Sasaki, K.; et al. Oral decitabine plus cedazuridine and venetoclax in patients with higher-risk myelodysplastic syndromes or chronic myelomonocytic leukaemia: A single-centre, phase 1/2 study. Lancet Haematol. 2024, 11, e186–e195. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, B.C.; Fathi, A.T.; Dinardo, C.D.; Pollyea, D.A.; Chan, S.M.; Swords, R. Isocitrate dehydrogenase mutations in myeloid malignancies. Leukemia 2017, 31, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Sebert, M.; Cluzeau, T.; Beyne-Rauzy, O.; Stamatoullas-Bastard, A.; Dimicoli-Salazar, S.; Thepot, S.; Peterlin, P.; Park, S.; Gourin, M.-P.; Brehar, O.; et al. Ivosidenib Monotherapy Is Effective in Patients with IDH1 Mutated Myelodysplastic Syndrome (MDS): The Idiome Phase 2 Study By the GFM Group. Blood 2021, 138 (Suppl. S1), 62. [Google Scholar] [CrossRef]
- Sebert, M.; Clappier, E.; Cluzeau, T.; Bergugnat, H.; Beyne-Rauzy, O.; Stamatoullas-Bastard, A.; Dimicoli-Salazar, S.; Larcher, L.; Goldwirt, L.; Thepot, S.; et al. Ivosidenib Monotherapy in IDH1 Mutated Myelodysplastic Syndrome, Final Results of the IDIOME Trial, a GFM Study. EHA Library, S182 2024. Available online: https://library.ehaweb.org/eha/2024/eha2024-congress/422286/marie.sbert.ivosidenib.monotherapy.in.idh1.mutated.myelodysplastic.syndrome.html (accessed on 12 May 2025).
- Sebert, M.; Platzbecker, U.; Valcárcel Ferreiras, D.; Santini, V.; Borate, U.; Kapsalis, S.M.; Simonot, L.; Yuan, W.; Patel, P.A.; Garcia-Manero, G. Phase 3 Study of Either Ivosidenib (IVO) Monotherapy or Azacitidine (AZA) Monotherapy in Patients with IDH1 Mutant Myelodysplastic Syndromes (MDS) Who Are Hypomethylating Agent (HMA) Naive (PyramIDH). Blood 2024, 114 (Suppl. S1), 1845.1841. [Google Scholar] [CrossRef]
- Forma Therapeutics, Inc. REZLIDHIA™ (Olutasidenib) Capsules, for Oral Use; Forma Therapeutics, Inc.: Greenville, NC, USA, 2025. [Google Scholar]
- Cortes, J.; Yang, J.; Lee, S.; Dinner, S.; Wang, E.S.; Baer, M.R.; Donnellan, W.; Watts, J.M. Olutasidenib Alone or in Combination with Azacitidine Induces Durable Complete Remissions in Patients with mIDH1 Myelodysplastic Syndromes/Neoplasms (MDS). Blood 2023, 142 (Suppl. S1), 1872. [Google Scholar] [CrossRef]
- Olutasidenib in Combination with Hypomethylation Therapy for the Treatment of IDH1-Mutated Higher-Risk Myelodysplastic Syndromes, Chronic Myelomonocytic Leukemia, and Myeloproliferative Neoplasms. ClinicalTrials.ov ID. Available online: https://www.cancer.gov/research/participate/clinical-trials-search/v?id=NCI-2024-07758&r=1 (accessed on 12 May 2025).
- Stein, E.M.; Fathi, A.T.; DiNardo, C.D.; Pollyea, D.A.; Roboz, G.J.; Collins, R.; Sekeres, M.A.; Stone, R.M.; Attar, E.C.; Frattini, M.G.; et al. Enasidenib in patients with mutant IDH2 myelodysplastic syndromes: A phase 1 subgroup analysis of the multicentre, AG221-C-001 trial. Lancet Haematol. 2020, 7, e309–e319. [Google Scholar] [CrossRef] [PubMed]
- Dinardo, C.D.; Venugopal, S.; Lachowiez, C.; Takahashi, K.; Loghavi, S.; Montalban-Bravo, G.; Wang, X.; Carraway, H.; Sekeres, M.; Sukkur, A.; et al. Targeted therapy with the mutant IDH2 inhibitor enasidenib for high-risk IDH2-mutant myelodysplastic syndrome. Blood Adv. 2023, 7, 2378–2387. [Google Scholar] [CrossRef] [PubMed]
- Bernard, E.; Nannya, Y.; Hasserjian, R.P.; Devlin, S.M.; Tuechler, H.; Medina-Martinez, J.S.; Yoshizato, T.; Shiozawa, Y.; Saiki, R.; Malcovati, L.; et al. Implications of TP53 allelic state for genome stability, clinical presentation and outcomes in myelodysplastic syndromes. Nat. Med. 2020, 26, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Cluzeau, T.; Sebert, M.; Rahmé, R.; Cuzzubbo, S.; Lehmann-Che, J.; Madelaine, I.; Peterlin, P.; Bève, B.; Attalah, H.; Chermat, F.; et al. Eprenetapopt Plus Azacitidine in TP53-Mutated Myelodysplastic Syndromes and Acute Myeloid Leukemia: A Phase II Study by the Groupe Francophone des Myélodysplasies (GFM). J. Clin. Oncol. 2021, 39, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Sallman, D.A.; Dezern, A.E.; Garcia-Manero, G.; Steensma, D.P.; Roboz, G.J.; Sekeres, M.A.; Cluzeau, T.; Sweet, K.L.; McLemore, A.; McGraw, K.L.; et al. Eprenetapopt (APR-246) and Azacitidine in TP53-Mutant Myelodysplastic Syndromes. J. Clin. Oncol. 2021, 39, 1584–1594. [Google Scholar] [CrossRef] [PubMed]
- Aprea Therapeutics, Press Release December 28, 2020. Aprea Therapeutics Announces Results of Primary Endpoint from Phase 3 Trial of Eprenetapopt in TP53 Mutant Myelodysplastic Syndromes (MDS). Available online: https://ir.aprea.com/news-releases/news-release-details/aprea-therapeutics-announces-results-primary-endpoint-phase-3 (accessed on 2 February 2024).
| Feature | CHRS (Clonal Hematopoiesis Risk Score) | CCRS (Clonal Cytopenia Risk Score) |
|---|---|---|
| Publication | Weeks et al., NEJM Evidence, 2023 [32] | Xie et al., Blood, 2024 [31] |
| Purpose | Predicts risk of progression to myeloid neoplasm malignancy in patients CHIP/CCUS | Stratifies CCUS patients based on progression risk to MDS or AML |
| Population | CHIP and CCUS (including individuals without cytopenias) | Specifically developed for CCUS |
| Key Components | 8 prognostic factors: Number of mutations, specific genes (high-risk mutations, DNMT3A), VAF, presence of cytopenia, RDW, MCV, age | 3 prognostic factors: Number of mutations (≥2), specific genes (splicing factor mutations), and platelet count < 100 × 109/L |
| Risk Scoring | Low risk: ≤9.5 points Intermediate risk: 10–12 points High risk: ≥12.5 points | Low risk: <2.5 points Intermediate risk: 2.5–≤5 points High risk: ≥5 points |
| Gene weighting | Higher risk for splicing factor mutations, AML-like (IDH1/2, FLT3, RUNX1), JAK2 and TP53 assigned score 2.5; if ≥2 mutations assigned score of 2 | Splicing factor mutations assigned score of 2; if ≥2 mutations assigned score of 3 |
| Risk for MN | 5-year CI for MN (±SD) (%): No CHIP/CCUS: 0.0740 (±0.0064) Low risk: 0.232 (±0.0484) Intermediate risk: 2.76 (±0.482) High risk: 24.4 (±4.12) | 2-year CI (%, 95% confidence interval) of MN progression: Low risk: 6.4 (13–11.4) Intermediate risk: 14.1 (7.9–22.2) High risk: 37.2 (19.8–54.7) |
| Strengths | Large population cohort; integrates age and VAF; validated | Only 3 prognostic factors; CCUS-specific; validated |
| Agent or Drug Combination | Mechanism of Action | Trial Phase | Population | Clinical Trial ID | References |
|---|---|---|---|---|---|
| Luspatercept vs. ESA | TGF-β superfamily ligand trap | III | LR-MDS (IPSS-R ≤ 3.5), RBC-TI | NCT05949684 | Zeidan et al., ASH, 2023 [49] |
| KER-050 (Elritercept) | Modified activin receptor type IIA ligand trap | II | LR-MDS (IPSS-R ≤ 3.5) | NCT04419649 | Diez-Campelo et al., ASH, 2023 [60]; Tan et al., ASH, 2024 [61] |
| RVU120 | CDK8/19 small molecule inhibitor | II | LR-MDS (IPSS-R ≤ 3.5), R/R or ineligible for other therapies | NCT06243458 [62] | ClinicalTrials.gov |
| Canakinumab | Anti-IL-1β human monoclonal antibody | II | LR-MDS (IPSS-R ≤ 3.5), R/R ESA, HMAs | NCT04239157 | Rodriguez Sevilla et al., ASH, 2023 [63] |
| Attenuated durations of HMAs | DNMT inhibitor | II | LR-MDS (IPSS ≤ 1) | NCT02269280 | Bouligny et al., EHA, 2025 [64] |
| Luspatercept + ESA | TGF-β superfamily ligand trap | I/II | LR-MDS (IPSS ≤ 1), non-RS, R/R ESA | 2021-000596-37 * | Ades et al., ASH, 2024 [65] |
| Oral decitabine + cedazuridine (ASTX727) | DNMT inhibitor | I/II | LR-MDS (IPSS ≤ 1), non-RS, R/R | NCT03502668 | Garcia-Manero et al., SOHO, 2023 [66] |
| LB-100 | Protein phosphatase 2A inhibitor | Ib/II | LR-MDS (IPSS ≤ 1), requiring treatment | NCT03886662 [67] | ClinicalTrials.gov |
| R289 | IRAK1/4 inhibitor | Ib | LR-MDS (IPSS-R ≤ 3.5), R/R, included del(5q) | NCT05308264 | Garcia-Manero et al., ASH, 2024 [68] |
| DFV890 | Selective NLRP3 inhibitor | Ib | LR-MDS (IPSS-R ≤ 3.5), previously treated | NCT05552469 | Garcia-Manero et al., ASH, 2024 [69] |
| SX-682 +/− Decitabine | CXCR1/2 inhibitor | I | LR-MDS (IPSS ≤1), R/R | NCT04245397 [70] | ClinicalTrials.gov |
| Agent/Drug Combination | Trial Phase | Status | Population | Results | Clinical Trial ID | Reference |
|---|---|---|---|---|---|---|
| Oral azacitidine vs. placebo | III | Active | AML, MDS | N/A | NCT04173533 | ClinicalTrials.gov [86] |
| IL-2 | I/Ib | Active | AML, MDS | N/A | NCT06138587 | ClinicalTrials.gov [88] |
| Decitabine and cedazuridine | I/II | Recruiting | MDS | N/A | NCT04742634 | ClinicalTrials.gov [89] |
| IDH1 inhibitor | I | Completed | AML, MDS | CIR 19% (95% CI: 4.0–41) 2y-PFS 81% (95% CI: 52–94) 2y-OS 88% (95% CI: 59–97) | NCT03564821 | Fathi et al., Clin Cancer Res, 2023 [90] |
| IDH2 inhibitor | I | Completed | AML, MDS | CIR 16% (95% CI: 3.7–36) 2y-PFS 69% (95% CI: 39–86) 2y-OS 74% (95% CI: 44–90) | NCT03515512 | Fathi et al., Blood Adv, 2022 [91] |
| Eprenetapopt + azacitidine | II | Completed | TP53-mutated AML, MDS | 1-y PFS 59.9% (95% CI: 41–74) 1y-OS 78.8% (95% CI: 60.6–89.3) MRD monitoring after transplant predicts outcome | NCT03931291 | Mishra et al., JCO, 2022 [92] |
| Azacitidine + DLI | II | Completed | AML, MDS | 2y-PFS 68.3% (95% CI: 58.3–80.1) 2y-OS 76% (95% CI:52–90) | NCT01541280 | Guillaume, et al., Transpl and Cel Ther, 2021 [93] |
| Panobinostat | I/II | Phase III Terminated | AML, MDS | Phase II results: CIR 20% (95% 7–33) 2y-RFS 75% (63–90). 2yr-OS 81% (95% CI: 69–95) | NCT04326764 [94] | Bug et al., Leukemia, 2017 [95] |
| Agent/Drug Combination | Trial PHASE | Target/MOA | Population | Results | Status | Clinical Trial ID |
|---|---|---|---|---|---|---|
| Venetoclax + AZA [102] | III | BCL-2 inhibitor + DNMTi | HR-MDS | Did not meet primary endpoint of OS; HR 0.908, p = 0.3772 | Active, not recruiting | NCT04401748 |
| APR-246 (Eprenetapopt) + AZA [103] | III | TP53 | TP53-mutant HR-MDS | APR-246 + AZA arm: CR 34.6% AZA arm: CR 22.4% | Completed | NCT03745716 |
| Rigosertib vs. BSC [104] | III | Microtubule-destabilizing agent | HR-MDS after failure of HMAs | Rigosertib: OS 8.2 months (95% CI: 6.1–10.1) Best supportive care: OS 5.9 months (95% CI: 4.1–9.3) (HR 0.87, 95% CI: 0.67–1.14; p = 0.33) | Completed | NCT02562443 |
| AZA and Cedazuridine (ASTX030) [105] | Multi-phase | Oral DNMTi | MDS, CMML, AML | NA | Active, recruiting | NCT04256317 |
| AZA + pevonedistat [106] | III | NEDD8-activating enzyme | HR-MDS, CMML or AML with 20–30% blasts | Pevonedistat + AZA arm: median EFS 17.7 months AZA arm: median EFS 15.7 months (HR 0.968; 95% CI: 0.757–1.238; p = 0.557) in the HR-MDS cohort | Completed | NCT03268954 |
| AZA + magrolimab [107] | III | Anti-CD47 monoclonal antibody + DNMTi | HR-MDS | Magrolimab + AZA arm: CR 21.3% AZA arm: CR 23.6% | Terminated | NCT04313881 |
| AZA + sabatolimab [108] | III | TIM-3 inhibitor + DNMTi | MDS, CMML-2 | Median PFS: Sabatolimab + AZA arm: 11.1 months AZA arm: 8.5 months (p = 0.102) CR rate: Sabatolimab + AZA arm: 21.5% AZA arm: 17.7% (p = 0.769) | Terminated | NCT04266301 |
| AZA + tamibarotene [109] | III | (RARα) agonist + DNMTi | HR-MDS | Tamibarotene + AZA arm: CR 23.8% AZA arm: CR 18.8% (p = 0.2084) | Terminated | NCT04797780 |
| AZA + Lisaftoclax [110] | III | BCL-2 inhibitor + DNMTi | HR-MDS | NA | Recruiting | NCT06641414 |
| AZA + AK117 [111] | I/II | Anti-CD47 monoclonal antibody + DNMTi | HR-MDS | NA | Active, not recruiting | NCT04900350 |
| AZA +/− BGB-11417 [112] | I/II | BCL-2 inhibitor + DNMTi | MDS, AML | NA | Recruiting | NCT04771130 |
| Ivosidenib [113] | I | IDH1 inhibitor +/− DNMTi | Hematologic malignancies with IDH1 mutations | ORR of 83.3% and CR rate of 38.9% in 18 patients | Active, recruiting | NCT02074839 |
| Enasidenib [114] | II | IDH2 inhibitor +/− DNMTi | R/R MDS, HR-MDS, LR-MDS resistant to ESA | Enasidenib monotherapy (cohort A):best OR 42.9% after 3–6 cycles | Active, not recruiting | NCT03744390 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stempel, J.M.; Kewan, T.; Zeidan, A.M. Advances and Challenges in the Management of Myelodysplastic Syndromes. Cancers 2025, 17, 2469. https://doi.org/10.3390/cancers17152469
Stempel JM, Kewan T, Zeidan AM. Advances and Challenges in the Management of Myelodysplastic Syndromes. Cancers. 2025; 17(15):2469. https://doi.org/10.3390/cancers17152469
Chicago/Turabian StyleStempel, Jessica M., Tariq Kewan, and Amer M. Zeidan. 2025. "Advances and Challenges in the Management of Myelodysplastic Syndromes" Cancers 17, no. 15: 2469. https://doi.org/10.3390/cancers17152469
APA StyleStempel, J. M., Kewan, T., & Zeidan, A. M. (2025). Advances and Challenges in the Management of Myelodysplastic Syndromes. Cancers, 17(15), 2469. https://doi.org/10.3390/cancers17152469







