Effect of a Probiotic Combination on Clinical and Microbiological Oral Parameters in Head and Neck Cancer Patients: A Randomised Clinical Trial
Simple Summary
Abstract
1. Introduction
- Can probiotic supplementation enhance salivary gland function in patients after radiotherapy?
- Does it reduce overall bacterial burden and the prevalence of periodontopathogens in the oral cavity?
- Is it a safe and well-tolerated adjuvant option in the context of supportive cancer care?
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Inclusion Criteria
- Adults aged over 18 years.
- Histological diagnosis of head and neck cancer.
- Radiotherapy completed within the previous year.
- Presence of at least six teeth in the oral cavity.
- Provided written informed consent to participate in the study.
2.4. Exclusion Criteria
- Declined to participate.
- Presence of osteonecrosis.
- Inability to take oral medication.
- Known allergy to probiotics.
- Active antibiotic treatment or use of antibiotics within the previous 30 days.
2.5. Outcomes
2.5.1. Primary Outcomes
- Change in unstimulated and stimulated salivary flow (mL/min).
- Change in total and specific bacterial loads (P. gingivalis, F. nucleatum, A. actinomycetemcomitans, C. rectus, and T. forsythia) assessed by culture and qPCR.
2.5.2. Secondary Outcomes
- Change in salivary pH.
- Adherence to treatment and adverse events, evaluated through a post-intervention survey.
2.6. Sample Size Calculation
2.7. Randomisation and Blinding
2.8. Intervention
2.9. Clinical Procedures
2.9.1. Sialometry (Unstimulated and Stimulated Salivary Flow)
- Unstimulated Salivary Flow:
- Stimulated Salivary Flow:
2.9.2. Salivary pH Measurement
2.9.3. Sampling for Microbiological Analysis
2.10. Oral Microbiological Evaluation
2.10.1. Standard Bacterial Culture
2.10.2. Real-Time Quantitative PCR (qPCR)
2.11. Statistical Analysis
- All statistical analyses were performed using Python (v3.9) with SciPy and Pandas libraries. The Shapiro–Wilk test was used to assess the normality of continuous variables. As most data were non-normally distributed, non-parametric tests were applied throughout.
- For paired data (e.g., baseline vs. post-intervention within the same group), the Wilcoxon signed-rank test was used. Between-group comparisons (probiotic vs. placebo) were evaluated using the Mann–Whitney U test. Associations between salivary and microbiological variables were explored using Spearman’s rank correlation coefficient.
- All tests were two-tailed, and a significance level of p < 0.05 was adopted. Where appropriate, results are presented with 95% confidence intervals. Statistical outputs were interpreted in the context of both clinical and biological relevance.
3. Results
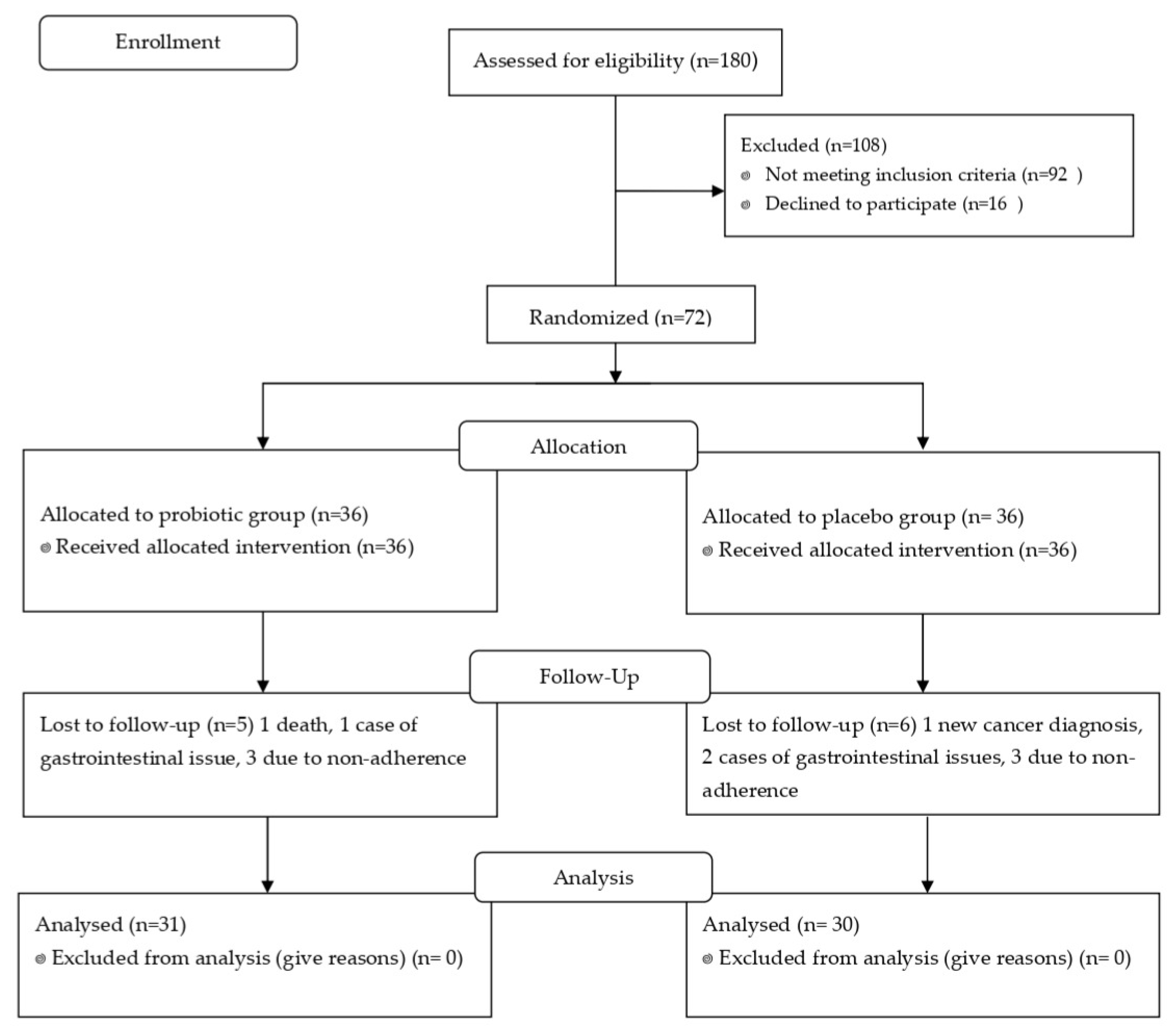
3.1. Participation and Baseline Characteristics
3.2. Salivary Parameters
3.3. Microbiological Analysis
3.4. Bacterial Detection Frequency
3.5. Correlation Between Microbiological Methods
3.6. Tolerability and Adverse Events
4. Discussion
4.1. Clinical Implications
4.2. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HNC | Head and Neck Cancer |
| IMRT | Intensity-Modulated Radiotherapy |
| CFU | Colony Forming Unit |
| GCF | Gingival Crevicular Fluid |
| qPCR | Quantitative Polymerase Chain Reaction |
| RTF | Reduced Transport Fluid |
| FAA | Fastidious Anaerobic Agar |
| BHI | Brain Heart Infusion |
| DNA | Deoxyribonucleic Acid |
| PCR | Polymerase Chain Reaction |
References
- Chow, L.Q.M. Head and Neck Cancer. N. Engl. J. Med. 2020, 382, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Barsouk, A.; Aluru, J.S.; Rawla, P.; Saginala, K.; Barsouk, A. Epidemiology, Risk Factors, and Prevention of Head and Neck Squamous Cell Carcinoma. Med. Sci. 2023, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and Neck Squamous Cell Carcinoma. Nat. Rev. Dis. Primer 2020, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zeng, N.; Yang, J.; He, J.; Zhu, F.; Liao, W.; Xiong, M.; Li, Y. Advancements of Radiotherapy for Recurrent Head and Neck Cancer in Modern Era. Radiat. Oncol. 2023, 18, 166. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.B.; Barasch, A. Oral and Dental Health in Head and Neck Cancer Patients. Cancer Treat. Res. 2018, 174, 43–57. [Google Scholar] [PubMed]
- Gharzai, L.A.; Mierzwa, M.L.; Peipert, J.D.; Kirtane, K.; Casper, K.; Yadav, P.; Rothrock, N.; Cella, D.; Shaunfield, S. Monitoring Adverse Effects of Radiation Therapy in Patients With Head and Neck Cancer: The FACT-HN-RAD Patient-Reported Outcome Measure. JAMA Otolaryngol. Neck Surg. 2023, 140, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.; Sonis, S.T. Pharmacotherapy for the Management of Cancer Regimen-Related Oral Mucositis. Expert Opin. Pharmacother. 2016, 17, 1801–1807. [Google Scholar] [CrossRef] [PubMed]
- Warwas, B.; Cremers, F.; Gerull, K.; Leichtle, A.; Bruchhage, K.L.; Hakim, S.G.; Schild, S.E.; Rades, D. Risk Factors for Xerostomia Following Radiotherapy of Head-and-Neck Cancers. Anticancer. Res. 2022, 42, 2657–2663. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Cheng, L.; You, Y.; Tang, C.; Ren, B.; Li, Y.; Xu, X.; Zhou, X. Oral Microbiota in Human Systematic Diseases. Int. J. Oral. Sci. 2022, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Gaetti-Jardim, E.; Jardim, E.C.G.; Schweitzer, C.M.; da Silva, J.C.L.; Oliveira, M.M.; Masocatto, D.C.; Dos Santos, C.M. Supragingival and Subgingival Microbiota from Patients with Poor Oral Hygiene Submitted to Radiotherapy for Head and Neck Cancer Treatment. Arch. Oral Biol. 2018, 90, 45–52. [Google Scholar] [CrossRef] [PubMed]
- de Freitas Neiva Lessa, A.; da Silva Amâncio, A.M.T.; de Oliveira, A.C.R.; de Sousa, S.F.; Caldeira, P.C.; De Aguiar, M.C.F.; Bispo, P.J.M. Assessing the Oral Microbiome of Head and Neck Cancer Patients before and during Radiotherapy. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2024, 32, 752. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-H.; Kim, S.; Kim, H.-J.; Jeong, H.-O.; Lee, J.; Jang, J.; Joo, J.-Y.; Shin, Y.; Kang, J.; Park, A.K.; et al. Prediction of Chronic Periodontitis Severity Using Machine Learning Models Based On Salivary Bacterial Copy Number. Front. Cell. Infect. Microbiol. 2020, 10, 571515. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Kodangala, S. Periodontal Pathogens as Potential Risk Factors for Systemic Diseases: An Overview. Acta Microbiol. Immunol. Hung. 2025, 72, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bui, F.Q.; Almeida-da-Silva, C.L.C.; Huynh, B.; Trinh, A.; Liu, J.; Woodward, J.; Asadi, H.; Ojcius, D.M. Association between Periodontal Pathogens and Systemic Disease. Biomed. J. 2019, 42, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert Consensus Document. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Sniffen, J.C.; McFarland, L.V.; Evans, C.T.; Goldstein, E.J.C. Choosing an Appropriate Probiotic Product for Your Patient: An Evidence-Based Practical Guide. PLoS ONE 2018, 13, e0209205. [Google Scholar] [CrossRef] [PubMed]
- Chugh, P.; Dutt, R.; Sharma, A.; Bhagat, N.; Dhar, M.S. A Critical Appraisal of the Effects of Probiotics on Oral Health. J. Funct. Foods 2020, 70, 103985. [Google Scholar] [CrossRef]
- Ishikawa, K.H.; Mita, D.; Kawamoto, D.; Nicoli, J.R.; Albuquerque-Souza, E.; Lorenzetti Simionato, M.R.; Mayer, M.P.A. Probiotics Alter Biofilm Formation and the Transcription of Porphyromonas gingivalis Virulence-Associated Genes. J. Oral. Microbiol. 2020, 12, 1805553. [Google Scholar] [CrossRef] [PubMed]
- Jaffar, N.; Ishikawa, Y.; Mizuno, K.; Okinaga, T.; Maeda, T. Mature Biofilm Degradation by Potential Probiotics: Aggregatibacter actinomycetemcomitans versus Lactobacillus Spp. PLoS ONE 2016, 11, e0159466. PLoS ONE 2016, 11, e0159466. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Sun, X.; Cao, S.; Zhao, C.; Wang, Y.; Wang, X. Heat-Killed Lactobacillus Acidophilus Mediates Fusobacterium nucleatum Induced pro-Inflammatory Responses in Epithelial Cells. FEMS Microbiol. Lett. 2021, 368, fnaa160. [Google Scholar] [CrossRef] [PubMed]
- Şahin, T.; Akca, G.; Özmeriç, N. The Role of Probiotics for Preventing Dysbiosis in Periodontal Disease: A Randomized Controlled Trial. Turk. J. Med. Sci. 2024, 54, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wang, D.; Zhou, S.; Huo, C.; Chen, H.; Li, F.; Ding, M.; Li, H.; Zhao, H.; He, J.; et al. Probiotics Combined with Prebiotics Alleviated Seasonal Allergic Rhinitis by Altering the Composition and Metabolic Function of Intestinal Microbiota: A Prospective, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Front. Immunol. 2024, 15, 1439830. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Feng, H.; Mao, X.-L.; Deng, Y.-J.; Wang, X.-B.; Zhang, Q.; Guo, Y.; Xiao, S.-M. The Effects of Probiotics Supplementation on Glycaemic Control among Adults with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomised Clinical Trials. J. Transl. Med. 2023, 21, 442. [Google Scholar] [CrossRef] [PubMed]
- Buhaș, M.C.; Candrea, R.; Gavrilaș, L.I.; Miere, D.; Tătaru, A.; Boca, A.; Cătinean, A. Transforming Psoriasis Care: Probiotics and Prebiotics as Novel Therapeutic Approaches. Int. J. Mol. Sci. 2023, 24, 11225. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Rath, G.K.; Chaudhary, S.P.; Thakar, A.; Mohanti, B.K.; Bahadur, S. Lactobacillus brevis CD2 Lozenges Reduce Radiation- and Chemotherapy-Induced Mucositis in Patients with Head and Neck Cancer: A Randomized Double-Blind Placebo-Controlled Study. Eur. J. Cancer Oxf. Engl. 1990 2012, 48, 875–881. [Google Scholar] [CrossRef] [PubMed]
- López-López, J.; Reuss, J.M.; Vinuesa-Aumedes, T.; Egido-Moreno, S.; Roselló-Llabres, X.; Pereira-Riveros, T.; Reuss, D.; Alonso-Gamo, L.; Rodríguez-Vilaboa, B. Rapid Reduction of Pro-Inflammatory Cytokines with an Oral Topical Composition Comprising Olive Oil, Trimethylglycine and Xylitol: A Randomized Double-Blind Controlled Trial. Int. J. Mol. Sci. 2025, 26, 4920. [Google Scholar] [CrossRef] [PubMed]
- Rubio, A.; Pereira, T.; Boj, J.R.; Vinuesa, T. Microbiological Analysis of Primary Molars Restored with Stainless Steel Crowns Compared to Healthy Molars. Microorganisms 2025, 13, 1294. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C.-A.O.; Asarkar, A.A.; Entezami, P.; Corry, J.; Strojan, P.; Poorten, V.V.; Makitie, A.; Eisbruch, A.; Robbins, K.T.; Smee, R.; et al. Current Management of Xerostomia in Head and Neck Cancer Patients. Am. J. Otolaryngol. 2023, 44, 103867. [Google Scholar] [CrossRef] [PubMed]
- Owosho, A.A.; Thor, M.; Oh, J.H.; Riaz, N.; Tsai, C.J.; Rosenberg, H.; Varthis, S.; Yom, S.H.K.; Huryn, J.M.; Lee, N.Y.; et al. The Role of Parotid Gland Irradiation in the Development of Severe Hyposalivation (Xerostomia) after Intensity-Modulated Radiation Therapy for Head and Neck Cancer: Temporal Patterns, Risk Factors, and Testing the QUANTEC Guidelines. J. Cranio-Maxillofac. Surg. 2017, 45, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Mojdami, Z.D.; Barbour, A.; Oveisi, M.; Sun, C.; Fine, N.; Saha, S.; Marks, C.; Elebyary, O.; Watson, E.; Tenenbaum, H.; et al. The Effect of Intensity-Modulated Radiotherapy to the Head and Neck Region on the Oral Innate Immune Response and Oral Microbiome: A Prospective Cohort Study of Head and Neck Tumour Patients. Int. J. Mol. Sci. 2022, 23, 9594. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.T.; Huang, X.; Ellepola, K.; Liao, S.; Li, Y. Lactobacilli and Human Dental Caries: More than Mechanical Retention. Microbiol. Read. Engl. 2022, 168, 001196. [Google Scholar] [CrossRef] [PubMed]
- Henne, K.; Rheinberg, A.; Melzer-Krick, B.; Conrads, G. Aciduric Microbial Taxa Including Scardovia wiggsiae and Bifidobacterium spp. in Caries and Caries Free Subjects. Anaerobe 2015, 35, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Dias, G.S.; Vieira, A.C.; Baioni E Silva, G.; Simões, N.F.; Milessi, T.S.; Saraiva, L.S.; Xavier, M.D.C.A.; Longati, A.A.; Rodrigues, M.F.A.; Fernandes, S.; et al. Fructooligosaccharides: A Comprehensive Review on Their Microbial Source, Functional Benefits, Production Technology, and Market Prospects. Processes 2025, 13, 1252. [Google Scholar] [CrossRef]
- Jiang, Q.; Stamatova, I.; Kainulainen, V.; Korpela, R.; Meurman, J.H. Interactions between Lactobacillus rhamnosus GG and Oral Microorganisms in an in Vitro Biofilm Model. BMC Microbiol. 2016, 16, 149. [Google Scholar] [CrossRef] [PubMed]
- Sanghvi, U.; Chhabra, T.; Sethuraman, R. Effect of Probiotics on the Amount and pH of Saliva in Edentulous Patients: A Prospective Study. J. Indian Prosthodont. Soc. 2018, 18, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Akbari, E.; Epstein, J.B.; Samim, F. Unveiling the Hidden Links: Periodontal Disease, Fusobacterium nucleatum, and Cancers. Curr. Oncol. Rep. 2024, 26, 1388–1397. [Google Scholar] [CrossRef] [PubMed]
- McIlvanna, E.; Linden, G.J.; Craig, S.G.; Lundy, F.T.; James, J.A. Fusobacterium nucleatum and Oral Cancer: A Critical Review. BMC Cancer 2021, 21, 1212. [Google Scholar] [CrossRef] [PubMed]
- Little, A.; Tangney, M.; Tunney, M.M.; Buckley, N.E. Fusobacterium nucleatum: A Novel Immune Modulator in Breast Cancer? Expert Rev. Mol. Med. 2023, 25, e15. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wei, Z.; Wang, Z.; Xu, F.; Yang, J.; Lin, B.; Chen, Y.; Wenren, H.; Wu, L.; Guo, X.; et al. Fusobacterium nucleatum Induces Oxaliplatin Resistance by Inhibiting Ferroptosis through E-Cadherin/β-Catenin/GPX4 Axis in Colorectal Cancer. Free Radic. Biol. Med. 2024, 220, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Guo, F.; Yu, Y.; Sun, T.; Ma, D.; Han, J.; Qian, Y.; Kryczek, I.; Sun, D.; Nagarsheth, N.; et al. Fusobacterium nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy. Cell 2017, 170, 548–563. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Wang, K.; Jia, Y.-P.; Zhu, P.; Fang, Y.; Zhang, Z.-J.; Mao, X.-H.; Li, Q.; Zeng, D.-Z. Fusobacterium nucleatum-Induced Impairment of Autophagic Flux Enhances the Expression of Proinflammatory Cytokines via ROS in Caco-2 Cells. PLoS ONE 2016, 11, e0165701. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Fang, J.-Y. Fusobacterium nucleatum, a Key Pathogenic Factor and Microbial Biomarker for Colorectal Cancer. Trends Microbiol. 2023, 31, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Shen, J.; Xu, Y. Fusobacterium nucleatum and Colorectal Cancer. Infect. Drug Resist. 2022, 15, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Vesty, A.; Gear, K.; Boutell, S.; Taylor, M.W.; Douglas, R.G.; Biswas, K. Randomised, Double-Blind, Placebo-Controlled Trial of Oral Probiotic Streptococcus salivarius M18 on Head and Neck Cancer Patients Post-Radiotherapy: A Pilot Study. Sci. Rep. 2020, 10, 13201. [Google Scholar] [CrossRef] [PubMed]
- Alanzi, A.; Honkala, S.; Honkala, E.; Varghese, A.; Tolvanen, M.; Söderling, E. Effect of Lactobacillus rhamnosus and Bifidobacterium lactis on Gingival Health, Dental Plaque, and Periodontopathogens in Adolescents: A Randomised Placebo-Controlled Clinical Trial. Benef. Microbes 2018, 9, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Vernon, J.J. Modulation of the Human Microbiome: Probiotics, Prebiotics, and Microbial Transplants. Adv. Exp. Med. Biol. 2025, 1472, 277–294. [Google Scholar] [PubMed]
- de Carvalho, K.L.K.; Porto, A.N.; Aranha, A.M.F.; Freitas, G.P.; Volpato, L.E.R. Evaluation of A. actinomycetemcomitans and P. gingivalis from the Mouth of Patients Irradiated in the Head. and Neck Region: A Cross-Sectional Study. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2024, 32, 770. [Google Scholar] [CrossRef] [PubMed]
- Ermongkonchai, T.; Khor, R.; Wada, M.; Lau, E.; Xing, D.T.; Ng, S.P. A Review of Diffusion-Weighted Magnetic Resonance Imaging in Head and Neck Cancer Patients for Treatment Evaluation and Prediction of Radiation-Induced Xerostomia. Radiat. Oncol. 2023, 18, 20. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, D.; Laszlo, M.; Provisor, A.; Yu, A. Nutrition Management for the Head and Neck Cancer Patient. Cancer Treat. Res. 2018, 174, 187–208. [Google Scholar] [PubMed]
- Mohd Fuad, A.S.; Amran, N.A.; Nasruddin, N.S.; Burhanudin, N.A.; Dashper, S.; Arzmi, M.H. The Mechanisms of Probiotics, Prebiotics, Synbiotics, and Postbiotics in Oral Cancer Management. Probiotics Antimicrob. Proteins 2023, 15, 1298–1311. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Peng, S.; Li, M.; Ao, X.; Liu, Z. The Efficacy and Safety of Probiotics for Allergic Rhinitis: A Systematic Review and Meta-Analysis. Front. Immunol. 2022, 13, 848279. [Google Scholar] [CrossRef] [PubMed]
- Moludi, J.; Fathollahi, P.; Khedmatgozar, H.; Pourteymour Fard Tabrizi, F.; Ghareaghaj Zare, A.; Razmi, H.; Amirpour, M. Probiotics Supplementation Improves Quality of Life, Clinical Symptoms, and Inflammatory Status in Patients With Psoriasis. J. Drugs Dermatol. JDD 2022, 21, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Wu, J.; Lam, Y.Y.; Kwan, H.Y.; Bian, Z.-X.; Wong, H.L.X. Gut-Microbial Metabolites, Probiotics and Their Roles in Type 2 Diabetes. Int. J. Mol. Sci. 2021, 22, 12846. [Google Scholar] [CrossRef] [PubMed]
- Doppalapudi, R.; Vundavalli, S.; Prabhat, M.P. Effect of Probiotic Bacteria on Oral Candida in Head- and Neck-Radiotherapy Patients: A Randomized Clinical Trial. J. Cancer Res. Ther. 2020, 16, 470–477. [Google Scholar] [CrossRef] [PubMed]
| Variable | Placebo (n = 30) | Probiotic (n = 31) | Total (n = 61) |
|---|---|---|---|
| Age, mean (SD), years | 57.9 (14.6) | 60.1 (12.0) | 59.0 (13.2) |
| Sex, n (%) | |||
| - Male | 19 (63.3%) | 19 (61.3%) | 38 (62.3%) |
| - Female | 11 (36.7%) | 12 (38.7%) | 23 (37.7%) |
| Tumour location, n (%): | |||
| - Hypopharynx | 0 (0.0%) | 1 (3.2%) | 1 (1.6%) |
| - Oral cavity | 10 (33.3%) | 6 (19.4%) | 16 (26.2%) |
| - Oropharynx | 10 (33.3%) | 14 (45.2%) | 24 (39.3%) |
| - Larynx | 7 (23.3%) | 2 (6.5%) | 9 (14.8%) |
| - Nasopharynx | 2 (6.7%) | 6 (19.4%), | 8(13.1%) |
| - Salivary gland | 0 (0.0%) | 1 (3.2%) | 1 (1.6%) |
| - Unknown primary (neck) | 0 (0.0%) | 1 (3.2%) | 1 (1.6%) |
| Adjuvant radiotherapy, n (%): | 100% | 100% | 100% |
| RT dose, mean (SD), Gy | 65.8 (5.4) | 67.2 (3.9) | 66.5 (4.7) |
| RT sessions, median (range) | 33 (25–35) | 33 (30–35) | 33 (25–35) |
| Time since RT, mean (SD), months | 2.77 ± 1.99 | 3.52 ± 2.53 | |
| Chemotherapy, n (%): | |||
| - Received QT | 19 (63.3%) | 16 (51.6%) | 35 (36.5%) |
| QT regimen: | |||
| - Cisplatin | 16 (53.3%) | 7 (22.6%) | 23 (24.0%) |
| - Cetuximab | 0 (0.0%) | 1 (3.2%) | 1 (1.0%) |
| - Other | 3 (10.0%) | 8 (25.8%) | 11 (11.5%) |
| Surgery, n (%) | 14 (46.7%) | 12 (38.7%) | 26 (27.1%) |
| Comorbidities, n (%) | |||
| - Diabetes mellitus | 6 (20.0%) | 7 (22.6%) | 13 (13.5%) |
| - Hypertension | 11 (36.7%) | 12 (38.7%) | 23 (24.0%) |
| - Cardiovascular disease | 3 (10.0%) | 1 (3.2%) | 4 (4.2%) |
| - Pulmonary disease | 1 (3.3%) | 2 (6.5%) | 3 (3.1%) |
| - Thyroid disease | 1 (3.3%) | 2 (6.5%) | 3 (3.1%) |
| Smoking status, n (%): | |||
| - Never smoker | 14 (46.7%) | 14 (45.2%) | 28 (29.2%) |
| - Former smoker | 13 (43.3%) | 16 (51.6%) | 29 (30.2%) |
| - Current smoker | 3 (10.0%) | 0 (0.0%) | 3 (3.1%) |
| Alcohol consumption, n (%): | |||
| - None | 22 (73.3%) | 23 (74.2%) | 45 (46.9%) |
| - Former consumer | 1 (3.3%) | 1 (3.2%) | 2 (2.1%) |
| - Occasional | 2 (6.7%) | 6 (19.4%) | 8 (8.3%) |
| - Chronic use | 5 (16.7%) | 1 (3.2%) | 6 (6.2%) |
| Parameter | Group | Test Statistic | p-Value |
|---|---|---|---|
| Unstimulated saliva | Probiotic | 0.0 | 0.0253 |
| Placebo | 4.5 | 0.0339 | |
| Stimulated saliva | Probiotic | 0.0 | 0.0016 |
| Placebo | 12.0 | 0.7055 | |
| pH | Probiotic | 13.0 | 0.0209 |
| Placebo | 20.0 | 0.4054 |
| Method | Bacterial Target | Group | Statistic | p-Value |
|---|---|---|---|---|
| Culture (log CFU/mL) | Total cultivable bacteria | Probiotic | 147.5 | 0.0502 |
| P. gingivalis | Probiotic | 43.0 | 0.1961 | |
| F. nucleatum | Probiotic | 99.0 | 0.0026 | |
| C. rectus | Probiotic | 87.0 | 0.5016 | |
| Total cultivable bacteria | Placebo | 172.0 | 0.2206 | |
| P. gingivalis | Placebo | 7.0 | 0.2367 | |
| F. nucleatum | Placebo | 184.0 | 0.3284 | |
| C. rectus | Placebo | 75.5 | 0.6627 | |
| q-PCR (log10 copies/mL) | Total bacterial load | Probiotic | 131.0 | 0.0209 |
| P. gingivalis | Probiotic | 37.0 | 0.1089 | |
| F. nucleatum | Probiotic | 115.0 | 0.0080 | |
| C. rectus | Probiotic | 109.0 | 0.8213 | |
| T. forsythia | Probiotic | 60.0 | 0.2668 | |
| Total bacterial load | Placebo | 201.5 | 0.5425 | |
| P. gingivalis | Placebo | 7.0 | 0.2367 | |
| F. nucleatum | Placebo | 194.0 | 0.4399 | |
| C. rectus | Placebo | 95.0 | 0.4761 | |
| T. forsythia | Placebo | 63.0 | 0.7960 |
| Bacterial Target | Group | Spearman r | p-Value | Interpretation |
|---|---|---|---|---|
| Total bacteria | Probiotic | 0.886 | 3.32 × 10−11 | Strong positive correlation |
| Placebo | 0.765 | 8.69 × 10−7 | Strong positive correlation | |
| P. gingivalis | Probiotic | 0.997 | 2.20 × 10−34 | Nearly perfect correlation |
| Placebo | 0.995 | 9.65 × 10−30 | Nearly perfect correlation | |
| F. nucleatum | Probiotic | 0.923 | 1.60 × 10−13 | Strong positive correlation |
| Placebo | 0.799 | 1.18 × 10−7 | Strong positive correlation | |
| C. rectus | Probiotic | 0.870 | 2.06 × 10−10 | Strong positive correlation |
| Placebo | 0.955 | 2.53 × 10−16 | Strong positive correlation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira Riveros, T.; Jané Salas, E.; Lozano Borbalas, A.; Aguilera, F.R.; Vinuesa Aumedes, T. Effect of a Probiotic Combination on Clinical and Microbiological Oral Parameters in Head and Neck Cancer Patients: A Randomised Clinical Trial. Cancers 2025, 17, 2459. https://doi.org/10.3390/cancers17152459
Pereira Riveros T, Jané Salas E, Lozano Borbalas A, Aguilera FR, Vinuesa Aumedes T. Effect of a Probiotic Combination on Clinical and Microbiological Oral Parameters in Head and Neck Cancer Patients: A Randomised Clinical Trial. Cancers. 2025; 17(15):2459. https://doi.org/10.3390/cancers17152459
Chicago/Turabian StylePereira Riveros, Tanya, Enric Jané Salas, Alicia Lozano Borbalas, Felipe Rodrigo Aguilera, and Teresa Vinuesa Aumedes. 2025. "Effect of a Probiotic Combination on Clinical and Microbiological Oral Parameters in Head and Neck Cancer Patients: A Randomised Clinical Trial" Cancers 17, no. 15: 2459. https://doi.org/10.3390/cancers17152459
APA StylePereira Riveros, T., Jané Salas, E., Lozano Borbalas, A., Aguilera, F. R., & Vinuesa Aumedes, T. (2025). Effect of a Probiotic Combination on Clinical and Microbiological Oral Parameters in Head and Neck Cancer Patients: A Randomised Clinical Trial. Cancers, 17(15), 2459. https://doi.org/10.3390/cancers17152459






