Modified Proximal Gastrectomy and D2 Lymphadenectomy Is an Oncologically Sound Operation for Locally Advanced Proximal and GEJ Adenocarcinoma
Simple Summary
Abstract
1. Introduction
2. Methods
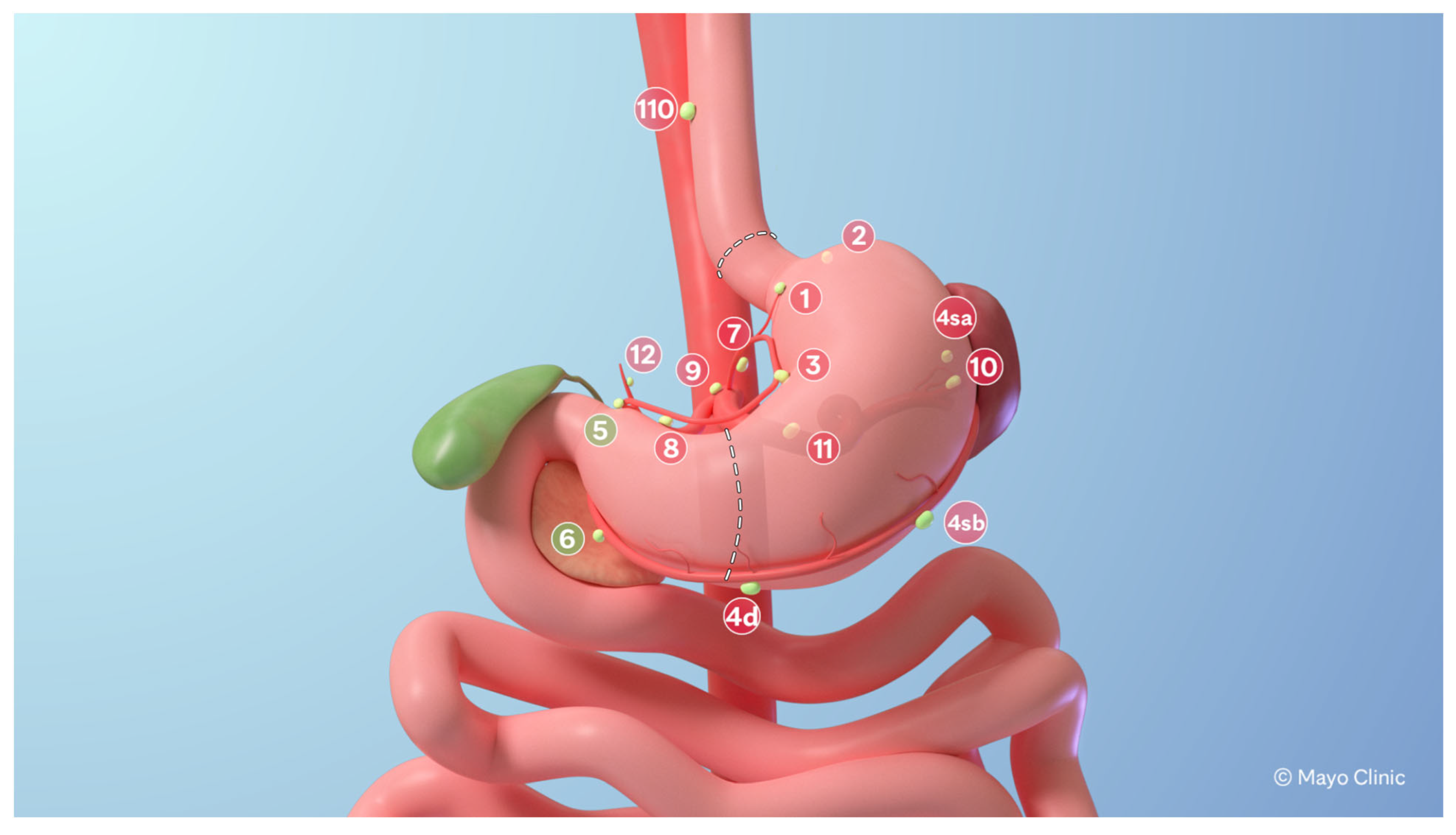
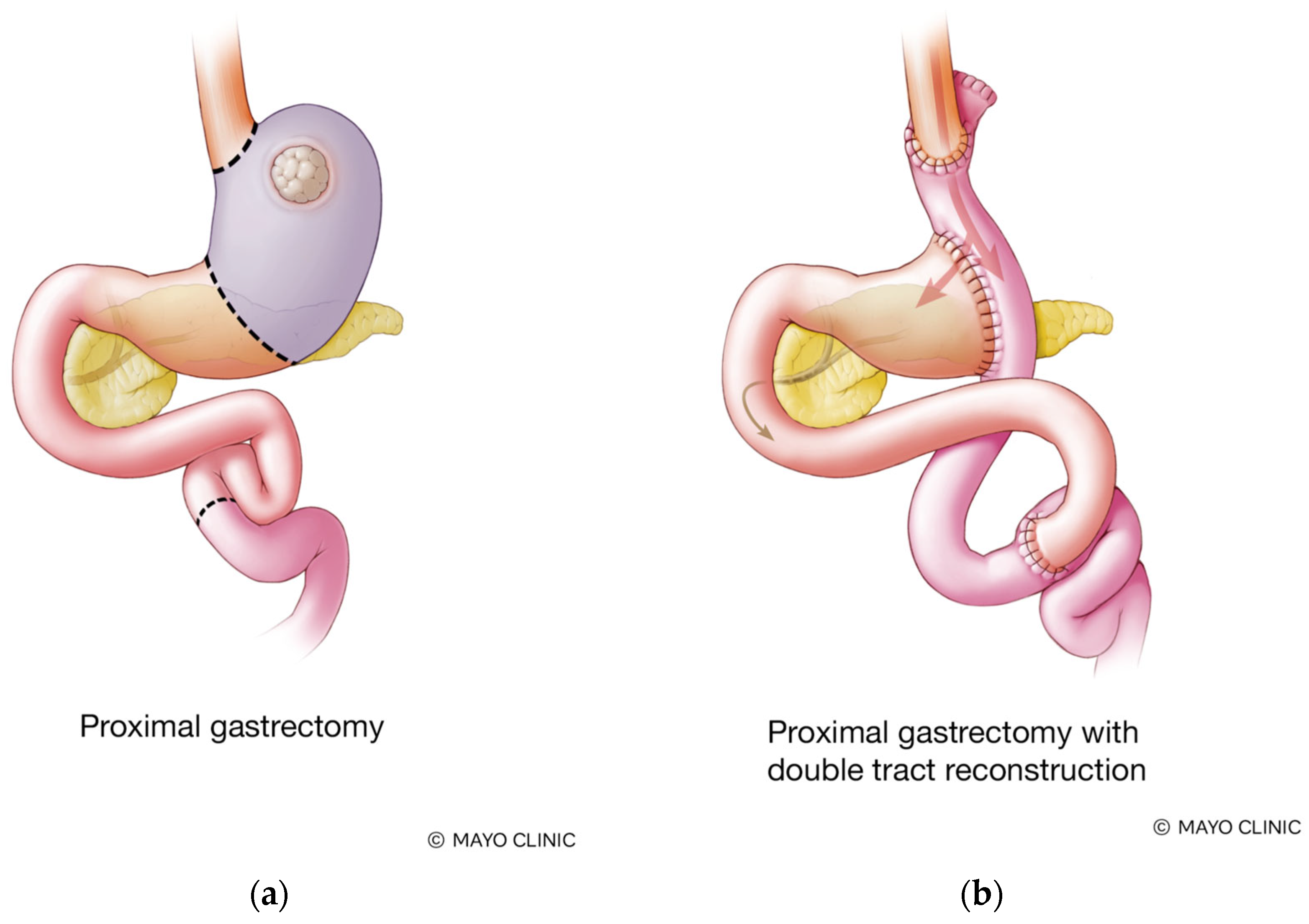
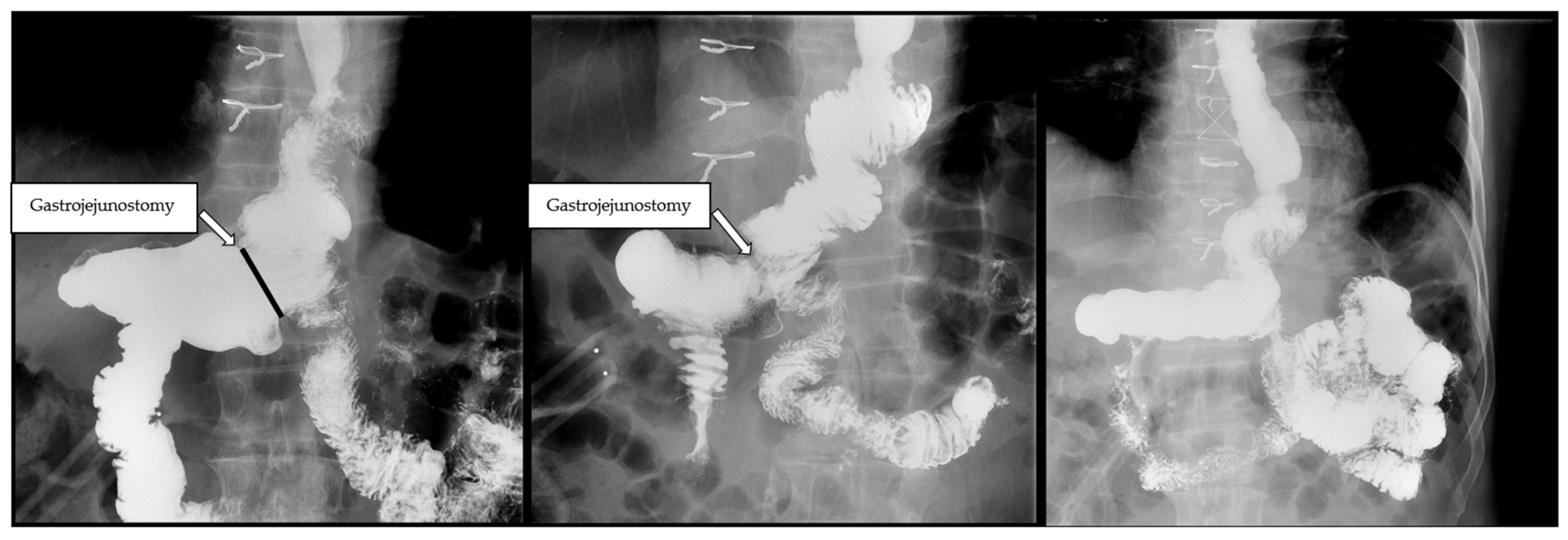
2.1. Surgical Technique
2.2. Data Analysis
3. Results
3.1. Baseline Characteristics
3.2. Perioperative Outcomes
3.3. Biliary Reflux
3.4. Oncologic Outcomes
3.5. Weight Loss
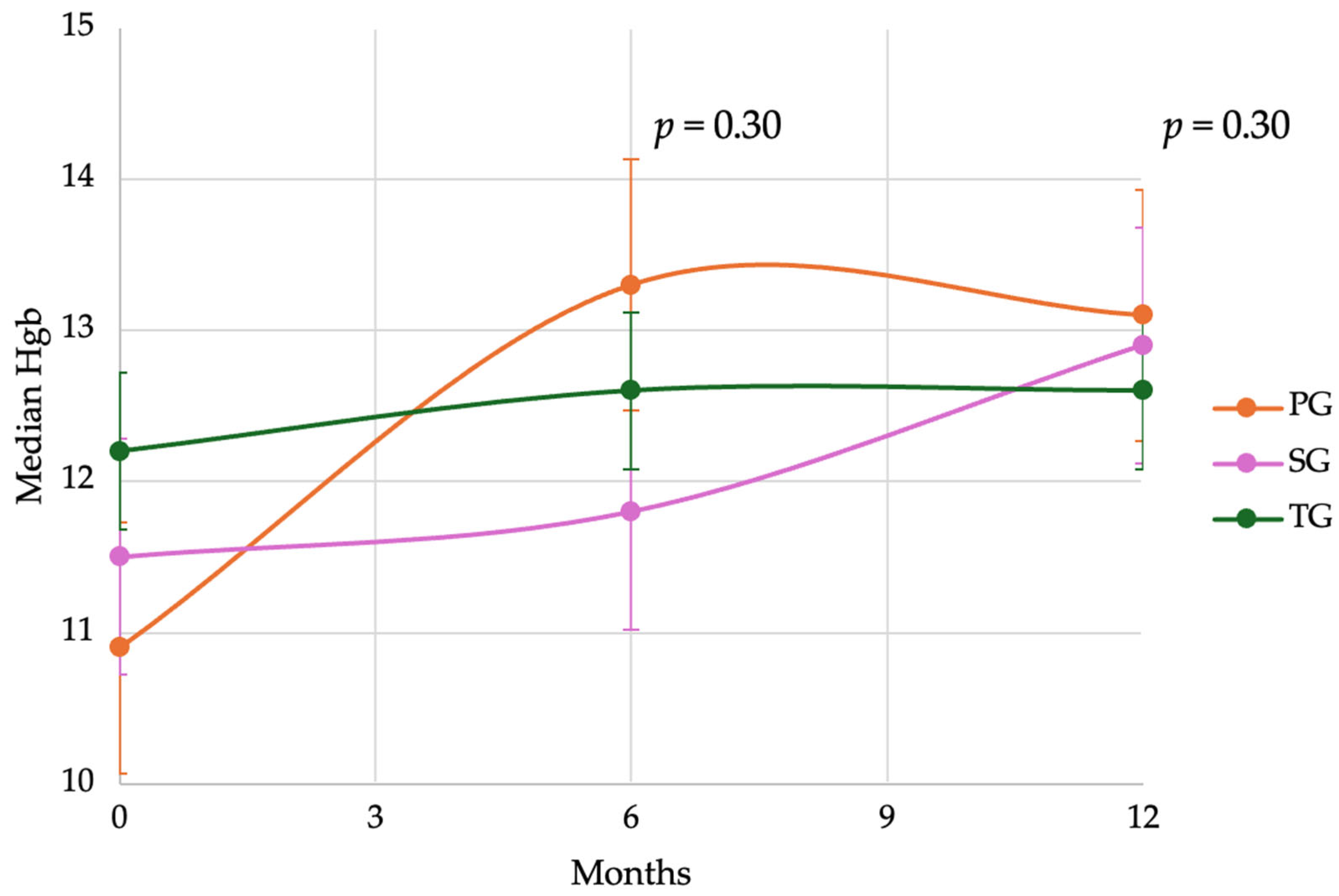
3.6. Anemia and Postoperative Hemoglobin
4. Discussion
4.1. Oncologic Safety of Partial Gastrectomy
4.2. Benefits of Preserving Distal Stomach in PG
4.3. Proposed Standardization of PG with DTR and D2 Lymphadenectomy for LAPGC
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, S.; Crane, T.E.; Goldberg, D.S. Trends in the Incidence of Upper Gastrointestinal Cancers Show Changing Dynamics. Clin. Gastroenterol. Hepatol. 2023, 21, 1365–1367 e1361. [Google Scholar] [CrossRef] [PubMed]
- Bozzetti, F.; Marubini, E.; Bonfanti, G.; Miceli, R.; Piano, C.; Gennari, L. Subtotal versus total gastrectomy for gastric cancer: Five-year survival rates in a multicenter randomized Italian trial. Italian Gastrointestinal Tumor Study Group. Ann. Surg. 1999, 230, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Berlth, F.; Kim, W.H.; Choi, J.H.; Park, S.H.; Kong, S.H.; Lee, H.J.; Yang, H.K. Prognostic Impact of Frozen Section Investigation and Extent of Proximal Safety Margin in Gastric Cancer Resection. Ann. Surg. 2020, 272, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Postlewait, L.M.; Squires, M.H., 3rd; Kooby, D.A.; Poultsides, G.A.; Weber, S.M.; Bloomston, M.; Fields, R.C.; Pawlik, T.M.; Votanopoulos, K.I.; Schmidt, C.R.; et al. The importance of the proximal resection margin distance for proximal gastric adenocarcinoma: A multi-institutional study of the US Gastric Cancer Collaborative. J. Surg. Oncol. 2015, 112, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Harrison, L.E.; Karpeh, M.S.; Brennan, M.F. Total gastrectomy is not necessary for proximal gastric cancer. Surgery 1998, 123, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Yoon, H.; Kong, S.H.; Kim, J.S.; Paeng, J.C.; Lee, H.J.; Lee, K.U.; Yang, H.K. Analysis of esophageal reflux after proximal gastrectomy measured by wireless ambulatory 24-hr esophageal pH monitoring and TC-99m diisopropyliminodiacetic acid (DISIDA) scan. J. Surg. Oncol. 2010, 101, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.H.; Park, D.J.; Kim, H.H.; Hyung, W.J.; Hur, H.; Yang, H.K.; Lee, H.J.; Kim, H.I.; Kong, S.H.; Kim, Y.W.; et al. Short-Term Outcomes of Laparoscopic Proximal Gastrectomy With Double-Tract Reconstruction Versus Laparoscopic Total Gastrectomy for Upper Early Gastric Cancer: A KLASS 05 Randomized Clinical Trial. J. Gastric Cancer 2022, 22, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Park, D.J.; Han, S.U.; Hyung, W.J.; Hwang, S.H.; Hur, H.; Yang, H.K.; Lee, H.J.; Kim, H.I.; Kong, S.H.; Kim, Y.W.; et al. Effect of Laparoscopic Proximal Gastrectomy With Double-Tract Reconstruction vs Total Gastrectomy on Hemoglobin Level and Vitamin B12 Supplementation in Upper-Third Early Gastric Cancer: A Randomized Clinical Trial. JAMA Netw. Open 2023, 6, e2256004. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Jiao, X.; Zhou, S.; Zhu, Q.; Yu, L.; Sun, Q.; Li, B.; Fu, H.; Huang, J.; Lang, W.; et al. Can proximal gastrectomy with double-tract reconstruction replace total gastrectomy? a meta-analysis of randomized controlled trials and propensity score-matched studies. BMC Gastroenterol. 2024, 24, 230. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Ling, R.; Ma, F.; Ren, H.; Zhou, H.; Wang, T.; Chen, Y.; Hu, S.; Zhao, D. Clinical outcomes of proximal gastrectomy versus total gastrectomy for locally advanced proximal gastric cancer: A propensity score matching analysis. Transl. Cancer Res. 2020, 9, 2769–2779. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.L.; Selby, L.V.; Chou, J.F.; Schattner, M.; Ilson, D.H.; Capanu, M.; Brennan, M.F.; Coit, D.G.; Strong, V.E. Patterns and Predictors of Weight Loss After Gastrectomy for Cancer. Ann. Surg. Oncol. 2016, 23, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Akad, F.; Filip, B.; Preda, C.; Zugun-Eloae, F.; Peiu, S.N.; Akad, N.; Crauciuc, D.V.; Vatavu, R.; Gavril, L.C.; Sufaru, R.F.; et al. Assessing Nutritional Status in Gastric Cancer Patients after Total versus Subtotal Gastrectomy: Cross-Sectional Study. Nutrients 2024, 16, 1485. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Kim, H.I.; Hyung, W.J.; Song, K.J.; Lee, J.H.; Kim, Y.M.; Noh, S.H. Vitamin B(12) deficiency after gastrectomy for gastric cancer: An analysis of clinical patterns and risk factors. Ann. Surg. 2013, 258, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Aikou, T.; Natsugoe, S.; Shimazu, H.; Nishi, M. Antrum preserving double tract method for reconstruction following proximal gastrectomy. Jpn. J. Surg. 1988, 18, 114–115. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Dong, J.; Huang, Q.; Zhang, W.; Tao, K. Comparison of three digestive tract reconstruction methods for the treatment of Siewert II and III adenocarcinoma of esophagogastric junction: A prospective, randomized controlled study. World J. Surg. Oncol. 2019, 17, 209. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, M.; Oki, E.; Ando, K.; Nakashima, Y.; Saeki, H.; Maehara, Y. Laparoscopic Proximal Gastrectomy Maintains Body Weight and Skeletal Muscle Better Than Total Gastrectomy. World J. Surg. 2018, 42, 3270–3276. [Google Scholar] [CrossRef] [PubMed]
- Kanda, M.; Tanaka, C.; Misawa, K.; Mochizuki, Y.; Watanabe, T.; Sueoka, S.; Ishiyama, A.; Yamada, T.; Oshima, T.; Hattori, M.; et al. A multi-institutional prospective observational study to compare postoperative quality of life of patients who undergo total or proximal gastrectomy for early gastric cancer (CCOG1602). World J. Surg. 2024, 48, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Japanese Gastric Cancer, A. Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer 2023, 26, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Squires, M.H., 3rd; Kooby, D.A.; Poultsides, G.A.; Pawlik, T.M.; Weber, S.M.; Schmidt, C.R.; Votanopoulos, K.I.; Fields, R.C.; Ejaz, A.; Acher, A.W.; et al. Is it time to abandon the 5-cm margin rule during resection of distal gastric adenocarcinoma? A multi-institution study of the U.S. Gastric Cancer Collaborative. Ann. Surg. Oncol. 2015, 22, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, Y.; Takeuchi, H.; Doki, Y.; Mine, S.; Terashima, M.; Yasuda, T.; Yoshida, K.; Daiko, H.; Sakuramoto, S.; Yoshikawa, T.; et al. Mapping of Lymph Node Metastasis From Esophagogastric Junction Tumors: A Prospective Nationwide Multicenter Study. Ann. Surg. 2021, 274, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Mansfield, P.; Badgwell, B.D.; Ikoma, N. Evidence-Based Surgical Approach to Gastroesophageal Junction Cancer: How We Do Robotic Transhiatal Lower Mediastinal Dissection and Esophagojejunostomy. Ann. Surg. Oncol. 2023, 30, 2956. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.M.; Giugliano, D.N.; Berger, A.C.; Pucci, M.J.; Palazzo, F. Surgical approaches to adenocarcinoma of the gastroesophageal junction: The Siewert II conundrum. Langenbecks Arch. Surg. 2017, 402, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Songun, I.; Putter, H.; Kranenbarg, E.M.; Sasako, M.; van de Velde, C.J. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010, 11, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Degiuli, M.; Reddavid, R.; Tomatis, M.; Ponti, A.; Morino, M.; Sasako, M.; Rebecchi, F.; Garino, M.; Vigano, L.; Scaglione, D.; et al. D2 dissection improves disease-specific survival in advanced gastric cancer patients: 15-year follow-up results of the Italian Gastric Cancer Study Group D1 versus D2 randomised controlled trial. Eur. J. Cancer 2021, 150, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Yura, M.; Yoshikawa, T.; Otsuki, S.; Yamagata, Y.; Morita, S.; Katai, H.; Nishida, T.; Yoshiaki, T. Oncological safety of proximal gastrectomy for T2/T3 proximal gastric cancer. Gastric Cancer 2019, 22, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Son, W.J.; Roh, Y.H.; Song, J.H.; Park, S.H.; Cho, M.; Kim, Y.M.; Hyung, W.J.; Kim, H.I. Indication of Proximal Gastrectomy for Advanced Proximal Gastric Cancer Based on Lymph Node Metastasis at the Distal Part of the Stomach. Ann. Surg. Open 2021, 2, e107. [Google Scholar] [CrossRef] [PubMed]
- Ri, M.; Kumagai, K.; Namikawa, K.; Atsumi, S.; Hayami, M.; Makuuchi, R.; Ida, S.; Ohashi, M.; Sano, T.; Nunobe, S. Is proximal gastrectomy indicated for locally advanced cancer in the upper third of the stomach? Ann. Gastroenterol. Surg. 2021, 5, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Haruta, S.; Shinohara, H.; Hosogi, H.; Ohkura, Y.; Kobayashi, N.; Mizuno, A.; Okamura, R.; Ueno, M.; Sakai, Y.; Udagawa, H. Proximal gastrectomy with exclusion of no. 3b lesser curvature lymph node dissection could be indicated for patients with advanced upper-third gastric cancer. Gastric Cancer 2017, 20, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.X.; Lin, J.P.; Wang, Z.K.; Li, P.; Xie, J.W.; Wang, J.B.; Lu, J.; Chen, Q.Y.; Cao, L.L.; Lin, M.; et al. Assessment of Laparoscopic Spleen-Preserving Hilar Lymphadenectomy for Advanced Proximal Gastric Cancer Without Invasion Into the Greater Curvature: A Randomized Clinical Trial. JAMA Surg. 2023, 158, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Maezawa, Y.; Aoyama, T.; Yamada, T.; Kano, K.; Hayashi, T.; Sato, T.; Oshima, T.; Rino, Y.; Masuda, M.; Ogata, T.; et al. Priority of lymph node dissection for proximal gastric cancer invading the greater curvature. Gastric Cancer 2018, 21, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Chen, Q.Y.; Xu, Y.C.; Zhao, G.; Cai, L.S.; Li, G.X.; Xu, Z.K.; Yan, S.; Wu, Z.G.; Xue, F.Q.; et al. Reappraise role of No. 10 lymphadenectomy for proximal gastric cancer in the era of minimal invasive surgery during total gastrectomy: A pooled analysis of 4 prospective trial. Gastric Cancer 2021, 24, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.X.; Xu, B.B.; Zheng, H.L.; Li, P.; Xie, J.W.; Wang, J.B.; Lu, J.; Chen, Q.Y.; Cao, L.L.; Lin, M.; et al. Laparoscopic Spleen-Preserving Hilar Lymphadenectomy for Advanced Proximal Gastric Cancer Without Greater Curvature Invasion: Five-Year Outcomes From the Fuges-02 Randomized Clinical Trial. JAMA Surg. 2024, 159, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.M.; Zhang, J.R.; Zheng, C.H.; Li, P.; Xie, J.W.; Wang, J.B.; Lin, J.X.; Lu, J.; Chen, Q.Y. A 346 case analysis for laparoscopic spleen-preserving no.10 lymph node dissection for proximal gastric cancer: A single center study. PLoS ONE 2014, 9, e108480. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Xu, Y.; Zhao, G.; Cai, L.; Li, G.; Xu, Z.; Yan, S.; Wu, Z.; Xue, F.; Sun, Y.; et al. Outcomes of Laparoscopic Total Gastrectomy Combined With Spleen-Preserving Hilar Lymphadenectomy for Locally Advanced Proximal Gastric Cancer: A Nonrandomized Clinical Trial. JAMA Netw. Open 2021, 4, e2139992. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.H.; Xu, Y.C.; Zhao, G.; Cai, L.S.; Li, G.X.; Xu, Z.K.; Yan, S.; Wu, Z.G.; Xue, F.Q.; Sun, Y.H.; et al. Safety and feasibility of laparoscopic spleen-preserving No. 10 lymph node dissection for locally advanced upper third gastric cancer: A prospective, multicenter clinical trial. Surg. Endosc. 2020, 34, 5062–5073. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, Y.; Sasako, M.; Sano, T.; Yoshikawa, T.; Iwasaki, Y.; Nashimoto, A.; Ito, S.; Kurita, A.; Mizusawa, J.; Nakamura, K.; et al. Ten-year follow-up results of a randomized clinical trial comparing left thoracoabdominal and abdominal transhiatal approaches to total gastrectomy for adenocarcinoma of the oesophagogastric junction or gastric cardia. Br. J. Surg. 2015, 102, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Yoshikawa, T.; Rino, Y.; Oshima, T.; Aoyama, T.; Hayashi, T.; Sato, T.; Yukawa, N.; Kameda, Y.; Sasaki, T.; et al. Priority of lymph node dissection for Siewert type II/III adenocarcinoma of the esophagogastric junction. Ann. Surg. Oncol. 2013, 20, 4252–4259. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.B.; Qi, W.X.; Chen, J.Y.; Xu, C.; Kirova, Y.M.; Cao, W.G.; Cai, R.; Cao, L.; Yan, M.; Cai, G. Competing risk nomogram predicting initial loco-regional recurrence in gastric cancer patients after D2 gastrectomy. Radiat. Oncol. 2019, 14, 128. [Google Scholar] [CrossRef] [PubMed]
- Alnoor, M.; Boys, J.A.; Worrell, S.G.; Oh, D.S.; Hagen, J.A.; DeMeester, S.R. Timing and Pattern of Recurrence after Gastrectomy for Adenocarcinoma. Am. Surg. 2015, 81, 1057–1060. [Google Scholar] [CrossRef] [PubMed]
- D’Angelica, M.; Gonen, M.; Brennan, M.F.; Turnbull, A.D.; Bains, M.; Karpeh, M.S. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann. Surg. 2004, 240, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Baiocchi, G.L.; Marrelli, D.; Verlato, G.; Morgagni, P.; Giacopuzzi, S.; Coniglio, A.; Marchet, A.; Rosa, F.; Capponi, M.G.; Di Leo, A.; et al. Follow-up after gastrectomy for cancer: An appraisal of the Italian research group for gastric cancer. Ann. Surg. Oncol. 2014, 21, 2005–2011. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Lee, Y.J.; Bae, K.; Park, J.H.; Hong, S.C.; Jung, E.J.; Ju, Y.T.; Jeong, C.Y.; Park, T.J.; Park, M.; et al. The investigation of diet recovery after distal gastrectomy. Medicine 2019, 98, e17543. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, Y.; Li, B.; Shan, F.; Li, Z. Short-term outcomes and long-term quality of life of reconstruction methods after proximal gastrectomy: A systematic review and meta-analysis. BMC Cancer 2024, 24, 56. [Google Scholar] [CrossRef] [PubMed]
- Shaibu, Z.; Chen, Z.; Mzee, S.A.S.; Theophilus, A.; Danbala, I.A. Effects of reconstruction techniques after proximal gastrectomy: A systematic review and meta-analysis. World J. Surg. Oncol. 2020, 18, 171. [Google Scholar] [CrossRef] [PubMed]

| Patient Demographics and Pathological Factors | ||||
|---|---|---|---|---|
| TG (N = 54) | SG (N = 34) | PG (N = 14) | p-Value | |
| Median Age | 63 (IQR 53–69) | 66 (IQR 55–76) | 64 (IQR 55–69) | 0.39 |
| Sex | 0.40 | |||
| Female | 24 (44%) | 17 (50%) | 4 (29%) | |
| Male | 30 (55%) | 17 (50%) | 10 (71%) | |
| Median BMI | 26.7 (IQR 24–30) | 26.5 (IQR 23–30) | 24.6 (IQR 22–27) | 0.09 |
| Clinical Stage (EUS) | 0.42 | |||
| Stage IB | 3 (6%) | 6 (18%) | 1 (7%) | |
| Stage IIA | 7 (13%) | 3 (9%) | 0 (0%) | |
| Stage IIB | 13 (24%) | 12 (35%) | 5 (36%) | |
| Stage III | 19 (35%) | 10 (29%) | 7 (50%) | |
| no/nondefinitive staging EUS | 12 (22%) | 3 (9%) | 1 (7%) | |
| Diffuse vs. Intestinal | 0.07 | |||
| Diffuse | 20 (37%) | 9 (26%) | 1 (7%) | |
| Intestinal | 9 (17%) | 14 (41%) | 3 (21%) | |
| Missing | 25 (46%) | 11 (32%) | 10 (71%) | |
| Signet Ring Cell | 30 (56%) | 16 (47%) | 1 (7%) | 0.01 * |
| Siewert Class (GEJ tumors) | 0.26 | |||
| Type II | 6 (12%) | n/a | 1 (7%) | |
| Type III | 14 (26%) | n/a | 9 (64%) | |
| Perioperative Treatment | 0.003 * | |||
| Chemotherapy only | 32 (59%) | 25 (74%) | 3 (21%) | |
| Chemo + Chemoradiation | 17 (31%) | 5 (15%) | 11 (79%) | |
| Chemo radiation | 3 (6%) | 3 (9%) | 0 (0%) | |
| Missing | 2 (4%) | 1 (3%) | 0 (0%) | |
| Histologic Grade | <0.01 * | |||
| Moderately differentiated | 11 (20%) | 5 (15%) | 4 (29%) | |
| Poorly differentiated | 35 (65%) | 25 (73%) | 4 (29%) | |
| unavailable | 8 (15%) | 4 (12%) | 6 (43%) | |
| Perioperative Outcomes | ||||
|---|---|---|---|---|
| TG | SG | PG | p-Value | |
| Median EBL (mL) | 150 | 150 | 175 | 0.56 |
| Mean OR time (hours) | 8.5 | 8.1 | 7.8 | 0.78 |
| Median hospital length of stay (days) | 5 | 5 | 4 | 0.19 |
| 30-day readmission rate (%) | 13.2 | 23.5 | 14 | 0.27 |
| 30-day reoperation rate (%) | 15 | 8.8 | 0 | 0.24 |
| 90-day major morbidity (%) | 26.4 | 12.9 | 14 | 0.28 |
| 90-day mortality (%) | 0 | 3.2 | 0 | 0.34 |
| R0 achieved (%) | 93 | 100 | 100 | 0.16 |
| Median LN (IQR) | 30 (25–38) | 30 (21–40) | 24 (18–38) | 0.86 |
| 18-month locoregional recurrence (%) | 7.5 | 12.5 | 0 | 0.50 |
| 18-month overall survival (%) | 77 | 68 | 78 | 0.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siegler, E.L.; Grotz, T.E. Modified Proximal Gastrectomy and D2 Lymphadenectomy Is an Oncologically Sound Operation for Locally Advanced Proximal and GEJ Adenocarcinoma. Cancers 2025, 17, 2455. https://doi.org/10.3390/cancers17152455
Siegler EL, Grotz TE. Modified Proximal Gastrectomy and D2 Lymphadenectomy Is an Oncologically Sound Operation for Locally Advanced Proximal and GEJ Adenocarcinoma. Cancers. 2025; 17(15):2455. https://doi.org/10.3390/cancers17152455
Chicago/Turabian StyleSiegler, Emily L., and Travis E. Grotz. 2025. "Modified Proximal Gastrectomy and D2 Lymphadenectomy Is an Oncologically Sound Operation for Locally Advanced Proximal and GEJ Adenocarcinoma" Cancers 17, no. 15: 2455. https://doi.org/10.3390/cancers17152455
APA StyleSiegler, E. L., & Grotz, T. E. (2025). Modified Proximal Gastrectomy and D2 Lymphadenectomy Is an Oncologically Sound Operation for Locally Advanced Proximal and GEJ Adenocarcinoma. Cancers, 17(15), 2455. https://doi.org/10.3390/cancers17152455








