Simple Summary
Genetic changes play an important role in the development of acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS). Some rare genetic mutations, specifically in the BCOR and BCORL1 genes, have been found in adult patients, but have not been well described in pediatric patients. In this report, we share a case series of pediatric and adolescent patients who have these specific mutations. While this helps shed light on a rare finding, more research with larger groups of patients is needed to truly understand what these mutations mean for young people with AML or MDS.
Abstract
Somatic and epigenetic alterations contribute to myeloid leukemogenesis and play an important role in risk stratification and the optimization of treatment for myeloid malignancies. The significance of rare genetic alterations, such B-cell lymphoma-6 corepressor (BCOR) and B-cell lymphoma-6 corepressor-like protein 1 (BCORL1) mutations, in pediatric acute myeloid leukemias (AML) and myelodysplastic syndrome (MDS) is unknown. We present a case series of pediatric and adolescent patients, with de novo AML, harboring BCOR/BCORL1 mutations. Studies involving larger cohorts of patients are needed to further elucidate the role of BCOR/BCORL1 mutations in pediatric AML and MDS.
1. Introduction
Several rare genetic alterations in pediatric leukemias impacting risk stratification and treatment have recently been identified using next-generation sequencing (NGS). NGS is a pivotal tool that allows comprehensive profiling, the identification of genetic mutations and epigenetic changes, and targeted treatment [1].
B-cell lymphoma-6 corepressor (BCOR) is a transcription factor, located on chromosome X, and plays a key role in hematopoiesis and stem cell function and pluripotency, and mutations can lead to hematopoietic malignancies such as de novo and secondary AML [2,3].
BCOR and its homolog, B-cell lymphoma-6 corepressor-like protein 1 (BCORL1), are rarely altered (<2% incidence) in pediatric leukemias [4,5,6,7]. Similarly, BCOR mutations rarely occur in adult leukemias, with one study reporting BCOR mutations in 4% of 262 adults with cytogenetically normal acute myeloid leukemia (CN-AML) [4]. In adults, loss-of-function mutations in BCOR are associated with poor prognoses, but the prognostic significance of BCORL1 has yet to be examined due to its relative rarity [5].
BCOR and BCORL1 are corepressors of the B-cell lymphoma-6 (BCL6) gene (Figure 1) [6] that function as a part of the non-canonical PRC1.1 complex, responsible for the repression of several antitumor genes through chromatin modifications and opposition of the differentiation of cells toward myeloid lineage, through the repression of HOX and Cepb family genes [4,8,9]. The disruption of this complex, e.g., BCOR loss of function, leads to multiple downstream effects, which include the promotion of clonal expansion, persistence of DNA damage, and absence of cellular differentiation, contributing to leukemogenesis [10,11,12].
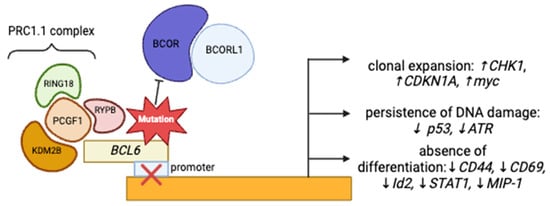
Figure 1.
Figure describing the mechanisms through which BCOR/BCORL1 could induce leukemogenesis.
The role of BCOR in promoting leukemogenesis is further elucidated by several murine models. P53 knockout mice harboring the BCOR exon 4 deletion (BCORΔE4/y) develop T-cell acute lymphoblastic leukemia (T-ALL) in a NOTCH1-dependent manner [13]. BCOR−/−DNMT3A−/− double-knockout mice develop an acute erythroid leukemia (AEL) phenotype [14]. Frameshift BCOR mutations in a nine-base-pair hotspot in exon 8 collaborate with oncogene NUP98-PHF23 (NP23) to generate an aggressive acute lymphoblastic leukemia of B-1 lymphocyte progenitor origin (pro-B1 ALL) [15].
Mice harboring only BCOR mutations do not develop leukemia, but have increased circulating peripheral blood neutrophils, without significant changes in lymphocyte, platelet, or erythrocyte counts [6]. BCOR knockout mice crossed with oncogenic KRAS-variant mice develop a lethal leukemic phenotype [14]. Notably, BCOR-/KRAS-variant mice have significantly worse survival than those with the oncogenic KRAS mutation alone [14]. In summary, these murine models support the notion that BCOR/BCORL1 mutations contribute to leukemogenesis but are likely insufficient to induce leukemogenesis alone.
Spliceosome mutations are found in the majority of MDS patients and are frequently the earliest mutations that are identified. Additional subclonal somatic lesions are acquired subsequently and often confer a resistant phenotype and drive the progression from MDS to AML [16]. Bernard et al. performed genomic profiling of 3233 patients with adult MDS and identified 3.5% (n = 114) of patients as having mutations in BCOR (85%), 33% as having those in BCORL1, and 17% as having those in both genes. Additionally, subclonal RUNX1 mutations were common (41%) in these patient samples. BCOR/L1-mutated MDS was characterized by severe thrombocytopenia, high blast counts, a shorter OS (median, 2.2 years), and a 24% 2-year incidence of AML transformation [17].
BCOR mutations confer an adverse prognosis in adult AML and were incorporated into the 2022 European Leukemia Net (ELN) classification as an adverse risk marker [18,19]. The impact of BCOR/BCORL1 mutation on the outcomes of AML/MDS patients that have received an allogeneic hematopoietic stem cell transplant has not been studied extensively [20].
The genetic landscape of pediatric AML differs from that of adult AML. Adult patients with AML often display increased mutational burden and fewer cytogenetic alterations when compared to children [21]. Currently, genetic abnormalities in pediatric AML are classified into three categories based on their prognostic significance: favorable risk, intermediate risk, and adverse risk. Favorable-risk genetic alterations include core-binding factor protein fusions like RUNX1:RUNX1T1, CBFB:MYH11, bi-allelic CEBPA mutations, and NPM1 mutations without FLT3-ITD. Favorable-risk genetic alterations portend better prognosis with 5-year overall survival rates surpassing 80%.
In contrast, adverse genetic changes have significantly worse prognosis, with 5-year overall survival rates less than 40% in some cases [22]. FLT3-ITD mutations occur in 20–25% of pediatric AMLs and are among the most clinically significant adverse alterations [23]. The prognosis associated with FLT3-ITD mutations varies based on the allelic ratio, with higher allelic ratios leading to worse prognosis [23]. Other well-established adverse genetic alterations include NUP98 gene fusions, RAS pathway alterations (NRAS, KRAS, PTPN11), and CSF3R mutations [24,25]. Additionally, karyotypic changes such as monosomy 7 and monosomy 5/del5q are associated with worse prognosis and lead to a rare subtype of AML, acute megakaryoblastic leukemia (AMKL). KMT2A rearrangements, formerly MLL rearrangements, represent an emerging adverse genetic change [26]. This rearrangement has over one hundred described fusion partners, but is more commonly observed in infants less than one year of age [21,27].
In between favorable- and adverse-risk genetic changes fall intermediate-risk mutations. There is no set number of intermediate-risk mutations [28]. By definition, these mutations encompass all those genetic changes that have not been well described as either favorable or adverse [29]. The prognosis for this group varies significantly based on concurrent mutations, and overall survival is near 50% [28].
Here, we review a case series of pediatric and adolescent patients, with de novo AML/MDS, harboring BCOR/BCORL1 mutations, adding to the growing body of research investigating the role of BCOR and BCORL1 mutations in pediatric myeloid malignancies.
2. Materials and Methods
We identified a cohort of patients diagnosed with acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), or myelodysplastic syndrome (MDS) at Riley Hospital for Children between January 2015 and December 2023 who had next-generation sequencing (NGS) performed on their bone marrow aspirate samples, utilizing the FoundationOne Heme (https://www.foundationmedicine.com/test/foundationone-heme, accessed on 1 January 2025) assay, and harbored mutations in BCL6, BCOR, and BCOR1, at diagnosis and/or relapse, and conducted a retrospective chart review. FoundationOne Heme utilizes DNA sequencing to interrogate the entire coding sequence of 406 oncogenes, selected introns of 31 genes involved in rearrangements, and the RNA sequencing of 265 genes known to be somatically altered in hematological malignancies (https://www.foundationmedicine.com/test/foundationone-heme, accessed on 1 January 2025). Relapse-free survival (RFS) was defined as survival without evidence of disease recurrence at three years off therapy. Kaplan–Meier survival curves were generated using MedCalc Software Limited© v22, MedCalc Software Ltd., Ostend, Belgium.
BCOR-like mutation variants were categorized by the type of mutation: intron variant, missense, gain of function, and synonymous. We queried large genetic databases [Catalogue of Somatic Mutations in Cancer (COSMIC) (https://cancer.sanger.ac.uk/cosmic, accessed on 1 January 2025), Clinical Knowledgebase Boost (CKB) (https://ckbhome.jax.org), the LifeOmic Precision Health Cloud (PHC) (https://www.medigy.com/offering/lifeomic-precision-health-cloud/, accessed on 16 July 2025), Online Mendelian Inheritance in Man (OMIM) (https://www.omim.org/), and Uniprot (https://www.uniprot.org/)] for each variant identified in our cohort of patients, to evaluate the likelihood of pathogenicity of each mutation. Variants were identified as potentially pathogenic if they were predicted to cause protein truncation. Additionally, using FoundationHeme, we identified co-mutations that occurred with BCOR and BCORL1 mutations.
3. Results
A total of 102 patients with acute lymphoblastic leukemia (ALL) and 82 patients with AML/MDS had FoundationOne Heme NGS performed during their treatment course on their marrow samples. Eight (4.3%) patients harbored BCOR, BCORL1, or BCL6 at diagnosis and/or relapse. In the ALL patient cohort, all mutations occurred at diagnosis (n = 3, 2.9%). In AML/MDS patients, mutations were identified at diagnosis (n = 2, 2.4%) and only at relapse (n = 3, 3.7%, but not at diagnosis) (Figure 2). Of the seventeen variants (BCOR n = 5, BCORL1 n = 5, BCL6 n = 7), we identified three variants (18%) as possibly pathogenic (R1532fs, V1687fs, E1655fs) in the AML/MDS cohort (Figure 2). Additionally, we identified 95 co-mutations in these patients (Figure 3). WT1 (n = 5), RUNX1 (n = 5), and KRAS (n = 4) mutations were the most common co-mutations identified among AML/MDS patients (Figure 3).
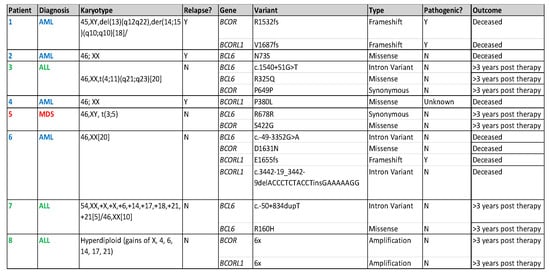
Figure 2.
Table describing location of variants and likelihood of pathogenicity for each identified mutation and patient outcomes.
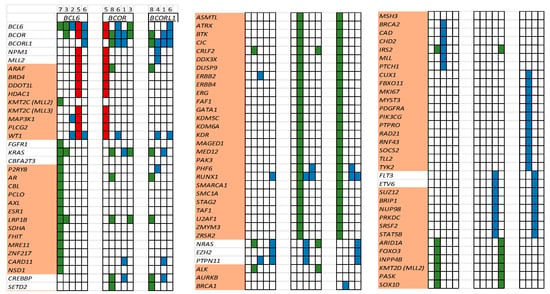
Figure 3.
List of co-occurring mutations for each patient (#1–8, red—MDS, green—ALL, blue—AML). Each column depicts one patient. A cell is colored if the corresponding gene (listed in rows) is mutated. Genes highlighted in orange are variants of unknown significance (VUSs).
The three patients with ALL harboring BCOR mutations, identified at diagnosis, had excellent RFS (100% were relapse-free at three years). The RFS for patients with AML/MDS (n = 5) harboring BCOR/BCORL1 mutations was only 20% (Figure 3), with the patient with MDS being the lone survivor. All AML/MDS patients received a stem cell transplant either as a part of their upfront consolidative therapy (n = 1) or for relapsed disease (n = 4).
4. Discussion
BCOR/BCORL1 mutations occur infrequently in pediatric AML/MDS [4]. Only 6.1% of our AML/MDS patient bone marrow samples harbored these mutations. It is important to note that in three patients of the AML/MDS cohort, BCOR-like mutations were only identified at relapse. This finding indicates that BCOR-like mutations may be acquired in pediatric AML during disease evolution, persistence, and progression. The four AML patients died of progression despite receiving a stem cell transplant (Figure 3), with only one patient (MDS, patient #5) alive at the last-known follow-up.
Our findings concur with the published literature in adults, which has reported BCOR mutations to frequently occur in exon 4 (Figure 4) [30,31,32]. However, the mutations in exon 4 in our patient cohort are unlikely to be pathogenic, as they are synonymous mutations, and do not occur in known mutational hotspots. Three of the seventeen (17.6%) frameshift mutations identified in AML patients #1 and #6 (Figure 2) are potentially pathogenic, as they likely lead to truncations in the C-terminus of BCOR (R1532fs) and BCORL1 (V1687fs, E1655fs) proteins, affecting the binding of BCOR with the PRC1.1 complex through its PUFD domain at the C-terminal end [6,31]. A truncation at the C-terminal end could prevent adequate binding and ultimately impair the function of the non-canonical PRC1.1 complex. Additionally, we identified two mutations (V1687fs, E1655fs) in the BCORL1 gene affecting the LXXLL nuclear receptor recruitment motif on the C-terminal end (Figure 2 and Figure 4). Previous studies have identified that BCORL1 mutations resulting in the absence of the LXXLL nuclear receptor impaired BCORL1 function [33]. We predict that frameshift mutations in this region resulting in protein truncation would similarly impair BCORL1 function.
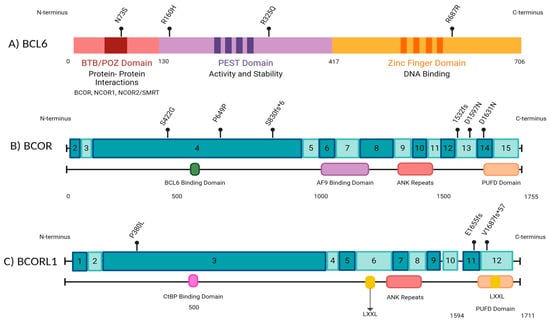
Figure 4.
A schematic representation of BCL6 and BCOR gene structures along with the locations of mutations identified by next-generation sequencing (NGS) in our cohort of pediatric leukemia patients. The numbers in the boxes indicate exons. Made with BioRender.com.
In summary, our findings are similar to those of previously published studies that have reported that the distribution of BCOR/BCORl1 mutation sites are varied, often within the same patient sample, and that frameshift, missense mutations are common. The prognostic impact of the location or type of mutation continues to be an area of research that is evolving [15,34]. It appears that frameshift mutations may be associated with an inferior outcome in patients with adult hematopoietic malignancies compared to other type of mutations [34,35]. Due to the limited number of patients in our study, and the rarity of BCOR mutations in pediatric myeloid leukemias, we cannot draw definitive conclusions.
When examining co-occurring mutations, we found that most identified co-mutations were variants of unknown significance (VUSs) (Figure 3). WT1 (patients #2, 4, and 6; no ALL patient), RUNX1 (patients #1, 6), and KRAS (patient #1) co-mutations were identified in our AML/MDS cohort of patients co-harboring BCOR/BCORL1/BCL6 mutations (Figure 3). WT1 is often overexpressed in AML, CML blast crisis, and ALL [36]. Additionally, several studies have identified somatic mutations in WT1 in AML [37]. Wt-1 alterations are associated with chemo-resistance, increased relapse risk, and poor survival [38,39]. Similarly, alterations in RAS in AML/MDS are heterogeneous, differentially impact outcomes, and are likely influenced by the presence of concurrent mutations such as BCOR, leading to resistance to chemotherapy, venetoclax, azacitidine, and targeted therapy such as tyrosine kinase and FLT3 inhibitors [40,41].
Germline RUNX1 mutations cause Familial platelet disorder with associated myeloid malignancies (FPDMM), which is characterized by an increased risk of developing hematologic malignancies. Interestingly, in terms of frameshift mutations, BCOR is the most frequently altered gene in this patient cohort [42].
The presence of these co-mutations underscores the complexity of dynamic changes that can occur during the clonal expansion and transformation of pediatric AML/MDS. While the clinical significance of these findings is unclear, larger studies would perhaps help clarify their role.
Mutations in BCL6, BCOR, and BCORL1 can contribute to leukemogenesis through various mechanisms and can be targeted with novel therapeutic strategies. BCL6 has been the most well-researched target in this setting, as it is crucial for the survival and self-renewal of AML cells [43]. High BCL6 expression in ALL and AML has been associated with treatment resistance and disease progression [10]. Zhang et al. recently identified WK499, a small-molecule compound that disrupts the interaction of BCL6 with corepressor proteins [44]. This disruption causes downstream changes in the effects of BCL6 and ultimately induces cell cycle arrest and apoptosis in AML cells [44]. Novel BCL6-targeting proteolysis-targeting chimeras (PROTACs) with effective antitumor activities against DLBCL in vitro and in vivo have been identified, and could be tested in human trials in the future [45]. In addition to its own anti-proliferative effects, BCL6 inhibition has been shown to augment the anti-proliferative effects of classical chemotherapeutic agents like cytarabine in in vivo studies, making it a promising adjunct therapy [10].
There is also a precedent for disrupting genes that target BCL6, as BCOR and BCORL1 do. In diffuse large B-cell lymphoma, small-molecule inhibition of STAT3, a transcriptional target of BCL6, has shown promise in chemotherapy-refractory activated B-cell-like DLBCL [46].
Menin interacts with KMT2A directly and regulates KMT2A target genes. Several clinical trials have established small-molecule menin inhibitors as novel agents that impact resistant myeloid leukemias, with promising results [38]. Menin inhibitors display efficacy in early therapy of AML, but resistance can develop through epigenetic reactivation of non-canonical menin targets [47]. Zhou et al. showed that the loss of PRC1.1 subunits, like BCOR and EZH, led to menin resistance, but with functional PRC1.1 complexes, AML cells remained sensitive to menin inhibitors [47]. Interestingly, they found that the loss of PRC1.1 sensitizes AML cells to BCL2 blockade. These findings underscore the hypothesis that BCOR/BCORL1 mutation-induced menin resistance may be overcome with bcl-2 inhibitors such as venetoclax [38]. While there are no current agents that target the PRC1.1 complex, inhibitors of EZH1/2 and EED, subunits of the PRC2 complex, could also be novel effective downstream therapeutic targets [9].
5. Conclusions
This report demonstrates that BCOR-like somatic mutations are rare in pediatric leukemias and may or may not be pathogenic. Larger cooperative group studies are needed to further investigate the prognostic impact of BCOR/BCORL1 mutations in pediatric AML and MDS.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers17152443/s1, Figure S1: Kaplan–Meier survival curve for AML/MDS patients (n = 5).
Author Contributions
Conceptualization, S.B.; Methodology, M.S.M.; Validation, S.B.; Formal analysis, T.C.F.-H., A.S., M.S.M. and S.B.; Investigation, T.C.F.-H., A.S. and S.B.; Resources, S.B.; Data curation, T.C.F.-H., A.S., T.B. and S.B.; Writing—original draft, T.C.F.-H., A.S. and S.B.; Writing—review & editing, M.S.M. and S.B.; Visualization, S.B.; Supervision, S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of Indiana University (IRB-exempt protocol #1011003233, active 2017–2025). Ethical review and approval were waived for this study due to its retrospective de-identified design.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material (Figure S1). Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Damm, F.; Chesnais, V.; Nagata, Y.; Yoshida, K.; Scourzic, L.; Okuno, Y.; Itzykson, R.; Sanada, M.; Shiraishi, Y.; Gelsi-Boyer, V.; et al. BCOR and BCORL1 mutations in myelodysplastic syndromes and related disorders. Blood 2013, 122, 3169–3177. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Marouf, A.; Kogure, Y.; Koya, J.; Liévin, R.; Bruneau, J.; Tabata, M.; Saito, Y.; Shingaki, S.; Yuasa, M.; et al. Comprehensive Genetic Profiling Reveals Frequent Alterations of Driver Genes on the X Chromosome in Extranodal NK/T-cell Lymphoma. Cancer Res. 2024, 84, 2181–2201. [Google Scholar] [CrossRef] [PubMed]
- Baranwal, A.; Gurney, M.; Basmaci, R.; Katamesh, B.; He, R.; Viswanatha, D.S.; Greipp, P.; Foran, J.; Badar, T.; Murthy, H.; et al. Genetic landscape and clinical outcomes of patients with BCOR mutated myeloid neoplasms. Haematologica 2024, 109, 1779–1791. [Google Scholar] [CrossRef] [PubMed]
- de Rooij, J.D.; van den Heuvel-Eibrink, M.M.; Hermkens, M.C.; Verboon, L.J.; Arentsen-Peters, S.T.; Fornerod, M.; Baruchel, A.; Stary, J.; Reinhardt, D.; de Haas, V.; et al. BCOR and BCORL1 mutations in pediatric acute myeloid leukemia. Haematologica 2015, 100, e194–e195. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eckardt, J.-N.; Stasik, S.; Kramer, M.; Röllig, C.; Krämer, A.; Scholl, S.; Hochhaus, A.; Crysandt, M.; Brümmendorf, T.H.; Naumann, R.; et al. Loss-of-Function Mutations of BCOR Are an Independent Marker of Adverse Outcomes in Intensively Treated Patients with Acute Myeloid Leukemia. Cancers 2021, 13, 2095. [Google Scholar] [CrossRef] [PubMed]
- Sportoletti, P.; Sorcini, D.; Falini, B. BCOR gene alterations in hematologic diseases. Blood 2021, 138, 2455–2468. [Google Scholar] [CrossRef] [PubMed]
- Umeda, M.; Liu, Y.-C.; Karol, S.E.; Klco, J.M. Novel classification system and high-risk categories of pediatric acute myeloid leukemia. Haematologica, 2025; ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Gearhart, M.D.; Corcoran, C.M.; Wamstad, J.A.; Bardwell, V.J. Polycomb group and SCF ubiquitin ligases are found in a novel BCOR complex that is recruited to BCL6 targets. Mol. Cell Biol. 2006, 26, 6880–6889. [Google Scholar] [CrossRef] [PubMed]
- Kaito, S.; Iwama, A. Pathogenic Impacts of Dysregulated Polycomb Repressive Complex Function in Hematological Malignancies. Int. J. Mol. Sci. 2020, 22, 74. [Google Scholar] [CrossRef] [PubMed]
- McLachlan, T.; Matthews, W.C.; Jackson, E.R.; Staudt, D.E.; Douglas, A.M.; Findlay, I.J.; Persson, M.L.; Duchatel, R.J.; Mannan, A.; Germon, Z.P.; et al. B-cell Lymphoma 6 (BCL6): From Master Regulator of Humoral Immunity to Oncogenic Driver in Pediatric Cancers. Mol. Cancer Res. 2022, 20, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Tara, S.; Isshiki, Y.; Nakajima-Takagi, Y.; Oshima, M.; Aoyama, K.; Tanaka, T.; Shinoda, D.; Koide, S.; Saraya, A.; Miyagi, S.; et al. Bcor insufficiency promotes initiation and progression of myelodysplastic syndrome. Blood 2018, 132, 2470–2483. [Google Scholar] [CrossRef] [PubMed]
- Bouligny, I.M.; Maher, K.R.; Grant, S. Secondary-Type Mutations in Acute Myeloid Leukemia: Updates from ELN 2022. Cancers 2023, 15, 3292. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Nakajima-Takagi, Y.; Aoyama, K.; Tara, S.; Oshima, M.; Saraya, A.; Koide, S.; Si, S.; Manabe, I.; Sanada, M.; et al. Internal deletion of BCOR reveals a tumor suppressor function for BCOR in T lymphocyte malignancies. J. Exp. Med. 2017, 214, 2901–2913. [Google Scholar] [CrossRef] [PubMed]
- Sportoletti, P.; Sorcini, D.; Guzman, A.G.; Reyes, J.M.; Stella, A.; Marra, A.; Sartori, S.; Brunetti, L.; Rossi, R.; Del Papa, B.; et al. Bcor deficiency perturbs erythro-megakaryopoiesis and cooperates with Dnmt3a loss in acute erythroid leukemia onset in mice. Leukemia 2021, 35, 1949–1963. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Chung, Y.J.; Lindsley, R.C.; Walker, R.L.; Zhu, Y.J.; Ebert, B.L.; Meltzer, P.S.; Aplan, P.D. Engineered Bcor mutations lead to acute leukemia of progenitor B-1 lymphocyte origin in a sensitized background. Blood 2019, 133, 2610–2614. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M. Myelodysplastic Syndromes. N. Engl. J. Med. 2020, 383, 1358–1374. [Google Scholar] [CrossRef] [PubMed]
- Bernard, E.; Hasserjian, R.P.; Greenberg, P.L.; Ossa, J.E.A.; Creignou, M.; Tuechler, H.; Gutierrez-Abril, J.; Domenico, D.; Medina-Martinez, J.S.; Levine, M.; et al. Molecular taxonomy of myelodysplastic syndromes and its clinical implications. Blood 2024, 144, 1617–1632. [Google Scholar] [CrossRef] [PubMed]
- Eisfeld, A.-K.; Kohlschmidt, J.; Mims, A.; Nicolet, D.; Walker, C.J.; Blachly, J.S.; Carroll, A.J.; Papaioannou, D.; Kolitz, J.E.; Powell, B.E.; et al. Additional gene mutations may refine the 2017 European LeukemiaNet classification in adult patients with de novo acute myeloid leukemia aged <60 years. Leukemia 2020, 34, 3215–3227. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Shen, K.; Guo, Y.; Bao, X.B.; Dong, N.; Chen, S. The clinical implications of BCOR mutations in a large cohort of acute myeloid leukemia patients: A 5-year single-center retrospective study. Leuk. Lymphoma 2024, 65, 1964–1973. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, H.; Zheng, X.; Zhang, R.; Chen, X.; Ma, Q.; Yang, D.; Wei, J.; Pang, A.; He, Y.; et al. Impact of BCOR/BCORL1 mutation on outcomes of allogeneic hematopoietic stem cell transplantation in acute myeloid leukemia patients. Ann. Hematol. 2025, 104, 2631–2642. [Google Scholar] [CrossRef] [PubMed]
- Bolouri, H.; Farrar, J.E.; Triche, T.; Ries, R.E.; Lim, E.L.; Alonzo, T.A.; Ma, Y.; Moore, R.; Mungall, A.J.; Marra, M.A.; et al. The molecular landscape of pediatric acute myeloid leukemia reveals recurrent structural alterations and age-specific mutational interactions. Nat. Med. 2018, 24, 103–112. [Google Scholar] [CrossRef] [PubMed]
- von Neuhoff, C.; Reinhardt, D.; Sander, A.; Zimmermann, M.; Bradtke, J.; Betts, D.R.; Zemanova, Z.; Stary, J.; Bourquin, J.P.; Haas, O.A.; et al. Prognostic impact of specific chromosomal aberrations in a large group of pediatric patients with acute myeloid leukemia treated uniformly according to trial AML-BFM 98. J. Clin. Oncol. 2010, 28, 2682–2689. [Google Scholar] [CrossRef] [PubMed]
- Elgarten, C.W.; Aplenc, R. Pediatric acute myeloid leukemia: Updates on biology, risk stratification, and therapy. Curr. Opin. Pediatr. 2020, 32, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Conneely, S.E.; Rau, R.E. The genomics of acute myeloid leukemia in children. Cancer Metastasis Rev. 2020, 39, 189–209. [Google Scholar] [CrossRef] [PubMed]
- Shiba, N. Comprehensive molecular understanding of pediatric acute myeloid leukemia. Int. J. Hematol. 2023, 117, 173–181. [Google Scholar] [CrossRef] [PubMed]
- de Rooij, J.D.; Masetti, R.; van den Heuvel-Eibrink, M.M.; Cayuela, J.M.; Trka, J.; Reinhardt, D.; Rasche, M.; Sonneveld, E.; Alonzo, T.A.; Fornerod, M.; et al. Recurrent abnormalities can be used for risk group stratification in pediatric AMKL: A retrospective intergroup study. Blood 2016, 127, 3424–3430. [Google Scholar] [CrossRef] [PubMed]
- Yuen, K.Y.; Liu, Y.; Zhou, Y.Z.; Wang, Y.; Zhou, D.H.; Fang, J.P.; Xu, L.H. Mutational landscape and clinical outcome of pediatric acute myeloid leukemia with 11q23/KMT2A rearrangements. Cancer Med. 2023, 12, 1418–1430. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, L.; Zhang, R.; Wan, Y.; Gong, X.; Zhang, L.; Yang, W.; Chen, X.; Zou, Y.; Chen, Y.; et al. Development and validation of a prognostic scoring model to risk stratify childhood acute myeloid leukaemia. Br. J. Haematol. 2022, 198, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Pérez, C.; de la Torre, E.P.; Sanchez-Garcia, J.; Martín-Calvo, C.; Martínez-Losada, C.; Casaño-Sanchez, J.; Serrano-López, J.; Serrano, J. Evolving Risk Classifications in AML in a Real-Life Scenario: After Changes upon Changes, Is It More and More Adverse? Cancers 2023, 15, 1425. [Google Scholar] [CrossRef] [PubMed]
- Terada, K.; Yamaguchi, H.; Ueki, T.; Usuki, K.; Kobayashi, Y.; Tajika, K.; Gomi, S.; Kurosawa, S.; Saito, R.; Furuta, Y.; et al. Usefulness of BCOR gene mutation as a prognostic factor in acute myeloid leukemia with intermediate cytogenetic prognosis. Genes Chromosom. Cancer 2018, 57, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Astolfi, A.; Fiore, M.; Melchionda, F.; Indio, V.; Bertuccio, S.N.; Pession, A. BCOR involvement in cancer. Epigenomics 2019, 11, 835–855. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, F.; Zhang, Z.; Guo, J.; He, Q.; Song, L.-X.; Wu, D.; Zhou, L.-Y.; Su, J.-Y.; Xiao, C.; et al. Dynamics of epigenetic regulator gene BCOR mutation and response predictive value for hypomethylating agents in patients with myelodysplastic syndrome. Clin. Epigenet. 2021, 13, 169. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Collins, R.; Jiao, Y.; Ouillette, P.; Bixby, D.; Erba, H.; Vogelstein, B.; Kinzler, K.W.; Papadopoulos, N.; Malek, S.N. Somatic mutations in the transcriptional corepressor gene BCORL1 in adult acute myelogenous leukemia. Blood 2011, 118, 5914–5917. [Google Scholar] [CrossRef] [PubMed]
- Abuhadra, N.; Mukherjee, S.; Al-Issa, K.; Adema, V.; Hirsch, C.M.; Advani, A.; Przychodzen, B.; Makhoul, A.; Awada, H.; Maciejewski, J.P.; et al. BCOR and BCORL1 mutations in myelodysplastic syndromes (MDS): Clonal architecture and impact on outcomes. Leuk. Lymphoma 2019, 60, 1587–1590. [Google Scholar] [CrossRef] [PubMed]
- Jallades, L.; Baseggio, L.; Sujobert, P.; Huet, S.; Chabane, K.; Callet-Bauchu, E.; Verney, A.; Hayette, S.; Desvignes, J.P.; Salgado, D.; et al. Exome sequencing identifies recurrent BCOR alterations and the absence of KLF2, TNFAIP3 and MYD88 mutations in splenic diffuse red pulp small B-cell lymphoma. Haematologica 2017, 102, 1758–1766. [Google Scholar] [CrossRef] [PubMed]
- Rampal, R.; Figueroa, M.E. Wilms tumor 1 mutations in the pathogenesis of acute myeloid leukemia. Haematologica 2016, 101, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Umeda, M.; Ma, J.; Westover, T.; Ni, Y.; Song, G.; Maciaszek, J.L.; Rusch, M.; Rahbarinia, D.; Foy, S.; Huang, B.J.; et al. A new genomic framework to categorize pediatric acute myeloid leukemia. Nat. Genet. 2024, 56, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Augsberger, C.; Hänel, G.; Xu, W.; Pulko, V.; Hanisch, L.J.; Augustin, A.; Challier, J.; Hunt, K.; Vick, B.; Rovatti, P.E.; et al. Targeting intracellular WT1 in AML with a novel RMF-peptide-MHC-specific T-cell bispecific antibody. Blood 2021, 138, 2655–2669. [Google Scholar] [CrossRef] [PubMed]
- Aydin, S.; Schmitz, J.; Dellacasa, C.M.; Dogliotti, I.; Giaccone, L.; Busca, A. WT1 Expression Is Associated with Poor Overall Survival after Azacytidine and DLI in a Cohort of Adult AML and MDS Patients. Cancers 2024, 16, 3070. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Liu, Y.; Zhu, Y.; Wang, Q.; Zhao, X.; Wang, Q.; Chen, Y.; Chen, S. Molecular, clinical, and prognostic implications of RAS pathway alterations in adult acute myeloid leukemia. Leuk. Lymphoma 2025, 66, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Alawieh, D.; Cysique-Foinlan, L.; Willekens, C.; Renneville, A. RAS mutations in myeloid malignancies: Revisiting old questions with novel insights and therapeutic perspectives. Blood Cancer J. 2024, 14, 72. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Deuitch, N.; Merguerian, M.; Cunningham, L.; Davis, J.; Bresciani, E.; Diemer, J.; Andrews, E.; Young, A.; Donovan, F.; et al. Genomic landscape of patients with germline RUNX1 variants and familial platelet disorder with myeloid malignancy. Blood Adv. 2024, 8, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.C.; Zong, H.; Meydan, C.; Wyman, S.; Wouters, B.J.; Sugita, M.; Goswami, S.; Albert, M.; Yip, W.; Roboz, G.J.; et al. BCL6 maintains survival and self-renewal of primary human acute myeloid leukemia cells. Blood 2021, 137, 812–825. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, M.; Guo, W.; Zhu, S.; Li, S.; Lv, S.; Li, Y.; Liu, L.; Xing, Y.; Chen, H.; et al. A small molecule BCL6 inhibitor as chemosensitizers in acute myeloid leukemia. Biomed. Pharmacother. 2023, 166, 115358. [Google Scholar] [CrossRef] [PubMed]
- Mi, D.; Li, C.; Li, Y.; Yao, M.; Li, Y.; Hong, K.; Xie, C.; Chen, Y. Discovery of novel BCL6-Targeting PROTACs with effective antitumor activities against DLBCL in vitro and in vivo. Eur. J. Med. Chem. 2024, 277, 116789. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.B.; Yu, J.J.; Yu, R.Y.-L.; Mendez, L.M.; Shaknovich, R.; Zhang, Y.; Cattoretti, G.; Ye, B.H. Constitutively activated STAT3 promotes cell proliferation and survival in the activated B-cell subtype of diffuse large B-cell lymphomas. Blood 2008, 111, 1515–1523. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, L.; Aryal, S.; Veasey, V.; Tajik, A.; Restelli, C.; Moreira, S.; Zhang, P.; Zhang, Y.; Hope, K.J.; et al. Epigenetic regulation of noncanonical menin targets modulates menin inhibitor response in acute myeloid leukemia. Blood 2024, 144, 2018–2032. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).