Optimizing Belantamab Mafodotin in Relapsed or Refractory Multiple Myeloma: Impact of Dose Modifications on Adverse Events and Hematologic Response in a Real-World Retrospective Study †
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Endpoints
2.2. Statistical Analysis
3. Results
3.1. Patients, Disease and Treatment Characteristics
3.2. Response to Belamaf
3.3. Predictive Markers for Belamaf Response
3.4. Adverse Events
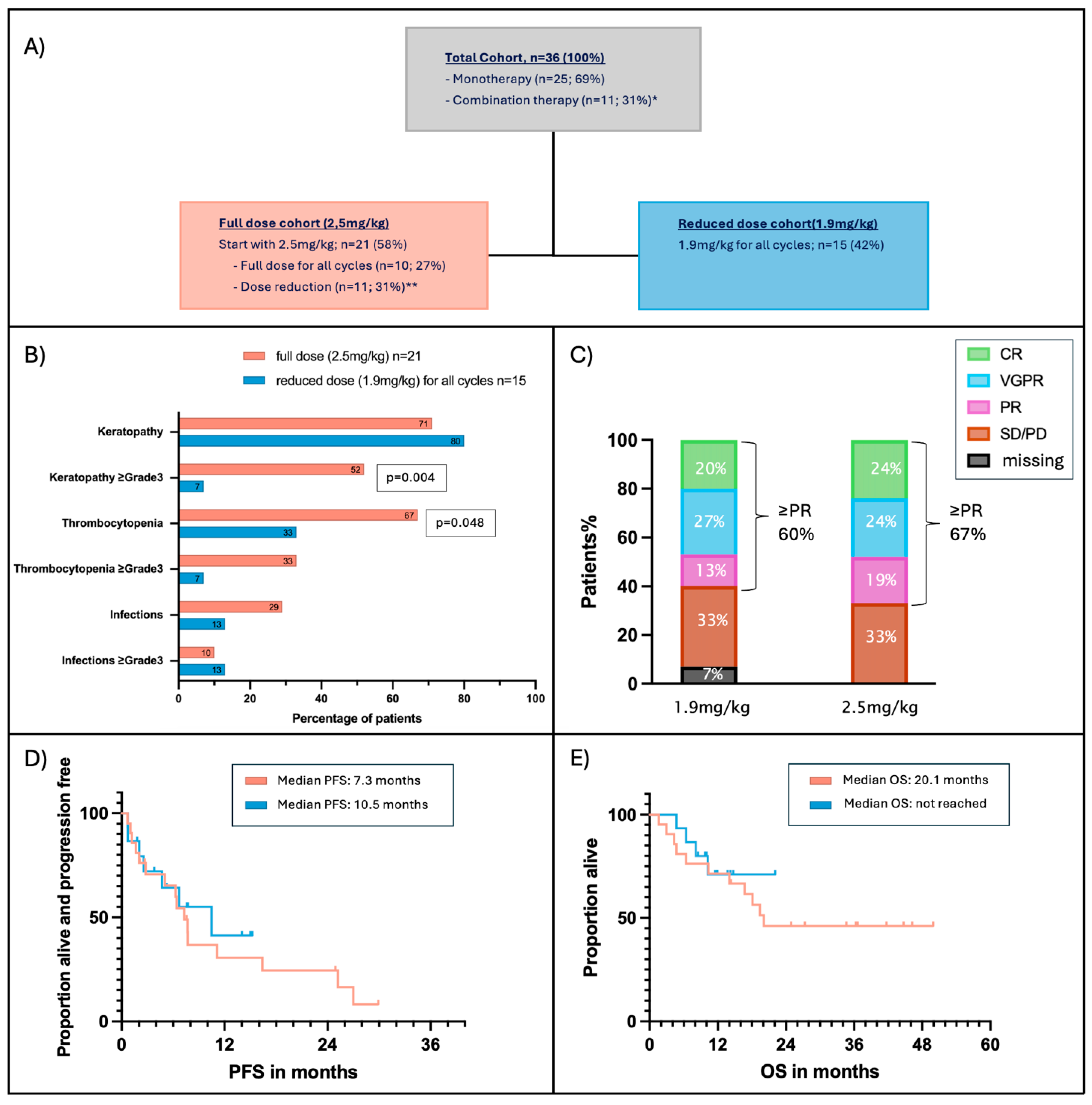
3.5. Effect of Dose Reduction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AEs | Adverse Events |
| ADC | Antibody–Drug Conjugate |
| BCMA | B-cell Maturation Antigen |
| BCVA | Best-Corrected Visual Acuity |
| CR | Complete Response |
| EK | Ethikkommission (German: Ethics Committee) |
| IMiDs | Immunomodulatory Drugs |
| IMWG | International Myeloma Working Group |
| KVA | Keratopathy and Visual Acuity (Scale) |
| MM | Multiple Myeloma |
| mAb | Monoclonal Antibody |
| MECs | Microcyst-like Epithelial Changes |
| MMAF | Monomethyl Auristatin F |
| MoAbs | Monoclonal Antibodies |
| NCI | National Cancer Institute |
| ORR | Overall Response Rate |
| OS | Overall Survival |
| PD | Progressive Disease |
| PFS | Progression-Free Survival |
| PIs | Proteasome Inhibitors |
| PR | Partial Response |
| RRMM | Relapsed/Refractory Multiple Myeloma |
| VGPR | Very Good Partial Response |
| χ2 | Chi-Square Test |
References
- Kumar, S.K.; Dispenzieri, A.; Lacy, M.Q.; Gertz, M.A.; Buadi, F.K.; Pandey, S.; Kapoor, P.; Dingli, D.; Hayman, S.R.; Leung, N.; et al. Continued improvement in survival in multiple myeloma: Changes in early mortality and outcomes in older patients. Leukemia 2014, 28, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Stalker, M.E.; Mark, T.M. Clinical Management of Triple-Class Refractory Multiple Myeloma: A Review of Current Strategies and Emerging Therapies. Curr. Oncol. 2022, 29, 4464–4477. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.J.; Hungria, V.; Mohty, M.; Mateos, M.V. How I treat triple-class refractory multiple myeloma. Br. J. Haematol. 2022, 198, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, R.; Abouzaid, S.; Bonafede, M.; Cai, Q.; Parikh, K.; Cosler, L.; Richardson, P. Trends in overall survival and costs of multiple myeloma, 2000–2014. Leukemia 2017, 31, 1915–1921. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V. Multiple myeloma: 2022 update on diagnosis, risk stratification, and management. Am. J. Hematol. 2022, 97, 1086–1107. [Google Scholar] [CrossRef] [PubMed]
- Sonneveld, P.; Dimopoulos, M.A.; Boccadoro, M.; Quach, H.; Ho, P.J.; Beksac, M.; Hulin, C.; Antonioli, E.; Leleu, X.; Mangiacavalli, S.; et al. Daratumumab, Bortezomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2024, 390, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Mikhael, J. Treatment Options for Triple-class Refractory Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2020, 20, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.K.; Dimopoulos, M.A.; Kastritis, E.; Terpos, E.; Nahi, H.; Goldschmidt, H.; Hillengass, J.; Leleu, X.; Beksac, M.; Alsina, M.; et al. Natural history of relapsed myeloma, refractory to immunomodulatory drugs and proteasome inhibitors: A multicenter IMWG study. Leukemia 2017, 31, 2443–2448. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.F.; Yee, C.W.; Gorsh, B.; Zichlin, M.L.; Paka, P.; Bhak, R.H.; Boytsov, N.; Khanal, A.; Noman, A.; DerSarkissian, M.; et al. Treatment patterns and overall survival of patients with double-class and triple-class refractory multiple myeloma: A US electronic health record database study. Leuk. Lymphoma 2023, 64, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Hungria, V.T.M.; Radinoff, A.; Delimpasi, S.; Mikala, G.; Masszi, T.; Li, J.; Capra, M.; Maiolino, A.; Pappa, V.; et al. Efficacy and safety of single-agent belantamab mafodotin versus pomalidomide plus low-dose dexamethasone in patients with relapsed or refractory multiple myeloma (DREAMM-3): A phase 3, open-label, randomised study. Lancet Haematol. 2023, 10, e801–e812. [Google Scholar] [CrossRef] [PubMed]
- Hungria, V.; Robak, P.; Hus, M.; Zherebtsova, V.; Ward, C.; Ho, P.J.; Ribas de Almeida, A.C.; Hajek, R.; Kim, K.; Grosicki, S.; et al. Belantamab Mafodotin, Bortezomib, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2024, 391, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Beksac, M.; Pour, L.; Delimpasi, S.; Vorobyev, V.; Quach, H.; Spicka, I.; Radocha, J.; Robak, P.; Kim, K.; et al. Belantamab Mafodotin, Pomalidomide, and Dexamethasone in Multiple Myeloma. N. Engl. J. Med. 2024, 391, 408–421. [Google Scholar] [CrossRef] [PubMed]
- Trudel, S.; McCurdy, A.; Louzada, M.L.; Parkin, S.; White, D.; Chu, M.P.; Kotb, R.; Mian, H.; Othman, I.; Su, J.; et al. Belantamab mafodotin, pomalidomide and dexamethasone in refractory multiple myeloma: A phase 1/2 trial. Nat. Med. 2024, 30, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Lonial, S.; Lee, H.C.; Badros, A.; Trudel, S.; Nooka, A.K.; Chari, A.; Abdallah, A.O.; Callander, N.; Lendvai, N.; Sborov, D.; et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): A two-arm, randomised, open-label, phase 2 study. Lancet Oncol. 2020, 21, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Abramson, H.N. B-Cell Maturation Antigen (BCMA) as a Target for New Drug Development in Relapsed and/or Refractory Multiple Myeloma. Int. J. Mol. Sci. 2020, 21, 5192. [Google Scholar] [CrossRef] [PubMed]
- Trudel, S.; Lendvai, N.; Popat, R.; Voorhees, P.M.; Reeves, B.; Libby, E.N.; Richardson, P.G.; Anderson, L.D., Jr.; Sutherland, H.J.; Yong, K.; et al. Targeting B-cell maturation antigen with GSK2857916 antibody-drug conjugate in relapsed or refractory multiple myeloma (BMA117159): A dose escalation and expansion phase 1 trial. Lancet Oncol. 2018, 19, 1641–1653. [Google Scholar] [CrossRef] [PubMed]
- Trudel, S.; Lendvai, N.; Popat, R.; Voorhees, P.M.; Reeves, B.; Libby, E.N.; Richardson, P.G.; Hoos, A.; Gupta, I.; Bragulat, V.; et al. Antibody-drug conjugate, GSK2857916, in relapsed/refractory multiple myeloma: An update on safety and efficacy from dose expansion phase I study. Blood Cancer. J. 2019, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Nooka, A.K.; Cohen, A.; Lee, H.C.; Badros, A.Z.; Suvannasankha, A.; Callander, N.; Abdallah, A.O.; Trudel, S.; Chari, A.; Libby, E.; et al. Single-Agent Belantamab Mafodotin in Patients with Relapsed or Refractory Multiple Myeloma: Final Analysis of the DREAMM-2 Trial. Blood 2022, 140, 7301–7303. [Google Scholar] [CrossRef]
- Farooq, A.V.; Degli Esposti, S.; Popat, R.; Thulasi, P.; Lonial, S.; Nooka, A.K.; Jakubowiak, A.; Sborov, D.; Zaugg, B.E.; Badros, A.Z.; et al. Corneal Epithelial Findings in Patients with Multiple Myeloma Treated with Antibody-Drug Conjugate Belantamab Mafodotin in the Pivotal, Randomized, DREAMM-2 Study. Ophthalmol. Ther. 2020, 9, 889–911. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Morphey, A.; Diaz, F.; Chen, J.; Razmandi, A.; Richards, T. Management of Ocular Toxicity in Patients Receiving Belantamab Mafodotin. J. Adv. Pr. Oncol. 2023, 14, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Lonial, S.; Nooka, A.K.; Thulasi, P.; Badros, A.Z.; Jeng, B.H.; Callander, N.S.; Potter, H.A.; Sborov, D.; Zaugg, B.E.; Popat, R.; et al. Management of belantamab mafodotin-associated corneal events in patients with relapsed or refractory multiple myeloma (RRMM). Blood Cancer J. 2021, 11, 103. [Google Scholar] [CrossRef] [PubMed]
- Author. In Proceedings of the Poser EHA 2025, Milan, Italy, 12–15 June 2025.
- Kumar, S.; Paiva, B.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.; Lonial, S.; Bladé, J.; Mateos, M.V.; et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016, 17, e328–e346. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V.; Richardson, P.; San Miguel, J.F. Guidelines for determination of the number of prior lines of therapy in multiple myeloma. Blood 2015, 126, 921–922. [Google Scholar] [CrossRef] [PubMed]
- Hultcrantz, M.; Kleinman, D.; Vij, R.; Escalante, F.; Delforge, M.; Kotowsky, N.; Bitetti, J.; Boytsov, N.; Camadoo-O’byrne, L.; Happ, L.P.; et al. PB2114: Belantamab mafodotin for relapsed/refractory multiple myeloma: A real-world observational study update. HemaSphere 2023, 7, e20009f20004. [Google Scholar] [CrossRef]
- Alegre, A.; Benzo, G.; Alonso, R.; Martínez-López, J.; Jimenez-Ubieto, A.; Cuéllar, C.; Askari, E.; Prieto, E.; Aláez, C.; Aguado, B.; et al. Real-World Outcomes of Belantamab Mafodotin for Relapsed/Refractory Multiple Myeloma (RRMM): Preliminary Results of a Spanish Expanded Access Program (EAP). Oncol. Ther. 2023, 11, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Abeykoon, J.P.; Vaxman, J.; Patel, S.V.; Kumar, S.; Malave, G.C.; Young, K.S.; Ailawadhi, S.; Larsen, J.T.; Dispenzieri, A.; Muchtar, E.; et al. Impact of belantamab mafodotin-induced ocular toxicity on outcomes of patients with advanced multiple myeloma. Br. J. Haematol. 2022, 199, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Shragai, T.; Magen, H.; Lavi, N.; Gatt, M.; Trestman, S.; Zektser, M.; Ganzel, C.; Jarchowsky, O.; Berger, T.; Tadmor, T.; et al. Real-world experience with belantamab mafodotin therapy for relapsed/refractory multiple myeloma: A multicentre retrospective study. Br. J. Haematol. 2023, 200, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Popat, R.; Augustson, B.; Gironella, M.; Lee, C.; Cannell, P.; Patel, N.; Kasinathan, R.S.; Rogers, R.; Shaikh, M.; Curry, A.; et al. Results from Arm A of Phase 1/2 DREAMM-6 trial: Belantamab mafodotin with lenalidomide plus dexamethasone in patients with relapsed/refractory multiple myeloma. Blood Cancer J. 2024, 14, 184. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Gavriatopoulou, M.; Ntanasis-Stathopoulos, I.; Malandrakis, P.; Fotiou, D.; Migkou, M.; Theodorakakou, F.; Spiliopoulou, V.; Kostopoulos, I.V.; Syrigou, R.E.; et al. Belantamab mafodotin, lenalidomide and dexamethasone in transplant-ineligible patients with newly diagnosed multiple myeloma: Part 1 results of a phase I/II study. Haematologica 2024, 109, 2594–2605. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Terpos, E.; Boccadoro, M.; Moreau, P.; Mateos, M.-V.; Zweegman, S.; Cook, G.; Engelhardt, M.; Delforge, M.; Hajek, R.; et al. EHA–EMN Evidence-Based Guidelines for diagnosis, treatment and follow-up of patients with multiple myeloma. Nat. Rev. Clin. Oncol. 2025. [Google Scholar] [CrossRef] [PubMed]

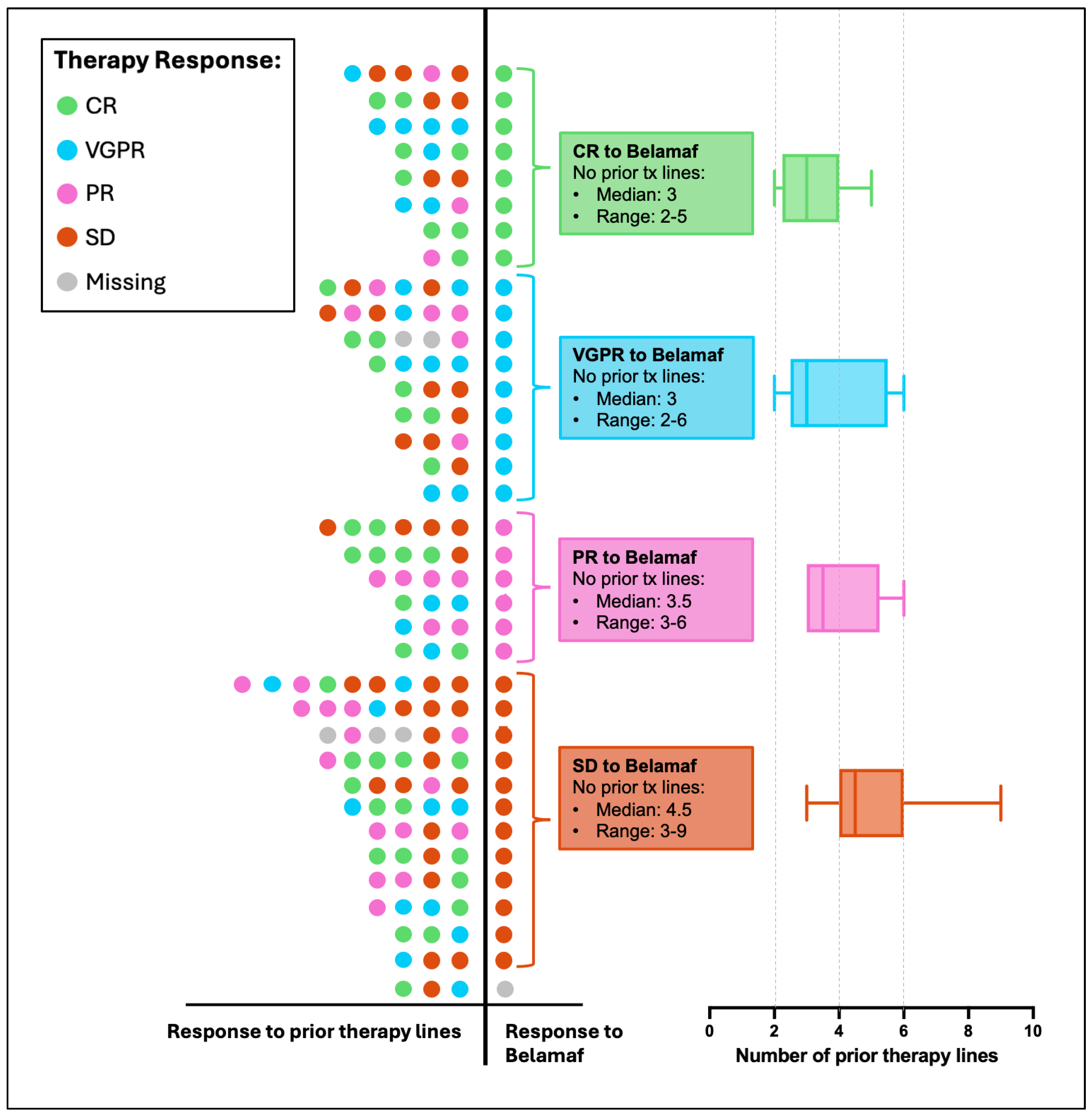
| Baseline Characteristic | Total Cohort n = 36 (100%) | |
|---|---|---|
| Patient | Age: Median (range) | 66 (54–87) |
| Sex: No (%) | ||
| Women | 22 (61%) | |
| Men | 14 (39%) | |
| Median time (in years) since diagnosis: Median (range) | 4.4 (1.2–21.7) | |
| Disease | ISS stage at screening: No (%) | |
| I | 10 (28%) | |
| II | 12 (33%) | |
| III | 12 (33%) | |
| Missing | 2 (6%) | |
| Multiple Myeloma type: No (%) * | ||
| Intact (Ig G/A/M/D) | 22 (61%) | |
| FLC | 13 (36%) | |
| Asecretory (no Paraprotein kown) | 1 (3%) | |
| High risk Cytogenetic markers: No (%) | ||
| High risk | 11 (31%) | |
| del(l17p) | 5 (14%) | |
| t(4;14) | 3 (8%) | |
| t(14;16) | 3 (8%) | |
| 1q21+ | 5 (14%) | |
| Standard risk | 9 (25%) | |
| Missing | 16 (44%) | |
| Soft tissue extramedullary disease: No (%) | 7 (19%) | |
| Bone-related paramedullary disease: No (%) | 11 (31%) | |
| Osteolytic lesions: No (%) | 32 (89%) | |
| Prior therapies | No of prior therapy lines: Median (range) | 4 (2–9) |
| Best prior observed response to any line: No (%) | ||
| Complete response (CR) | 24 (67%) | |
| Very good partial response (≥VGPR) | 32 (89%) | |
| Partial response (≥PR) | 36 (100%) | |
| Previous PIs: No (%) | 36 (100%) | |
| n = 1 | 4 (11%) | |
| n = 2 | 28 (78%) | |
| n = 3 | 4 (11%) | |
| Previous Imids: No (%) | 36 (100%) | |
| n = 1 | 9 (25%) | |
| n = 2 | 20 (56%) | |
| n = 3 | 7 (19%) | |
| Previous CD38 **: No (%) | 36 (100%) | |
| Prior PACE: No (%) *** | 6 (17%) | |
| Prior Transplant: No (%) **** | 23 (64%) | |
| Mono | 12 (33%) | |
| Tandem | 3 (8%) | |
| 2× (Second salvage ASCT) | 7 (19%) | |
| Allo (1× autologous; 1× allogenic) | 1 (3%) | |
| ORR to most recent tx line: No (%) | 24 (67%) | |
| Complete response (CR) | 8 (22%) | |
| Very good partial response (VGPR) | 8 (22%) | |
| Partial response (PR) | 8 (22%) | |
| Stable disease/Progressive disease (SD/PD) | 12 (33%) | |
| PFS to last tx line in months: Median (range) | 11.5 (0.5–36.9) | |
| Therapy free interval prior belamaf in days: median (range) | 42 (2–181) | |
| Belamaf | Therapy regimen used: No (%) | |
| Belamaf Mono | 25 (69%) | |
| Belamaf in Combination with other therapies ***** | 11 (31%) | |
| Cycles of belamaf: Median (range) | 5 (1–27) | |
| Treatment holiday (one dose only) >21 days: No (%) | 6 (17%) | |
| Therapy interval: No (%) | ||
| 3 weeks | 22 (61%) | |
| ≥4 weeks ****** | 14 (39%) | |
| Dosing interval in days: Median (range) | 31 (21–80) | |
| Percentage of cycles with prolonged tx interval: median (range) | 79% (20–100) | |
| Reason for modified therapy intervals: No (%) | ||
| Keratopathy | 9 (25%) | |
| Keratopathy prophylaxis | 3 (8%) | |
| Thrombocytopenia | 1 (3%) | |
| Treatment dose: No (%) | ||
| 2.5 mg/kg start dosis and for all cycles | 21 (58%) | |
| 2.5 mg/kg start dosis, reduction to 1.9 mg/kg | 11 (31%) | |
| 1.9 mg/kg (for all cycles) | 15 (42%) | |
| Percentage of therapy cycles in reduced treatment dose: | ||
| Median (range) | 100 (3.7–100) | |
| Reason for dose reduction: No (%) | ||
| Keratopathy | 6 (17%) | |
| Keratopathy prophylaxis | 19 (53%) | |
| Thrombocytopenia | 1 (3%) | |
| Characteristic: | Total Cohort n = 36 (100%) |
|---|---|
| Overall response rate (≥PR): No (%) | 23 (64%) |
| Complete response (CR) | 8 (22%) * |
| Very good partial response (VGPR) | 9 (25%) |
| Partial response (PR) | 6 (17%) |
| Stable disease/Progressive disease (SD/PD) | 12 (33%) |
| Response missing ** | 1 (3%) |
| Time to first observed response in days: Median (range) | 28 (8–102) |
| Complete response (CR) | 29 (8–66) |
| Very good partial response (VGPR) | 20 (12–86) |
| Partial response (PR) | 71.5 (18–102) |
| Time to best observed response in days: Median (range) | 72 (14–347) |
| Complete response (CR) | 66 (14–347) |
| Very good partial response (VGPR) | 84 (46–218) |
| Partial response (PR) | 72 (27–102) |
| Time from first to best observed response: Median (range) | 60 (9–327) |
| Primary Reason for Termination of belamaf: No (%) *** | |
| PD | 21 (58%) |
| Keratopathy | 8 (22%) |
| Thrombocytopenia | 1 (3%) |
| Reason unrelated to belamaf or PD **** | 2 (6%) |
| Belamaf ongoing | 4 (11%) |
| Characteristic: | Total Cohort n = 36 (100%) |
|---|---|
| Degree of Keratopathy described (KVA-Scale): No (%) | |
| 0 | 9 (25%) |
| 1 | 6 (17%) |
| 2 | 9 (25%) |
| 3 | 5 (14%) |
| 4 | 7 (19%) |
| Time from belamaf start to first documented visual acuity impairment in days: Median (range) | 41 (18–96) |
| Belamaf cycles until keratopathy: Median (range) | 2 (1–3) |
| Thrombocytopenia (CTCAE): No (%) | |
| 0 | 17 (47%) |
| 1 | 11 (31%) |
| 2 | 0 |
| 3 | 4 (11%) |
| 4 | 4 (11%) |
| Infections (CTCAE): No (%) * | |
| 0 | 28 (78%) |
| 1 | 2 (6%) |
| 2 | 2 (6%) |
| 3 | 4 (11%) |
| 4 | 0 |
| Hospitalization ≥1 time: No (%) | 8 (22%) ** |
| Other AE | 6 (17%) *** |
| DREAMM-1 PMID: 30442502 | DREAMM-2 PMID: 31859245 | DREAMM-3 PMID: 37793771 | DREAMM-6 PMID: 39433730 | ALGONQUIN trial PMID: 38177852 | Hultcrantz et al. [25] PMCID: PMC10429714 | Alegre et al. [26] PMID: 36509945 | Abeykoon et. al. [27] PMID: 35694818 | Shragai et al. [28] PMID: 36205375 | Our Data | |
|---|---|---|---|---|---|---|---|---|---|---|
| Design | Prospective | Prospective | Prospective | Prospective | Prospective | Retrospective | Retrospective | Retrospective | Retrospective | Retrospective |
| Protocol | 3.4 mg/kg Belamaf Single Agent | Cohort 1: 2.5 mg/kg Single Agent | Cohort 1: Belamaf 2.5 mg Single Agent | 4 Cohorts * (1.9 mg/kg and 2.5 mg/kg in different intervals + Lenalidomide + Dexamethasone) | *** Belamaf (Dose: 1.9 mg; 2.5 mg or 3.4 mg /kg; Q4 W or Q8 W) + Pomalidomide + Dexamethasone | n.a. | 2.5 mg/kg Single Agent | 2.5 mg/kg (no further information) | 2.5 mg/kg and 3.4 mg/kg initial dose | 2.5 mg/kg and 1.9 mg/kg; single agent (69%) and combination therapies (31%) |
| n | 35 (Part 2) | 95 (Cohort1) | 218 (Belamaf Cohort) | 45 (all patients) | 87 (all patients) | 184 | 33 | 38 | 106 | 36 |
| Age | 60 (46–75) | 65 (60–70) | 68 (IQR: 59–74) | 68 (36–80) | 67 (36–35) | 69 (n.a.) | 70 (46–79) | 67 (49–90) | 69 | 66 (54–87) |
| No of prior therapy lines | n.a. ≥5 lines (57%) | 7 (3–21) | 4 IQR (3–4) | 3 (1–10) | 3 (1–6) | n.a. 62% ≥5 prior ty lines | 5 (3–8) | 8 (2–15) | 6 (2–11) | 4 (2–9) |
| ORR (≥PR) | 60% | 31% | 41% | 67% | 88% | 74% | 42% | 29% | 46% | 64% |
| Median PFS | 7.9 months | 2.9 months | 11.2 months | 18.4 months | 21.8 months | 4.5 months | 3 months | 2 months | 4.7 months (8.8 if ≥PR) | 7.3 months |
| Median OS | not yet sufficiently mature | not yet sufficiently mature | 21 months | n.a. | 34 months | 7.9 months | 13 | 7.2 months | 14.5 months | 20.1 months |
| Keratopathy/ ocular toxicity any grade | 63% | 71% | 12% ** | 78% (Keratopathy) | 71% | 41% (Keratopathy) | 52% (Keratopathy) | 69% (Keratopathy) | 68% (Keratopathy) | 75% |
| Keratopathy/ ocular toxicity Grade 3–4 | 9% | 27% | 4% | n.a. “Ocular AE Grade 3–4: 69%” | 55% | n.a. | 21% | 14% | 41% | 33% |
| Time to onset of keratopathy | 23 (1–84) | 36 (19–143) | n.a. | n.a. | n.a. | 39 (n.a.) | n.a. | 42 | n.a. | 41 (18–96) |
| Time to resolution of keratopathy | 35 (5–442) | 71 (57–99) | n.a. | n.a. | n.a. | n.a. | n.a. | 72 (15–126) | n.a. | 76 (36–380) |
| Thrombocytopenia any grade | 57% | 35% | 34% | 53% | 44% | n.a. | 21% | n.a. | 27% | 53% |
| Thrombocytopenia Grade 3–4 | 34% | 20% | 22% | 29% | 33% | n.a. | n.a. | n.a. | 18% | 22% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rüsing, L.Z.; Schweighofer, J.; Aschauer, J.; Jeryczynski, G.; Vospernik, L.; Gisslinger, H.; Bumberger, A.M.; Cserna, J.; Riedl, J.; Agis, H.; et al. Optimizing Belantamab Mafodotin in Relapsed or Refractory Multiple Myeloma: Impact of Dose Modifications on Adverse Events and Hematologic Response in a Real-World Retrospective Study. Cancers 2025, 17, 2398. https://doi.org/10.3390/cancers17142398
Rüsing LZ, Schweighofer J, Aschauer J, Jeryczynski G, Vospernik L, Gisslinger H, Bumberger AM, Cserna J, Riedl J, Agis H, et al. Optimizing Belantamab Mafodotin in Relapsed or Refractory Multiple Myeloma: Impact of Dose Modifications on Adverse Events and Hematologic Response in a Real-World Retrospective Study. Cancers. 2025; 17(14):2398. https://doi.org/10.3390/cancers17142398
Chicago/Turabian StyleRüsing, Lina Zoe, Jakob Schweighofer, Julia Aschauer, Georg Jeryczynski, Lea Vospernik, Heinz Gisslinger, Armin Marcus Bumberger, Julia Cserna, Julia Riedl, Hermine Agis, and et al. 2025. "Optimizing Belantamab Mafodotin in Relapsed or Refractory Multiple Myeloma: Impact of Dose Modifications on Adverse Events and Hematologic Response in a Real-World Retrospective Study" Cancers 17, no. 14: 2398. https://doi.org/10.3390/cancers17142398
APA StyleRüsing, L. Z., Schweighofer, J., Aschauer, J., Jeryczynski, G., Vospernik, L., Gisslinger, H., Bumberger, A. M., Cserna, J., Riedl, J., Agis, H., & Krauth, M.-T. (2025). Optimizing Belantamab Mafodotin in Relapsed or Refractory Multiple Myeloma: Impact of Dose Modifications on Adverse Events and Hematologic Response in a Real-World Retrospective Study. Cancers, 17(14), 2398. https://doi.org/10.3390/cancers17142398





