Enhancing Hepatocellular Carcinoma Surveillance: Comparative Evaluation of AFP, AFP-L3, DCP and Composite Models in a Biobank-Based Case-Control Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
2.2. Demographic and Clinical Data Collection
2.3. Serum Biomarker Measurements and Calculation of Scoring Models
2.4. Statistical Analysis
- (1)
- Optimal cut-offs: optimal thresholds for each biomarker and scoring model were determined using the Youden index, which maximizes the sum of sensitivity and specificity.
- (2)
- Established cut-offs: performance was also assessed using previously validated cut-offs from the literature, allowing for comparison with established thresholds for clinical use.
- (3)
- 90% specificity cut-off: to evaluate the ability of biomarkers and models to maintain high sensitivity, performance was assessed at cut-offs where specificity was strictly set at 90%.
2.5. Ethical Statement
3. Results
3.1. Baseline Characteritics
3.2. Biomarkers and Composite Model Scores
3.3. Logistic Regression Analyses
3.4. Diagnostic Performance of Biomakers and Scoring Models
3.4.1. Performance Based on Optimal Cut-Offs
3.4.2. Performance Used in Established Cut-Offs
3.4.3. Performance at the 90% Specificity Threshold
3.5. Receiver Operating Characteristic (ROC) Curve Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFP | Alpha-fetoprotein |
| AFP-L3 | Lens culinaris agglutinin-reactive AFP |
| ALBI | Albumin-bilirubin |
| ALD | Alcohol-related liver disease |
| ALP | Alkaline phosphatase |
| ALT | Alanine aminotransferase |
| AUC | Area under the curve |
| BCLC | Barcelona Clinic Liver Cancer |
| BMI | Body mass index |
| CLD | Chronic liver disease |
| CT | Computed tomography |
| DCP | Des-gamma-carboxy prothrombin |
| EASL | European Association for the Study of the Liver |
| HBV | Hepatitis B virus |
| HCC | Hepatocellular carcinoma |
| HCV | Hepatitis C virus |
| INR | International normalized ratio |
| MASLD | Metabolic-associated steatotic liver disease |
| MRI | Magnetic resonance imaging |
| NPV | Negative predictive value |
| PIVKA-II | Protein Induced by Vitamin K Absence or Antagonist-II |
| PPV | Positive predictive value |
| PVT | Portal vein thrombosis |
| ROC | Receiver operating characteristic |
| USG | Ultrasonography |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.Y.; Danpanichkul, P.; Yong, J.N.; Yu, Z.; Tan, D.J.H.; Lim, W.H.; Koh, B.; Lim, R.Y.Z.; Tham, E.K.J.; Mitra, K.; et al. Liver cancer in 2021: Global Burden of Disease study. J. Hepatol. 2025, 82, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ding, C.; Cao, M.; Yang, F.; Yan, X.; He, S.; Cao, M.; Zhang, S.; Teng, Y.; Tan, N.; et al. Global epidemiology of liver cancer 2022: An emphasis on geographic disparities. Chin. Med. J. 2024, 137, 2334–2342. [Google Scholar] [CrossRef] [PubMed]
- Sangro, B.; Argemi, J.; Ronot, M.; Paradis, V.; Meyer, T.; Mazzaferro, V.; Jepsen, P.; Golfieri, R.; Galle, P.; Dawson, L.; et al. EASL Clinical Practice Guidelines on the management of hepatocellular carcinoma. J. Hepatol. 2025, 82, 315–374. [Google Scholar] [CrossRef] [PubMed]
- Taddei, T.H.; Brown, D.B.; Yarchoan, M.; Mendiratta-Lala, M.; Llovet, J.M. Critical Update: AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology 2025, 82, 272–274. [Google Scholar] [CrossRef] [PubMed]
- Omata, M.; Cheng, A.L.; Kokudo, N.; Kudo, M.; Lee, J.M.; Jia, J.; Tateishi, R.; Han, K.H.; Chawla, Y.K.; Shiina, S.; et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol. Int. 2017, 11, 317–370. [Google Scholar] [CrossRef] [PubMed]
- Demirtas, C.O.; Brunetto, M.R. Surveillance for hepatocellular carcinoma in chronic viral hepatitis: Is it time to personalize it? World J. Gastroenterol. 2021, 27, 5536–5554. [Google Scholar] [CrossRef] [PubMed]
- Atiq, O.; Tiro, J.; Yopp, A.C.; Muffler, A.; Marrero, J.A.; Parikh, N.D.; Murphy, C.; McCallister, K.; Singal, A.G. An assessment of benefits and harms of hepatocellular carcinoma surveillance in patients with cirrhosis. Hepatology 2017, 65, 1196–1205. [Google Scholar] [CrossRef] [PubMed]
- Demirtas, C.O.; Gunduz, F.; Tuney, D.; Baltacioglu, F.; Kani, H.T.; Bugdayci, O.; Alahdab, Y.O.; Ozdogan, O.C. Annual contrast-enhanced magnetic resonance imaging is highly effective in the surveillance of hepatocellular carcinoma among cirrhotic patients. Eur. J. Gastroenterol. Hepatol. 2020, 32, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Demirtas, C.O.; Ozdogan, O.C. Surveillance of hepatocellular carcinoma in cirrhotic patients: Current knowledge and future directions. Hepatol. Forum. 2020, 1, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Bertino, G.; Ardiri, A.; Malaguarnera, M.; Malaguarnera, G.; Bertino, N.; Calvagno, G.S. Hepatocellualar carcinoma serum markers. Semin. Oncol. 2012, 39, 410–433. [Google Scholar] [CrossRef] [PubMed]
- Schotten, C.; Ostertag, B.; Sowa, J.-P.; Manka, P.; Bechmann, L.P.; Hilgard, G.; Marquardt, C.; Wichert, M.; Toyoda, H.; Lange, C.M.; et al. GALAD Score Detects Early-Stage Hepatocellular Carcinoma in a European Cohort of Chronic Hepatitis B and C Patients. Pharmaceuticals 2021, 14, 735. [Google Scholar] [CrossRef] [PubMed]
- Miyaaki, H.; Nakashima, O.; Kurogi, M.; Eguchi, K.; Kojiro, M. Lens culinaris agglutinin-reactive alpha-fetoprotein and protein induced by vitamin K absence II are potential indicators of a poor prognosis: A histopathological study of surgically resected hepatocellular carcinoma. J. Gastroenterol. 2007, 42, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Yang, J.; Xu, L.; Dai, W.; Wang, F.; Shen, M.; Zhang, Y.; Zhang, H.; Chen, K.; Cheng, P.; et al. Diagnostic Performance of Des-gamma-carboxy Prothrombin for Hepatocellular Carcinoma: A Meta-Analysis. Gastroenterol. Res. Pract. 2014, 2014, 529314. [Google Scholar] [CrossRef] [PubMed]
- Marrero, J.A.; Feng, Z.; Wang, Y.; Nguyen, M.H.; Befeler, A.S.; Roberts, L.R.; Reddy, K.R.; Harnois, D.; Llovet, J.M.; Normolle, D.; et al. Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology 2009, 137, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Tayob, N.; Mehta, A.; Marrero, J.A.; El-Serag, H.; Jin, Q.; de Viteri, C.S.; Fobar, A.; Parikh, N.D. GALAD demonstrates high sensitivity for HCC surveillance in a cohort of patients with cirrhosis. Hepatology 2022, 75, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.-C.; Zhang, S.-Y.; Ding, Q.; Li, N.; Fu, T.-T.; Zhang, G.-X.; He, Q.-Q.; Shen, F.; Yang, T.; Zhu, H. The Performance of GALAD Score for Diagnosing Hepatocellular Carcinoma in Patients with Chronic Liver Diseases: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 949. [Google Scholar] [CrossRef] [PubMed]
- Villa, E.; Donghia, R.; Baldaccini, V.; Tedesco, C.C.; Shahini, E.; Cozzolongo, R.; Ascari, S.; Pesole, P.L.; Coletta, S.; Critelli, R.M.; et al. GALAD outperforms aMAP and ALBI for predicting HCC in patients with compensated advanced chronic liver disease: A 12-year prospective study. Hepatol Commun. 2023, 7, e0262. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wu, R.; Liu, X.; Xu, H.; Chi, X.; Wang, X.; Zhan, M.; Wang, B.; Peng, F.; Gao, X.; et al. Validation of the GALAD Model and Establishment of GAAP Model for Diagnosis of Hepatocellular Carcinoma in Chinese Patients. J. Hepatocell. Carcinoma 2020, 7, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhao, Y.; Cai, W.; Ming, A.; Li, H. Validation and update of a multivariable prediction model for the identification and management of patients at risk for hepatocellular carcinoma. Clin. Proteomics 2021, 18, 21. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Papatheodoridis, G.; Sun, J.; Innes, H.; Toyoda, H.; Xie, Q.; Mo, S.; Sypsa, V.; Guha, I.N.; Kumada, T.; et al. aMAP risk score predicts hepatocellular carcinoma development in patients with chronic hepatitis. J. Hepatol. 2020, 73, 1368–1378. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Devarajan, K.; Singal, A.G.; Marrero, J.A.; Dai, J.; Feng, Z.; Rinaudo, J.A.S.; Srivastava, S.; Evans, A.; Hann, H.-W.; et al. The Doylestown Algorithm: A Test to Improve the Performance of AFP in the Detection of Hepatocellular Carcinoma. Cancer Prev. Res. 2016, 9, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Kagebayashi, C.; Yamaguchi, I.; Akinaga, A.; Kitano, H.; Yokoyama, K.; Satomura, M.; Kurosawa, T.; Watanabe, M.; Kawabata, T.; Chang, W.; et al. Automated immunoassay system for AFP-L3% using on-chip electrokinetic reaction and separation by affinity electrophoresis. Anal. Biochem. 2009, 388, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.J.; Pirrie, S.J.; Cox, T.F.; Berhane, S.; Teng, M.; Palmer, D.; Morse, J.; Hull, D.; Patman, G.; Kagebayashi, C.; et al. The detection of hepatocellular carcinoma using a prospectively developed and validated model based on serological biomarkers. Cancer Epidemiol. Biomarkers Prev. 2014, 23, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.M.; Wang, T.; Zhang, K.H. AFP-L3 for the diagnosis of early hepatocellular carcinoma: A meta-analysis. Medicine 2021, 100, e27673. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Li, M.; Tian, Q.B.; Liu, D.W. Diagnostic performance of des-gamma-carboxy prothrombin (DCP) for hepatocellular carcinoma: A bivariate meta-analysis. Neoplasma 2012, 59, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Fang, M.; Xiao, X.; Wang, H.; Gao, Z.; Ji, J.; Liu, L.; Gu, E.; Li, Y.; Wang, M.; et al. Validation of the GALAD model for early diagnosis and monitoring of hepatocellular carcinoma in Chinese multicenter study. Liver Int. 2022, 42, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Best, J.; Bilgi, H.; Heider, D.; Schotten, C.; Manka, P.; Bedreli, S.; Gorray, M.; Ertle, J.; van Grunsven, L.A.; Dechêne, A. The GALAD scoring algorithm based on AFP, AFP-L3, and DCP significantly improves detection of BCLC early stage hepatocellular carcinoma. Z. Gastroenterol. 2016, 54, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.D.; Mai, S.H.; Lam, H.T. Evaluating the GALAD Score for Detection of Hepatocellular Carcinoma in Patients With Cirrhosis. J. Clin. Gastroenterol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Berhane, S.; Toyoda, H.; Tada, T.; Kumada, T.; Kagebayashi, C.; Satomura, S.; Schweitzer, N.; Vogel, A.; Manns, M.P.; Benckert, J.; et al. Role of the GALAD and BALAD-2 Serologic Models in Diagnosis of Hepatocellular Carcinoma and Prediction of Survival in Patients. Clin. Gastroenterol. Hepatol. 2016, 14, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Caviglia, G.P.; Abate, M.L.; Petrini, E.; Gaia, S.; Rizzetto, M.; Smedile, A. Highly sensitive alpha-fetoprotein, Lens culinaris agglutinin-reactive fraction of alpha-fetoprotein and des-gamma-carboxyprothrombin for hepatocellular carcinoma detection. Hepatol Res. 2016, 46, E130–E135. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.D.; Addissie, B.D.; Lavu, S.; Cvinar, J.L.; Giama, N.H.; Moser, C.D.; Miyabe, K.; Allotey, L.K.; Algeciras-Schimnich, A.; Theobald, J.P.; et al. GALAD Score for Hepatocellular Carcinoma Detection in Comparison with Liver Ultrasound and Proposal of GALADUS Score. Cancer Epidemiol. Biomarkers Prev. 2019, 28, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Best, J.; Bechmann, L.P.; Sowa, J.P.; Sydor, S.; Dechêne, A.; Pflanz, K.; Bedreli, S.; Schotten, C.; Geier, A.; Berg, T.; et al. GALAD Score Detects Early Hepatocellular Carcinoma in an International Cohort of Patients With Nonalcoholic Steatohepatitis. Clin. Gastroenterol. Hepatol. 2020, 18, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Beudeker, B.J.; Fu, S.; Balderramo, D.; Mattos, A.Z.; Carrera, E.; Diaz, J.; Prieto, J.; Banales, J.; Vogel, A.; Arrese, M.; et al. Validation and optimization of AFP-based biomarker panels for early HCC detection in Latin America and Europe. Hepatol Commun. 2023, 7, e0264. [Google Scholar] [CrossRef] [PubMed]
- Tayob, N.; Kanwal, F.; Alsarraj, A.; Hernaez, R.; El-Serag, H.B. The Performance of AFP, AFP-3, DCP as Biomarkers for Detection of Hepatocellular Carcinoma (HCC): A Phase 3 Biomarker Study in the United States. Clin. Gastroenterol. Hepatol. 2023, 21, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Marsh, T.L.; Parikh, N.D.; Roberts, L.R.; Schwartz, M.E.; Nguyen, M.H.; Befeler, A.; Page-Lester, S.; Tayob, N.; Srivastava, S.; Rinaudo, J.A.; et al. Phase 3 Biomarker Validation of GALAD for the Detection of Hepatocellular Carcinoma in Cirrhosis. Gastroenterology 2025, 168, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Liou, W.; Tan, S.; Yamada, H.; Krishnamoorthy, T.; Chang, J.P.; Yeo, C.; Tan, C. Performance of the GALAD Model in an Asian Cohort Undergoing Hepatocellular Carcinoma Surveillance: A Prospective Cohort Study. J. Gastroenterol. Hepatol. 2025, 40, 1818–1824. [Google Scholar] [CrossRef] [PubMed]
- Safri, F.; Nguyen, R.; Zerehpooshnesfchi, S.; George, J.; Qiao, L. Heterogeneity of hepatocellular carcinoma: From mechanisms to clinical implications. Cancer Gene Ther. 2024, 31, 1105–1112. [Google Scholar] [CrossRef] [PubMed]

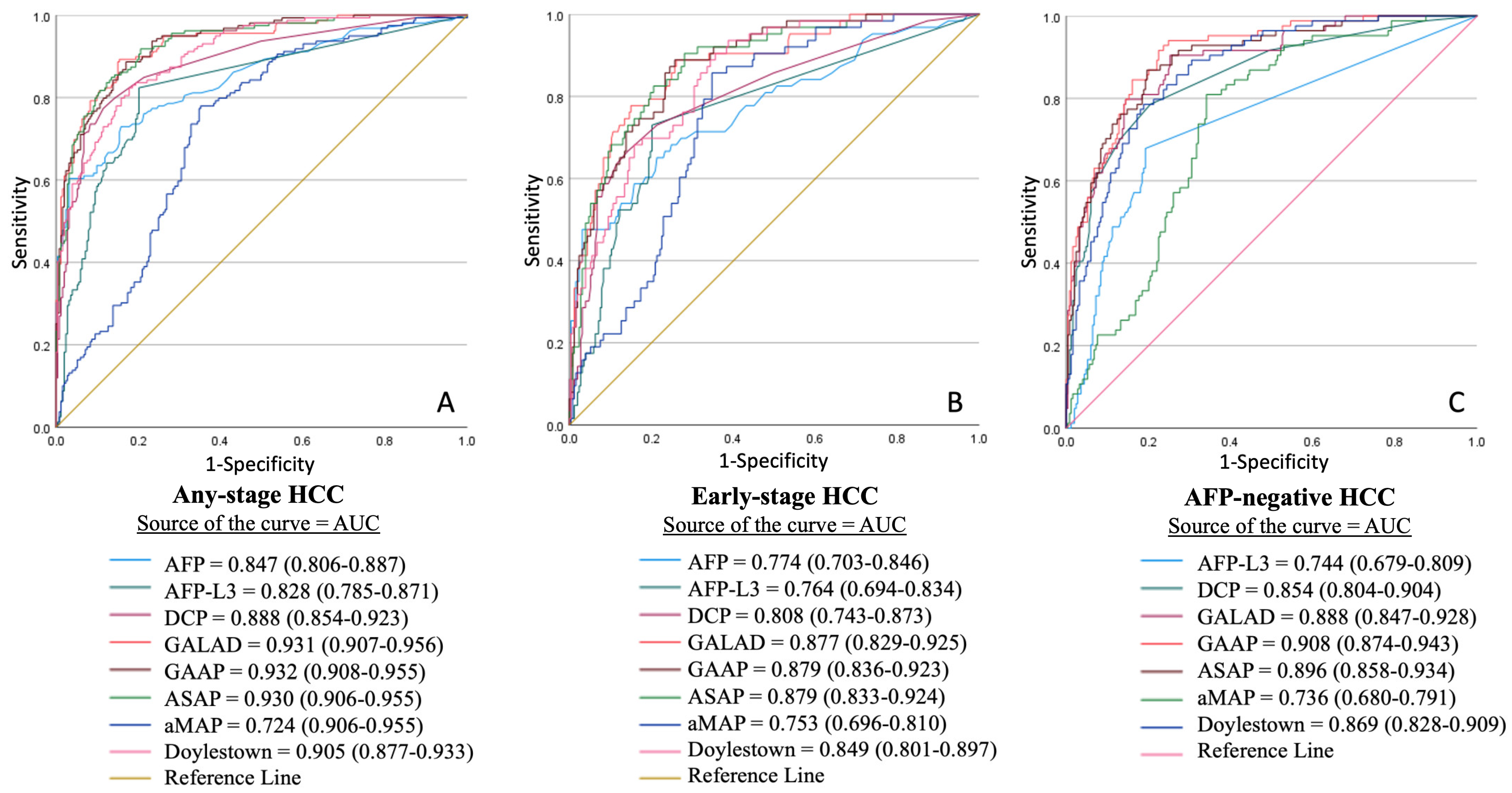
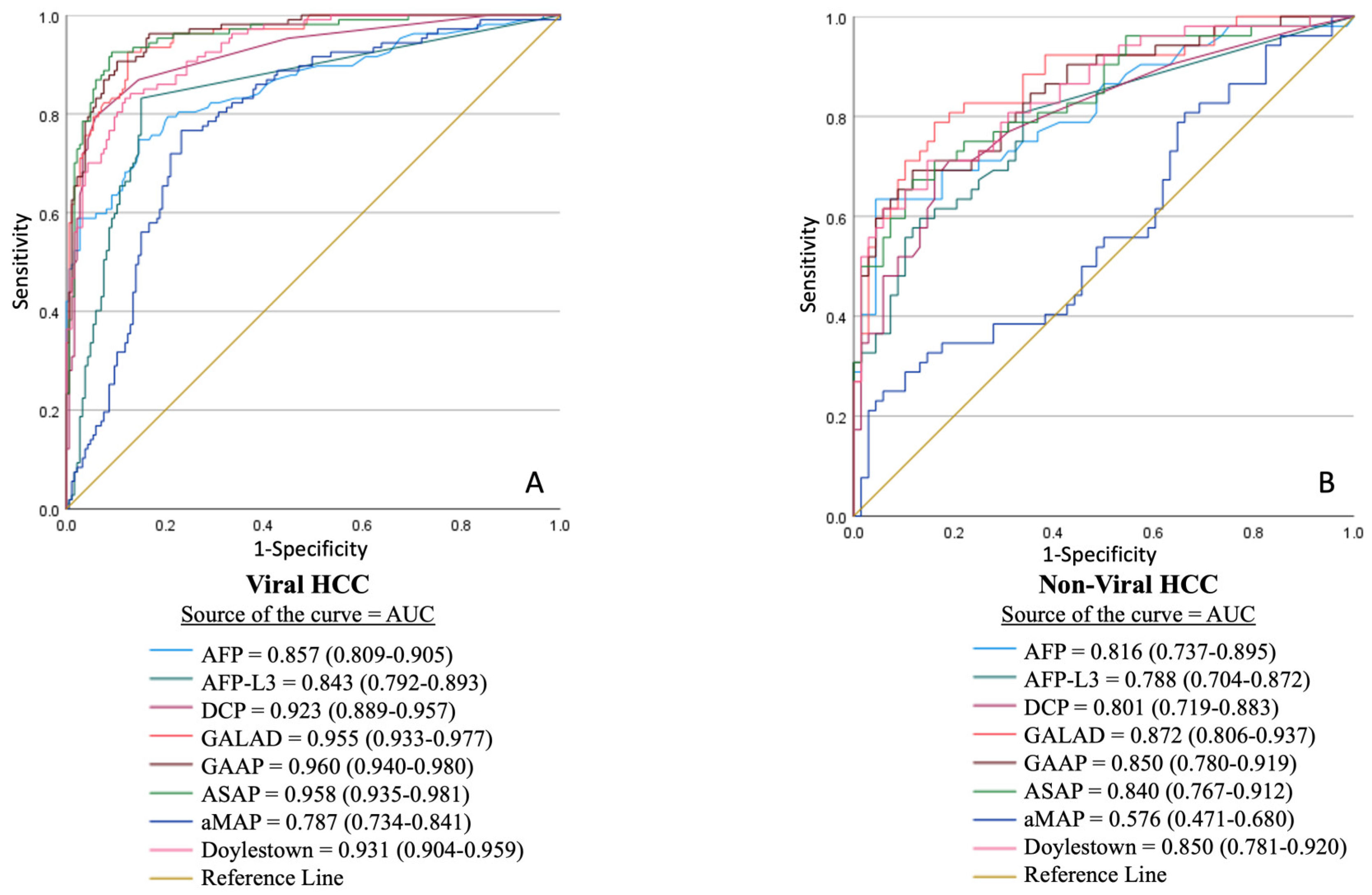
| Overall Cohort (n = 562) | Controls (n = 120) | CLD (n = 277) | HCC (n = 165) | p-Value | |
|---|---|---|---|---|---|
| Age, median (IQR), years | 59 (19–88) | 45 (18–89) | 54 (19–81) | 65 (37–88) | <0.001 * |
| Gender, n (%) | <0.001 * | ||||
| - Male | 292 (66.1) | 64 (53.3) | 165 (59.6) | 127 (77.0) | |
| - Female | 150 (33.9) | 56 (46.7) | 112 (40.4) | 38 (23.0) | |
| Body mass index, median (IQR),kg/m2 | 27.7 (16.5–46.7) | 24.7 (18.9–29.7) | 27.7 (16.9–46.7) | 26.9 (16.5–45.7) | 0.284 |
| Diabetes mellitus, n (%) | 136 (30.8) | - | 75 (27.2) | 61 (37.2) | 0.033 * |
| Hypertension, n (%) | 120 (27.1) | - | 61 (22.2) | 59 (36.2) | 0.002 * |
| Hyperlipidemia, n (%) | 36 (8.1) | - | 18 (6.6) | 18 (11.5) | 0.103 |
| Etiology, n (%) | 0.002 * | ||||
| - HBV | 277 (62.7) | - | 189 (68.2) | 88 (53.3) | |
| - MASLD-cryptogenic | 105 (23.8) | - | 57 (20.6) | 48 (29.1) | |
| - HCV | 34 (7.7) | - | 12 (4.3) | 22 (13.3) | |
| - ALD | 14 (3.2) | - | 10 (3.6) | 4 (2.4) | |
| - Autoimmune—PBC | 10 (2.3) | - | 7 (2.5) | 3 (1.8) | |
| - Wilson’s disease | 1 (0.2) | - | 1 (0.4) | - | |
| - Budd–Chiari | 1 (0.2) | - | 1 (0.4) | - | |
| Cirrhosis, n (%) | 296 (67.0) | - | 146 (52.7) | 150 (90.9) | <0.001 * |
| CTP score, median (IQR) | 5 (5–12) | - | 5 (5–10) | 6 (5–12) | <0.001 * |
| MELD score, median (IQR) | 8 (6–28) | - | 7 (6–28) | 10 (6–28) | <0.001 * |
| ALBI score, median (IQR) | −2.80 (−4.39–1.03) | - | −2.98 (−3.76–0.71) | −2.39 (−4.39–1.03) | <0.001 * |
| Ascites, n (%) | 101 (22.9) | - | 44 (16.0) | 57 (35.2) | <0.001 * |
| Esophageal varices, n (%) | 143 (32.4) | - | 79 (30.6) | 64 (44.4) | 0.007 * |
| Variceal bleeding, n (%) | 27 (6.1) | - | 11 (4.0) | 16 (9.9) | 0.022 * |
| Albumin, median (IQR), gr/dL | 4.2 (1.8–6.4) | - | 3.9 (1.8–5.1) | 3.7 (2.1–6.4) | <0.001 * |
| Total bilirubin, median (IQR), mg/dL | 0.865 (0.1–29.0) | - | 1.1 (0.3–18.2) | 1.2 (0.2–29.0) | <0.001 * |
| Creatinine, median (IQR), mg/dL | 0.8 (0.3–4.4) | - | 0.7 (0.3–2.0) | 0.8 (0.4–4.4) | 0.610 |
| Sodium, median (IQR), mEq/L | 140 (121–146) | - | 140.0 (122.0–146.0) | 138.0 (121.0–145.0) | <0.001 * |
| Platelet count, median (IQR), ×1000/m3 | 164 (28–838) | - | 103 (28–430) | 147.5 (36–838) | 0.041 * |
| INR, median (IQR) | 1.2 (0.9–3.5) | - | 1.2 (0.9–3.5) | 1.2 (0.9–3.5) | <0.001 * |
| AFP, median (IQR), ng/mL | 3.2 (0.3–200,000.0) | 2.1 (0.3–5.8) | 2.9 (0.7–253.4) | 16.8 (1.1–200,000.0) | <0.001 * |
| AFP-L3, median (IQR), % | 0.5 (0.5–94.4) | 0.5 (0.5–0.5) | 0.5 (0.5–90.6) | 15.9 (0.5–94.4) | <0.001 * |
| DCP, median (IQR), ng/mL | 0.3 (0.1–14,980.0) | 0.3 (0.1–0.6) | 0.2 (0.1–413.2) | 4.8 (0.1–14,980.0) | <0.001 * |
| GALAD | −3.1 (−9.8–16.7) | −5.0 (−9.8–−1.1) | −3.7 (−8.9–6.1) | 2.5 (−4.8–16.7) | <0.001 * |
| GAAP | −6.1 (−12.6–10.4) | −7.6 (−10.1–−4.1) | −6.6 (−12.6–2.1) | −1.2 (−7.6–10.4) | <0.001 * |
| ASAP | −5.9 (−10.4–9.8) | −6.7 (−9.0–−4.7) | −6.4 (−10.4–2.0) | −1.7 (−7.0–9.8) | <0.001 * |
| aMAP | 55.3 (19.8–80.3) | 44.0 (23.3–64.5) | 55.1 (24.7–80.3) | 63.4 (19.8–80.2) | <0.001 * |
| Doylestown | 0.2 (0.0–1.0) | 0.0 (0.0–0.5) | 0.1 (0.0–1.0) | 0.8 (0.1–1.0) | <0.001 * |
| Univariate | Multivariate | ||||
|---|---|---|---|---|---|
| p-Value | Beta-Coefficient | aOR | 95% CI | p-Value | |
| AFP | <0.001 * | 0.039 | 1.040 + | 1.016–1.064 | <0.001 * |
| AFP-L3 | <0.001 * | 0.069 | 1.071 # | 1.051–1.092 | <0.001 * |
| DCP | <0.001 * | 0.015 | 1.015 ˆ | 1.003–1.027 | 0.011 * |
| GALAD | <0.001 * | 0.773 | 2.166 ¶ | 1.769–2.651 | <0.001 * |
| ASAP | <0.001 * | 0.728 | 2.072 † | 1.704–2.519 | <0.001 * |
| GAAP | <0.001 * | 0.769 | 2.157 † | 1.759–2.646 | <0.001 * |
| aMAP | <0.001 * | −0.023 | 0.977 ¶ | 0.935–1.021 | 0.298 |
| Doylestown | <0.001 * | 4.874 | 130.81 ⊠ | 37.257–459.277 | <0.001 * |
| Any-Stage HCC (n = 165) | Early-Stage HCC (n = 64) | AFP-Negative HCC (n = 88) | Viral HCC (n = 110) | Non-Viral HCC (n = 55) | |
|---|---|---|---|---|---|
| Optimal cut-off | |||||
| AFP | 6.15 | 5.25 | - | 6.30 | 13.20 |
| AFP-L3 | 0.90 | 0.95 | 7.15 | 0.95 | 9.95 |
| DCP | 0.35 | 0.35 | 0.45 | 0.55 | 1.15 |
| GALAD | −1.96 | −2.55 | −1.70 | −1.70 | −0.43 |
| GAAP | −5.09 | −5.49 | −5.09 | −4.9 | −1.81 |
| ASAP | −5.32 | −5.67 | −5.17 | −5.0 | −4.19 |
| aMAP | 60.19 | 60.44 | 61.96 | 60.20 | 67.39 |
| Doylestown | 0.33 | 0.23 | 0.38 | 0.48 | 0.63 |
| Sensitivity, (%) | |||||
| AFP | 72.1 (64.6–78.8) | 64.1 (51.1–75.7) | - | 73.6 (64.4–81.6) | 63.6 (49.6–76.2) |
| AFP-L3 | 82.4 (75.7–87.9) | 73.4 (60.9–83.7) | 58.0 (47.0–68.4) | 82.7 (74.4–89.3) | 61.8 (47.7–74.6) |
| DCP | 85.5 (79.13–90.45) | 73.4 (60.9–83.7) | 71.6 (61.0–80.7) | 80.0 (71.3–87.0) | 70.9 (57.1–82.4) |
| GALAD | 90.3 (84.73–94.36) | 89.1 (78.6–95.5) | 80.7 (70.9–88.3) | 92.7 (86.2–96.8) | 72.7 (59.0–83.9) |
| GAAP | 88.8 (82.8–93.2) | 85.7 (74.6–93.3) | 84.7 (75.3–91.6) | 87.0 (79.2–92.7) | 59.6 (45.1–73.0) |
| ASAP | 87.5 (81.4–92.2) | 84.1 (72.7–92.1) | 77.4 (67.3–86.0) | 87.0 (79.2–92.7) | 71.2 (56.9–82.9) |
| aMAP | 72.8 (65.3–79.5) | 79.4 (67.3–88.5) | 57.0 (45.9–67.6) | 70.6 (61.2–79.0) | 37.7 (24.8–52.1) |
| Doylestown | 83.8 (77.1–89.1) | 76.2 (63.8–86.0) | 72.9 (62.2–82.0) | 70.4 (60.8–78.8) | 71.2 (56.9–82.9) |
| Specificity, (%) | |||||
| AFP | 84.8 (80.1–88.8) | 78.7 (73.4–83.4) | - | 86.1 (80.5–90.5) | 94.7 (87.1–98.6) |
| AFP-L3 | 80.5 (75.3–85.0) | 80.5 (75.3–85.0) | 84.6 (79.7–88.6) | 85.1 (79.4–89.7) | 86.8 (77.1–93.5) |
| DCP | 78.7 (73.40–83.37) | 78.7 (73.4–83.4) | 87.5 (83.0–91.2) | 94.5 (90.4–97.2) | 84.2 (74.0–91.6) |
| GALAD | 80.5 (75.3–85.0) | 73.6 (68.0–78.7) | 86.0 (81.3–89.9) | 88.6 (83.3–92.6) | 88.2 (78.7–94.4) |
| GAAP | 83.1 (80.0–87.5) | 76.5 (70.8–81.5) | 84.1 (78.9–88.4) | 91.4 (86.5–95.0) | 95.6 (87.6–99.1) |
| ASAP | 82.0 (76.7–86.5) | 76.1 (70.3–81.2) | 85.3 (80.3–89.4) | 94.1 (89.7–97.0) | 83.8 (72.9–91.6) |
| aMAP | 67.0 (61.1–72.6) | 67.4 (61.5–73.0) | 72.9 (67.2–78.2) | 79.1 (72.7–84.6) | 73.0 (61.4–82.7) |
| Doylestown | 80.0 (74.6–84.7) | 71.8 (65.8–77.2) | 83.7 (78.5–88.0) | 95.2 (91.1–97.8) | 83.8 (72.9–91.6) |
| PPV, (%) | |||||
| AFP | 73.9 (67.8–79.1) | 41.0 (34.2–48.2) | - | 74.3 (66.8–80.6) | 89.7 (76.6–95.9) |
| AFP-L3 | 71.5 (66.2–76.4) | 46.5 (39.7–53.6) | 54.8 (46.6–62.8) | 75.2 (68.3–81.0) | 77.3 (64.8–86.3) |
| DCP | 70.5 (65.4–75.1) | 44.3 (37.8–51.1) | 65.0 (56.9–72.3) | 88.9 (81.7–93.5) | 76.5 (65.3–84.9) |
| GALAD | 73.4 (68.4–77.9) | 43.9 (38.7–49.2) | 65.1 (57.8–71.9) | 81.6 (75.1–86.7) | 81.6 (70.2–89.3) |
| GAAP | 76.7 (71.4–81.4) | 47.4 (41.4–53.4) | 64.3 (57.2–70.8) | 85.5 (78.5–90.4) | 91.2 (77.0–97.0) |
| ASAP | 75.3 (70.0–79.9) | 46.5 (40.5–52.6) | 64.1 (56.5–71.0) | 89.5 (82.7–93.8) | 77.1 (65.6–85.6) |
| aMAP | 57.0 (52.2–61.7) | 36.2 (31.5–41.3) | 41.0 (34.2–47.1) | 65.3 (58.2–71.7) | 50.0 (37.5–62.5) |
| Doylestown | 72.4 (67.1–77.2) | 40.0 (34.4–45.9) | 60.2 (52.6–67.3) | 89.4 (81.5–94.2) | 77.1 (65.6–85.6) |
| NPV, (%) | |||||
| AFP | 83.6 (79.9–86.7) | 90.5 (87.2–92.9) | - | 85.6 (81.3–89.1) | 78.3 (71.7–83.7) |
| AFP-L3 | 88.5 (84.6–91.5) | 92.9 (89.7–95.2) | 86.1 (82.9–88.9) | 90.0 (85.6–93.2) | 75.9 (69.0–81.7) |
| DCP | 90.1 (86.2–92.9) | 92.8 (89.5–95.1) | 90.5 (87.2–93.0) | 89.6 (85.6–92.6) | 80.0 (72.4–85.9) |
| GALAD | 93.3 (89.7–95.7) | 96.7 (93.5–98.3) | 93.2 (90.0–95.5) | 95.7 (91.9–97.8) | 81.7 (74.2–87.4) |
| GAAP | 92.2 (88.4–94.8) | 95.6 (92.2–97.6) | 94.2 (90.8–96.4) | 92.4 (88.2–95.2) | 75.6 (68.9–81.2) |
| ASAP | 91.3 (87.3–94.1) | 95.1 (91.6–97.2) | 91.9 (88.3–94.4) | 92.6 (88.5–95.4) | 79.2 (71.0–85.5) |
| aMAP | 80.4 (75.9–84.3) | 93.3 (89.5–95.8) | 84.0 (80.3–87.1) | 82.9 (78.2–86.7) | 62.1 (56.0–67.8) |
| Doylestown | 88.7 (84.6–91.9) | 92.4 (88.6–95.0) | 90.1 (86.5–92.9) | 84.8 (80.6–88.2) | 79.2 (71.0–85.5) |
| Any-Stage HCC (n = 165) | Early-Stage HCC (n = 64) | AFP-Negative HCC (n = 88) | Viral HCC (n = 110) | Non-Viral HCC (n = 55) | |
|---|---|---|---|---|---|
| Established cut-off | |||||
| AFP | 20 | 20 | - | 20 | 20 |
| AFP-L3 | 10 | 10 | 10 | 10 | 10 |
| DCP | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 |
| GALAD | −0.63 | −0.63 | −0.63 | −0.63 | −0.63 |
| GAAP | −0.65 | −0.65 | −0.65 | −0.65 | −0.65 |
| ASAP | 0.56 | 0.56 | 0.56 | 0.56 | 0.56 |
| aMAP | 60 | 60 | 60 | 60 | 60 |
| Doylestown | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Sensitivity, (%) | |||||
| AFP | 46.7 (38.9–54.6) | 34.4 (23.0–47.3) | - | 47.3 (37.7–57.0) | 45.5 (32.0–59.5) |
| AFP-L3 | 61.2 (53.3–68.7) | 46.9 (34.3–59.8) | 44.3 (33.7–55.3) | 60.9 (51.1–70.1) | 61.8 (47.7–74.6) |
| DCP | 43.6 (35.9–51.6) | 15.6 (7.8–26.9) | 31.8 (22.3–42.6) | 44.6 (35.1–54.3) | 41.8 (28.7–55.9) |
| GALAD | 75.8 (68.5–82.1) | 57.8 (44.8–70.1) | 58.0 (47.0–68.4) | 76.4 (67.3–83.9) | 74.6 (61.0–85.3) |
| GAAP | 43.8 (35.9–51.8) | 19.1 (10.3–30.9) | 24.7 (16.0–35.3) | 41.7 (32.3–51.6) | 48.1 (31.0–62.4) |
| ASAP | 32.5 (25.3–40.3) | 12.7 (5.7–23.5) | 16.5 (9.3–26.1) | 32.4 (23.7–42.1) | 32.7 (20.3–47.1) |
| aMAP | 72.8 (65.3–79.5) | 79.4 (67.3–88.5) | 72.1 (61.4–81.2) | 70.6 (61.2–79.0) | 77.4 (63.8–87.7) |
| Doylestown | 71.3 (63.6–78.1) | 54.0 (40.9–66.6) | 58.8 (47.6–69.4) | 69.4 (59.8–78.0) | 75.0 (61.1–86.0) |
| Specificity, (%) | |||||
| AFP | 98.2 (95.8–99.4) | 98.2 (95.8–99.4) | - | 99.5 (97.3–100.0) | 94.7 (87.1–98.6) |
| AFP-L3 | 89.2 (84.9–92.6) | 89.2 (84.9–92.6) | 90.4 (86.3–93.7) | 90.1 (85.1–93.8) | 86.8 (77.1–93.5) |
| DCP | 97.5 (94.9–99.0) | 97.5 (94.9–99.0) | 98.2 (95.8–99.4) | 98.5 (95.7–99.7) | 94.7 (87.1–98.6) |
| GALAD | 93.5 (89.9–96.1) | 93.5 (89.9–96.1) | 94.9 (91.5–97.2) | 96.5 (93.0–98.6) | 85.5 (75.6–92.6) |
| GAAP | 98.8 (96.6–99.8) | 98.8 (96.6–99.8) | 99.6 (97.8–100.0) | 99.5 (97.1–100.0) | 97.1 (89.8–99.6) |
| ASAP | 99.2 (97.2–99.9) | 99.2 (97.2–99.9) | 99.6 (97.8–100.0) | 99.5 (97.1–100.0) | 98.5 (92.1–100.0) |
| aMAP | 65.9 (59.9–71.6) | 65.9 (59.9–71.6) | 66.5 (60.5–72.2) | 77.6 (71.1–83.2) | 35.1 (24.9–47.1) |
| Doylestown | 89.0 (84.5–92.6) | 89.0 (84.5–92.6) | 89.6 (85.2–93.1) | 95.7 (91.7–98.1) | 70.6 (58.3–81.0) |
| PPV, (%) | |||||
| AFP | 93.9 (86.4–97.4) | 81.5 (63.4–91.8) | - | 98.1 (87.9–99.7) | 86.2 (69.8–94.4) |
| AFP-L3 | 77.1 (70.2–82.8) | 50.0 (39.5–60.5) | 60.0 (49.3–69.8) | 77.0 (68.3–83.9) | 77.3 (64.8–86.3) |
| DCP | 91.1 (82.9–95.6) | 58.8 (36.1–78.3) | 84.9 (69.0–93.4) | 94.2 (83.9–98.1) | 85.2 (67.8–94.0) |
| GALAD | 87.4 (81.5–91.6) | 67.3 (55.7–77.1) | 78.5 (68.0–86.2) | 92.3 (85.2–96.2) | 78.9 (67.9–86.8) |
| GAAP | 95.9 (88.2–98.7) | 80.0 (53.8–93.2) | 95.5 (74.2–99.4) | 97.8 (86.3–99.7) | 92.6 (75.6–98.1) |
| ASAP | 96.3 (86.5–99.1) | 80.0 (46.5–94.8) | 93.3 (65.1–99.1) | 97.2 (82.9–99.6) | 94.4 (70.0–99.2) |
| aMAP | 56.2 (51.5–60.8) | 35.2 (30.6–40.1) | 41.1 (36.0–46.3) | 63.6 (56.8–70.0) | 46.1 (40.6–51.6) |
| Doylestown | 80.3 (73.9–85.4) | 54.8 (44.4–64.8) | 65.8 (56.2–74.3) | 90.4 (82.5–94.9) | 66.1 (56.7–74.4) |
| NPV, (%) | |||||
| AFP | 75.6 (72.8–78.1) | 86.6 (84.4–88.6) | - | 77.5 (74.3–80.5) | 70.6 (65.2–75.4) |
| AFP-L3 | 79.4 (76.0–82.4) | 87.9 (85.2–90.2) | 83.4 (80.6–85.9) | 88.8 (76.8–84.2) | 75.9 (69.0–81.7) |
| DCP | 74.4 (71.7–76.9) | 83.3 (81.8–84.8) | 81.7 (79.4–83.7) | 76.5 (73.3–79.3) | 69.2 (64.1–73.9) |
| GALAD | 86.6 (83.2–89.5) | 90.6 (87.8–92.8) | 87.5 (84.5–89.9) | 88.2 (84.2–91.3) | 82.3 (74.5–88.1) |
| GAAP | 73.7 (70.9–76.3) | 83.2 (81.4–84.8) | 79.6 (77.6–81.5) | 74.7 (71.6–77.6) | 71.0 (65.2–76.1) |
| ASAP | 70.1 (67.8–72.3) | 82.1 (80.7–83.5) | 77.9 (76.2–79.5) | 71.8 (69.1–74.4) | 65.7 (61.3–69.9) |
| aMAP | 80.2 (75.6–84.1) | 93.2 (89.3–95.7) | 88.1 (83.9–91.3) | 82.6 (77.9–86.5) | 68.4 (54.7–79.6) |
| Doylestown | 83.2 (79.4–86.3) | 88.7 (85.7–91.1) | 86.5 (83.3–89.3) | 84.4 (80.3–87.8) | 78.7 (69.2–85.8) |
| Any-Stage HCC (n = 165) | Early-Stage HCC (n = 64) | AFP-Negative HCC (n = 88) | Viral HCC (n = 110) | Non-Viral HCC (n = 55) | |
|---|---|---|---|---|---|
| 90% specificity cut-off | |||||
| AFP | 8.70 | 8.80 | - | 8.25 | 9.80 |
| AFP-L3 | 11.65 | 11.65 | 10.50 | 11.15 | 16.45 |
| DCP | 0.65 | 0.65 | 0.55 | 0.45 | 3.15 |
| GALAD | −1.22 | −1.22 | −1.31 | −1.35 | −0.02 |
| GAAP | −4.27 | −4.24 | −4.38 | −5.06 | −3.02 |
| ASAP | −4.64 | −4.63 | −4.81 | −5.52 | −2.86 |
| aMAP | 69.11 | 69.11 | 68.67 | 66.58 | 71.17 |
| Doylestown | 0.52 | 0.52 | 0.51 | 0.38 | 0.73 |
| Sensitivity, (%) | |||||
| AFP | 63.0 (55.2–70.4) | 48.4 (35.8–61.3) | - | 62.7 (53.0–71.8) | 63.6 (49.6–76.2) |
| AFP-L3 | 57.6 (49.7–65.2) | 40.6 (28.5–53.6) | 43.2 (32.7–54.2) | 59.1 (49.3–68.4) | 49.1 (35.4–62.9) |
| DCP | 74.6 (67.2–81.0) | 59.4 (46.4–71.5) | 67.1 (65.2–76.7) | 81.8 (73.3–88.5) | 52.7 (38.8–66.4) |
| GALAD | 81.2 (74.4–86.9) | 68.8 (55.9–79.8) | 68.2 (57.4–77.7) | 83.6 (75.4–90.0) | 69.1 (55.2–80.9) |
| GAAP | 77.5 (70.2–83.7) | 60.3 (47.2–72.4) | 65.9 (54.8–75.8) | 88.9 (81.4–94.1) | 65.4 (50.9–78.0) |
| ASAP | 80.0 (73.0–85.9) | 66.7 (53.7–78.1) | 70.6 (59.7–80.0) | 92.6 (85.9–96.8) | 59.6 (45.1–73.0) |
| aMAP | 22.2 (16.1–29.4) | 22.2 (12.7–34.5) | 22.1 (13.9–32.3) | 28.4 (20.2–38.9) | 28.3 (16.8–42.4) |
| Doylestown | 70.0 (62.3–77.0) | 52.4 (39.4–65.1) | 57.8 (46.5–68.3) | 80.6 (71.8–87.5) | 63.5 (49.0–76.4) |
| Specificity, (%) | |||||
| AFP | 90% (89.5–90.5%) | 90% (89.5–90.5%) | - | 90% (89.5–90.5%) | 90% (89.5–90.5%) |
| AFP-L3 | 90% (89.5–90.5%) | 90% (89.5–90.5%) | 90% (89.5–90.5%) | 90% (89.5–90.5%) | 90% (89.5–90.5%) |
| DCP | 90% (89.5–90.5%) | 90% (89.5–90.5%) | 90% (89.5–90.5%) | 90% (89.5–90.5%) | 90% (89.5–90.5%) |
| GALAD | 90% (89.5–90.5%) | 90% (89.5–90.5%) | 90% (89.5–90.5%) | 90% (89.5–90.5%) | 90% (89.5–90.5%) |
| GAAP | 90% (89.5–90.5%) | 90% (89.5–90.5%) | 90% (89.5–90.5%) | 90% (89.5–90.5%) | 90% (89.5–90.5%) |
| ASAP | 90% (89.5–90.5%) | 90% (89.5–90.5%) | 90% (89.5–90.5%) | 90% (89.5–90.5%) | 90% (89.5–90.5%) |
| aMAP | 90% (89.5–90.5%) | 90% (89.5–90.5%) | 90% (89.5–90.5%) | 90% (89.5–90.5%) | 90% (89.5–90.5%) |
| Doylestown | 90% (89.5–90.5%) | 90% (89.5–90.5%) | 90% (89.5–90.5%) | 90% (89.5–90.5%) | 90% (89.5–90.5%) |
| PPV, (%) | |||||
| AFP | 79.4 (72.5–84.9) | 53.5 (42.6–64.0) | - | 78.4 (69.8–85.1) | 81.4 (68.8–89.7) |
| AFP-L3 | 78.5 (71.2–84.4) | 50.0 (38.4–61.6) | 60.3 (49.4–70.3) | 78.3 (69.4–85.2) | 77.1 (62.4–87.3) |
| DCP | 83.1 (77.0–87.8) | 60.3 (49.8–69.9) | 68.6 (59.8–76.3) | 85.7 (78.5–90.8) | 78.4 (64.3–88.0) |
| GALAD | 83.2 (77.5–87.7) | 62.0 (52.3–70.7) | 69.0 (60.2–76.6) | 83.6 (76.6–88.9) | 82.6 (70.7–90.4) |
| GAAP | 83.2 (77.2–87.9) | 60.3 (49.9–69.9) | 69.1 (60.0–77.0) | 84.2 (77.4–89.3) | 82.9 (70.1–91.0) |
| ASAP | 83.7 (77.8–88.2) | 62.7 (52.7–71.7) | 70.6 (61.8–78.1) | 84.8 (78.1–89.6) | 81.6 (68.0–90.2) |
| aMAP | 58.1 (46.5–68.8) | 35.0 (23.0–49.3) | 42.2 (29.9–55.6) | 62.0 (49.2–73.3) | 65.2 (46.2–80.4) |
| Doylestown | 80.6 (74.1–85.7) | 55.0 (44.4–65.2) | 65.3 (55.6–79.9) | 81.3 (74.0–86.9) | 82.5 (69.4–90.7) |
| NPV, (%) | |||||
| AFP | 80.4 (77.0–83.4) | 88.3 (85.6–90.6) | - | 81.6 (77.6–85.0) | 77.3 (70.4–83.0) |
| AFP-L3 | 78.2 (74.9–81.1) | 86.9 (84.3–89.0) | 83.2 (80.4–85.6) | 80.3 (76.4–83.6) | 70.8 (64.9–76.1) |
| DCP | 85.7 (82.2–88.7) | 90.7 (87.8–92.9) | 89.4 (86.2–91.9) | 90.3 (86.2–93.3) | 72.3 (66.2–77.8) |
| GALAD | 89.0 (85.4–91.7) | 92.6 (89.7–94.7) | 89.7 (86.5–92.3) | 91.0 (86.9–94.0) | 80.0 (72.8–85.7) |
| GAAP | 86.5 (82.7–89.5) | 90.2 (87.1–92.6) | 88.6 (85.3–91.3) | 93.4 (89.2–96.0) | 77.3 (69.8–83.2) |
| ASAP | 87.8 (84.0–90.8) | 91.6 (88.5–94.0) | 90.0 (86.6–92.7) | 95.5 (91.5–97.6) | 74.4 (67.4–80.3) |
| aMAP | 66.0 (83.9–68.0) | 83.3 (81.3–85.1) | 78.2 (76.1–80.1) | 69.4 (66.7–72.0) | 63.5 (59.0–67.7) |
| Doylestown | 82.6 (79.0–85.8) | 88.4 (85.4–90.8) | 86.2 (82.9–88.9) | 88.8 (84.4–92.1) | 76.3 (69.0–82.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demirtas, C.O.; Akin, S.; Yilmaz Karadag, D.; Yilmaz, T.; Ciftci, U.; Huseynov, J.; Tolu Bulte, T.; Armutcuoglu Kaldirim, Y.; Dilber, F.; Ozdogan, O.C.; et al. Enhancing Hepatocellular Carcinoma Surveillance: Comparative Evaluation of AFP, AFP-L3, DCP and Composite Models in a Biobank-Based Case-Control Study. Cancers 2025, 17, 2390. https://doi.org/10.3390/cancers17142390
Demirtas CO, Akin S, Yilmaz Karadag D, Yilmaz T, Ciftci U, Huseynov J, Tolu Bulte T, Armutcuoglu Kaldirim Y, Dilber F, Ozdogan OC, et al. Enhancing Hepatocellular Carcinoma Surveillance: Comparative Evaluation of AFP, AFP-L3, DCP and Composite Models in a Biobank-Based Case-Control Study. Cancers. 2025; 17(14):2390. https://doi.org/10.3390/cancers17142390
Chicago/Turabian StyleDemirtas, Coskun O., Sehnaz Akin, Demet Yilmaz Karadag, Tuba Yilmaz, Ugur Ciftci, Javid Huseynov, Tugba Tolu Bulte, Yasemin Armutcuoglu Kaldirim, Feyza Dilber, Osman Cavit Ozdogan, and et al. 2025. "Enhancing Hepatocellular Carcinoma Surveillance: Comparative Evaluation of AFP, AFP-L3, DCP and Composite Models in a Biobank-Based Case-Control Study" Cancers 17, no. 14: 2390. https://doi.org/10.3390/cancers17142390
APA StyleDemirtas, C. O., Akin, S., Yilmaz Karadag, D., Yilmaz, T., Ciftci, U., Huseynov, J., Tolu Bulte, T., Armutcuoglu Kaldirim, Y., Dilber, F., Ozdogan, O. C., & Eren, F. (2025). Enhancing Hepatocellular Carcinoma Surveillance: Comparative Evaluation of AFP, AFP-L3, DCP and Composite Models in a Biobank-Based Case-Control Study. Cancers, 17(14), 2390. https://doi.org/10.3390/cancers17142390





