Metastatic Patterns of Apical Lymph Node and Prognostic Analysis in Rectal and Sigmoid Colon Cancer—A Multicenter Retrospective Cohort Study of 2809 Cases
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
- (1)
- Histopathologically confirmed as adenocarcinoma, mucinous adenocarcinoma or signet ring cell carcinoma.
- (2)
- The lesion location was in the sigmoid colon and rectum.
- (3)
- Intention-to-treat was curative and apical lymph node was dissected.
- (4)
- Clinicopathological data was completed.
- (1)
- Patients with history of colorectal cancer.
- (2)
- Patients underwent neoadjuvant therapy.
- (3)
- Patients with multiple primary colorectal cancers.
- (4)
- Patients with family history of hereditary diseases.
- (5)
- Patients with extra-regional lymph node metastasis.
- (6)
- Patients with distant metastasis.
2.2. Data Collection and Analysis
2.3. Surgical Procedures
2.4. Follow-Up and Postoperative Therapy
2.5. Statistical Analysis
3. Results
3.1. Clinicopathological Characteristics
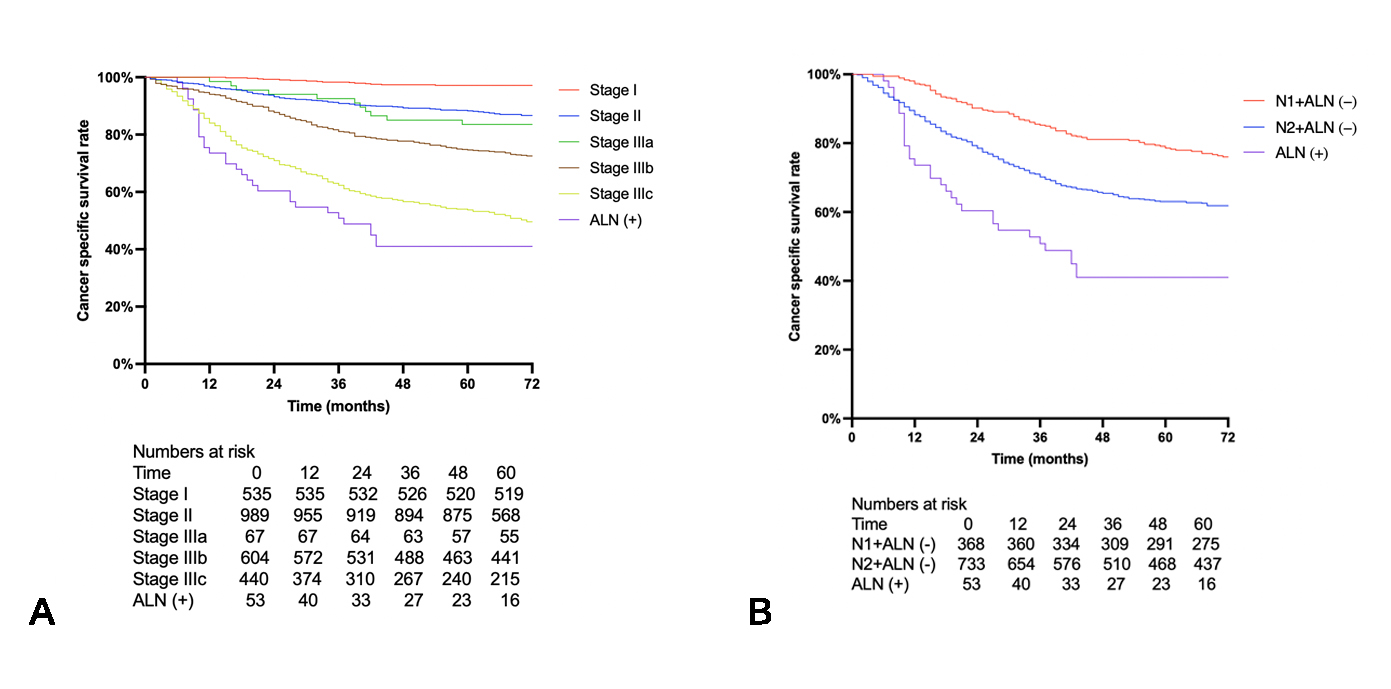
3.2. Survival Analysis
3.3. Risk Factors of ALN Metastasis
3.4. Nomogram Development
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ALN | Apical lymph node |
| EMVI | Extramural venous invasion |
| CRM | Circumferential resection margin |
| OR | Odd ratio |
| CI | Confidence interval |
| IMA | Inferior mesenteric artery |
| HL | High ligation |
| CME | Complete mesocolic excision |
| TME | Total mesorectal excision |
| OS | Overall survival |
| CSS | Cancer-specific survival |
Appendix A

Appendix B
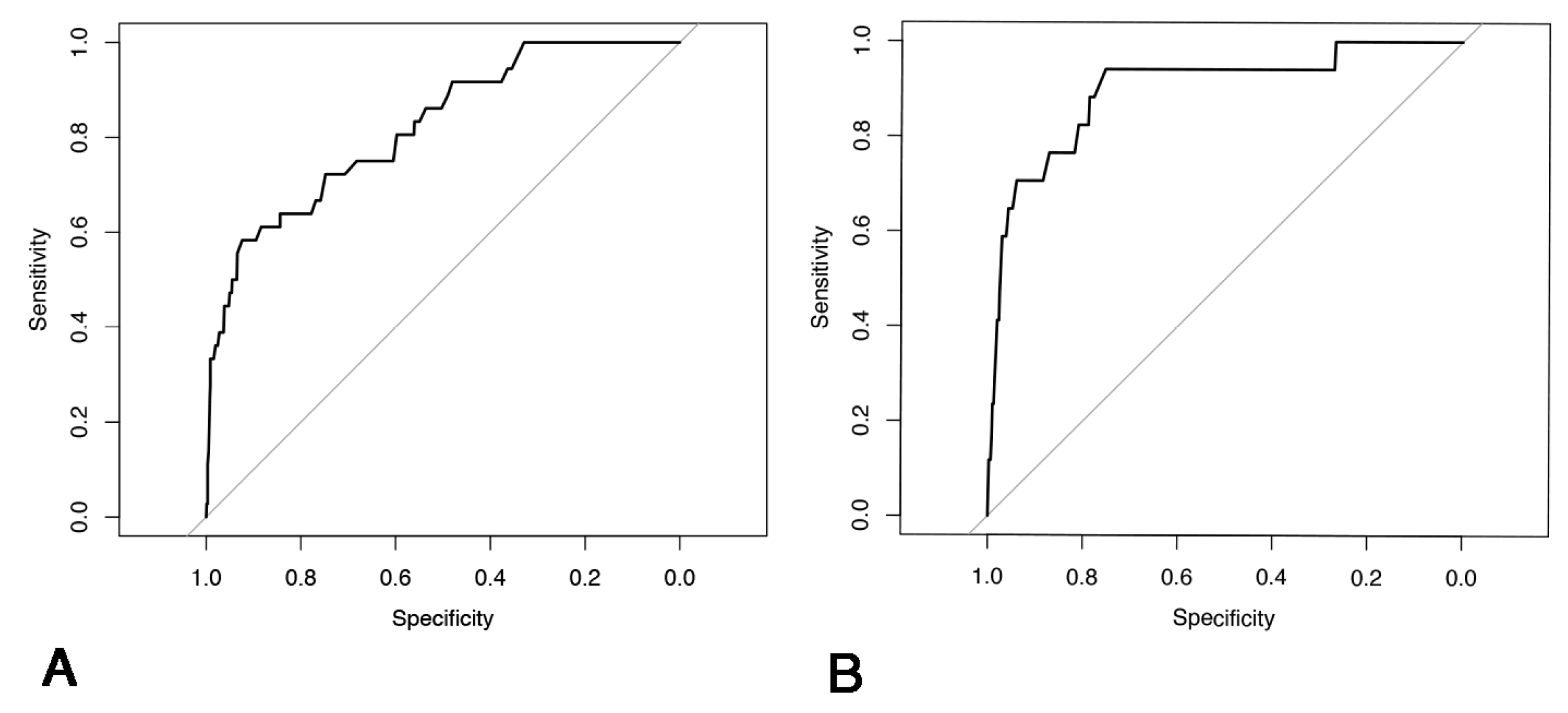
Appendix C
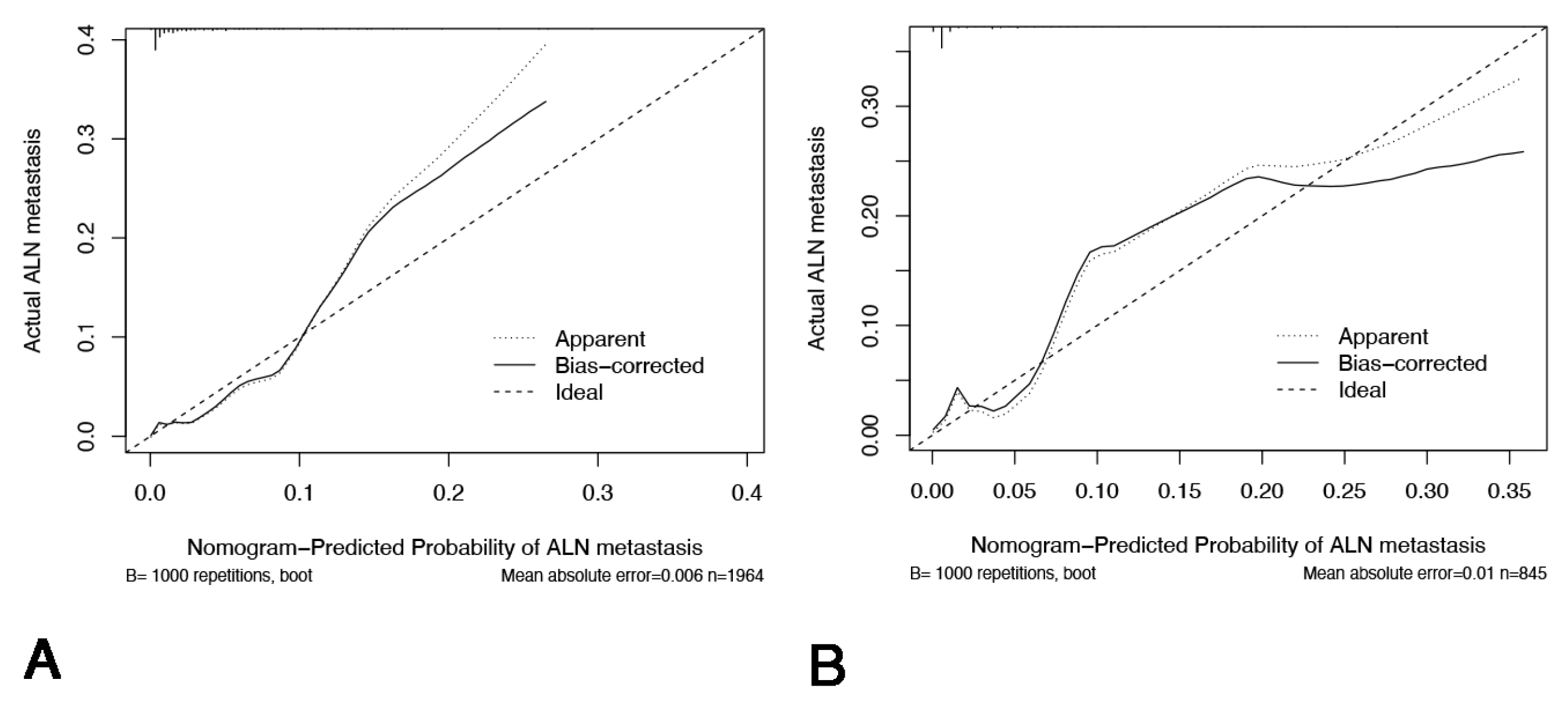
Appendix D
| Risk Factors | T4 | N2 | Tumor Size > 5 cm | Sigmoid/Upper Rectum | Mucinous Adenocarcinoma/Signet Ring Cell Carcinoma | Vascular or Lymphatic Invasion |
|---|---|---|---|---|---|---|
| Total patients | 855 | 815 | 766 | 2164 | 410 | 1083 |
| ALN (+) patients | 32 | 41 | 27 | 49 | 24 | 36 |
| ALN (+) % | 3.7% | 5.0% | 3.5% | 2.3% | 5.9% | 3.3% |
Appendix E
| C-Index | Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|---|
| Training set | 0.834 | 0.639 | 0.93 | 0.146 | 0.993 |
| Validation set | 0.890 | 0.882 | 0.781 | 0.077 | 0.997 |
References
- Zhang, C.; Zhang, L.; Xu, T.; Xue, R.; Yu, L.; Zhu, Y.; Wu, Y.; Zhang, Q.; Li, D.; Shen, S.; et al. Mapping the spreading routes of lymphatic metastases in human colorectal cancer. Nat. Commun. 2020, 11, 1993. [Google Scholar] [CrossRef]
- Kim, J.C.; Lee, K.H.; Yu, C.S.; Kim, H.C.; Kim, J.R.; Chang, H.M.; Kim, J.H.; Kim, J.S.; Kim, T.W. The clinicopathological significance of inferior mesenteric lymph node metastasis in colorectal cancer. Eur. J. Surg. Oncol. 2004, 30, 271–279. [Google Scholar] [CrossRef]
- Ang, C.W.; Tweedle, E.M.; Campbell, F.; Rooney, P.S. Apical node metastasis independently predicts poor survival in Dukes C colorectal cancer. Colorectal Dis. 2011, 13, 526–531. [Google Scholar] [CrossRef]
- Malassagne, B.; Valleur, P.; Serra, J.; Sarnacki, S.; Galian, A.; Hoang, C.; Hautefeuille, P. Relationship of apical lymph node involvement to survival in resected colon carcinoma. Dis. Colon. Rectum. 1993, 36, 645–653. [Google Scholar] [CrossRef]
- Yi, J.W.; Lee, T.G.; Lee, H.S.; Heo, S.C.; Jeong, S.Y.; Park, K.J.; Kang, S.B. Apical-node metastasis in sigmoid colon or rectal cancer: Is it a factor that indicates a poor prognosis after high ligation? Int. J. Colorectal Dis. 2012, 27, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Azad, N.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Garrido-Laguna, I.; et al. Rectal Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 1139–1167. [Google Scholar] [CrossRef] [PubMed]
- Tomita, N.; Ishida, H.; Tanakaya, K.; Yamaguchi, T.; Kumamoto, K.; Tanaka, T.; Hinoi, T.; Miyakura, Y.; Hasegawa, H.; Takayama, T.; et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2020 for the Clinical Practice of Hereditary Colorectal Cancer. Int. J. Clin. Oncol. 2021, 26, 1353–1419. [Google Scholar] [CrossRef] [PubMed]
- Hinoi, T.; Okajima, M.; Shimomura, M.; Egi, H.; Ohdan, H.; Konishi, F.; Sugihara, K.; Watanabe, M. Effect of left colonic artery preservation on anastomotic leakage in laparoscopic anterior resection for middle and low rectal cancer. World J. Surg. 2013, 37, 2935–2943. [Google Scholar] [CrossRef]
- Cirocchi, R.; Trastulli, S.; Farinella, E.; Desiderio, J.; Vettoretto, N.; Parisi, A.; Boselli, C.; Noya, G. High tie versus low tie of the inferior mesenteric artery in colorectal cancer: A RCT is needed. Surg. Oncol. 2012, 21, e111–e123. [Google Scholar] [CrossRef]
- Komen, N.; Slieker, J.; de Kort, P.; de Wilt, J.H.W.; van der Harst, E.; Coene, P.-P.; Gosselink, M.; Tetteroo, G.; de Graaf, E.; van Beek, T.; et al. High tie versus low tie in rectal surgery: Comparison of anastomotic perfusion. Int. J. Colorectal Dis. 2011, 26, 1075–1078. [Google Scholar] [CrossRef]
- Kanemitsu, Y.; Hirai, T.; Komori, K.; Kato, T. Survival benefit of high ligation of the inferior mesenteric artery in sigmoid colon or rectal cancer surgery. Br. J. Surg. 2006, 93, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Hida, J.; Okuno, K. High ligation of the inferior mesenteric artery in rectal cancer surgery. Surg. Today 2013, 43, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.C.; Su, W.C.; Chen, P.J.; Chang, T.-K.; Chen, Y.-C.; Li, C.-C.; Hsieh, Y.-C.; Tsai, H.-L.; Huang, C.-W.; Wang, J.-Y. Oncological Outcomes of Robotic-Assisted Surgery with High Dissection and Selective Ligation Technique for Sigmoid Colon and Rectal Cancer. Front. Oncol. 2020, 10, 570376. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Zhang, J.; Liu, T.; Wu, Y.; Jiang, Y.; Wang, P.; Chen, G.; Pan, Y.; Wu, T.; Liu, Y.; et al. Prognostic value of inferior mesenteric artery lymph node metastasis in cancer of the descending colon, sigmoid colon and rectum. Colorectal Dis. 2018, 20, O135–O142. [Google Scholar] [CrossRef]
- Peng, J.; Wu, H.; Li, X.; Sheng, W.; Huang, D.; Guan, Z.; Wang, M.; Cai, S. Prognostic significance of apical lymph node metastasis in patients with node-positive rectal cancer. Colorectal Dis. 2013, 15, e13–e20. [Google Scholar] [CrossRef]
- Zhao, X.; Ma, J.; Fu, Z.; Hong, H.; Zhang, L.; Xue, P.; Cai, Z.; He, Z.; Zang, L.; Zheng, M. Prognostic value of apical lymph node metastasis at the inferior mesenteric artery in sigmoid and rectal cancer patients who undergo laparoscopic surgery. J. Surg. Oncol. 2021, 123 (Suppl. S1), S88–S94. [Google Scholar] [CrossRef]
- Kang, J.; Hur, H.; Min, B.S.; Kim, N.K.; Lee, K.Y. Prognostic impact of inferior mesenteric artery lymph node metastasis in colorectal cancer. Ann. Surg. Oncol. 2011, 18, 704–710. [Google Scholar] [CrossRef]
- Huh, J.W.; Kim, Y.J.; Kim, H.R. Distribution of lymph node metastases is an independent predictor of survival for sigmoid colon and rectal cancer. Ann. Surg. 2012, 255, 70–78. [Google Scholar] [CrossRef]
- Kobayashi, H.; Ueno, H.; Hashiguchi, Y.; Mochizuki, H. Distribution of lymph node metastasis is a prognostic index in patients with stage III colon cancer. Surgery 2006, 139, 516–522. [Google Scholar] [CrossRef]
- Beahrs, O.H.; Henson, D.E.; Hutter, R.V.P.; Myers, M.H. Manual for staging of cancer. Am. J. Clin. Oncol. 1988, 11, 686. [Google Scholar] [CrossRef]
- Tang, R.; Wang, J.Y.; Chen, J.S.; Chang-Chien, C.R.; Tang, S.; E Lin, S.; You, Y.T.; Hsu, K.C.; Ho, Y.S.; Fan, H. Survival impact of lymph node metastasis in TNM stage III carcinoma of the colon and rectum. J. Am. Coll. Surg. 1995, 180, 705–712. [Google Scholar] [PubMed]
- Yang, Y.; Wang, G.; He, J.; Zhang, J.; Xi, J.; Wang, F. High tie versus low tie of the inferior mesenteric artery in colorectal cancer: A meta-analysis. Int. J. Surg. 2018, 52, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Boström, P.; Hultberg, D.K.; Häggström, J.; Haapamäki, M.M.; Matthiessen, P.; Rutegård, J.; Rutegård, M. Oncological Impact of High Vascular Tie After Surgery for Rectal Cancer: A Nationwide Cohort Study. Ann. Surg. 2021, 274, e236–e244. [Google Scholar] [CrossRef]
- Steup, W.H.; Moriya, Y.; van de Velde, C.J. Patterns of lymphatic spread in rectal cancer. A topographical analysis on lymph node metastases. Eur. J. Cancer 2002, 38, 911–918. [Google Scholar] [CrossRef]
- Chin, C.C.; Yeh, C.Y.; Tang, R.; Changchien, C.-R.; Huang, W.-S.; Wang, J.-Y. The oncologic benefit of high ligation of the inferior mesenteric artery in the surgical treatment of rectal or sigmoid colon cancer. Int. J. Colorectal Dis. 2008, 23, 783–788. [Google Scholar] [CrossRef]
- Wang, L.; Hirano, Y.; Heng, G.; Ishii, T.; Kondo, H.; Hara, K.; Obara, N.; Asari, M.; Yamaguchi, S. Prognostic Utility of Apical Lymph Node Metastasis in Patients with Left-sided Colorectal Cancer. Vivo 2020, 34, 2981–2989. [Google Scholar] [CrossRef]
- Mari, G.M.; Crippa, J.; Cocozza, E.; Berselli, M.; Livraghi, L.; Carzaniga, P.; Valenti, F.; Roscio, F.; Ferrari, G.; Mazzola, M.; et al. Low Ligation of Inferior Mesenteric Artery in Laparoscopic Anterior Resection for Rectal Cancer Reduces Genitourinary Dysfunction: Results from a Randomized Controlled Trial (HIGHLOW Trial). Ann. Surg. 2019, 269, 1018–1024. [Google Scholar] [CrossRef]
- Hu, S.; Li, S.; Teng, D.; Yan, Y.; Lin, H.; Liu, B.; Du, X. Analysis of risk factors and prognosis of 253 lymph node metastasis in colorectal cancer patients. BMC Surg. 2021, 21, 280. [Google Scholar] [CrossRef]
- Wang, X.J.; Chi, P.; Lin, H.M.; Lu, X.-R.; Huang, Y.; Xu, Z.-B.; Huang, S.-H.; Sun, Y.-W. A scoring system to predict inferior mesenteric artery lymph node metastasis and prognostic value of its involvement in rectal cancer. Int. J. Colorectal Dis. 2014, 29, 293–300. [Google Scholar] [CrossRef]

| Variables | N = 2809 |
|---|---|
| Age | 61.60 ± 11.19 |
| Gender | |
| Male | 1721 (61.3%) |
| Female | 1088 (38.8%) |
| Location | |
| Sigmoid colon | 967 (34.4%) |
| Higher rectum | 1198 (42.7%) |
| Lower rectum | 644 (22.9%) |
| Histological subtype | |
| Signet ring cell cancer/Mucinous adenocarcinoma | 410 (14.6%) |
| Poorly differentiated | 494 (17.6%) |
| Moderate to well differentiated | 1905 (67.8%) |
| T stage | |
| 1 | 179 (6.4%) |
| 2 | 544 (19.4%) |
| 3 | 1233 (43.9%) |
| 4 | 853 (30.4%) |
| N stage | |
| 0 | 1581 (56.4%) |
| 1 | 413 (14.7%) |
| 2 | 815 (29.0%) |
| TNM stage | |
| 1 | 554 (19.7%) |
| 2 | 1027 (36.6%) |
| 3 | 1228 (43.7%) |
| Tumor size | |
| Less than 5 cm | 2043 (72.7%) |
| More than 5 cm | 766 (27.2%) |
| Apical lymph node | |
| Positive | 53 (1.9%) |
| Negative | 2756 (98.1%) |
| EMVI | |
| Positive | 681 (24.2%) |
| Negative | 2128 (75.8%) |
| BRAF V600 | |
| Wild-type | 2624 (93.4%) |
| Mutant | 185 (6.6%) |
| Mismatch repair | |
| Proficient | 2577 (91.7%) |
| Deficient | 232 (8.3%) |
| Adjuvant therapy | |
| No | 859 (30.6%) |
| Yes | 1950 (69.4%) |
| OS | CSS | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | p | HR | 95% CI | p |
| Gender | ||||||
| Male | 1.00 | 1.00 | ||||
| Female | 1.13 | 0.93 to 1.37 | 0.228 | 1.18 | 0.97 to 1.45 | 0.100 |
| Age | ||||||
| <65 | 1.00 | 1.00 | ||||
| ≥65 | 1.06 | 0.87 to 1.28 | 0.582 | 1.04 | 0.85 to 1.28 | 0.678 |
| Tumor location | ||||||
| Rectum | 1.00 | 1.00 | ||||
| Sigmoid colon | 1.23 | 0.97 to 1.56 | 0.089 | 1.27 | 0.99 to 1.62 | 0.058 |
| Histological subtype | ||||||
| Signet ring cell cancer/Mucinous adenocarcinoma | 1.00 | 1.00 | ||||
| Poor differentiated adenocarcinoma | 0.87 | 0.65 to 1.16 | 0.336 | 0.95 | 0.70 to 1.29 | 0.752 |
| Moderate to well differentiated adenocarcinoma | 0.76 | 0.58 to 0.99 | 0.040 * | 0.78 | 0.59 to 1.04 | 0.092 |
| Tumor size | ||||||
| <5 cm | 1.00 | 1.00 | ||||
| ≥5 cm | 0.93 | 0.75 to 1.16 | 0.528 | 0.98 | 0.78 to 1.22 | 0.843 |
| T stage | ||||||
| T1–3 | 1.00 | 1.00 | ||||
| T4 | 1.11 | 0.90 to 1.37 | 0.342 | 1.11 | 0.89 to 1.39 | 0.343 |
| N stage | ||||||
| N0–1 | 1.00 | 1.00 | ||||
| N2 | 1.71 | 1.37 to 2.14 | <0.001 * | 1.75 | 1.38 to 2.22 | <0.001 * |
| Perineural invasion | ||||||
| Negative | 1.00 | 1.00 | ||||
| Positive | 1.25 | 1.01 to 1.54 | 0.041 * | 1.26 | 1.01 to 1.56 | 0.041 * |
| Vascular invasion | ||||||
| Negative | 1.00 | 1.00 | ||||
| Positive | 1.13 | 0.92 to 1.39 | 0.247 | 1.13 | 0.91 to 1.41 | 0.258 |
| Apical lymph node | ||||||
| Negative | 1.00 | 1.00 | ||||
| Positive | 2.02 | 1.39 to 2.95 | <0.001 * | 2.06 | 1.40 to 3.03 | <0.001 * |
| Adjuvant therapy | ||||||
| No | 1.00 | 1.00 | ||||
| Yes | 0.81 | 0.43 to 1.52 | 0.501 | 1.08 | 0.51 to 2.30 | 0.840 |
| Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|
| Variable | Apical Lymph Node (−) n = 2756 | Apical Lymph Node (+) n = 53 | Univariate p | OR | 95% CI | p |
| Age | 0.974 | — | — | — | ||
| <65 | 1034 (37.5%) | 20 (37.7%) | ||||
| ≥65 | 1722 (62.5%) | 33 (62.3%) | ||||
| Gender | 0.482 | — | — | — | ||
| Male | 1691 (61.4%) | 30 (56.6%) | ||||
| Female | 1065 (38.6%) | 23 (43.4%) | ||||
| Tumor location | 0.007 * | 0.110 | ||||
| Sigmoid/Upper. rectum | 2115 (76.7%) | 50 (94.3%) | 1.00 | |||
| Lower rectum | 641 (23.3%) | 3 (5.7%) | 0.42 | 0.14 to 1.22 | ||
| Tumor size (cm) | <0.001 * | 0.004 * | ||||
| <5 cm | 2017 (73.2%) | 26 (49.1%) | 1.00 | |||
| ≥5 cm | 739 (26.8%) | 27 (50.9%) | 2.32 | 1.30 to 4.13 | ||
| Histological type | <0.001 * | <0.001 * | ||||
| Signet ring cell cancer/Mucinous adenocarcinoma | 386 (14.0%) | 24 (45.3%) | 1.00 | |||
| Poor differentiated adenocarcinoma | 485 (17.6%) | 9 (17.0%) | 0.19 | 0.08 to 0.44 | ||
| Moderate to well differentiated adenocarcinoma | 1885 (68.4%) | 20 (37.7%) | 0.22 | 0.11 to 0.42 | ||
| T stage | <0.001 * | 0.034 * | ||||
| T1–3 | 1933 (70.1%) | 23 (43.4%) | 1.00 | |||
| T4 | 823 (29.9%) | 30 (56.6%) | 1.93 | 1.05 to 3.55 | ||
| N stage | <0.001 * | <0.001 * | ||||
| N0–1 | 1982 (71.9%) | 12 (22.6%) | 1.00 | |||
| N2 | 774 (28.1%) | 41 (77.4%) | 8.86 | 4.45 to 17.65 | ||
| EMVI | <0.001 * | 0.040 * | ||||
| Negative | 2104 (98.9%) | 652 (95.7%) | 1.00 | |||
| Positive | 24 (1.1%) | 29 (4.3%) | 1.88 | 1.03 to 3.42 | ||
| BRAF V600 | 0.776 | — | — | — | ||
| Wild-type | 2575 (93.4%) | 49 (92.5%) | ||||
| Mutant | 181 (6.6%) | 4 (7.5%) | ||||
| Mismatch repair | 0.443 | — | — | — | ||
| Proficient | 2530 (91.8%) | 47 (88.7%) | ||||
| Deficient | 226 (8.2%) | 6 (11.3%) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Zhao, F.; Wu, A.; Du, X.; Zhou, L.; Mei, S.; Wei, F.; Hu, S.; Liu, X.; Yang, H.; et al. Metastatic Patterns of Apical Lymph Node and Prognostic Analysis in Rectal and Sigmoid Colon Cancer—A Multicenter Retrospective Cohort Study of 2809 Cases. Cancers 2025, 17, 2389. https://doi.org/10.3390/cancers17142389
Zhang M, Zhao F, Wu A, Du X, Zhou L, Mei S, Wei F, Hu S, Liu X, Yang H, et al. Metastatic Patterns of Apical Lymph Node and Prognostic Analysis in Rectal and Sigmoid Colon Cancer—A Multicenter Retrospective Cohort Study of 2809 Cases. Cancers. 2025; 17(14):2389. https://doi.org/10.3390/cancers17142389
Chicago/Turabian StyleZhang, Mingguang, Fuqiang Zhao, Aiwen Wu, Xiaohui Du, Lei Zhou, Shiwen Mei, Fangze Wei, Shidong Hu, Xinzhi Liu, Hua Yang, and et al. 2025. "Metastatic Patterns of Apical Lymph Node and Prognostic Analysis in Rectal and Sigmoid Colon Cancer—A Multicenter Retrospective Cohort Study of 2809 Cases" Cancers 17, no. 14: 2389. https://doi.org/10.3390/cancers17142389
APA StyleZhang, M., Zhao, F., Wu, A., Du, X., Zhou, L., Mei, S., Wei, F., Hu, S., Liu, X., Yang, H., Xu, L., Xiao, Y., Wang, X., Liu, Q., & on behalf of the Chinese Apical Lymph Node Study Consortium. (2025). Metastatic Patterns of Apical Lymph Node and Prognostic Analysis in Rectal and Sigmoid Colon Cancer—A Multicenter Retrospective Cohort Study of 2809 Cases. Cancers, 17(14), 2389. https://doi.org/10.3390/cancers17142389





