Cell-Free DNA as a Prognostic Biomarker in Oral Carcinogenesis and Oral Squamous Cell Carcinoma: A Translational Perspective
Simple Summary
Abstract
1. Introduction
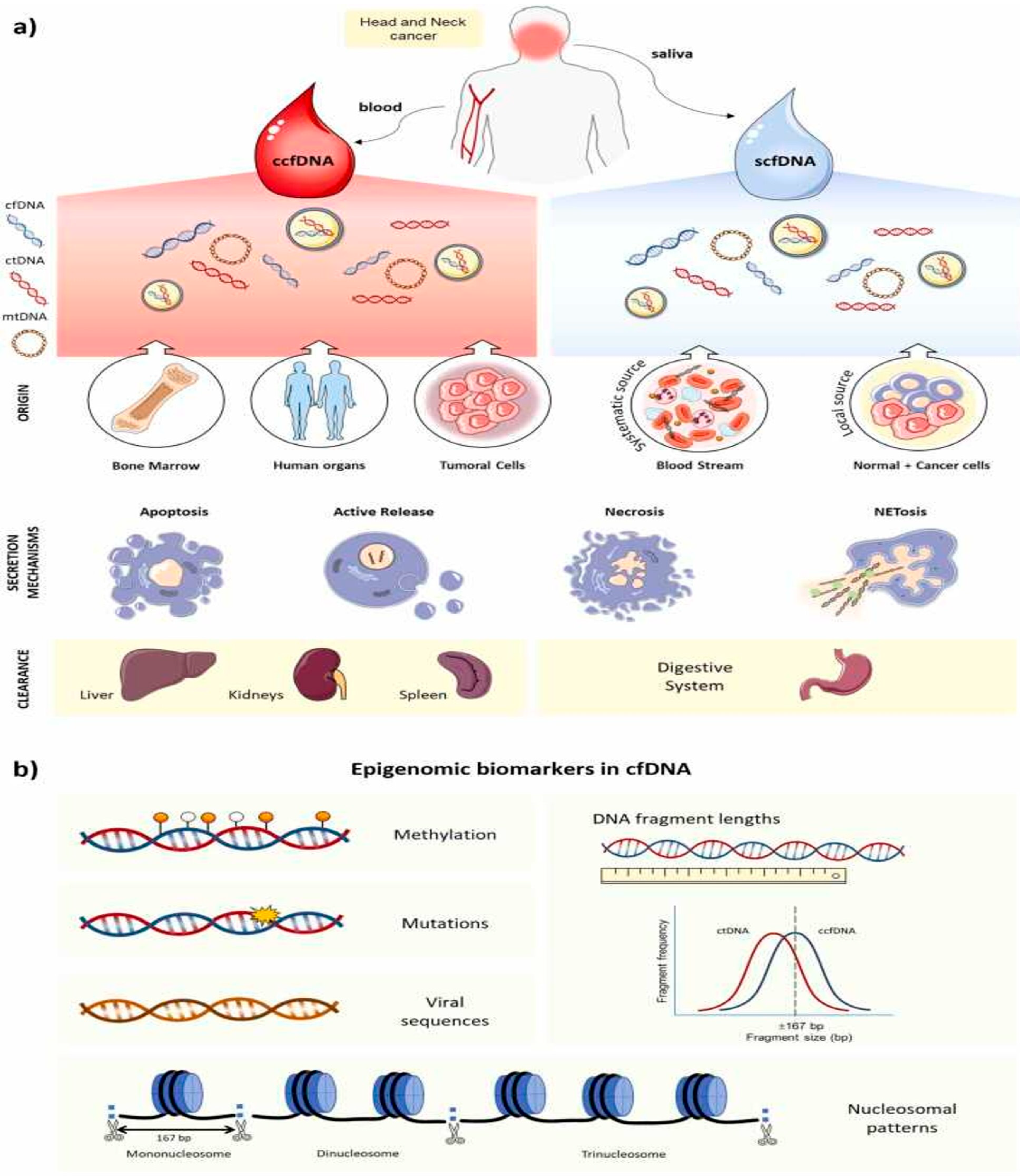
2. Overview of Cell-Free DNA
2.1. Origin, Biological Features, and Mechanism Release in Cancer of cfDNA
2.2. cfDNA Detection in Blood vs. Saliva: Advantages and Disadvantages
3. CfDNA in Oral Squamous Cell Carcinoma
3.1. cfDNA Levels and Tumor Burden Correlation
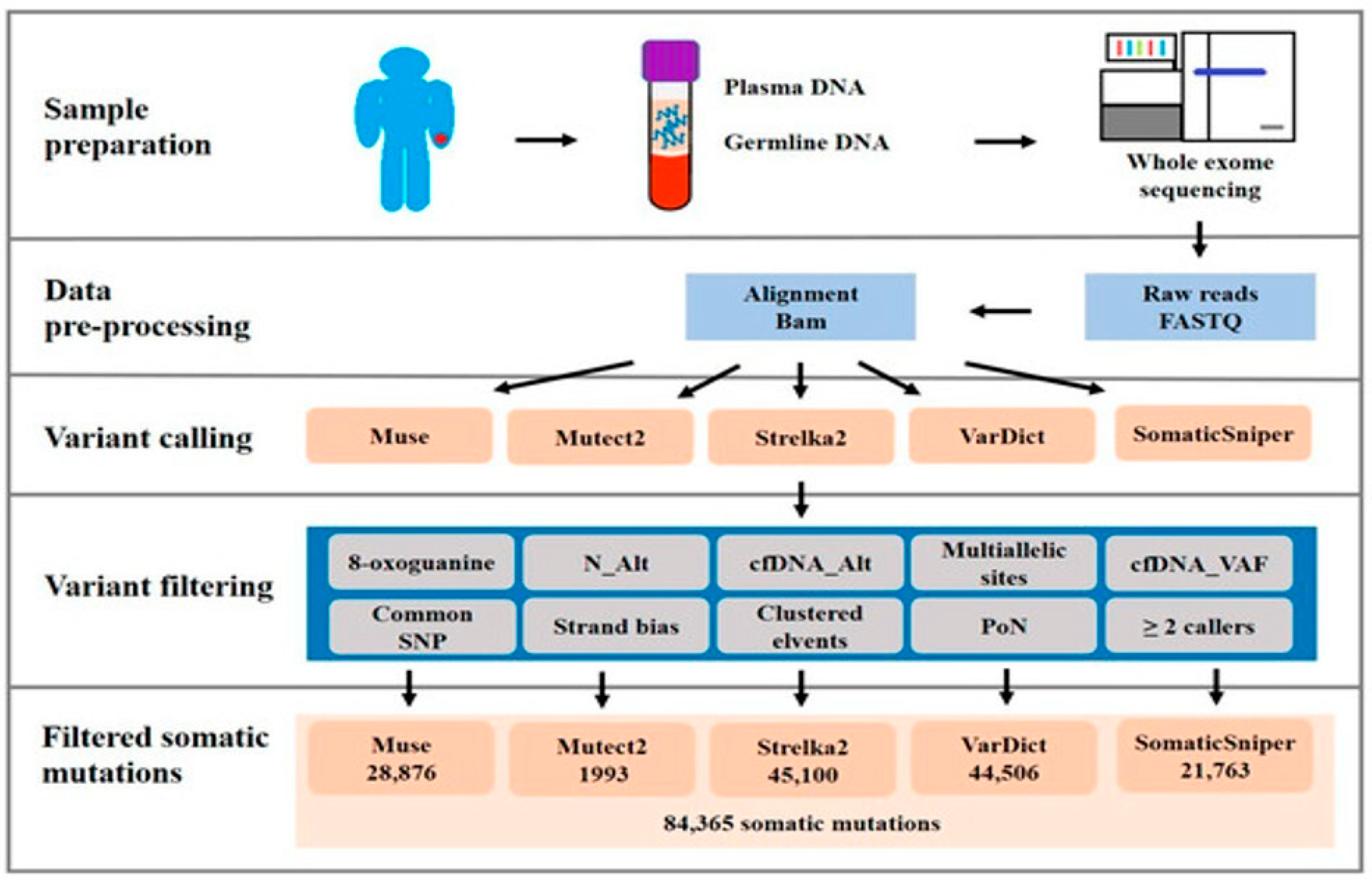
3.2. Gene Mutations of cfDNA
3.3. CfDNA Fragmentation and Integrity as Prognostic Markers
3.4. DNA Methylation Patterns and Epigenetic Signatures in cfDNA
4. Clinical Applications and Translational Relevance
4.1. CfDNA for Early Detection and Prognostic Stratification
4.2. Monitoring Therapeutic Response and Recurrence
4.3. CfDNA as a Companion Diagnostic Tool in Personalized Oncology
5. Challenges, Limitations, and Future Directions
Techniques and Challenges for cfDNA-Containing Sample Handling in Liquid Biopsy
6. The Emerging Role of Proteomics and Metabolomics in Oral Cancer Biomarker Discovery
6.1. Proteomics in Oral Cancer Biomarker Discovery
6.2. Metabolomics in Oral Cancer Biomarker Discovery
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OPMDs | Oral potentially malignant disorders |
| OSCC | Oral squamous cell carcinoma |
| Cf-DNA | Cell-free DNA |
| Ct-DNA | Circulating tumor DNA |
| OLK | Oral leukoplakia |
| OLP | Oral lichen planus |
| OSF | Oral submucous fibrosis |
| CSF | Cerebrospinal fluid |
| HNSCC | Head and Neck Squamous Cell Carcinoma |
| ddPCR | Droplet digital polymerase chain reaction |
| WES | Whole-exome sequencing |
| MRD | Minimal residual disease |
| dPCR | Digital PCR |
| TME | Tumor microenvironment |
| MS | Mass spectrometry |
| iTRAQ | Isobaric tags for relative and absolute quantitation |
| LC-MRM/MS | Liquid Chromatography Multiple-Reaction-Monitoring Mass Spectrometry |
| MALDI-MSI | Matrix-assisted laser desorption/ionization mass spectrometry imaging |
| SELDI-TOF/MS | Surface-enhanced laser desorption/ionization-time-of-flight/mass spectrometry |
| CFH | Complement factor H |
| FGA | Fibrinogen alpha chain |
| SERPINA1 | Alpha-1-antitrypsin |
| GC-MS | Gas chromatography–mass spectrometry |
| UHPLC-MS/MS | Ultra-high-performance liquid chromatography–tandem mass spectrometry |
| CE-MS | Capillary electrophoresis-mass spectrometry |
| NMR | Nuclear magnetic resonance |
References
- Gupta, A.; Devi, A.; Kamboj, M.; Narwal, A.; Kumar, A.; Singh, S. Immunohistochemical Expression of PARK2 and YAP in Oral Epithelial Dysplasia and Oral Squamous Cell Carcinoma. Head Neck Pathol. 2025, 19, 50. [Google Scholar] [CrossRef] [PubMed]
- Tarrad, N.A.F.; Hassan, S.; Shaker, O.G.; AbdelKawy, M. Salivary LINC00657 and miRNA-106a as Diagnostic Biomarkers for Oral Squamous Cell Carcinoma, an Observational Diagnostic Study. BMC Oral Health 2023, 23, 994. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Urs, A.B.; Koner, B.C.; Augustine, J.; Guru, S.A. Evaluation of Diagnostic Significance of Salivary miRNA-184 and miRNA-21 in Oral Squamous Cell Carcinoma and Oral Potentially Malignant Disorders. Head Neck Pathol. 2023, 17, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Farshbaf, A.; Mohtasham, N.; Shamshiri, A.K.; Khazaeni, K.; Aghaee-Bakhtiari, S.H.; Arab, F.; Cheshomi, M.; Hassanabadi, M.; Mohajertehran, F. Clinical Value and Potential Circulating of miR-99a as Tumor Suppressor Biomarker in Serum of Oral Squamous Cell Carcinoma and Erosive Atrophic Lichen Planus. J. Stomatol. Oral Maxillofac. Surg. 2024, 125, 101806. [Google Scholar] [CrossRef] [PubMed]
- Radaic, A.; Kamarajan, P.; Cho, A.; Wang, S.; Hung, G.; Najarzadegan, F.; Wong, D.T.; Ton-That, H.; Wang, C.; Kapila, Y.L. Biological Biomarkers of Oral Cancer. Periodontology 2000 2024, 96, 250–280. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Luo, T.; Yang, J.; Wang, K.; Liu, S.; Wei, Y.; Liu, H.; Xu, J.; Zheng, J.; Zeng, Y. Assessment of Serum Synuclein-γ and Squamous Cell Carcinoma Antigen as Diagnostic Biomarkers in Patients with Oral Squamous Cell Carcinoma and Oral Potentially Malignant Disorders. J. Oral Pathol. Med. 2021, 50, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.; Sekar, R.; Priya, M.D.L.; Varma, S.R.; Karobari, M.I. A New Perspective on Diagnostic Strategies Concerning the Potential of Saliva-Based miRNA Signatures in Oral Cancer. Diagn. Pathol. 2024, 19, 147. [Google Scholar] [CrossRef] [PubMed]
- Pekarek, L.; Garrido-Gil, M.; Sánchez-Cendra, A.; Cassinello, J.; Pekarek, T.; Fraile-Martinez, O.; García-Montero, C.; Lopez-Gonzalez, L.; Rios-Parra, A.; Álvarez-Mon, M.; et al. Emerging Histological and Serological Biomarkers in Oral Squamous Cell Carcinoma: Applications in Diagnosis, Prognosis Evaluation and Personalized Therapeutics (Review). Oncol. Rep. 2023, 50, 213. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Qian, B.; Xu, J.; Yang, J.; Wu, G.; Zhao, Y.; Liu, Q.; Yuan, Z.; Fan, Y.; Li, H. Clinical Relevance of Serum Lipids in the Carcinogenesis of Oral Squamous Cell Carcinoma. BMC Oral Health 2023, 23, 200. [Google Scholar] [CrossRef] [PubMed]
- Rahman, R.; Shaikh, M.H.; Gopinath, D.; Idris, A.; Johnson, N.W. Human Papillomavirus and Epstein-Barr Virus Co-infection in Oral and Oropharyngeal Squamous Cell Carcinomas: A Systematic Review and Meta-analysis. Mol. Oral Microbiol. 2023, 38, 259–274. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, F.A.; Da Silva Bittencourt, L.; Barbosa, S.; Diel, L.F.; Bernardi, L.; Matte, C.; Lamers, M.L. Energy Metabolic Profile in Oral Potentially Malignant Disorders and Oral Squamous Cell Carcinoma: A Preliminary Landscape of Warburg Effect in Oral Cancer. Mol. Carcinog. 2025, 64, 126–137. [Google Scholar] [CrossRef] [PubMed]
- López-Pintor, R.M.; González-Serrano, J.; Vallina, C.; Ivaylova Serkedzhieva, K.; Virto, L.; Nuevo, P.; Caponio, V.C.A.; Iniesta, M.; Rodríguez Santamarta, T.; Lequerica Fernández, P.; et al. Factors Influencing Salivary Lactate Dehydrogenase Levels in Oral Squamous Cell Carcinoma and Oral Potentially Malignant Disorders. Front. Oral Health 2025, 5, 1525936. [Google Scholar] [CrossRef] [PubMed]
- Soparlo, J.; McLean, L.; McCord, C.; Jackson-Boeters, L.; Shimizu, M.; Robinson, M.; Tilakaratne, W.; Darling, M. S100A7 as a Predictive Biomarker in Malignant Transformation of Oral Epithelial Dysplastic Lesions. Discov. Oncol. 2025, 16, 610. [Google Scholar] [CrossRef] [PubMed]
- Khijmatgar, S.; Yong, J.; Rübsamen, N.; Lorusso, F.; Rai, P.; Cenzato, N.; Gaffuri, F.; Del Fabbro, M.; Tartaglia, G.M. Salivary Biomarkers for Early Detection of Oral Squamous Cell Carcinoma (OSCC) and Head/Neck Squamous Cell Carcinoma (HNSCC): A Systematic Review and Network Meta-Analysis. Jpn. Dent. Sci. Rev. 2024, 60, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Contaldo, M.; Di Spirito, F.; Di Palo, M.P.; Amato, A.; Fiori, F.; Serpico, R. Fractal Analysis Applied to the Diagnosis of Oral Cancer and Oral Potentially Malignant Disorders: A Comprehensive Review. Appl. Sci. 2024, 14, 777. [Google Scholar] [CrossRef]
- Smriti, K.; Ray, M.; Chatterjee, T.; Shenoy, R.-P.; Gadicherla, S.; Pentapati, K.-C.; Rustaqi, N. Salivary MMP-9 as a Biomarker for the Diagnosis of Oral Potentially Malignant Disorders and Oral Squamous Cell Carcinoma. Asian Pac. J. Cancer Prev. 2020, 21, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Campagna, R.; Pozzi, V.; Salvucci, A.; Togni, L.; Mascitti, M.; Sartini, D.; Salvolini, E.; Santarelli, A.; Lo Muzio, L.; Emanuelli, M. Paraoxonase-2 Expression in Oral Squamous Cell Carcinoma. Hum. Cell 2023, 36, 1211–1213. [Google Scholar] [CrossRef] [PubMed]
- Gattuso, G.; Crimi, S.; Lavoro, A.; Rizzo, R.; Musumarra, G.; Gallo, S.; Facciponte, F.; Paratore, S.; Russo, A.; Bordonaro, R.; et al. Liquid Biopsy and Circulating Biomarkers for the Diagnosis of Precancerous and Cancerous Oral Lesions. ncRNA 2022, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Kinane, D.F.; Gabert, J.; Xynopoulos, G.; Guzeldemir-Akcakanat, E. Strategic Approaches in Oral Squamous Cell Carcinoma Diagnostics Using Liquid Biopsy. Periodontology 2000 2024, 96, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Roi, A.; Boia, S.; Rusu, L.-C.; Roi, C.I.; Boia, E.R.; Riviș, M. Circulating miRNA as a Biomarker in Oral Cancer Liquid Biopsy. Biomedicines 2023, 11, 965. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cheng, L.; Wang, G.; Lv, J.; He, Y.; Shao, P.-L.; Hu, R.; Xiao, H.; Tang, J.; Niu, D.; et al. A Nano-Magnetic Size Selective cfDNA Extraction Platform for Liquid Biopsy with Enhanced Precision. J. Chromatogr. B 2022, 1199, 123236. [Google Scholar] [CrossRef] [PubMed]
- Loy, C.; Ahmann, L.; De Vlaminck, I.; Gu, W. Liquid Biopsy Based on Cell-Free DNA and RNA. Annu. Rev. Biomed. Eng. 2024, 26, 169–195. [Google Scholar] [CrossRef] [PubMed]
- Anitha, K.; Posinasetty, B.; Naveen Kumari, K.; Chenchula, S.; Padmavathi, R.; Prakash, S.; Radhika, C. Liquid Biopsy for Precision Diagnostics and Therapeutics. Clin. Chim. Acta 2024, 554, 117746. [Google Scholar] [CrossRef] [PubMed]
- Rapado-González, Ó.; Rodríguez-Ces, A.M.; López-López, R.; Suárez-Cunqueiro, M.M. Liquid Biopsies Based on Cell-Free DNA as a Potential Biomarker in Head and Neck Cancer. Jpn. Dent. Sci. Rev. 2023, 59, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Avssn, R.; Chittela, R.K. A Phenol-Chloroform Free Method for cfDNA Isolation from Cell Conditioned Media: Development, Optimization and Comparative Analysis. Anal. Biochem. 2024, 687, 115454. [Google Scholar] [CrossRef] [PubMed]
- Bronkhorst, A.J.; Holdenrieder, S. A Pocket Companion to Cell-Free DNA (cfDNA) Preanalytics. Tumour Biol. 2024, 46, S297–S308. [Google Scholar] [CrossRef] [PubMed]
- Sorbini, M.; Carradori, T.; Togliatto, G.M.; Vaisitti, T.; Deaglio, S. Technical Advances in Circulating Cell-Free DNA Detection and Analysis for Personalized Medicine in Patients’ Care. Biomolecules 2024, 14, 498. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Zhao, X.; An, Y.; Yang, M.; Zhang, Z.; Liu, X.; Hu, D.; Wang, W.; Pan, Y.; Xia, Z.; et al. Cell-Free DNA End Characteristics Enable Accurate and Sensitive Cancer Diagnosis. Cell Rep. Methods 2024, 4, 100877. [Google Scholar] [CrossRef] [PubMed]
- Birknerova, N.; Mancikova, V.; Paul, E.D.; Matyasovsky, J.; Cekan, P.; Palicka, V.; Parova, H. Circulating Cell-Free DNA-Based Methylation Pattern in Saliva for Early Diagnosis of Head and Neck Cancer. Cancers 2022, 14, 4882. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhang, H.; Li, L.; Lin, Y.; Wang, X.; Chen, W.; Tao, Y.; Ou, R.; Zhou, W.; Zheng, F.; et al. Heat Inactivation Does Not Alter Host Plasma Cell-Free DNA Characteristics in Infectious Disease Research. Clin. Chim. Acta 2024, 553, 117751. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Chen, H.; Long, Y.; Li, P.; Gu, Y. The Main Sources of Circulating Cell-Free DNA: Apoptosis, Necrosis and Active Secretion. Crit. Rev. Oncol./Hematol. 2021, 157, 103166. [Google Scholar] [CrossRef] [PubMed]
- Nafar, S.; Hosseini, K.; Shokrgozar, N.; Farahmandi, A.Y.; Alamdari-Palangi, V.; Saber Sichani, A.; Fallahi, J. An Investigation into Cell-Free DNA in Different Common Cancers. Mol. Biotechnol. 2024, 66, 3462–3474. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, Z.; Zhang, W.; Liu, S. Advances in Biomarker Discovery Using Circulating Cell-free DNA for Early Detection of Hepatocellular Carcinoma. WIREs Mech. Dis. 2023, 15, e1598. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Kong, P.; Ma, G.; Li, L.; Zhu, J.; Xia, T.; Xie, H.; Zhou, W.; Wang, S. Characterization of the Release and Biological Significance of Cell-Free DNA from Breast Cancer Cell Lines. Oncotarget 2017, 8, 43180–43191. [Google Scholar] [CrossRef] [PubMed]
- Viglianisi, G.; Santonocito, S.; Polizzi, A.; Troiano, G.; Amato, M.; Zhurakivska, K.; Pesce, P.; Isola, G. Impact of Circulating Cell-Free DNA (cfDNA) as a Biomarker of the Development and Evolution of Periodontitis. Int. J. Mol. Sci. 2023, 24, 9981. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Z.; Ji, K.; Li, X.; Wang, C.; Ren, Z.; Liu, Y.; Chen, X.; Han, X.; Meng, L.; et al. Clinical Application Value of Circulating Cell-Free DNA in Hepatocellular Carcinoma. Front. Mol. Biosci. 2021, 8, 736330. [Google Scholar] [CrossRef] [PubMed]
- Brooks, P.J.; Malkin, E.Z.; De Michino, S.; Bratman, S.V. Isolation of Salivary Cell-Free DNA for Cancer Detection. PLoS ONE 2023, 18, e0285214. [Google Scholar] [CrossRef] [PubMed]
- Salfer, B.; Havo, D.; Kuppinger, S.; Wong, D.; Li, F.; Zhang, L. Evaluating Pre-Analytical Variables for Saliva Cell-Free DNA Liquid Biopsy. Diagnostics 2023, 13, 1665. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Song, X.; Geng, X.; Liu, L.; Ma, H.; Wang, X.; Wei, L.; Xie, L.; Song, X. Saliva-derived cfDNA Is Applicable for EGFR Mutation Detection but Not for Quantitation Analysis in Non-small Cell Lung Cancer. Thorac. Cancer 2019, 10, 1973–1983. [Google Scholar] [CrossRef] [PubMed]
- Rapado-González, Ó.; López-Cedrún, J.L.; Lago-Lestón, R.M.; Abalo, A.; Rubin-Roger, G.; Salgado-Barreira, Á.; López-López, R.; Muinelo-Romay, L.; Suárez-Cunqueiro, M.M. Integrity and Quantity of Salivary Cell-free DNA as a Potential Molecular Biomarker in Oral Cancer: A Preliminary Study. J. Oral Pathol. Med. 2022, 51, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-H.; Chang, K.-W.; Cheng, H.-W.; Liu, C.-J. Identification of Somatic Mutations in Plasma Cell-Free DNA from Patients with Metastatic Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2023, 24, 10408. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-H.; Chang, K.-W.; Kao, S.-Y.; Cheng, H.-W.; Liu, C.-J. Increased Plasma Circulating Cell-Free DNA Could Be a Potential Marker for Oral Cancer. Int. J. Mol. Sci. 2018, 19, 3303. [Google Scholar] [CrossRef] [PubMed]
- Hudečková, M.; Koucký, V.; Rottenberg, J.; Gál, B. Gene Mutations in Circulating Tumour DNA as a Diagnostic and Prognostic Marker in Head and Neck Cancer—A Systematic Review. Biomedicines 2021, 9, 1548. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-H.; Cheng, H.-W.; Liu, C.-J. Droplet Digital Polymerase Chain Reaction for Detection and Quantification of Cell-Free DNA TP53 Target Somatic Mutations in Oral Cancer. CBM 2022, 33, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, A.; Hariharan, A.K.; Hasina, R.; Nair, J.R.; Katragadda, S.; Irusappan, S.; Ravichandran, A.; Veeramachaneni, V.; Bettadapura, R.; Bhati, M.; et al. Ultrasensitive Detection of Tumor-specific Mutations in Saliva of Patients with Oral Cavity Squamous Cell Carcinoma. Cancer 2021, 127, 1576–1589. [Google Scholar] [CrossRef] [PubMed]
- Azab, N.; Zahran, F.; Amin, A.; Rady, N. DNA Integrity in Diagnosis of Premalignant Lesions. Med. Oral 2021, 26, e445–e450. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Ji, M.; Li, F.; Li, Y.; Zhou, X.; Hsueh, C.; Zhou, L. Diagnostic and Prognostic Value of Plasma Cell-Free DNA Combined with VEGF-C in Laryngeal Squamous Cell Carcinoma. Mol. Cell. Probes 2023, 67, 101895. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Mishra, S.; Anand, N.; Hadi, R.; Rastogi, M.; Husain, N. Circulating Free DNA Integrity Index and Promoter Methylation of Tumor Suppressor Gene P16, DAPK and RASSF1A as a Biomarker for Oropharyngeal Squamous Cell Carcinoma. Pathol.—Res. Pract. 2023, 246, 154489. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Z.; Gao, Q.; Peng, Y.; Yu, Y.; Hu, T.; Wang, W. Correlation of DNA Methylation of DNMT3A and TET2 with Oral Squamous Cell Carcinoma. Discov. Oncol. 2024, 15, 15. [Google Scholar] [CrossRef] [PubMed]
- Mesgari, H.; Esmaelian, S.; Nasiri, K.; Ghasemzadeh, S.; Doroudgar, P.; Payandeh, Z. Epigenetic Regulation in Oral Squamous Cell Carcinoma Microenvironment: A Comprehensive Review. Cancers 2023, 15, 5600. [Google Scholar] [CrossRef] [PubMed]
- Rapado-González, Ó.; Salta, S.; López-López, R.; Henrique, R.; Suárez-Cunqueiro, M.M.; Jerónimo, C. DNA Methylation Markers for Oral Cancer Detection in Non- and Minimally Invasive Samples: A Systematic Review. Clin. Epigenetics 2024, 16, 105. [Google Scholar] [CrossRef] [PubMed]
- Burassakarn, A.; Pientong, C.; Sunthamala, N.; Chuerduangphui, J.; Vatanasapt, P.; Patarapadungkit, N.; Kongyingyoes, B.; Ekalaksananan, T. Aberrant Gene Promoter Methylation of E-Cadherin, P16 INK4a, P14 ARF, and MGMT in Epstein–Barr Virus-Associated Oral Squamous Cell Carcinomas. Med. Oncol. 2017, 34, 128. [Google Scholar] [CrossRef] [PubMed]
- Rettori, M.M.; De Carvalho, A.C.; Bomfim Longo, A.L.; De Oliveira, C.Z.; Kowalski, L.P.; Carvalho, A.L.; Vettore, A.L. Prognostic Significance of TIMP3 Hypermethylation in Post-Treatment Salivary Rinse from Head and Neck Squamous Cell Carcinoma Patients. Carcinogenesis 2013, 34, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Chang, C.; Ko, H.; Lee, J.; Chen, H.; Wang, H.; Lin, H.; Chiang, C. Hypermethylated ZNF582 and PAX1 Genes in Mouth Rinse Samples as Biomarkers for Oral Dysplasia and Oral Cancer Detection. Head Neck 2018, 40, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-C. Circulating Tumor DNA in Liquid Biopsy: Current Diagnostic Limitation. World J. Gastroenterol. 2024, 30, 2175–2178. [Google Scholar] [CrossRef] [PubMed]
- Ghiyasimoghaddam, N.; Shayan, N.; Mirkatuli, H.A.; Baghbani, M.; Ameli, N.; Ashari, Z.; Mohtasham, N. Does Circulating Tumor DNA Apply as a Reliable Biomarker for the Diagnosis and Prognosis of Head and Neck Squamous Cell Carcinoma? Discov. Oncol. 2024, 15, 427. [Google Scholar] [CrossRef] [PubMed]
- Aulakh, S.S.; Silverman, D.A.; Young, K.; Dennis, S.K.; Birkeland, A.C. The Promise of Circulating Tumor DNA in Head and Neck Cancer. Cancers 2022, 14, 2968. [Google Scholar] [CrossRef] [PubMed]
- Rendek, T.; Pos, O.; Duranova, T.; Saade, R.; Budis, J.; Repiska, V.; Szemes, T. Current Challenges of Methylation-Based Liquid Biopsies in Cancer Diagnostics. Cancers 2024, 16, 2001. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.; Ahmed, R.; Das, D.K.; Pramanik, D.D.; Dash, S.K.; Pramanik, A. Recent Advancements in the Application of Circulating Tumor DNA as Biomarkers for Early Detection of Cancers. ACS Biomater. Sci. Eng. 2024, 10, 4740–4756. [Google Scholar] [CrossRef] [PubMed]
- Markou, A.; Londra, D.; Tserpeli, V.; Kollias, Ι.; Tsaroucha, E.; Vamvakaris, I.; Potaris, K.; Pateras, I.; Kotsakis, A.; Georgoulias, V.; et al. DNA Methylation Analysis of Tumor Suppressor Genes in Liquid Biopsy Components of Early Stage NSCLC: A Promising Tool for Early Detection. Clin. Epigenetics 2022, 14, 427. [Google Scholar] [CrossRef] [PubMed]
- Bie, Z.; Ping, Y.; Li, X.; Lan, X.; Wang, L. Accurate Early Detection and EGFR Mutation Status Prediction of Lung Cancer Using Plasma cfDNA Coverage Patterns: A Proof-of-Concept Study. Biomolecules 2024, 14, 716. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, S.; Ullah, I.; Ali, A.; Jan, Z.; Aleem, B.; Khan, M.; Naseem, W. Role of Circulating Tumor DNA and Cell-Free DNA Biomarkers in Diagnosis and Prognosis of Oral Cancer—A Systematic Review. BMC Oral Health 2025, 25, 522. [Google Scholar] [CrossRef] [PubMed]
- Varzaru, B.; Iacob, R.A.; Bunduc, S.; Manea, I.; Sorop, A.; Spiridon, A.; Chelaru, R.; Croitoru, A.; Topala, M.; Becheanu, G.; et al. Prognostic Value of Circulating Cell-Free DNA Concentration and Neutrophil-to-Lymphocyte Ratio in Patients with Pancreatic Ductal Adenocarcinoma: A Prospective Cohort Study. Int. J. Mol. Sci. 2024, 25, 2854. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Gao, Y.; Vafaei, S.; Gu, X.; Zhong, X. The Prognostic Value of Plasma Cell-Free DNA Concentration in the Prostate Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 599602. [Google Scholar] [CrossRef] [PubMed]
- Uchibori, M.; Hosomichi, K.; Hoshimoto, Y.; Sasaki, M.; Aoki, T.; Tajima, A.; Ota, Y.; Kimura, M. The Efficacy of Liquid Biopsy of Total cfDNA for Predicting Systemic Metastasis in Japanese Patients With Oral Squamous Cell Carcinoma. Head Neck 2025, 47, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, M.; Chernenko, P.; Breder, V.; Laktionov, K.; Rozhavskaya, E.; Musienko, S.; Baranova, A.; Mileyko, V. Utility of cfDNA Fragmentation Patterns in Designing the Liquid Biopsy Profiling Panels to Improve Their Sensitivity. Front. Genet. 2019, 10, 194. [Google Scholar] [CrossRef] [PubMed]
- Bittla, P.; Kaur, S.; Sojitra, V.; Zahra, A.; Hutchinson, J.; Folawemi, O.; Khan, S. Exploring Circulating Tumor DNA (CtDNA) and Its Role in Early Detection of Cancer: A Systematic Review. Cureus 2023, 15, e45784. [Google Scholar] [CrossRef] [PubMed]
- Honoré, N.; Van Marcke, C.; Galot, R.; Helaers, R.; Ambroise, J.; Van Maanen, A.; Mendola, A.; Dahou, H.; Marbaix, E.; Van Eeckhout, P.; et al. Tumor-Agnostic Plasma Assay for Circulating Tumor DNA Detects Minimal Residual Disease and Predicts Outcome in Locally Advanced Squamous Cell Carcinoma of the Head and Neck. Ann. Oncol. 2023, 34, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Kogo, R.; Manako, T.; Iwaya, T.; Nishizuka, S.; Hiraki, H.; Sasaki, Y.; Idogawa, M.; Tokino, T.; Koide, A.; Komune, N.; et al. Individualized Circulating Tumor DNA Monitoring in Head and Neck Squamous Cell Carcinoma. Cancer Med. 2022, 11, 3960–3968. [Google Scholar] [CrossRef] [PubMed]
- Chera, B.S.; Kumar, S.; Beaty, B.T.; Marron, D.; Jefferys, S.; Green, R.; Goldman, E.C.; Amdur, R.; Sheets, N.; Dagan, R.; et al. Rapid Clearance Profile of Plasma Circulating Tumor HPV Type 16 DNA during Chemoradiotherapy Correlates with Disease Control in HPV-Associated Oropharyngeal Cancer. Clin. Cancer Res. 2019, 25, 4682–4690. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.; Zou, J.; Magalhaes, M.; Oliva, M.; Spreafico, A.; Hansen, A.R.; McDade, S.S.; Coyle, V.M.; Lawler, M.; Elimova, E.; et al. Circulating Tumour DNA Kinetics in Recurrent/Metastatic Head and Neck Squamous Cell Cancer Patients. Eur. J. Cancer 2023, 188, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Lianidou, E. Detection and Relevance of Epigenetic Markers on ctDNA: Recent Advances and Future Outlook. Mol. Oncol. 2021, 15, 1683–1700. [Google Scholar] [CrossRef] [PubMed]
- Maansson, C.T.; Thomsen, L.S.; Meldgaard, P.; Nielsen, A.L.; Sorensen, B.S. Integration of Cell-Free DNA End Motifs and Fragment Lengths Can Identify Active Genes in Liquid Biopsies. Int. J. Mol. Sci. 2024, 25, 1243. [Google Scholar] [CrossRef] [PubMed]
- Markou, A.; Tzanikou, E.; Lianidou, E. The Potential of Liquid Biopsy in the Management of Cancer Patients. Semin. Cancer Biol. 2022, 84, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Torresan, S.; De Scordilli, M.; Bortolot, M.; Di Nardo, P.; Foltran, L.; Fumagalli, A.; Guardascione, M.; Ongaro, E.; Puglisi, F. Liquid Biopsy in Colorectal Cancer: Onward and Upward. Crit. Rev. Oncol./Hematol. 2024, 194, 104242. [Google Scholar] [CrossRef] [PubMed]
- Ignatiadis, M.; Sledge, G.W.; Jeffrey, S.S. Liquid Biopsy Enters the Clinic—Implementation Issues and Future Challenges. Nat. Rev. Clin. Oncol. 2021, 18, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Wilson, H.L.; D’Agostino, R.B.; Meegalla, N.; Petro, R.; Commander, S.; Topaloglu, U.; Zhang, W.; Porosnicu, M. The Prognostic and Therapeutic Value of the Mutational Profile of Blood and Tumor Tissue in Head and Neck Squamous Cell Carcinoma. Oncologist 2021, 26, e279–e289. [Google Scholar] [CrossRef] [PubMed]
- Romani, C.; Baronchelli, M.; Assoni, C.; Mattavelli, D.; Calza, S.; Piazza, C.; Bossi, P. Stability of Circulating miRNA in Saliva: The Influence of Sample Associated Pre-Analytical Variables. Clin. Chim. Acta 2024, 553, 117702. [Google Scholar] [CrossRef] [PubMed]
- Bartolomucci, A.; Nobrega, M.; Ferrier, T.; Dickinson, K.; Kaorey, N.; Nadeau, A.; Castillo, A.; Burnier, J.V. Circulating Tumor DNA to Monitor Treatment Response in Solid Tumors and Advance Precision Oncology. NPJ Precis. Onc. 2025, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- Sanesi, L.; Mori, G.; Troiano, G.; Ballini, A.; Valzano, F.; Dioguardi, M.; Muzio, L.L.; Magalhaes, M.; Caponio, V.C.A. Salivary Exosomal microRNA Profile as Biomonitoring Tool for Diagnosis and Prognosis of Patients with Head and Neck Squamous Cell Carcinoma: A Systematic Review. Arch. Oral Biol. 2024, 165, 106012. [Google Scholar] [CrossRef] [PubMed]
- Togni, L.; Mascitti, M.; Sartini, D.; Campagna, R.; Pozzi, V.; Salvolini, E.; Offidani, A.; Santarelli, A.; Emanuelli, M. Nicotinamide N-Methyltransferase in Head and Neck Tumors: A Comprehensive Review. Biomolecules 2021, 11, 1594. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Wang, Z.; Xu, M.; Li, B.; Huang, Z.; Qin, S.; Nice, E.C.; Tang, J.; Huang, C. Oral Squamous Cell Carcinomas: State of the Field and Emerging Directions. Int. J. Oral Sci. 2023, 15, 44. [Google Scholar] [CrossRef] [PubMed]
- Silvoniemi, A.; Laine, J.; Aro, K.; Nissi, L.; Bäck, L.; Schildt, J.; Hirvonen, J.; Hagström, J.; Irjala, H.; Aaltonen, L.-M.; et al. Circulating Tumor DNA in Head and Neck Squamous Cell Carcinoma: Association with Metabolic Tumor Burden Determined with FDG-PET/CT. Cancers 2023, 15, 3970. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Yang, L.; Zhang, H.; Beeraka, N.M.; Zhang, D.; Wang, Q.; Wang, M.; Pr, H.V.; Sethi, G.; Wang, G. Decoding Tumor Microenvironment: EMT Modulation in Breast Cancer Metastasis and Therapeutic Resistance, and Implications of Novel Immune Checkpoint Blockers. Biomed. Pharmacother. 2024, 181, 117714. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Hu, S.; Li, X.; Pei, S.; Jin, L.; Zhang, L.; Chen, X.; Min, A.; Yin, M. SPP1+ TAM Regulates the Metastatic Colonization of CXCR4+ Metastasis-Associated Tumor Cells by Remodeling the Lymph Node Microenvironment. Adv. Sci. 2024, 11, 2400524. [Google Scholar] [CrossRef] [PubMed]
- Schiavoni, V.; Emanuelli, M.; Sartini, D.; Salvolini, E.; Pozzi, V.; Campagna, R. Curcumin and Its Analogues in Oral Squamous Cell Carcinoma: State-of-the-Art and Therapeutic Potential. Anti-Cancer Agents Med. Chem. 2025, 25, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Leo, P.; Jones, L.; Duijf, P.H.G.; Hartel, G.; Kenny, L.; Vasani, S.; Punyadeera, C. A Comparison between Mutational Profiles in Tumour Tissue DNA and Circulating Tumour DNA in Head and Neck Squamous Cell Carcinoma—A Systematic Review. Mutat. Res.—Rev. Mutat. Res. 2024, 793, 108477. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, Z.; Pu, J.J.; Zhong, J.; Khoo, U.; Su, Y.; Zhang, G. Proteogenomic Characterisation of Primary Oral Cancer Unveils Extracellular Matrix Remodelling and Immunosuppressive Microenvironment Linked to Lymph Node Metastasis. Clin. Transl. Med. 2025, 15, e70261. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Birkeland, A.C. Liquid Biopsies in Head and Neck Cancer: Current State and Future Challenges. Cancers 2021, 13, 1874. [Google Scholar] [CrossRef] [PubMed]
- Moon, G.Y.; Dalkiran, B.; Park, H.S.; Shin, D.; Son, C.; Choi, J.H.; Bang, S.; Lee, H.; Doh, I.; Kim, D.H.; et al. Dual Biomarker Strategies for Liquid Biopsy: Integrating Circulating Tumor Cells and Circulating Tumor DNA for Enhanced Tumor Monitoring. Biosensors 2025, 15, 74. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.A.; Sborchia, M.; Bye, H.; Roman-Escorza, M.; Amar, A.; Henley-Smith, R.; Odell, E.; McGurk, M.; Simpson, M.; Ng, T.; et al. Mutation Detection in Saliva from Oral Cancer Patients. Oral Oncol. 2024, 151, 106717. [Google Scholar] [CrossRef] [PubMed]
- Hitchen, N.; Shahnam, A.; Tie, J. Circulating Tumor DNA: A Pan-Cancer Biomarker in Solid Tumors with Prognostic and Predictive Value. Annu. Rev. Med. 2025, 76, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.; Rubio-Alarcón, C.; Van Den Broek, D.; Vessies, D.C.L.; Van’T Erve, I.; Meijer, G.A.; Vink, G.R.; Schuuring, E.; Fijneman, R.J.A.; Coupé, V.M.H.; et al. A Scenario-drafting Study to Explore Potential Future Implementation Pathways of Circulating Tumor DNA Testing in Oncology. Mol. Oncol. 2024, 18, 2730–2742. [Google Scholar] [CrossRef] [PubMed]
- Rashid, S.; Puttagunta, P.; Pamulapati, S.; Yang, J.; Pocha, S.; Saba, N.F.; Teng, Y. Leveraging Saliva for Insights into Head and Neck Cancer. Int. J. Mol. Sci. 2024, 25, 13514. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Wang, S.; Lu, W.; Ju, W.; Li, J.; Tan, X.; Zhao, H.; Han, W.; Yang, X. Application of Metabolomics in Oral Squamous Cell Carcinoma. Oral Dis. 2024, 30, 3719–3731. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.S.; Singh, R.P.; Karsauliya, K.; Sonker, A.K.; Reddy, P.J.; Mehrotra, D.; Gupta, S.; Singh, S.; Kumar, R.; Singh, S.P. Label-Free Plasma Proteomics for the Identification of the Putative Biomarkers of Oral Squamous Cell Carcinoma. J. Proteom. 2022, 259, 104541. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-T.; Chen, H.-W.; Wu, C.-F.; Chu, L.J.; Chiang, W.-F.; Wu, C.-C.; Yu, J.-S.; Tsai, C.-H.; Liang, K.-H.; Chang, Y.-S.; et al. Development of a Multiplexed Liquid Chromatography Multiple-Reaction-Monitoring Mass Spectrometry (LC-MRM/MS) Method for Evaluation of Salivary Proteins as Oral Cancer Biomarkers. Mol. Cell Proteom. 2017, 16, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Ardito, F.; Perrone, D.; Cocchi, R.; Lo Russo, L.; DE Lillo, A.; Giannatempo, G.; Lo Muzio, L. Novel Possibilities in the Study of the Salivary Proteomic Profile Using SELDI-TOF/MS Technology. Oncol. Lett. 2016, 11, 1967–1972. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.-W.; Chang, K.-P.; Hsu, C.-W.; Chang, I.Y.-F.; Liu, H.-P.; Chen, Y.-T.; Wu, C.-C. Identification of Salivary Biomarkers for Oral Cancer Detection with Untargeted and Targeted Quantitative Proteomics Approaches. Mol. Cell. Proteom. 2019, 18, 1796–1806. [Google Scholar] [CrossRef] [PubMed]
- Pillai, J.; Chincholkar, T.; Dixit, R.; Pandey, M. A Systematic Review of Proteomic Biomarkers in Oral Squamous Cell Cancer. World J. Surg. Oncol. 2021, 19, 315. [Google Scholar] [CrossRef] [PubMed]
- Pomella, S.; Melaiu, O.; Cifaldi, L.; Bei, R.; Gargari, M.; Campanella, V.; Barillari, G. Biomarkers Identification in the Microenvironment of Oral Squamous Cell Carcinoma: A Systematic Review of Proteomic Studies. Int. J. Mol. Sci. 2024, 25, 8929. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, B. Updates of Liquid Biopsy in Oral Cancer and Multiomics Analysis. Oral Dis. 2023, 29, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Garcia, P.N.; De Souza, M.M.; Izidoro, M.A.; Juliano, L.; Lourenço, S.V.; Camillo, C.M.C. Saliva Metabolomics: Concepts and Applications in Oral Disorders. Clin. Oral Investig. 2024, 28, 579. [Google Scholar] [CrossRef] [PubMed]
- Drouin, N.; Ramautar, R. Capillary Electrophoresis-Mass Spectrometry for Metabolomics: Possibilities and Perspectives. Adv. Exp. Med. Biol. 2021, 1336, 159–178. [Google Scholar] [CrossRef] [PubMed]
- Nagana Gowda, G.A.; Raftery, D. NMR-Based Metabolomics. Adv. Exp. Med. Biol. 2021, 1280, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Nijakowski, K.; Gruszczyński, D.; Kopała, D.; Surdacka, A. Salivary Metabolomics for Oral Squamous Cell Carcinoma Diagnosis: A Systematic Review. Metabolites 2022, 12, 294. [Google Scholar] [CrossRef] [PubMed]
- Nazar, N.S.B.M.; Ramanathan, A.; Ghani, W.M.N.; Rokhani, F.B.; Jacob, P.S.; Sabri, N.E.B.; Hassan, M.S.; Kadir, K.; Dharmarajan, L. Salivary Metabolomics in Oral Potentially Malignant Disorders and Oral Cancer Patients—A Systematic Review with Meta-Analysis. Clin. Oral Investig. 2024, 28, 98. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, S.; Sugimoto, M.; Edamatsu, K.; Sugano, A.; Kitabatake, K.; Iino, M. Discrimination of Oral Squamous Cell Carcinoma from Oral Lichen Planus by Salivary Metabolomics. Oral Dis. 2020, 26, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.-Y.; Zhu, X.-Y.; Wei, C.-L.; Sun, P.; Zhang, H.; Deng, J. Study of salivary metabonomics in patients with oral squamous cell carcinoma and leukoplakia. Shanghai Kou Qiang Yi Xue 2021, 30, 201–205. [Google Scholar] [PubMed]
- Ferrarini, A.; Di Poto, C.; He, S.; Tu, C.; Varghese, R.S.; Kara Balla, A.; Jayatilake, M.; Li, Z.; Ghaffari, K.; Fan, Z.; et al. Metabolomic Analysis of Liver Tissues for Characterization of Hepatocellular Carcinoma. J. Proteome Res. 2019, 18, 3067–3076. [Google Scholar] [CrossRef] [PubMed]
- Tantray, S.; Sharma, S.; Prabhat, K.; Nasrullah, N.; Gupta, M. Salivary Metabolite Signatures of Oral Cancer and Leukoplakia through Gas Chromatography-Mass Spectrometry. J. Oral Maxillofac. Pathol. 2022, 26, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Yang, X.; Narayanan, R.; Shankar, V.; Ethiraj, S.; Wang, X.; Duan, N.; Ni, Y.-H.; Hu, Q.; Zare, R.N. Oral Squamous Cell Carcinoma Diagnosed from Saliva Metabolic Profiling. Proc. Natl. Acad. Sci. USA 2020, 117, 16167–16173. [Google Scholar] [CrossRef] [PubMed]
- Li, X.B.; Liu, L.W.; Li, N.; Jia, Q.Q.; Wang, X.S.; Long, J.L.; Xue, P.; Sun, Z.; Zhao, H.Y. Identification of serum biomarkers and evaluation of metabolism disorders in patients with oral squamous cell carcinoma. Zhonghua Kou Qiang Yi Xue Za Zhi 2021, 56, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Song, X.; Yang, X.; Han, W.; Fu, Y.; Wang, S.; Zhang, X.; Sun, G.; Lu, Y.; Wang, Z.; et al. Big Cohort Metabolomic Profiling of Serum for Oral Squamous Cell Carcinoma Screening and Diagnosis. Nat. Sci. 2022, 2, e20210071. [Google Scholar] [CrossRef]
- Zuo, L.; Chen, Z.; Chen, L.; Kang, J.; Shi, Y.; Liu, L.; Zhang, S.; Jia, Q.; Huang, Y.; Sun, Z. Integrative Analysis of Metabolomics and Transcriptomics Data Identifies Prognostic Biomarkers Associated With Oral Squamous Cell Carcinoma. Front. Oncol. 2021, 11, 750794. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-H.; Jing, Y.; Wang, S.; Ding, F.; Zhang, X.-X.; Chen, S.; Zhang, L.; Hu, Q.-G.; Ni, Y.-H. Integrated Non-Targeted and Targeted Metabolomics Uncovers Amino Acid Markers of Oral Squamous Cell Carcinoma. Front. Oncol. 2020, 10, 426. [Google Scholar] [CrossRef] [PubMed]
- Musharraf, S.G.; Shahid, N.; Naqvi, S.M.A.; Saleem, M.; Siddiqui, A.J.; Ali, A. Metabolite Profiling of Preneoplastic and Neoplastic Lesions of Oral Cavity Tissue Samples Revealed a Biomarker Pattern. Sci. Rep. 2016, 6, 38985. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Washio, J.; Takahashi, T.; Echigo, S.; Takahashi, N. Glucose and Glutamine Metabolism in Oral Squamous Cell Carcinoma: Insight from a Quantitative Metabolomic Approach. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 118, 218–225. [Google Scholar] [CrossRef] [PubMed]

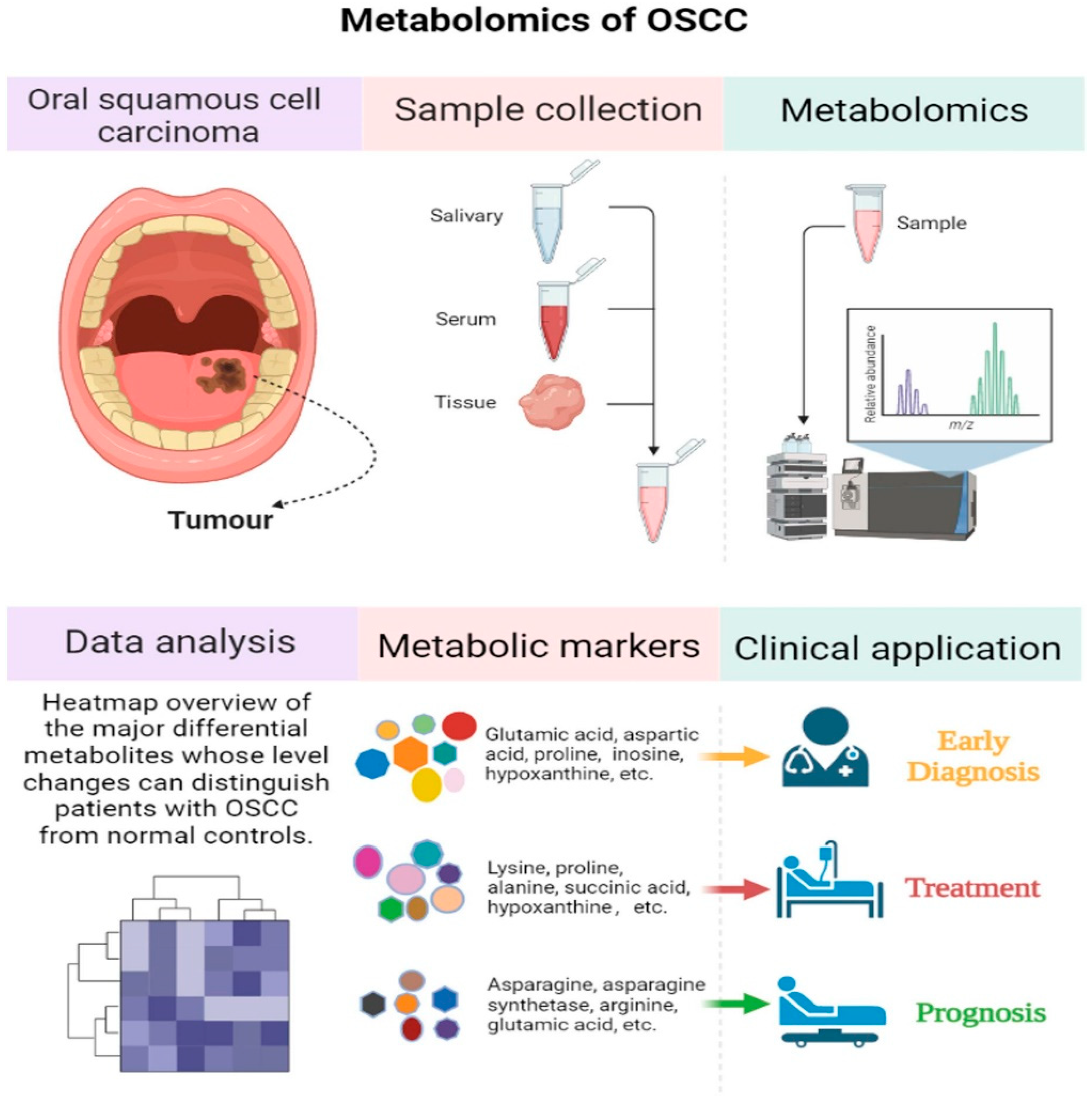
| Sample | Comparison | Metabolite Analysis Technique | Differential Metabolites | References |
|---|---|---|---|---|
| Saliva | OSCC versus OLK | GC-TOF-MS | Tyrosine, malic acid, tyrosine, lactic acid, 2-hydroxybutyric acid, prostaglandin E2 | Xue et al. [109] |
| OSCC versus HC | GC-TOF-MS | Prostaglandin E2, lactic acid, tyrosine, lactic acid, 2-hydroxybutyric acid, prostaglandin E2 | Ferrarini et al. [110] | |
| OLK versus HC | GC-TOF-MS | Tyrosine, lactic acid, 2-hydroxybutanedioic acid, prostaglandin E2 | Xue et al. [109] | |
| OSCC versus OLK | GC-MS | Decanedioic acid, 2-methyloctadecane, eicosanoic acid, octane, 3,5-dimethyl | Tantray et al. [111] | |
| OSCC versus HC | CPSI-MS | Polyamines (e.g., spermidine, spermine) and amino acids (e.g., arginine, lysine, histidine, glutamine, leucine) Metabolites related to energy metabolism (e.g., glucose, creatine, creatinine) and purine metabolites (e.g., inosine, hypoxanthine) | Song et al. [112] | |
| HC versus PML | CPSI-MS | Inosine, hypoxanthine, adenosine, thymidine, uridine, guanosine, cytosine, choline, sphingolipids, etc. | Song et al. [112] | |
| Serum | OSCC versus HC | UHPLC-Q-Orbitrap HRMS | LysoPC, taurine, and glutamate | Li et al. [113] |
| OSCC versus HC | CPSI-MS | GPC, lyso-GPC, acylcarnitine, DG, sphingolipids, etc. | Yang, Song, Yang, et al. [114] | |
| OSCC preoperative versus postoperative | UHPLC-Q-Orbitrap HRMS | Succinic acid, arginine, L-carnitine, NAcetyl-L-tyrosine, glutamine, xanthine, sphingosine, palmitoyl ethanolamide, hexanoyl carnitine, whey acid, uric acid, vanillylmandelic acid, ethyl acetate, thromboxane B2 | Zuo et al. [115] | |
| Tissue | OSCC versus HC | GC–MS UHPLC–MS/MS | Glutamic acid, aspartic acid, proline | Yang et al. [116] |
| OSCC versus PML versus HC | GC-MS Analyses | Glycine, threonine, glutamine, lysine, proline, alanine, glutamic acid, leucine, serine | Musharraf et al. [117] | |
| OSCC versus HC | CE-TOF-MS | Glucose, glycerol triphosphate, glycerol diphosphate, lactic acid | Ogawa et al. [118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rigotti, P.; Polizzi, A.; Quinzi, V.; Blasi, A.; Lombardi, T.; Lo Muzio, E.; Isola, G. Cell-Free DNA as a Prognostic Biomarker in Oral Carcinogenesis and Oral Squamous Cell Carcinoma: A Translational Perspective. Cancers 2025, 17, 2366. https://doi.org/10.3390/cancers17142366
Rigotti P, Polizzi A, Quinzi V, Blasi A, Lombardi T, Lo Muzio E, Isola G. Cell-Free DNA as a Prognostic Biomarker in Oral Carcinogenesis and Oral Squamous Cell Carcinoma: A Translational Perspective. Cancers. 2025; 17(14):2366. https://doi.org/10.3390/cancers17142366
Chicago/Turabian StyleRigotti, Pietro, Alessandro Polizzi, Vincenzo Quinzi, Andrea Blasi, Teresa Lombardi, Eleonora Lo Muzio, and Gaetano Isola. 2025. "Cell-Free DNA as a Prognostic Biomarker in Oral Carcinogenesis and Oral Squamous Cell Carcinoma: A Translational Perspective" Cancers 17, no. 14: 2366. https://doi.org/10.3390/cancers17142366
APA StyleRigotti, P., Polizzi, A., Quinzi, V., Blasi, A., Lombardi, T., Lo Muzio, E., & Isola, G. (2025). Cell-Free DNA as a Prognostic Biomarker in Oral Carcinogenesis and Oral Squamous Cell Carcinoma: A Translational Perspective. Cancers, 17(14), 2366. https://doi.org/10.3390/cancers17142366










