Simple Summary
Autoimmune diseases (AIDs) are associated with the development of B-cell non-Hodgkin lymphomas (B-NHLs). Autologous chimeric antigen receptor T-cell therapy (CART) is an effective therapy approved for the treatment of lymphoma; however, patients with AIDs were excluded from trials that led to CART approval. The goal of this retrospective study was to compare clinical outcomes for patients treated with CART for aggressive B-NHL with and without underlying AIDs. We found that the safety profile and efficacy of CART were comparable between these two cohorts. We also provide data on the impact of CART on AID control. This provides real-world information on the utility of CART as treatment for lymphoma in patients with AIDs, as well as insight into its possible use in the treatment of AIDs alone.
Abstract
Background/Objectives: There is an association between autoimmune disease (AID) and non-Hodgkin lymphoma (NHL). Chimeric antigen receptor T-cell therapy (CART) is approved for the treatment of aggressive B-cell NHL (B-NHL) based on pivotal trials that excluded patients with active AID and/or on chronic immunosuppressive therapy (IST). Thus, the efficacy of CART in patients with AID warrants further investigation. Methods: We identified patients with and without AID from a cohort of 727 patients treated with CART for aggressive B-NHL across 12 institutions. Overall survival (OS) and progression-free survival (PFS) were estimated using Kaplan-Meier survival curves. AID vs. non-AID patients were compared using log-rank tests. Results: Among our patients with aggressive B-NHL, 47 (6.4%) had concurrent AID. Rates of cytokine release syndrome and neurotoxicity were not significantly different between patients with and without AID (p = 0.5), while infection rates were higher in the AID cohort. For AID vs. non-AID patients, complete response rates were similar between the groups (38% vs. 36.0%, p = 0.36). The rates of relapse/progression post-CART were not significantly different (p = 0.07). Three-year PFS rates were 49% and 32%, respectively (p = 0.18); 3-year OS rates were 57% and 44%, respectively (p = 0.25). Prior to CART, 32% of patients with AID were on IST; 6 (13%) patients with AID either remained on IST during CART or resumed IST post-CART, and 1 patient newly started IST following CART. Conclusions: In the real-world setting, despite inherent T-cell dysfunction expected in B-NHL patients with concurrent AID, the safety and efficacy of CART is comparable with durable responses.
1. Introduction
Autoimmune diseases (AIDs) have been shown to be a risk factor for the development of non-Hodgkin lymphoma (NHL), with more than 15 million Americans diagnosed with AIDs between 2011 and 2022 [1,2,3,4,5,6,7]. The underlying mechanism for this association unclear but is thought to be related, in part, to inflammation, increased cytokines, and use of immunosuppressant medications in patients with AIDs. Given the heterogeneity of these diseases, it is likely multifactorial, and no single mechanism has emerged [2].
Pivotal prospective trials evaluating autologous CD19-directed chimeric antigen receptor T-cell therapy (CART) for the management of B-NHL have typically excluded patients with AIDs with concerns raised for the potential to harvest dysfunctional T-cells, thus impacting efficacy, or to exacerbate the underlying AID with infusion of auto-reactive T-cells [8,9,10,11]. Data addressing the safety and efficacy of CART for the treatment of lymphoma in patients with underlying AIDs is therefore limited. There is also concern that the use of immunosuppressive therapy (IST) in these patients may impact T-cell function. AIDs in clinical practice, however, are not exclusionary for the use of CART. In fact, more recently, CART has been studied for the treatment of AIDs with the realization that these diseases are often driven by B-cells. CART leads to B-cell depletion and is therefore a rational area of research in AID treatment and disease control [12,13,14,15]. As such, CART is currently being studied in a variety of rheumatologic and neurologic AIDs [12]. Our objective for this paper was to explore outcomes for patients undergoing CART for aggressive B-NHL with and without underlying AIDs. We provide real-world insight on the safety and efficacy of CART for patients with concurrent aggressive B-NHL and AID and the impact of CART on disease control for both.
2. Materials and Methods
We identified patients with and without AIDs from a cohort of 727 patients treated with CD19 autologous CART for aggressive B-cell NHL across 12 academic institutions in the United States between 2018 and 2024. The breakdown of lymphoma histologies is as follows: diffuse large B-cell lymphoma (547 patients), transformed follicular lymphoma (138 patients), primary mediastinal B-cell lymphoma (14 patients), Richter’s transformation (23 patients), transformed marginal zone lymphoma (24 patients), post-transplant lymphoproliferative disorder (8 patients), grey zone (2 patients), transformed mantle cell lymphoma (3 patients), Burkitt’s lymphoma (3 patients), T-cell/histiocyte-rich large B-cell lymphoma (5 patients), plasmablastic (2 patients), primary central nervous system lymphoma (1 patient), and other (23 patients). The study was approved by the respective institutional review boards and conducted in accordance with the Declaration of Helsinki.
2.1. Endpoints and Assessments
Cytokine release syndrome (CRS) and immune effector-cell neurologic syndrome/neurotoxicity (ICANS) were defined according to the ASTCT Consensus criteria [16]. Survival time was calculated from infusion to date of death or last follow-up. Progression was defined as the earliest of the latter or documented relapse/progression. Clinically significant infection data were collected on patients post-CART infusion. Patients with a formal diagnosis of an autoimmune condition managed with or without immunosuppression were identified, including but not limited to seropositive inflammatory arthropathy and/or vasculitis, inflammatory bowel disease, thyroid disorders, celiac disease, sarcoidosis, multiple sclerosis (MS), and myasthenia gravis (MG). Immune thrombocytopenic purpura was excluded as this was thought likely to be a result of lymphoma rather than a primary autoimmune condition. This excluded 3 patients.
2.2. Statistical Analyses
Data were compared using the Chi-square/Fisher’s exact tests for categorical variables and the ANOVA test for continuous variables. Overall survival (OS) and progression-free survival (PFS) were estimated using Kaplan–Meier survival curves. AIDs vs. non-AIDs patients were compared using log-rank tests. All statistical tests were performed using SAS 9.4 (Cary, NC, USA), and a p-value < 0.05 was considered statistically significant.
3. Results
Of the 727 patients, 47 patients had an underlying AID (6.4%), with baseline demographic and clinical information for these patients noted in Table 1. There was no significant difference in median age or sex between groups (p = 0.15 and 0.72, respectively). The most common AID was rheumatoid arthritis (RA, 29.8%), followed by systemic lupus erythematosus (SLE), Sjogren’s syndrome, Crohn’s disease, and psoriasis (10.6% each).

Table 1.
Clinical and demographic features among patients at time of CART by autoimmune disease status (n = 727).
For AID vs. non-AID patients, there was no difference in rates of double-hit lymphoma (DHL; p = 0.39), double-expressor lymphoma (DEL; p = 0.67), primary refractory disease (p = 0.48), or median lines of prior therapy, including prior autologous stem cell transplant (p = 0.32). International prognostic index score, ECOG performance status, incidence of stage 4 disease, and elevated LDH at the time of CART collection were similar between cohorts (p = 0.21–1.0). After apheresis, there was no difference in the use of bridging therapy (p = 0.67). The median time from CART collection to infusion for non-AID and AID groups was 34 days and 36 days, respectively (p = 0.96). There was no difference in CART construct used (p = 0.07), with axicabtagene autoleucel being most commonly used in both groups.
3.1. Clinical Outcomes for B-NHL
Median follow-up for survivors was 26.5 months. For AID vs. non-AID groups, complete remission rates (CRs) were similar (38.3 vs. 36%, p = 0.36). The rates of relapse/progression post-CART were not significantly different—52.6% of the non-AID group and 38.3% in the AID group (p = 0.07). Three-year PFS rates from CART infusion were 49% in AID patients and 32% in non-AID patients (p = 0.18, Figure 1). Three-year OS rates from CART infusion were 57% in AID patients and 44% in non-AID patients (p = 0.25, Figure 2). There were no differences in PFS and OS between groups for primary refractory disease (p = 0.69 and 0.73), DHL (p = 0.91 and 0.61), DEL (p = 0.46 and 0.81), or patients requiring bridging (p = 0.87 and 0.7). All AIDs patients who relapsed received salvage therapy post-CART. Details regarding salvage therapies can be found in Supplemental Table S1.
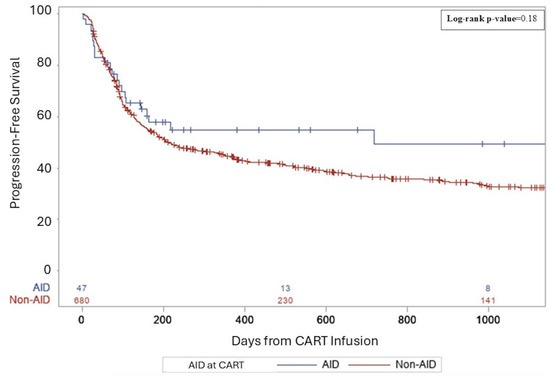
Figure 1.
A three-year progression-free survival following CART for aggressive lymphoma.
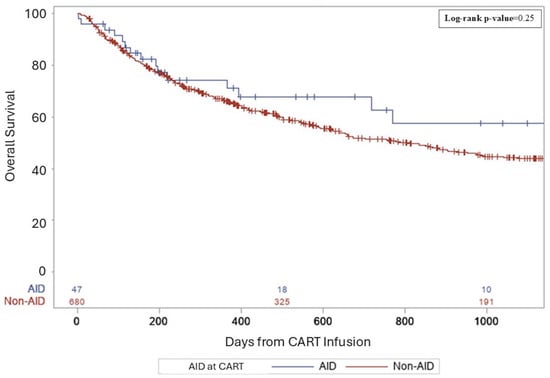
Figure 2.
A three-year overall survival following CART for aggressive lymphoma.
3.2. Clinical Impact of CART on the Underlying AID
Data regarding IST resumption was available in 36 patients. Notably, 15 (31.9%) patients with AID were on immunosuppressive therapy (IST) prior to CART. Seven (14.9%) patients with AID were placed on IST following CART, only one of whom was not on IST prior to CART; three (6.4%) of these patients restarted following CART, and three (6.4%) remained on IST throughout their CART treatment process and after treatment. Of the six patients that were on IST following CART, five received IST for active AIDs and one remained on IST as prophylaxis. Four patients were documented to have flares of their AID following CART, two of which were within the first 90 days following CART. Supplemental Table S2 provides further detail on IST utilization in patients with AIDs before and after CART.
3.3. Toxicity
Rates of CRS were similar between non-AID and AID groups, including grade 3+ (13.2% and 12.1%, respectively, p = 0.5). Median time to CRS from infusion was 3 days, and duration of CRS was 4 days for both cohorts. However, tocilizumab was used more frequently in patients with AID (p = 0.02). Rates of ICANS were also similar between non-AID and AID groups including grade 3+ (42.4% and 40%, respectively). Time to ICANS was 6 days in both groups. Duration of symptoms was 5 days in the non-AID group and 3 days in the AID group. Persistent neutropenia (ANC < 500) and thrombocytopenia (platelets < 50) at 30 days or greater post-CART were documented in five (10.6%) and three (6.4%) AID patients, respectively, compared to 50 (7.4%) and 78 (11.4%) in non-AID (not statistically different).
Within 90 days post-CART infusion, more AID patients incurred infections. Twenty AID patients (42.6%) versus 191 non-AID patients (28.1%) had documented infections (p = 0.02). Four patients in the AID group experienced multiple infections during this 90-day period; the most common initial infections were bacterial (65%) and viral (35%). No patients in the AID group and eight patients in the non-AID group were diagnosed with COVID-19 as their initial infection during this time period. Two patients with AID passed away as a result of infections.
Among the 20 AID patients with infections, neutropenia post-CART was present in 16 (80%). Immunoglobulin G (IgG) levels <400 were present in 6 AID patients (30%) and 61 non-AID patients (31.9%) who developed an infection within 90 days post-CART. In the AID patients, the mean IgG level was 431.4 (range 87–646) at 30 days and 355.4 (range 115–682) at 90 days post-CART. For prophylaxis administration in AID patients, 19 (95%) were on acyclovir and 15 (75%) were on Pneumocystis jirovecii pneumonia (PJP) prophylaxis following CART.
4. Discussion
AIDs are associated with T-cell dysfunction that can be exhausted by continuous activation by self-antigen. Therefore, whether CART is just as effective in such patients for the treatment of lymphoma is an important question. In this study, we report toxicity and survival data on the largest number of patients with concurrent AID and aggressive BNHL (de novo diffuse large B-cell lymphoma or transformed indolent lymphoma) to date with follow-up of >2 years. We show that there are no significant differences in outcomes in patients with and without AID treated with CART for aggressive B-cell NHL. While not statistically significant, relapse rates were numerically lower in patients with AID. Despite underlying immune dysregulation expected in patients with AID, we demonstrate that the efficacy of CART does not appear to be diminished, and durable responses are feasible. This is despite previous studies illustrating that T-cells in patients with autoimmune disorders showed decreased exhaustion and cell death markers during CART manufacturing as well as in the final product. This discrepancy between our clinical results and this preclinical data may be a result of decreased signaling during CART production that leads to restoration in T-cell function [17,18].
Wang et al. found similar results in their study of matched patients with and without underlying rheumatic AID with comparable survival outcomes and toxicity rates between groups treated with CD19 CART for a heterogenous group of aggressive or indolent lymphoma. They found both AID and non-AID groups had similar times to next treatment and overall survival. They showed >50% overall survival in both groups at 1000 days post-CART, similar to our findings [17]. Aside from this data, the application of CART in patients with concurrent lymphoma and autoimmune conditions has been limited to case reports [19].
In terms of AID control post-CART, the aforementioned study also demonstrated that patients achieved better AID control of underlying rheumatic diseases, based on improvement in biochemical markers and low rates of resumption of IST. We also show low rates of flares and resumption of IST in our AID population though it was a small sample size. Though, a larger study may confirm this information and support CART use for AID control and treatment. We recognize that a limitation of our data is the lack of serologic studies that would support AID control. Unlike Wang et al., our study characterized response to CART in a more homogenous population of aggressive B-NHL. We also provided survival data in a larger variety of rheumatologic AIDs, including neurologic disorders such as multiple sclerosis, supporting CART applicability to a broader population [19]. This is particularly important when factoring in neurologic toxicities associated with CART.
These collective findings align with an emerging interest in using CART primarily for the treatment of rheumatologic and neurologic AID in patients without underlying malignancy [20,21,22,23,24]. To date, there have been case reports of CART use in a variety of AIDs, such as SLE, systemic sclerosis, RA, myasthenia gravis, and multiple sclerosis [25,26,27,28,29]. Additionally, phase I clinical trials using compound CART cells in SLE and neuromyelitis optica spectrum disorders found that this treatment was both safe and effective [30,31].
There are currently numerous clinical trials underway for the treatment of various autoimmune conditions, including SLE, MS, MG, myopathies, dermatomyositis, and others, as described in Supplemental Table S2 [12,32].
Despite the inherent limitations of a retrospective study, our data further adds to the field by providing granularity on short- and long-term toxicities of CART in patients with concurrent lymphoma and AID. Although rates of CRS and ICANS were similar, we note a higher use of tocilizumab in our AID cohort—this may have been impacted by the use of IST in the peri-CART period in 12 patients. Although rates of neutropenia were comparable between groups, the significantly higher rates of infections in the AID group following CART may also be a result of peri-CART IST. Thus, patients with AIDs undergoing CART should be managed with antibacterial prophylaxis and close monitoring of immunoglobulin levels with quick initiation of secondary prophylaxis with intravenous immunoglobulin G (IVIG) in the post-CART period.
5. Conclusions
In summary, despite underlying immune dysregulation expected in patients with AID, autologous CART efficacy does not appear to be diminished, and durable responses are feasible. CART should be considered a curative treatment option for patients treated for aggressive B-cell NHL despite underlying AIDs without concern for higher signals of exacerbation of the underlying AID. Biologic correlatives of T-cell function and AID status are warranted to enhance our understanding of CART impact in patients with concurrent B-NHL and AID.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/cancers17142358/s1: Table S1: Post-CART salvage regimens; Table S2: AID conditions and IST resumption; Table S3: Current clinical trials investigating the use of CART in AID.
Author Contributions
Conceptualization, R.K. and N.J.A.; methodology, R.K.; formal analysis, N.J.A., M.M.H., N.M.N., D.M.S., J.B.C., N.E., M.C., R.B., T.K.M., V.K., T.O., B.H., L.F., G.S., J.A.D., C.J., A.P., J.M., A.L., S.M., J.N.W., A.D., S.K.B., L.I.G., J.R., N.S.G. and R.K.; investigation, R.K. and N.J.A.; resources, R.K.; data curation, N.J.A., M.M.H., N.M.N., D.M.S., J.B.C., N.E., M.C., R.B., T.K.M., V.K., T.O., B.H., L.F., G.S., J.A.D., C.J., A.P., J.M., A.L., S.M., J.N.W., A.D., S.K.B., L.I.G., J.R., N.S.G. and R.K.; writing—original draft preparation, R.K. and N.J.A.; writing—review and editing, M.M.H., N.M.N., D.M.S., J.B.C., N.E., M.C., R.B., T.K.M., V.K., T.O., B.H., L.F., G.S., J.A.D., C.J., A.P., J.M., A.L., S.M., J.N.W., A.D., S.K.B., L.I.G., J.R. and N.S.G.; supervision, R.K.; project administration, R.K.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Northwestern University (IRB # STU00214126, approved 1/2021).
Informed Consent Statement
Patient consent was waived given this was a retrospective analysis.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Conflicts of Interest
Epperla: Research funding (institution): Lilly, ADC Therapeutics, Ipsen, Incyte, and Beigene; Advisory Board: Ipsen, Genentech, CRISPR Therapeutics. Grover: Seagen: Honoraria; Novartis: Honoraria; Regeneron: Honoraria, Research Funding; ADC Therapeutics: Honoraria; BMS: Honoraria, Research Funding; Caribou: Honoraria; Ono Pharma: Honoraria; Genentech: Honoraria; Cabaletta: Research Funding; Janssen: Honoraria; Kite: Honoraria; Sangamo: Current holder of stock options in a privately-held company. Moyo: Century Therapeutics: Research Funding; J&J: Research Funding; Genmab: Research Funding; Kite Pharmaceuticals: Membership on an entity’s Board of Directors or advisory committees. Kenkre: Ipsen: Research Funding. Ollila: Lilly: Research Funding; Ono Pharmaceuticals: Membership on an entity’s Board of Directors or advisory committees, Research Funding; ADC Therapeutics: Membership on an entity’s Board of Directors or advisory committees. Shouse: Astra Zeneca: Honoraria; Abbvie: Consultancy; Beigene, Inc: Consultancy, Honoraria, Speakers Bureau; Kite Pharmaceuticals: Consultancy, Honoraria, Speakers Bureau. Ma: AbbVie: Consultancy, Honoraria, Research Funding; Juno: Research Funding; Janssen: Consultancy; Lilly: Research Funding, Speakers Bureau; BeiGene: Consultancy, Research Funding, Speakers Bureau; AstraZeneca: Consultancy, Research Funding, Speakers Bureau. Winter: Merck: Consultancy, Research Funding; BMS: Consultancy; Genetech: Consultancy. Danilov: Genentech: Consultancy; Cyclacel: Research Funding; GenMab: Consultancy, Research Funding; Incyte: Consultancy; Janssen: Consultancy; Lilly Oncology: Consultancy, Research Funding; Merck: Consultancy; Morphosys: Consultancy; Takeda Oncology: Research Funding; Bristol Meyers Squibb: Consultancy, Research Funding; MEI Pharma: Consultancy, Research Funding; TG Therapeutics: Research Funding; ADCT: Consultancy; AstraZeneca: Consultancy, Research Funding; BeiGene: Consultancy; Bayer Oncology: Research Funding; Abbvie: Consultancy, Research Funding; Nurix: Consultancy, Research Funding; Prelude: Consultancy. Gordon: Nanoparticles for cancer therapy (HDL NP As Inducers of Ferroptosis in Cancer, PCT/US2020/051549; b) Nanostructures for Treating Cancer and Other Conditions, PCT/UlllllLS2013/027431): Patents and Royalties: nanoparticles for cancer therapy (HDL NP As Inducers of Ferroptosis in Cancer, PCT/US2020/051549; b) Nanostructures for Treating Cancer and Other Conditions, PCT/UlllllLS2013/027431); Janssen: Other: data and safety monitoring board; Ono Pharmaceuticals: Consultancy, Membership on an entity’s Board of Directors or advisory committees; Bristol Meyers Squibb, Kite Pharmaceuticals: Other: Advisory board. Stephens: AstraZeneca, Beigene, Novartis: Research Funding; Abbvie, AstraZeneca, Beigene, BMS, Celegene, Eli Lilly, Genentech, Janssen, Pharmacyclics: Consultancy. Karmali: Abbvie: Consultancy; Genentech/Roche: Consultancy; BMS: Consultancy, Speakers Bureau; BeiGene: Speakers Bureau; Genmab: Consultancy; AstraZeneca: Speakers Bureau; AvenCell: Consultancy; Kite/Gilead: Consultancy.
Abbreviations
The following abbreviations are used in this manuscript.
| AID | Autoimmune disease |
| B-NHL | B-cell non-Hodgkin lymphoma |
| CART | Autologous chimeric antigen receptor T-cell therapy |
| IST | Immunosuppressive therapy |
| SLE | Systemic lupus erythematous |
| RA | Rheumatoid arthritis |
| MS | Multiple sclerosis |
| MG | Myasthenia gravis |
| ICANS | Immune effector cell-associated neurotoxicity syndrome |
| CRS | Cytokine release syndrome |
| IVIG | intravenous immunoglobulin G |
| OS | Overall survival |
| PFS | Progression free survival |
References
- Kleinstern, G.; Maurer, M.J.; Liebow, M.; Habermann, T.M.; Koff, J.L.; Allmer, C.; Witzig, T.E.; Nowakowski, G.S.; Micallef, I.N.; Johnston, P.B.; et al. History of autoimmune conditions and lymphoma prognosis. Blood Cancer J. 2018, 8, 73. [Google Scholar] [CrossRef] [PubMed]
- Zintzaras, E.; Voulgarelis, M.; Moutsopoulos, H.M. The risk of lymphoma development in autoimmune diseases: A meta-analysis. Arch. Intern. Med. 2005, 165, 2337–2344. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.A.; Gadalla, S.; Morton, L.M.; Landgren, O.; Pfeiffer, R.; Warren, J.L.; Berndt, S.I.; Ricker, W.; Parsons, R.; Engels, E.A. Population-based study of autoimmune conditions and the risk of specific lymphoid malignancies. Int. J. Cancer 2009, 125, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Abend, A.H.; He, I.; Bahroos, N.; Christianakis, S.; Crew, A.B.; Wise, L.M.; Lipori, G.P.; He, X.; Murphy, S.N.; Herrick, C.D.; et al. Estimation of prevalence of autoimmune diseases in the United States using electronic health record data. J. Clin. Investig. 2024, 135, e178722. [Google Scholar] [CrossRef]
- Hayter, S.M.; Cook, M.C. Updated assessment of the prevalence, spectrum and case definition of autoimmune disease. Autoimmun. Rev. 2012, 11, 754–765. [Google Scholar] [CrossRef]
- Smedby, K.E.; Vajdic, C.M.; Falster, M.; Engels, E.A.; Martínez-Maza, O.; Turner, J.; Hjalgrim, H.; Vineis, P.; Costantini, A.S.; Bracci, P.M.; et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: A pooled analysis within the InterLymph Consortium. Blood 2008, 111, 4029–4038. [Google Scholar] [CrossRef]
- Azrielant, S.; Tiosano, S.; Watad, A.; Mahroum, N.; Whitby, A.; Comaneshter, D.; Cohen, A.D.; Amital, H. Correlation between systemic lupus erythematosus and malignancies: A cross-sectional population-based study. Immunol. Res. 2017, 65, 464–469. [Google Scholar] [CrossRef]
- Khanmohammadi, S.; Shabani, M.; Tabary, M.; Rayzan, E.; Rezaei, N. Lymphoma in the setting of autoimmune diseases: A review of association and mechanisms. Crit. Rev. Oncol. Hematol. 2020, 150, 102945. [Google Scholar] [CrossRef]
- Westin, J.R.; Oluwole, O.O.; Kersten, M.J.; Miklos, D.B.; Perales, M.-A.; Ghobadi, A.; Rapoport, A.P.; Sureda, A.; Jacobson, C.A.; Farooq, U.; et al. Survival with axicabtagene ciloleucel in large B-cell lymphoma. N. Engl. J. Med. 2023, 389, 148–157. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef]
- Kamdar, M.; Solomon, S.R.; Arnason, J. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): Results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet 2022, 400, 2294–2308. [Google Scholar]
- Thieblemont, C.; Karimi, Y.; Jurczak, W.; Cheah, C.Y.; Clausen, M.R.; Cunningham, D.; Do, Y.R.; Lewis, D.J.; Gasiorowski, R.; Kim, T.M.; et al. Subcutaneous epcoritamab induces deep, durable complete remissions in relapsed/refractory large b-cell lymphoma: Longer follow-up from the pivotal epcore NHL-1 trial. Hematol. Oncol. 2023, 41 (Suppl. S2), 142–144. [Google Scholar] [CrossRef]
- Rampotas, A.; Richter, J.; Isenberg, D.; Roddie, C. CAR-T cell therapy embarks on autoimmune disease. Bone Marrow Transplant. 2025, 60, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.H.; Ha, Y.J.; Lee, Y.J. Autoantibody biomarkers in rheumatic diseases. Int. J. Mol. Sci. 2020, 21, 1382. [Google Scholar] [CrossRef]
- Wise, L.M.; Stohl, W. Belimumab and rituximab in systemic lupus erythematosus: A tale of two B cell-targeting agents. Front. Med. 2020, 7, 303. [Google Scholar] [CrossRef]
- Lee, D.W.; Santomasso, B.D.; Locke, F.L.; Ghobadi, A.; Turtle, C.J.; Brudno, J.N.; Maus, M.V.; Park, J.H.; Mead, E.; Pavletic, S.; et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol. Blood Marrow Transplant. 2019, 25, 625–638. [Google Scholar] [CrossRef]
- Wang, J.; Alkrekshi, A.; Dasari, S.; Lin, H.T.C.; Elantably, D.; Armashi, A.R.A. CD19-targeted chimeric antigen receptor T-cell therapy in patients with concurrent B-cell Non-Hodgkin lymphoma and rheumatic autoimmune diseases: A propensity score matching study. Bone Marrow Transplant. 2023, 58, 1223–1228. [Google Scholar] [CrossRef]
- Mackensen, A.; Müller, F.; Mougiakakos, D.; Böltz, S.; Wilhelm, A.; Aigner, M.; Völkl, S.; Simon, D.; Kleyer, A.; Munoz, L.; et al. Anti-CD19 CAR T cell therapy for refractory systemic lupus erythematosus. Nat. Med. 2022, 28, 2124–2132. [Google Scholar] [CrossRef]
- Jhaveri, K.S.; Schlam, I.; Holtzman, N.G.; Peravali, M.; Richardson, P.K.; Dahiya, S.; Malkovska, V.; Rapoport, A.P. Safety and efficacy of CAR T cells in a patient with lymphoma and a coexisting autoimmune neuropathy. Blood Adv. 2020, 4, 6019–6022. [Google Scholar] [CrossRef]
- Habib, A.; Ciurea, S.; Kamboh, H.; Miljkovic, M.; Mozaffar, T. Chimeric antigen receptor (CAR) T-cell therapy in neurological disorders. Neurology 2024, 103 (Suppl. 1), S119–S120. [Google Scholar] [CrossRef]
- Mougiakakos, D.; Meyer, E.H.; Schett, G. CAR T cells in autoimmunity: Game changer or stepping stone? Blood 2025, 145, 1841–1849. [Google Scholar] [CrossRef] [PubMed]
- Schett, G.; Mackensen, A.; Mougiakakos, D. CAR T-cell therapy in autoimmune diseases. Lancet 2023, 402, 2034–2044. [Google Scholar] [CrossRef] [PubMed]
- Haghikia, A.; Schett, G.; Mougiakakos, D. B cell-targeting chimeric antigen receptor T cells as an emerging therapy in neuroimmunological diseases. Lancet Neurol. 2024, 23, 615–624. [Google Scholar] [CrossRef]
- Mougiakakos, D.; Krönke, G.; Völkl, S.; Kretschmann, S.; Aigner, M.; Kharboutli, S.; Böltz, S.; Manger, B.; Mackensen, A.; Schett, G. CD19-targeted CAR T cells in refractory systemic lupus erythematosus. N. Engl. J. Med. 2021, 385, 567–569. [Google Scholar] [CrossRef] [PubMed]
- Müller, F.; Taubmann, J.; Bucci, L.; Wilhelm, A.; Bergmann, C.; Völkl, S.; Aigner, M.; Rothe, T.; Minopoulou, I.; Tur, C.; et al. CD19 CAR T-cell therapy in autoimmune disease—A case series with follow-up. N. Engl. J. Med. 2024, 390, 687–700. [Google Scholar] [CrossRef]
- Merkt, W.; Freitag, M.; Claus, M.; Kolb, P.; Falcone, V.; Röhrich, M.; Rodon, L.; Deicher, F.; Andreeva, I.; Tretter, T.; et al. Third-generation CD19.CAR-T cell-containing combination therapy in Scl70+ systemic sclerosis. Ann. Rheum. Dis. 2024, 83, 543–546. [Google Scholar] [CrossRef]
- Haghikia, A.; Hegelmaier, T.; Wolleschak, D.; Böttcher, M.; Pappa, V.; Motte, J.; Borie, D.; Gold, R.; Feist, E.; Schett, G.; et al. Clinical efficacy and autoantibody seroconversion with CD19-CAR T cell therapy in a patient with rheumatoid arthritis and coexisting myasthenia gravis. Ann. Rheum. Dis. 2024, 83, 1597–1598. [Google Scholar] [CrossRef]
- Haghikia, A.; Hegelmaier, T.; Wolleschak, D.; Böttcher, M.; Desel, C.; Borie, D.; Motte, J.; Schett, G.; Schroers, R.; Gold, R.; et al. Anti-CD19 CAR T cells for refractory myasthenia gravis. Lancet Neurol. 2023, 22, 1104–1105. [Google Scholar] [CrossRef]
- Fischbach, F.; Richter, J.; Pfeffer, L.K.; Fehse, B.; Berger, S.C.; Reinhardt, S.; Kuhle, J.; Badbaran, A.; Rathje, K.; Gagelmann, N.; et al. CD19-targeted chimeric antigen receptor T cell therapy in two patients with multiple sclerosis. Med 2024, 5, 550–558.e2. [Google Scholar] [CrossRef]
- Wang, W.; He, S.; Zhang, W.; Zhang, H.; DeStefano, V.M.; Wada, M.; Pinz, K.; Deener, G.; Shah, D.; Hagag, N.; et al. BCMA-CD19 compound CAR T cells for systemic lupus erythematosus: A phase 1 open-label clinical trial. Ann. Rheum. Dis. 2024, 83, 1304–1314. [Google Scholar] [CrossRef]
- Qin, C.; Tian, D.S.; Zhou, L.Q.; Shang, K.; Huang, L.; Dong, M.-H.; You, Y.-F.; Xiao, J.; Xiong, Y.; Wang, W.; et al. Anti-BCMA CAR T-cell therapy CT103A in relapsed or refractory AQP4-IgG seropositive neuromyelitis optica spectrum disorders: Phase 1 trial interim results. Signal Transduct. Target. Ther. 2023, 8, 5. [Google Scholar] [CrossRef]
- Blache, U.; Tretbar, S.; Koehl, U.; Mougiakakos, D.; Fricke, S. CAR T cells for treating autoimmune diseases. RMD Open 2023, 9, e002907. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).