Validation of the HFA-ICOS Score for Carfilzomib-Induced Cardiotoxicity in Multiple Myeloma: A Real-Life Perspective Study
Simple Summary
Abstract
1. Introduction
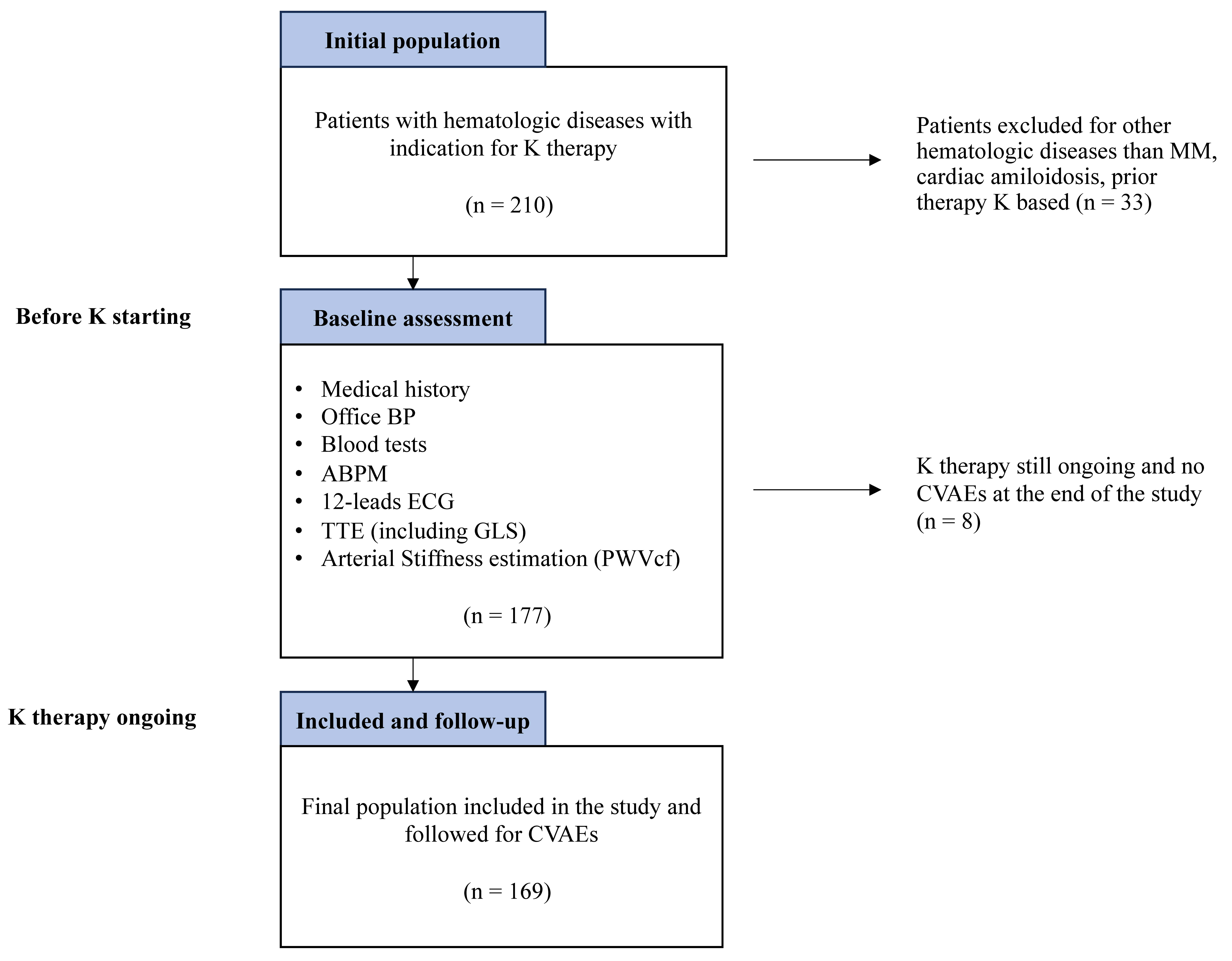
2. Materials and Methods
2.1. Baseline Assessment
2.2. Application of the HFO-ICOS Score
2.3. Follow-Up
2.4. Statistical Analysis
3. Results
3.1. Baseline Parameters and Cardiovascular Risk Factors
3.2. Stratification by the HFA-ICOS Score
3.3. Incidence of CVAEs During K Therapy
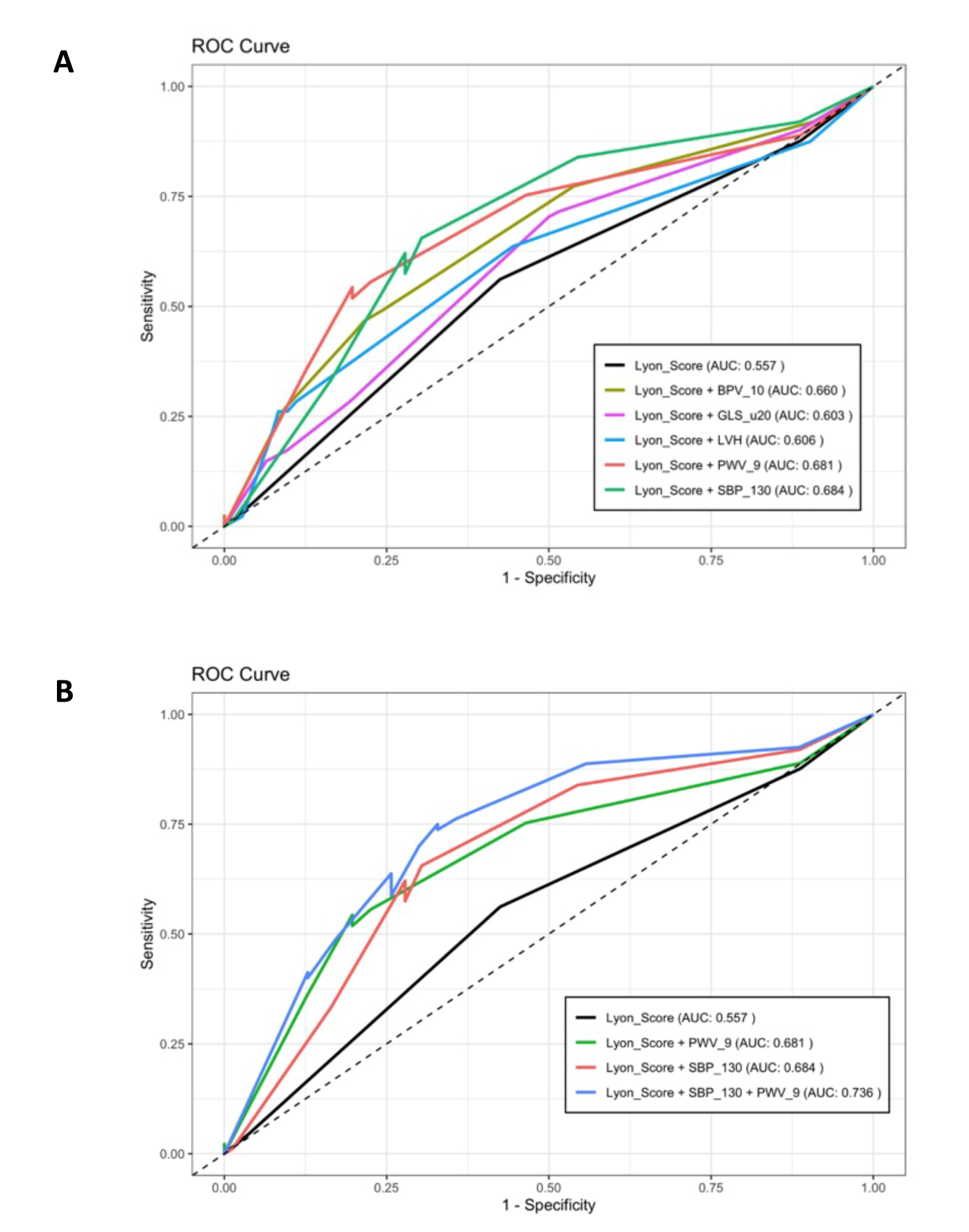
3.4. Accuracy of HFO-ICOS Score and the Role of Other Baseline Parameters as Predictors of CVAEs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Cardiovascular Adverse Events Definitions
Appendix A.2. Hypertensive Adverse Events Definitions
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [PubMed]
- Bringhen, S.; Milan, A.; D’Agostino, M.; Ferri, C.; Wäsch, R.; Gay, F.; LaRocca, A.; Offidani, M.; Zweegman, S.; Terpos, E.; et al. Prevention, monitoring and treatment of cardiovascular adverse events in myeloma patients receiving carfilzomib A consensus paper by the European Myeloma Network and the Italian Society of Arterial Hypertension. J. Intern. Med. 2019, 286, 63–74. [Google Scholar] [PubMed]
- Kistler, K.D.; Kalman, J.; Sahni, G.; Murphy, B.; Werther, W.; Rajangam, K.; Chari, A. Incidence and Risk of Cardiac Events in Patients with Previously Treated Multiple Myeloma Versus Matched Patients Without Multiple Myeloma: An Observational, Retrospective, Cohort Study. Clin. Lymphoma Myeloma Leuk. 2017, 17, 89–96. [Google Scholar]
- Georgiopoulos, G.; Makris, N.; Laina, A.; Theodorakakou, F.; Briasoulis, A.; Trougakos, I.P.; Dimopoulos, M.A.; Kastritis, E.; Stamatelopoulos, K. Cardiovascular Toxicity of Proteasome Inhibitors: Underlying Mechanisms and Management Strategies: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol. 2023, 5, 1–21. [Google Scholar]
- Siegel, D.S.; Martin, T.; Wang, M.; Vij, R.; Jakubowiak, A.J.; Lonial, S.; Trudel, S.; Kukreti, V.; Bahlis, N.; Alsina, M.; et al. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood 2012, 120, 2817–2825. [Google Scholar] [PubMed]
- Chari, A.; Stewart, A.K.; Russell, S.D.; Moreau, P.; Herrmann, J.; Banchs, J.; Hajek, R.; Groarke, J.; Lyon, A.R.; Batty, G.N.; et al. Analysis of carfilzomib cardiovascular safety profile across relapsed and/or refractory multiple myeloma clinical trials. Blood Adv. 2018, 2, 1633–1644. [Google Scholar]
- Astarita, A.; Mingrone, G.; Airale, L.; Cesareo, M.; Colomba, A.; Catarinella, C.; Leone, D.; Gay, F.; Bringhen, S.; Veglio, F.; et al. Carfilzomib-Based Regimen and Cardiotoxicity in Multiple Myeloma: Incidence of Cardiovascular Events and Organ Damage in Carfilzomib-Dexamethasone versus Carfilzomib-Lenalidomide-Dexamethasone. A Real-Life Prospective Study. Cancers 2023, 15, 955. [Google Scholar] [CrossRef]
- Cornell, R.F.; Ky, B.; Weiss, B.M.; Dahm, C.N.; Gupta, D.K.; Du, L.; Carver, J.R.; Cohen, A.D.; Engelhardt, B.G.; Garfall, A.L.; et al. Prospective Study of Cardiac Events During Proteasome Inhibitor Therapy for Relapsed Multiple Myeloma. J. Clin. Oncol. 2019, 37, 1946–1955. [Google Scholar]
- Astarita, A.; Mingrone, G.; Airale, L.; Vallelonga, F.; Covella, M.; Catarinella, C.; Cesareo, M.; Bruno, G.; Leone, D.; Giordana, C.; et al. Multiple Myeloma Patients Undergoing Carfilzomib: Development and Validation of a Risk Score for Cardiovascular Adverse Events Prediction. Cancers 2021, 13, 1631. [Google Scholar] [CrossRef]
- Lyon, A.R.; Dent, S.; Stanway, S.; Earl, H.; Brezden-Masley, C.; Cohen-Solal, A.; Tocchetti, C.G.; Moslehi, J.J.; Groarke, J.D.; Bergler-Klein, J.; et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: A position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur. J. Heart Fail. 2020, 22, 1945–1960. [Google Scholar]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [PubMed]
- Rashed, E.R.; Margulies, K.B. New Cardiotoxicity Risk Assessment Guidelines: Searching for Validation. JACC CardioOncol. 2023, 5, 638–640. [Google Scholar]
- McEvoy, J.W.; McCarthy, C.P.; Bruno, R.M.; Brouwers, S.; Canavan, M.D.; Ceconi, C.; Christodorescu, R.M.; Daskalopoulou, S.S.; Ferro, C.J.; Gerdts, E.; et al. 2024 ESC Guidelines for the management of elevated blood pressure and hypertension. Eur. Heart J. 2024, 45, 3912–4018. [Google Scholar] [PubMed]
- Waxman, A.J.; Clasen, S.; Hwang, W.-T.; Garfall, A.; Vogl, D.T.; Carver, J.; O’Quinn, R.; Cohen, A.D.; Stadtmauer, E.A.; Ky, B.; et al. Carfilzomib-Associated Cardiovascular Adverse Events. JAMA Oncol. 2018, 4, e174519. [Google Scholar]
- Atrash, S.; Tullos, A.; Panozzo, S.; Bhutani, M.; Van Rhee, F.; Barlogie, B.; Usmani, S.Z. Cardiac complications in relapsed and refractory multiple myeloma patients treated with carfilzomib. Blood Cancer J. 2015, 5, e272. [Google Scholar]
- Danhof, S.; Schreder, M.; Rasche, L.; Strifler, S.; Einsele, H.; Knop, S. ‘Real-life’ experience of preapproval carfilzomib-based therapy in myeloma—Analysis of cardiac toxicity and predisposing factors. Eur. J. Haematol. 2016, 97, 25–32. [Google Scholar]
- Chen, J.H.; Lenihan, D.J.; Phillips, S.E.; Harrell, S.L.; Cornell, R.F. Cardiac events during treatment with proteasome inhibitor therapy for multiple myeloma. Cardiooncology 2017, 3, 4. [Google Scholar]
- cGov.UK. Lenalidomide: Risk of Thrombosis and Thromboembolism. February 2011. Available online: https://www.gov.uk/drug-safety-update/lenalidomide-risk-of-thrombosis-and-thromboembolism (accessed on 3 June 2020).
- Libourel, E.J.; Sonneveld, P.; van der Holt, B.; de Maat, M.P.; Leebeek, F.W. High incidence of arterial thrombosis in young patients treated for multiple myeloma: Results of a prospective cohort study. Blood 2010, 116, 22–26. [Google Scholar]
- Suntheralingam, S.; Fan, C.S.; Calvillo-Argüelles, O.; Abdel-Qadir, H.; Amir, E.; Thavendiranathan, P. Evaluation of Risk Prediction Models to Identify Cancer Therapeutics Related Cardiac Dysfunction in Women with HER2+ Breast Cancer. J. Clin. Med. 2022, 11, 847. [Google Scholar]
- Battisti, N.M.L.; Andres, M.S.; Lee, K.A.; Ramalingam, S.; Nash, T.; Mappouridou, S.; Senthivel, N.; Asavisanu, K.; Obeid, M.; Tripodaki, E.S.; et al. Incidence of cardiotoxicity and validation of the Heart Failure Association-International Cardio-Oncology Society risk stratification tool in patients treated with trastuzumab for HER2-positive early breast cancer. Breast Cancer Res. Treat. 2021, 188, 149–163. [Google Scholar]
- Cronin, M.; Crowley, A.; Davey, M.G.; Ryan, P.; Abdelshafy, M.; Elkoumy, A.; Elzomor, H.; Arsang-Jang, S.; Ganly, S.; Nash, P.; et al. Heart Failure Association-International Cardio-Oncology Society Risk Score Validation in HER2-Positive Breast Cancer. J. Clin. Med. 2023, 12, 1278. [Google Scholar] [PubMed]
- Tini, G.; Cuomo, A.; Battistoni, A.; Sarocchi, M.; Mercurio, V.; Ameri, P.; Volpe, M.; Porto, I.; Tocchetti, C.G.; Spallarossa, P. Baseline cardio-oncologic risk assessment in breast cancer women and occurrence of cardiovascular events: The HFA/ICOS risk tool in real-world practice. Int. J. Cardiol. 2022, 349, 134–137. [Google Scholar] [PubMed]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [PubMed]
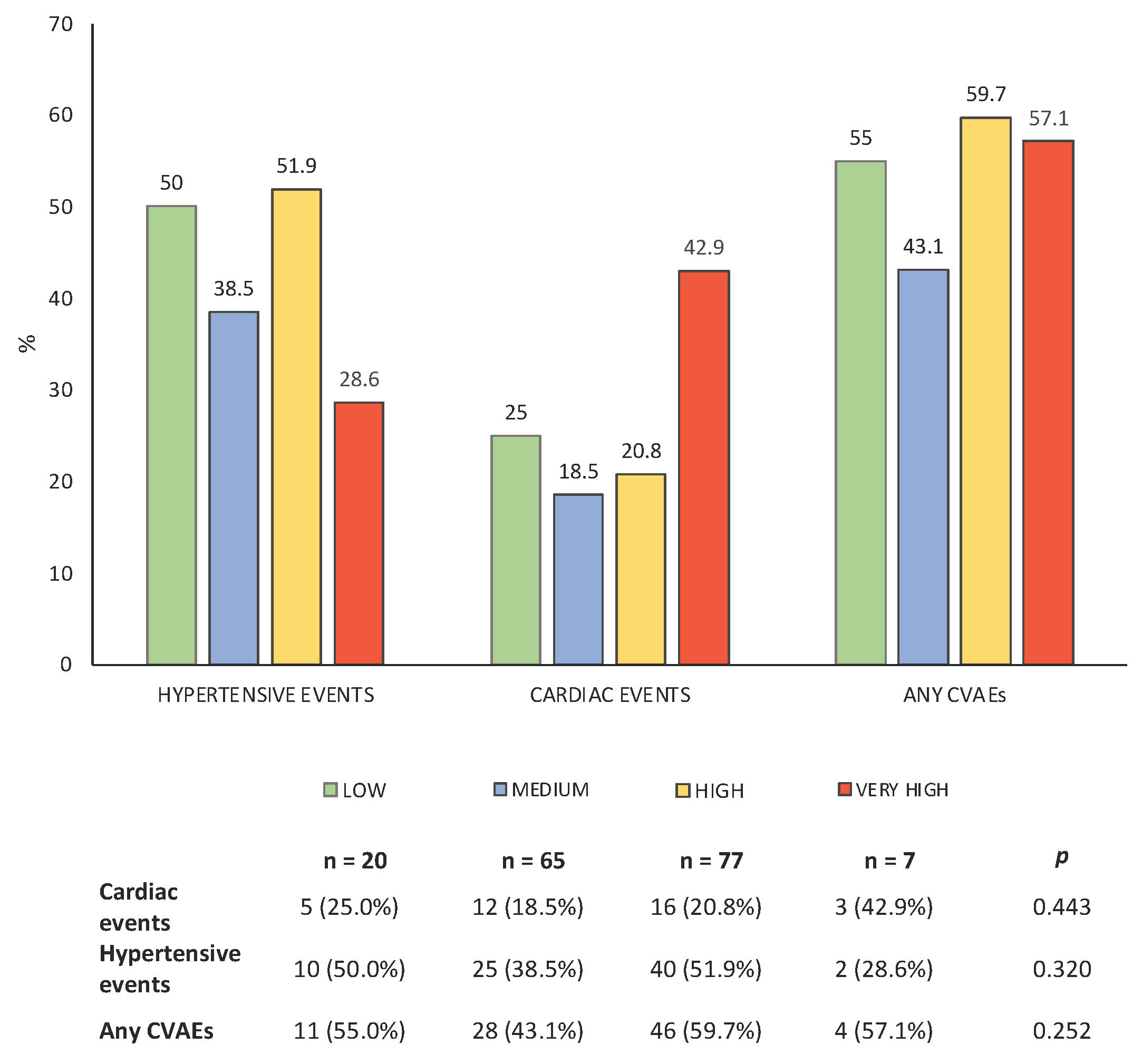
| Parameter | N = 169 (100%) |
|---|---|
| General | |
| Age, y | 70.0 (63.0; 74.2) |
| Male sex | 77 (45.6%) |
| BMI, kg/m2 | 26.7 (23.9; 30.3) |
| Individual CV risk factors and comorbidities | |
| Tobacco use (prior/current) | 90 (53.3) |
| Obesity (BMI ≥ 30) | 49 (29) |
| Known arterial hypertension | 80 (47.3) |
| Diabetes mellitus | 16 (9) |
| Chronic renal failure (eGFR < 60 mL/m) | 41 (26.3) |
| Ischemic heart disease | 4 (2.3) |
| Atrial fibrillation | 5 (2.9) |
| Dyslipidemia | 25 (14.8) |
| Previous stroke | 3 (1.8) |
| Office BP values | |
| SBP, mmHg | 128 (116; 141) |
| DBP, mmHg | 76.0 (70.0; 84.0) |
| ABPM 1 , mean (SD) | |
| Daytime SBP, mmHg | 124 (116; 132) |
| Daytime DBP, mmHg | 74.0 (69.0; 80.0) |
| Daytime MBP, mmHg | 92.0 (86.0; 96.5) |
| 24 h SBP, mmHg | 120 (112; 129) |
| 24 h DBP, mmHg | 71.0 (65.0; 76.5) |
| 24 h MBP, mmHg | 88.0 (82.0; 93.0) |
| Nighttime SBP, mmHg | 109 (102; 120) |
| Nighttime DBP, mmHg | 62.0 (57.0; 69.0) |
| Night MBP, mmHg | 78.0 (73.0; 86.0) |
| Blood pressure variability | 9.00 (7.0; 10.0) |
| Echocardiographic parameters 2 | |
| LAVi, ml/m2 | 49.0 (38.4; 61.2) |
| LVMi, g/m2 | 86.4 (74.4; 102.0) |
| LVH | 30 (18.8) |
| Diastolic dysfunction | 9 (6.4) |
| LVEF, % | 62.2 (58.3; 65.2) |
| Stroke volume, mL/m2 | 47.4 (39.1; 57.9) |
| GLS value, % | −21.50 (−23.40; −20.10) |
| GLS value ≥ −20 % | 33 (23.1) |
| Arterial stiffness evaluation 3 | |
| cfPWV value, m/s | 8.00 (7.0; 9.0) |
| cfPWV value ≥ 9 m/s | 49 (32.2) |
| Oncological history | |
| Median MM disease duration, months | 11.2 (5.23; 23.8) |
| Line of K therapy | 2.85 (1; 9) |
| Patients (n) | Score | |
|---|---|---|
| Previouscardiovascular diseases | ||
| Heart failure or cardiomyopathy | 2 (1.2) | Very High |
| Prior proteasome inhibitor cardiotoxicity | 0 (0) | Very High |
| Venous thrombosis (DVT or PE) | 0 (0) | Very High |
| Cardiac amyloidosis | na | Very High |
| Arterial vascular disease (IHD, PCI, CABG, stable angina, TIA, stroke, PVD) | 7 (4.1) | Very High |
| Prior immunomodulatory CV toxicity | 0 (0) | High |
| Baseline LVEF < 50% | 5 (3) | High |
| Borderline LVEF 50–54% | 5 (3) | Medium 1 |
| Arrhythmia | 5 (3) | Medium 2 |
| Left ventricular hypertrophy 1 | 30 (17.8) | Medium 1 |
| Cardiac biomarkers 2 | ||
| Elevated baseline troponin | 0 (0) | Medium 2 |
| Elevated baseline BNP or NT-proBNP | 2 (1.2) | High |
| Demographic and cardiovascular risk factors | ||
| Age ≥ 75 years | 40 (23.7) | High |
| Age 65–74 years | 79 (46.7) | Medium 1 |
| Arterial hypertension | 80 (47.3) | Medium 1 |
| Diabetes mellitus | 16 (9.5) | Medium 1 |
| Hyperlipidemia | 25 (14.8) | Medium 1 |
| Chronic kidney disease | 40 (23.7) | Medium 1 |
| Family history of thrombophilia | na | Medium 1 |
| Previous cardiotoxic cancer treatment | ||
| Prior anthracycline exposure | 34 (20.1) | High |
| Prior thoracic spine radiotherapy | 7 (4.1) | Medium 1 |
| Current myeloma treatment | ||
| High-dose of dexamethasone > 160 mg/month | 0 (0) | Medium 1 |
| Lifestyle risk factors | ||
| Current smoker or significant smoking history | 90 (53.3) | Medium 1 |
| Obesity (BMI > 30 kg/m2) | 49 (29) | Medium 1 |
| Parameter | Levels of Risk | |||||
|---|---|---|---|---|---|---|
| 1. Low N = 20 | 2. Medium N = 65 | 3. High N = 77 | 4. Very High N = 7 | p | Comparison | |
| General | ||||||
| Age, y | 60.0 (54.0; 65.0) | 69.0 (63.0; 72.0) | 75.0 (68.0; 77.0) | 68.0 (66.5; 68.5) | <0.001 | 1 vs. 2/3/4; 2 vs. 3 |
| Male sex | 8 (40.0) | 27 (41.5) | 38 (49.4) | 4 (57.1) | 0.677 | |
| BMI, kg/m2 | 24.8 (21.8; 27.0) | 28.1 (25.2; 31.6) | 26.4 (23.7; 30.1) | 27.6 (27.3; 31.4) | 0.012 | 1 vs. 2 |
| Individual CV risk factors | ||||||
| Tobacco use (prior/current) | 7 (35.0) | 37 (56.9) | 41 (53.2) | 5 (71.4) | 0.275 | |
| Obesity (BMI ≥ 30) | 0 (0.0) | 22 (33.8) | 24 (31.2) | 3 (42.9) | 0.005 | 1 vs. 2/3/4 |
| Known arterial hypertension | 2 (10.0) | 30 (46.2) | 42 (54.5) | 6 (85.7) | <0.001 | 1 vs. 2/3/4 |
| Diabetes mellitus | 0 (0) | 5 (7.7) | 10 (13.0) | 1 (14.3) | 0.253 | |
| Chronic renal failure (eGFR < 60 mL/m) | 92.9 (77.3; 106) | 80.7 (64.7; 99.0) | 67.8 (54.8; 93.5) | 81.5 (68.3; 89.0) | 0.033 | 1 vs. 3 |
| Ischemic heart disease | 0 (0) | 0 (0) | 0 (0) | 4 (57.1) | <0.001 | 4 vs. 1/2/3 |
| Atrial fibrillation | 0 (0) | 1 (1.5) | 3 (3.9) | 1 (14.3) | 0.277 | |
| Dyslipidaemia | 0 (0) | 13 (20) | 12 (15.6) | 0 (0) | 0.099 | |
| Previous stroke | 0 (0) | 0 (0) | 0 (0) | 3 (42.9) | <0.001 | 4 vs. 1/2/3 |
| Office BP values, mmHg | ||||||
| SBP | 120 (114; 128) | 128 (119; 141) | 130 (119; 142) | 122 (104; 131) | 0.081 | |
| DBP | 74.5 (70.0; 80.0) | 77.5 (70.8; 87.2) | 75.0 (69.0; 82.0) | 79.0 (65.5; 81.5) | 0.501 | |
| ABPM, mmHg | ||||||
| Daytime SBP | 120 (114; 130) | 126 (118; 133) | 124 (116; 132) | 114 (107; 116) | 0.095 | |
| Daytime DBP | 74.0 (70.0; 80.5) | 76.5 (69.0; 82.0) | 73.0 (68.0; 78.0) | 66.5 (60.5; 72.5) | 0.066 | |
| Daytime MBP | 88.5 (84.8; 96.8) | 93.0 (88.8; 99.0) | 91.0 (86.0; 95.5) | 84.5 (77.0; 92.0) | 0.104 | |
| 24 h SBP | 114 (107; 124) | 122 (114; 129) | 120 (112; 129) | 108 (104; 115) | 0.101 | |
| 24 h DBP | 70.0 (65.2; 76.8) | 73.0 (67.0; 77.5) | 70.5 (64.8; 75.0) | 64.5 (58.0; 71.0) | 0.250 | |
| 24 h MBP | 85.0 (79.5; 92.2) | 89.0 (84.0; 93.0) | 89.0 (82.0; 93.0) | 81.0 (72.8; 90.0) | 0.334 | |
| Nighttime SBP | 104 (96.8; 112) | 110 (102; 121) | 112 (103; 121) | 99.5 (95.5; 109) | 0.093 | |
| Nighttime DBP | 59.5 (56.2; 66.0) | 63.5 (59.0; 69.0) | 63.0 (58.0; 70.0) | 60.0 (53.5; 70.2) | 0.484 | |
| Night MBP, | 73.5 (71.2; 80.8) | 79.0 (74.0; 86.0) | 79.0 (74.0; 88.8) | 74.5 (69.2; 82.8) | 0.147 | |
| Blood pressure variability | 8.00 (7.00; 9.00) | 9.00 (7.50; 11.0) | 8.50 (7.00; 10.0) | 8.50 (6.50; 11.2) | 0.597 | |
| Echocardiographic parameters | ||||||
| LAVi, mL/m2 | 39.0 (33.3; 65.0) | 49.4 (41.2; 63.0) | 48.8 (38.3; 58.1) | 57.7 (49.2; 65.5) | 0.341 | |
| LVMi, g/m2 | 74.5 (66.7; 84.8) | 82.7 (70.2; 95.7) | 90.4 (76.9; 104) | 115 (86.1; 119) | 0.002 | 1 vs. 3/4 |
| LVH, | 1 (5.26%) | 7 (11.5%) | 18 (24.7%) | 4 (57.1%) | 0.007 | 1/2 vs. 4 |
| Diastolic dysfunction | 0 (0.00%) | 4 (7.69%) | 4 (5.97%) | 1 (16.7%) | 0.451 | |
| LVEF, % | 60.2 (57.6; 64.3) | 62.7 (58.9; 65.6) | 62.2 (58.6; 64.7) | 60.8 (55.0; 62.2) | 0.491 | |
| Stroke volume, mL/m2 | 53.0 (39.7; 60.8) | 50.2 (40.7; 61.5) | 43.5 (36.1; 53.1) | 54.9 (53.5; 68.8) | 0.023 | |
| GLS value, % | −21.90 (−23.40; −20.30) | −21.60 (−22.70; −19.60) | −21.60 (−23.90; −20.25) | −21.00 (−21.50; −21.00) | 0.707 | |
| GLS value ≥ −20% | 2 (11.8%) | 15 (28.3%) | 15 (22.1%) | 1 (20.0%) | 0.580 | |
| Arterial stiffness evaluation | ||||||
| cfPWV value, m/s | 7.00 (6.00; 7.50) | 8.00 (7.00; 9.00) | 9.00 (7.00; 10.0) | 8.00 (7.00; 9.00) | 0.001 | 1/2 vs. 3 |
| cfPWV value ≥ 9 m/s | 2 (10.5%) | 18 (30.5%) | 36 (52.2%) | 2 (40.0%) | 0.002 | 1 vs. 3 |
| Events | N = 169 (100%) | CTCAE | |
|---|---|---|---|
| Grade 1–2 | Grade ≥ 3 | ||
| Cardiovascular adverse events 1 | 36 (21.3) | ||
| ACS (STEMI) | 2 (1.2) | 0 | 2 |
| ACS (NSTEMI) | 4 (2.4) | 0 | 4 |
| Angina | 9 (5.3) | 9 | 0 |
| Heart failure | 10 (5.9) | 8 | 2 |
| Arrhythmias | 12 (7.1) | 11 | 1 |
| Sudden death | 1 (0.6) | na | 0 |
| LVEF impairment | 5 (3) | 0 | 3 |
| GLS impairment | 6 (3.6) | na | na |
| Hypertensive events 1 | 77 (45.6) | ||
| New onset/worsened hypertension | 67 (39.6) | 67 | 0 |
| Masked hypertension | 5 (3) | 5 | 0 |
| K pre-infusion hypertension permissive K infusion | 34 (20.1) | 27 | 7 |
| K pre-infusion hypertension not permissive K infusion | 18 (10.7) | 11 | 7 |
| K post-infusion hypertension | 20 (11.8) | 13 | 7 |
| Hypertensive urgency | 5 (3) | 0 | 5 |
| Hypertensive emergency | 0 (0) | 0 | 0 |
| Any CVAEs | 89 (52.7) | ||
| Baseline Parameter | Beta | OR (SD) | Wald Test | Valore di p |
|---|---|---|---|---|
| General | ||||
| Age | 0.02 | 1.02 (0.98–1.06) | 1.12 | 0.264 |
| Male sex | 0.44 | 1.55 (0.84–2.86) | 1.40 | 0.160 |
| BMI | 0.03 | 1.03 (0.96–1.10) | 0.81 | 0.419 |
| Office BP | ||||
| SBP | 0.04 | 1.04 (1.02–1.07) | 3.94 | <0.001 |
| DBP | 0.03 | 1.03 (1.00–1.06) | 2.03 | 0.042 |
| ABPM | ||||
| 24 h SBP | 0.03 | 1.03 (1.00–1.06) | 1.97 | 0.048 |
| 24 h DBP | 0.02 | 1.02 (0.98–1.06) | 0.92 | 0.358 |
| 24 h MBP | 0.02 | 1.02 (0.99–1.06) | 1.15 | 0.249 |
| Daytime SBP | 0.03 | 1.03 (1.01–1.06) | 2.41 | 0.016 |
| Daytime DBP | 0.02 | 1.02 (0.99–1.06) | 1.21 | 0.225 |
| Daytime MBP | 0.03 | 1.03 (1.00–1.07) | 1.85 | 0.065 |
| Nighttime SBP | 0.01 | 1.01 (0.98–1.03) | 0.42 | 0.674 |
| Nighttime DBP | −0.00 | 1.00 (0.96–1.04) | −0.16 | 0.872 |
| Night MBP | 0.00 | 1.00 (0.97–1.04) | 0.08 | 0.936 |
| Blood pressure Variability | 0.18 | 1.19 (1.07–1.35) | 3.06 | 0.002 |
| Echocardiographic parameters | ||||
| LAVi | 0.00 | 1.00 (0.98–1.02) | 0.17 | 0.865 |
| LVEF | 0.02 | 1.02 (0.97–1.07) | 0.77 | 0.441 |
| LVMi | 0.02 | 1.02 (1.01–1.04) | 2.69 | 0.007 |
| LVH | 1.19 | 3.29 (1.38–8.77) | 2.55 | 0.011 |
| Diastolic dysfunction | −0.94 | 0.39 (0.08–1.55) | −1.29 | 0.199 |
| GLS value | 0.10 | 1.10 (0.97–1.26) | 1.47 | 0.142 |
| GLS value ≥ −20 | 0.55 | 1.73 (0.78–4.02) | 1.32 | 0.188 |
| Arterial stiffness evaluation | ||||
| cfPWV | 0.40 | 1.49 (1.23–1.84) | 3.87 | <0.001 |
| cfPWV ≥ 9 m/s | 1.58 | 4.84 (2.38–10.32) | 4.23 | <0.001 |
| HFA-ICOS score | ||||
| Level of risk | 0.24 | 1.27 (0.85–1.92) | 1.15 | 0.251 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Astarita, A.; Mingrone, G.; Airale, L.; Colomba, A.; Catarinella, C.; Cesareo, M.; Vallelonga, F.; Paladino, A.; Bruno, G.; Leone, D.; et al. Validation of the HFA-ICOS Score for Carfilzomib-Induced Cardiotoxicity in Multiple Myeloma: A Real-Life Perspective Study. Cancers 2025, 17, 2353. https://doi.org/10.3390/cancers17142353
Astarita A, Mingrone G, Airale L, Colomba A, Catarinella C, Cesareo M, Vallelonga F, Paladino A, Bruno G, Leone D, et al. Validation of the HFA-ICOS Score for Carfilzomib-Induced Cardiotoxicity in Multiple Myeloma: A Real-Life Perspective Study. Cancers. 2025; 17(14):2353. https://doi.org/10.3390/cancers17142353
Chicago/Turabian StyleAstarita, Anna, Giulia Mingrone, Lorenzo Airale, Anna Colomba, Cinzia Catarinella, Marco Cesareo, Fabrizio Vallelonga, Arianna Paladino, Giulia Bruno, Dario Leone, and et al. 2025. "Validation of the HFA-ICOS Score for Carfilzomib-Induced Cardiotoxicity in Multiple Myeloma: A Real-Life Perspective Study" Cancers 17, no. 14: 2353. https://doi.org/10.3390/cancers17142353
APA StyleAstarita, A., Mingrone, G., Airale, L., Colomba, A., Catarinella, C., Cesareo, M., Vallelonga, F., Paladino, A., Bruno, G., Leone, D., Gay, F., Bringhen, S., Veglio, F., & Milan, A. (2025). Validation of the HFA-ICOS Score for Carfilzomib-Induced Cardiotoxicity in Multiple Myeloma: A Real-Life Perspective Study. Cancers, 17(14), 2353. https://doi.org/10.3390/cancers17142353









