Fluorescent In Situ Hybridization Testing Allows the Diagnosis of NRG1 Gene Fusions in Lung and Pancreas Cancers with No Other Identified Oncogenic Driver
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Cases Selection
2.2. Molecular Testing in PDADK
2.3. Molecular Testing in LADK
2.4. Statistical Analyses
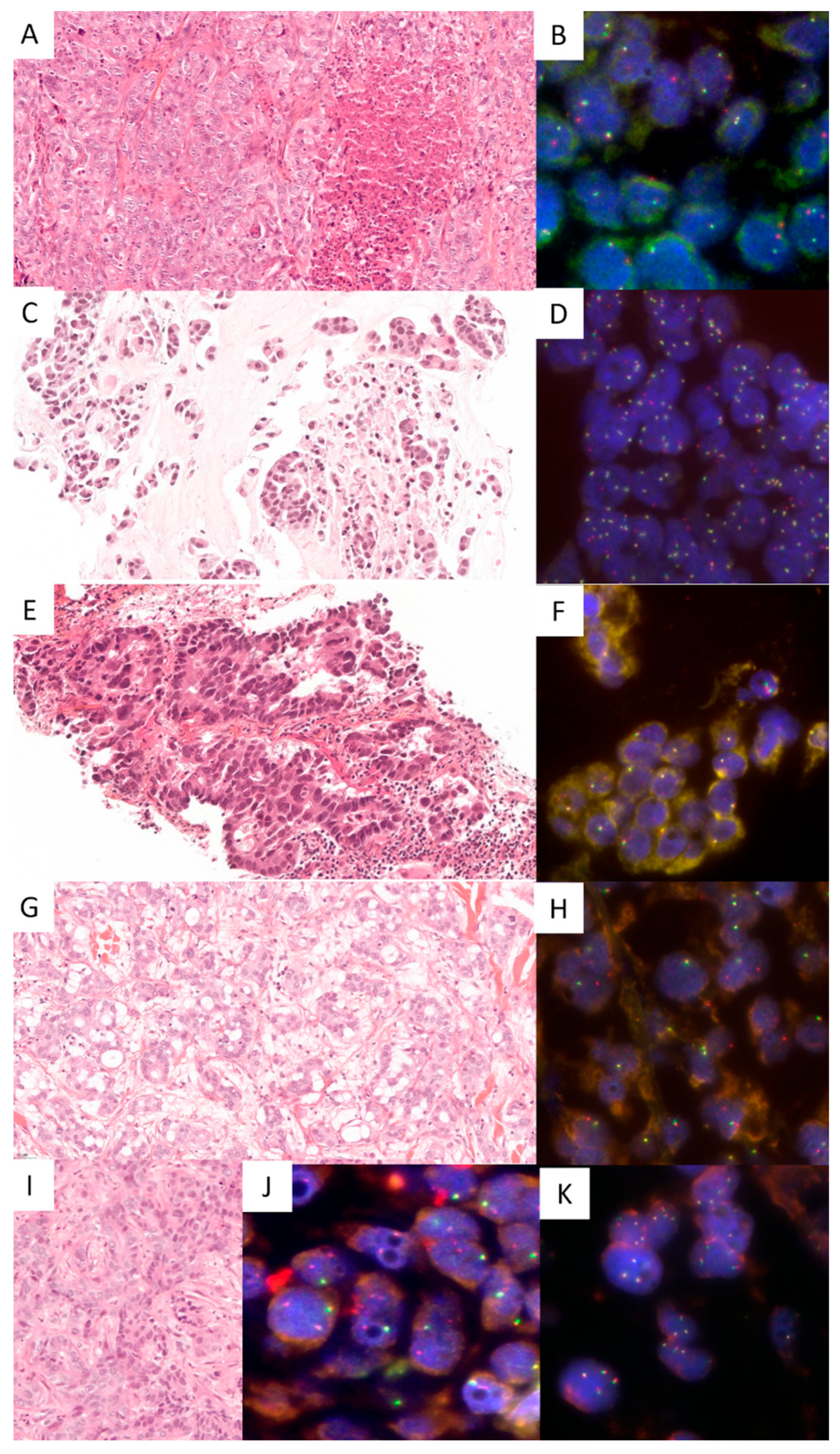
3. Results
3.1. Cases Included
3.2. Molecular Testing in PDADK
3.3. Molecular Testing in LADK
3.4. Specificity and Sensitivity of NRG1 FISH Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef]
- Li, D.; Xie, K.; Wolff, R.; Abbruzzese, J.L. Pancreatic cancer. Lancet 2004, 363, 1049–1057. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- American Cancer Society. Available online: https://www.cancer.org/cancer/lung-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 12 August 2022).
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 2.2021. J. Natl. Compr. Cancer Netw. 2021, 19, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Heining, C.; Horak, P.; Uhrig, S.; Codo, P.L.; Klink, B.; Hutter, B.; Fröhlich, M.; Bonekamp, D.; Richter, D.; Steiger, K.; et al. NRG1 Fusions in KRAS Wild-Type Pancreatic Cancer. Cancer Discov. 2018, 8, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.R.; Williamson, L.M.; Topham, J.T.; Lee, M.K.C.; Goytain, A.; Ho, J.; Denroche, R.E.; Jang, G.; Pleasance, E.; Shen, Y.; et al. NRG1 Gene Fusions Are Recurrent, Clinically Actionable Gene Rearrangements in KRAS Wild-Type Pancreatic Ductal Adenocarcinoma. Clin. Cancer Res. 2019, 25, 4674–4681. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.R.; Lim, H.; Shen, Y.; Pleasance, E.; Ch’ng, C.; Reisle, C.; Leelakumari, S.; Zhao, C.; Yip, S.; Ho, J.; et al. Successful targeting of the NRG1 pathway indicates novel treatment strategy for metastatic cancer. Ann. Oncol. 2017, 28, 3092–3097. [Google Scholar] [CrossRef] [PubMed]
- Schram, A.M.; Odintsov, I.; Espinosa-Cotton, M.; Khodos, I.; Sisso, W.J.; Mattar, M.S.; Lui, A.J.W.; Vojnic, M.; Shameem, S.H.; Chauhan, T.; et al. Zenocutuzumab, a HER2xHER3 Bispecific Antibody, Is Effective Therapy for Tumors Driven by NRG1 Gene Rearrangements. Cancer Discov. 2022, 12, 1233–1247. [Google Scholar] [CrossRef]
- Cadranel, J.; Liu, S.V.; Duruisseaux, M.; Branden, E.; Goto, Y.; Weinberg, B.A.; Heining, C.; Schlenk, R.F.; Cheema, P.; Jones, M.R.; et al. Therapeutic Potential of Afatinib in NRG1 Fusion-Driven Solid Tumors: A Case Series. Oncologist 2021, 26, 7–16. [Google Scholar] [CrossRef]
- Schram, A.M.; Goto, K.; Kim, D.W.; Macarulla, T.; Hollebecque, A.; O’Reilly, E.M.; Ou, S.I.; Rodon, J.; Rha, S.Y.; Nishino, K.; et al. Efficacy of Zenocutuzumab in NRG1 Fusion-Positive Cancer. N. Engl. J. Med. 2025, 392, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Stalbovskaya, V.; Wasserman, E.; Fryzek, J.; Bylsma, L.; Sirulnik, L.A. NRG1 fusion-driven cancers: A systematic literature review and meta-analysis. J. Clin. Oncol. 2020, 38, e15605. [Google Scholar] [CrossRef]
- Fernandez-Cuesta, L.; Plenker, D.; Osada, H.; Sun, R.; Menon, R.; Leenders, F.; Ortiz-Cuaran, S.; Peifer, M.; Bos, M.; Daßler, J.; et al. CD74-NRG1 fusions in lung adenocarcinoma. Cancer Discov. 2014, 4, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Rosas, D.; Raez, L.E.; Russo, A.; Rolfo, C. Neuregulin 1 Gene (NRG1). A Potentially New Targetable Alteration for the Treatment of Lung Cancer. Cancers 2021, 13, 5038. [Google Scholar] [CrossRef]
- Tempero, M.A.; Malafa, M.P.; Al-Hawary, M.; Behrman, S.W.; Benson, A.B.; Cardin, D.B.; Chiorean, E.G.; Chung, V.; Czito, B.; Del Chiaro, M.; et al. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 439–457. [Google Scholar] [CrossRef]
- O’Reilly, E.M.; Hechtman, J.F. Tumour response to TRK inhibition in a patient with pancreatic adenocarcinoma harbouring an NTRK gene fusion. Ann. Oncol. 2019, 30, viii36–viii40. [Google Scholar] [CrossRef]
- Trombetta, D.; Graziano, P.; Scarpa, A.; Sparaneo, A.; Rossi, G.; Rossi, A.; Di Maio, M.; Antonello, D.; Mafficini, A.; Fabrizio, F.P.; et al. Frequent NRG1 fusions in Caucasian pulmonary mucinous adenocarcinoma predicted by Phospho-ErbB3 expression. Oncotarget 2018, 9, 9661–9671. [Google Scholar] [CrossRef]
- Duruisseaux, M.; McLeer-Florin, A.; Antoine, M.; Alavizadeh, S.; Poulot, V.; Lacave, R.; Rabbe, N.; Cadranel, J.; Wislez, M. NRG1 fusion in a French cohort of invasive mucinous lung adenocarcinoma. Cancer Med. 2016, 5, 3579–3585. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Gao, F.; Liu, B.; Li, J.; Ren, S.; Peng, S.; Qiu, W.; Pu, X.; Ye, Q. Fluorescent in situ hybridization has limitations in screening NRG1 gene rearrangements. Diagn. Pathol. 2024, 19, 1. [Google Scholar] [CrossRef]
- Bocciarelli, C.; Caumont, C.; Samaison, L.; Cariou, M.; Aline-Fardin, A.; Doucet, L.; Roudié, J.; Terris, B.; Merlio, J.P.; Marcorelles, P.; et al. MSI-High RAS-BRAF wild-type colorectal adenocarcinomas with MLH1 loss have a high frequency of targetable oncogenic gene fusions whose diagnoses are feasible using methods easy-to-implement in pathology laboratories. Hum. Pathol. 2021, 114, 99–109. [Google Scholar] [CrossRef]
- Jonna, S.; Feldman, R.A.; Swensen, J.; Gatalica, Z.; Korn, W.M.; Borghaei, H.; Ma, P.C.; Nieva, J.J.; Spira, A.I.; Vanderwalde, A.M.; et al. Detection of NRG1 Gene Fusions in Solid Tumors. Clin. Cancer Res. 2019, 25, 4966–4972. [Google Scholar] [CrossRef]
- Drilon, A.; Somwar, R.; Mangatt, B.P.; Edgren, H.; Desmeules, P.; Ruusulehto, A.; Smith, R.S.; Delasos, L.; Vojnic, M.; Plodkowski, A.J.; et al. Response to ERBB3-Directed Targeted Therapy in NRG1-Rearranged Cancers. Cancer Discov. 2018, 8, 686–695. [Google Scholar] [CrossRef]
- Gupta, B.; Gosa Barrett, L.; Liu, S.V. NRG1 Fusions in NSCLC: Being eNRGy Conscious. Lung Cancer 2024, 15, 143–148. [Google Scholar] [CrossRef]
| Pancreatic Ductal Adenocarcinomas | n = 199 |
|---|---|
| Men | 112 (56.3%) |
| Women | 87 (43.7%) |
| Ages | mean 68.4 years (range from 36 to 87 years) |
| Pancreatic biopsies | 95 (47.7%) |
| Pancreatic surgical specimens | 104 (52.3%) |
| Lung Adenocarcinomas | n = 446 |
| Men | 251 (56.3%) |
| Women | 195 (43.7%) |
| Ages | mean 68.4 years (range from 36 to 87 years) |
| Lung samples | 304 (68.2%) |
| Nodal metastases | 39 (8.7%) |
| Other distant metastases | 103 (23.1%) |
| Cases | Sex (M/F) | Age Range (Years) | Smoking Habit | Clinical Stage * | Tumor Type and Immunohistochemistry Results | NRG1 FISH Result ** | NRG1 RNA Seq Result |
|---|---|---|---|---|---|---|---|
| Lung #1 | F | 82 | No | IVB | Lung solid ADK TTF-1− | 50% single 3′ | NA |
| Lung #2 | M | 78 | Yes | IIA | Lung invasive mucinous ADK TTF-1+ | 70% single 3′ | NA |
| Lung #3 | M | 71 | Yes | IB | Lung acinar ADK TTF-1+ | 70% single 3′ | NA |
| Lung #4 | M | 62 | NA | IVB | Lung acinar ADK TTF-1− | 80% single 3′ | NA |
| Lung #5 | M | 85 | Yes | IVB | Lung acinar ADK TTF-1− | 60% single 3′ | CD74-NRG1 |
| Lung #6 | F | 44 | No | IIIA | Lung acinar ADK TTF-1+ | 50% single 3′ | ADAM9-NRG1 |
| Lung #7 | M | 53 | No | IVB | Lung papillary ADK | 80% single 3′ | CD74-NRG1 |
| Pancreas #1 | F | 40 | NA | IV | Ductal pancreatic ADK TTF1−, CK7+,CK20+, CK19+, Bcl10− | 80% single 3′ | ATP1B1-NRG1 |
| Pancreas #2 | M | 50 | NA | IV | Ductal pancreatic ADK TTF1−, CK7+,CK20+ | 60% single 3′ | CDH1-NRG1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bastard, C.; Caumont, C.; Samaison, L.; Quintin-Roué, I.; Doucet, L.; Marcorelles, P.; Le Maréchal, C.; Merlio, J.-P.; Cappellen, D.; Uguen, A. Fluorescent In Situ Hybridization Testing Allows the Diagnosis of NRG1 Gene Fusions in Lung and Pancreas Cancers with No Other Identified Oncogenic Driver. Cancers 2025, 17, 2347. https://doi.org/10.3390/cancers17142347
Bastard C, Caumont C, Samaison L, Quintin-Roué I, Doucet L, Marcorelles P, Le Maréchal C, Merlio J-P, Cappellen D, Uguen A. Fluorescent In Situ Hybridization Testing Allows the Diagnosis of NRG1 Gene Fusions in Lung and Pancreas Cancers with No Other Identified Oncogenic Driver. Cancers. 2025; 17(14):2347. https://doi.org/10.3390/cancers17142347
Chicago/Turabian StyleBastard, Clara, Charline Caumont, Laura Samaison, Isabelle Quintin-Roué, Laurent Doucet, Pascale Marcorelles, Cédric Le Maréchal, Jean-Philippe Merlio, David Cappellen, and Arnaud Uguen. 2025. "Fluorescent In Situ Hybridization Testing Allows the Diagnosis of NRG1 Gene Fusions in Lung and Pancreas Cancers with No Other Identified Oncogenic Driver" Cancers 17, no. 14: 2347. https://doi.org/10.3390/cancers17142347
APA StyleBastard, C., Caumont, C., Samaison, L., Quintin-Roué, I., Doucet, L., Marcorelles, P., Le Maréchal, C., Merlio, J.-P., Cappellen, D., & Uguen, A. (2025). Fluorescent In Situ Hybridization Testing Allows the Diagnosis of NRG1 Gene Fusions in Lung and Pancreas Cancers with No Other Identified Oncogenic Driver. Cancers, 17(14), 2347. https://doi.org/10.3390/cancers17142347





