Oxygen-Enhanced R2* Weighted MRI and Diffusion Weighted MRI of Head and Neck Squamous Cell Cancer Lymph Nodes in Prediction of 2-Year Outcome Following Chemoradiotherapy
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Multiparametric Magnetic Resonance Imaging
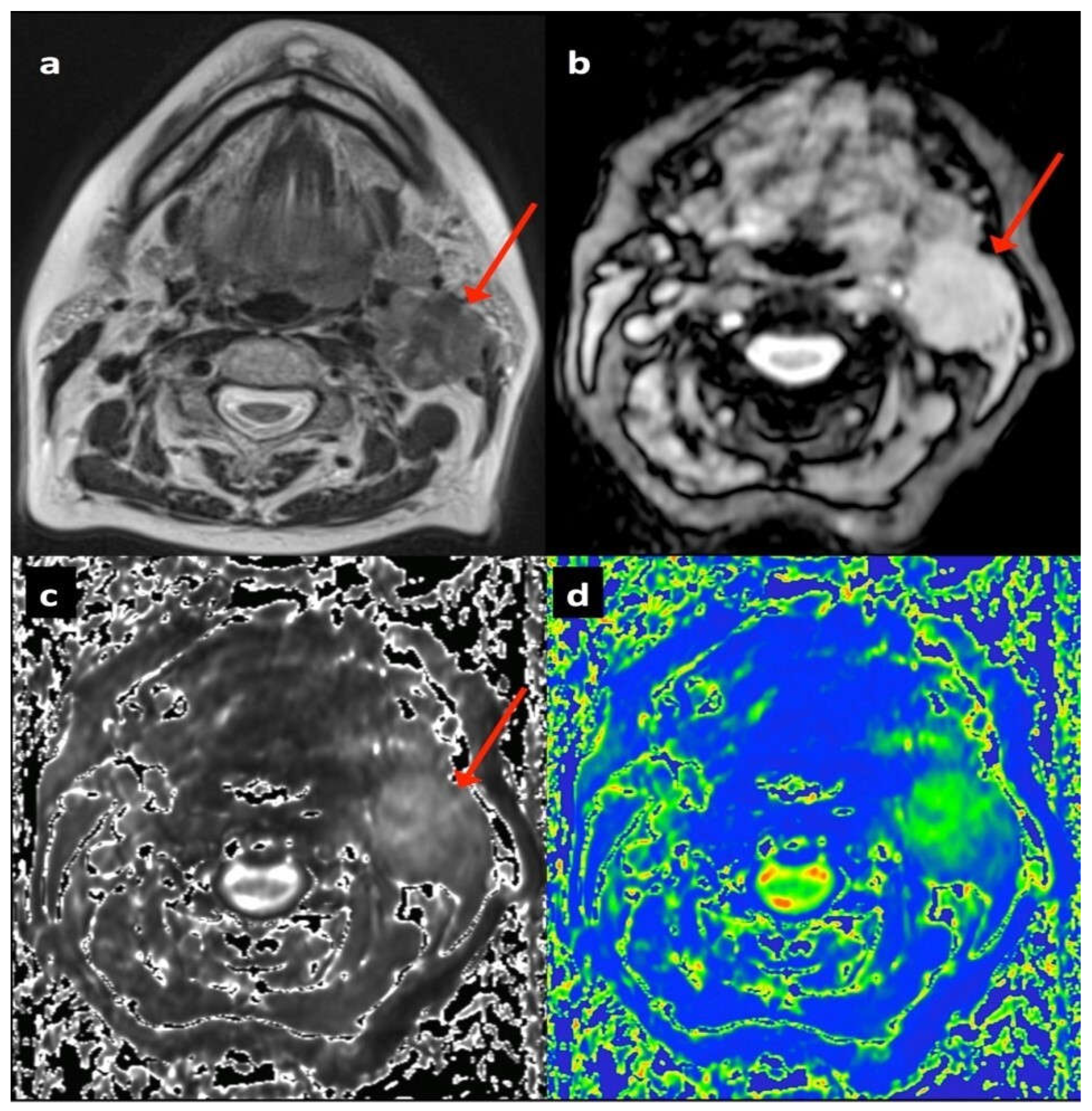
2.3. Image Analysis
2.4. Treatment Outcome Categorisation
2.5. Statistical Analyses
3. Results
3.1. Patient Cohort
3.2. Largest Node per Patient Analysis
3.2.1. Short Axis Diameter and Qualitative Descriptors
3.2.2. R2* Parameters
3.2.3. Diffusion Weighted Imaging Parameters
3.3. All-Node Analysis
3.3.1. Short Axis Diameter and Qualitative Descriptors
3.3.2. R2* Parameters
3.3.3. Diffusion Weighted Imaging Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Leemans, C.R.; Tiwari, R.; Nauta, J.J.; Waal, L.V.D.; Snow, G.B. Recurrence at the primary site in head and neck cancer and the significance of neck lymph node metastases as a prognostic factor. Cancer 1994, 73, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, J.L.; Chevalier, D.; Luboinski, B.; Kirkpatrick, A.; Collette, L.; Sahmoud, T. Larynx preservation in pyriform sinus cancer: Preliminary results of a European Organization for Research and Treatment of Cancer phase III trial. EORTC Head and Neck Cancer Cooperative Group. J. Natl. Cancer Inst. 1996, 88, 890–899. [Google Scholar] [CrossRef]
- Blanchard, P.; Baujat, B.; Holostenco, V.; Bourredjem, A.; Baey, C.; Bourhis, J.; Pignon, J.-P. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): A comprehensive analysis by tumour site. Radiother. Oncol. 2011, 100, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.M.; Daly, M.E.; Farwell, D.G.; Vazquez, E.; Courquin, J.; Lau, D.H.; Purdy, J.A. Quality of life among long-term survivors of head and neck cancer treated by intensity-modulated radiotherapy. JAMA Otolaryngol. Head Neck Surg. 2014, 140, 129–133. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, R.G.; Franssen, E.; Balogh, J.M.; Gilbert, R.W.; Birt, D.; Davidson, J. Comparing treatment outcomes of radiotherapy and surgery in locally advanced carcinoma of the larynx: A comparison limited to patients eligible for surgery. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 65–71. [Google Scholar] [CrossRef]
- Leoncini, E.; Vukovic, V.; Cadoni, G.; Pastorino, R.; Arzani, D.; Bosetti, C.; Canova, C.; Garavello, W.; Vecchia, L.; Maule, M.; et al. Clinical features and prognostic factors in patients with head and neck cancer: Results from a multicentric study. Cancer Epidemiol. 2015, 39, 367–374. [Google Scholar] [CrossRef]
- Chakravarthy, A.; Henderson, S.; Thirdborough, S.M.; Ottensmeier, C.H.; Su, X.; Lechner, M.; Feber, A.; Thomas, G.J.; Fenton, T.R. Human Papillomavirus Drives Tumor Development Throughout the Head and Neck: Improved Prognosis Is Associated With an Immune Response Largely Restricted to the Oropharynx. J. Clin. Oncol. 2016, 34, 4132–4141. [Google Scholar] [CrossRef]
- Bondt, D.; Nelemans, P.J.; Hofman, P.A.; Casselman, J.W.; Kremer, B.; van Engelshoven, J.M.; Beets-Tan, R.G. Detection of lymph node metastases in head and neck cancer: A meta-analysis comparing US, USgFNAC, CT and MR imaging. Eur. J. Radiol. 2007, 64, 266–272. [Google Scholar] [CrossRef]
- de Bondt, R.B.; Nelemans, P.J.; Bakers, F.; Casselman, J.W.; Peutz-Kootstra, C.; Kremer, B.; Hofman, P.A.; Beets-Tan, R.G. Morphological MRI criteria improve the detection of lymph node metastases in head and neck squamous cell carcinoma: Multivariate logistic regression analysis of MRI features of cervical lymph nodes. Eur. Radiol. 2009, 19, 626–633. [Google Scholar] [CrossRef]
- Taabazuing, C.Y.; Hangasky, J.A.; Knapp, M.J. Oxygen sensing strategies in mammals and bacteria. J. Inorg. Biochem. 2014, 133, 63–72. [Google Scholar] [CrossRef]
- Nguyen, L.K.; Cavadas, M.A.; Scholz, C.C.; Fitzpatrick, S.F.; Bruning, C.E.P.; Tambuwala, M.M.; Manresa, M.C.; Kholodenko, B.N.; Taylor, C.T.; Cheong, A. A dynamic model of the hypoxia-inducible factor 1 (HIF-1) network. J. Cell Sci. 2013, 126, 1454–1463. [Google Scholar] [CrossRef] [PubMed]
- Powers, W.E.; Tolmach, L.J. Demonstration of an anoxic component in mouse tumour cell population by in vivo assay of survival following irradiation. Radiology 1964, 83, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Fleckenstein, W.; Weiss, C.; Heinrich, R.; Schomerus, H.; Kersting, T. A new method for the bed-side recording of tissue pO2 histograms. Verh. Dtsch. Ges. Inn. Med. 1984, 90, 439–443. [Google Scholar]
- Terris, D.J.; Dunphy, E.P. Oxygen tension measurements of head and neck cancers. Arch. Otolaryngol. Head Neck Surg. 1994, 120, 283–287. [Google Scholar] [CrossRef]
- Nordsmark, M.; Bentzen, S.M.; Rudat, V.; Brizel, D.; Lartigau, E.; Stadler, P.; Becker, A.; Adam, M.; Molls, M.; Dunst, J.; et al. Prognostic value of tumor oxygenation in 397 head and neck tumors after primary radiation therapy. An international multi-center study. Radiother. Oncol. 2005, 77, 18–24. [Google Scholar] [CrossRef]
- Terris, D.J. Head and neck cancer: The importance of oxygen. Laryngoscope 2000, 110 Pt 1, 697–707. [Google Scholar] [CrossRef]
- Brown, J.M. Tumor hypoxia in cancer therapy. Methods Enzymol. 2007, 435, 297–321. [Google Scholar]
- Tadamura, E.; Hatabu, H.; Li, W.; Prasad, P.V.; Edelman, R.R. Effect of oxygen inhalation on relaxation times in various tissues. J. Magn. Reason. Imaging 1997, 7, 220–225. [Google Scholar] [CrossRef]
- Kim, C.K.; Park, S.Y.; Park, B.K.; Park, W.; Huh, S.J. Blood oxygenation level-dependent MR imaging as a predictor of therapeutic response to concurrent chemoradiotherapy in cervical cancer: A preliminary experience. Eur. Radiol. 2014, 24, 1514–1520. [Google Scholar] [CrossRef]
- Kotas, M.; Schmitt, P.; Jakob, P.M.; Flentje, M. Monitoring of tumor oxy- genation changes in head-and-neck carcinoma patients breathing a hyperoxic hypercapnic gas mixture with a noninvasive MRI technique. Strahlenther. Onkol. 2009, 185, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Li, X.S.; Fan, H.X.; Fang, H.; Song, Y.L.; Zhou, C.W. Value of R2* obtained from T2*-weighted imaging in predicting the prognosis of advanced cervical squamous carcinoma treated with concurrent chemoradiotherapy. J. Magn. Reson. Imaging 2015, 42, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Krishna, M.C.; Subramanian, S.; Kuppusamy, P.; Mitchell, J.B. Magnetic resonance imaging for in vivo assessment of tissue oxygen concentration. Semin. Radiat. Oncol. 2001, 11, 58–69. [Google Scholar] [CrossRef]
- Christen, T.; Bolar, D.S.; Zaharchuk, G. Imaging brain oxygenation with MRI using blood oxygenation approaches: Methods, validation, and clinical applications. AJNR Am. J. Neuroradiol. 2013, 34, 1113–1123. [Google Scholar] [CrossRef]
- Panek, R.; Welsh, L.; Dunlop, A.; Wong, K.H.; Riddell, A.M.; Koh, D.-M.; Schmidt, M.A.; Doran, S.; McQuaid, D.; Hopkinson, G.; et al. Repeatability and sensitivity of T2* measurements in patients with head and neck squamous cell carcinoma at 3T. J. Magn. Reason. Imaging 2016, 44, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Weatherall, P.T.; McColl, R.W.; Tripathy, D.; Mason, R.P. Blood oxy- genation level-dependent (BOLD) contrast magnetic resonance imaging (MRI) for prediction of breast cancer chemotherapy response: A pilot study. J. Magn. Reson. Imaging 2013, 37, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Padhani, A.R. Science to practice: What does MR oxygenation imaging tell us about human breast cancer hypoxia? Radiology 2010, 254, 1–3. [Google Scholar] [CrossRef]
- King, A.D.; Thoeny, H.C.; Functional, M.R. I for the prediction of treatment response in head and neck squamous cell carcinoma: Potential and limitations. Cancer Imaging 2016, 16, 23. [Google Scholar] [CrossRef]
- Edge, S.B.; Byrd, D.R.; Compton, C.C.; Fritz, A.G.; Greene, F.L.; Trotti, A. (Eds.) American Joint Committee on Cancer (AJCC) Cancer Staging Manual, 7th ed.; Springer: New York, NY, USA, 2009. [Google Scholar]
- McCabe, A.; Martin, S.; Rowe, S.; Shah, J.; Morgan, P.S.; Borys, D.; Panek, R. Oxygen-enhanced MRI assessment of tumour hypoxia in head and neck cancer is feasible and well tolerated in the clinical setting. Eur. Radiol. Exp. 2024, 8, 27. [Google Scholar] [CrossRef]
- Hagberg, G.E.; Indovina, I.; Sanes, J.N.; Posse, S. Real-time quantification of T2* changes using multiecho planar imaging and numerical methods. Magn. Reson. Med. 2002, 48, 877–882. [Google Scholar] [CrossRef]
- Zhao, J.M.; Clingman, C.S.; Narvainen, M.J.; Kauppinen, R.A.; van Zijl, P.C. Oxygenation and hematocrit dependence of transverse relaxation rates of blood at 3T. Magn. Reson. Med. 2007, 58, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Baudelet, C.; Ansiaux, R.; Jordan, B.F.; Havaux, X.; Macq, B.; Gallez, B. Physiological noise in murine solid tumors using T2*-weighted gradient- echo imaging: A marker of tumor acute hypoxia? Phys. Med. Biol. 2004, 49, 3389–3411. [Google Scholar] [CrossRef]
- Rijpkema, M.; Kaanders, J.H.; Joosten, F.B.; van der Kogel, A.J.; Heerschap, A. Effects of breathing a hyperoxic hypercapnic gas mixture on blood oxygenation and vascularity of head-and-neck tumors as measured by magnetic resonance imaging. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Neeman, M.; Dafni, H.; Bukhari, O.; Braun, R.D.; Dewhirst, M.W. In vivo BOLD contrast MRI mapping of subcutaneous vascular function and matura- tion: Validation by intravital microscopy. Magn. Reson. Med. 2001, 45, 887–898. [Google Scholar] [CrossRef]
- Al Hallaq, H.A.; River, J.N.; Zamora, M.; Oikawa, H.; Karczmar, G.S. Correlation of magnetic resonance and oxygen microelectrode measurements of carbogen-induced changes in tumour oxygenation. Int. J. Radiat. Oncol. Biol. Phys. 1998, 41, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Lebon, V.; Carlier, P.G.; Brillault-Salvat, C.; Leroy-Willig, A. Simultaneous measurement of perfusion and oxygenation changes using a multiple gradient-echo sequence: Application to human muscle study. Magn. Reson. Imaging 1998, 16, 721–729. [Google Scholar] [CrossRef]
- Howe, F.A.; Robinson, S.P.; McIntyre, D.J.O.; Stubbs, M.; Griffiths, J.R. Issues in flow and oxygenation dependent contrast (FLOOD) imaging of tumours. NMR Biomed. 2001, 14, 497–506. [Google Scholar] [CrossRef]
- McPhail, L.D.; Robinson, S.P. Intrinsic susceptibility MR imaging of chemically-induced rat mammary tumors: Relationship to histological assessment of hypoxia and fibrosis. Radiology 2009, 254, 110–118. [Google Scholar] [CrossRef]
- Padhani, A.R.; Ah-See, M.L.W.; Taylor, N.J.; Stirling, J.J.; Daley, F.M. An investigation of histological and DC-MRI correlates of intrinsic susceptibility contrast relaxivity (R2*) in human breast cancer [abstr]. In Proceedings of the Thirteenth Meeting of the International Society for Magnetic Resonance in Medicine, Miami Beach, FL, USA, 7–13 May 2005; International Society for Magnetic Resonance in Medicine: Berkeley, CA, USA, 2005; Volume 1846. [Google Scholar]
- Alonzi, R.; Padhani, A.R.; Maxwell, R.J.; Taylor, N.J.; Stirling, J.J.; Wilson, J.I.; Collins, D.J.; Saunders, M.I.; Hoskin, P.J. Carbogen breathing increases prostate cancer oxygenation: A translational MRI study in murine xenografts and humans. Br. J. Cancer 2009, 100, 644–648. [Google Scholar] [CrossRef]
- Hoskin, P.J.; Carnell, D.M.; Taylor, N.J.; Smith, R.E.; Stirling, J.J.; Daley, F.M.; Saunders, M.I.; Bentzen, S.M.; Collins, D.J.; d’Arcy, J.A.; et al. Hypoxia in prostate cancer: Correlation of BOLD-MRI with pimonidazole immuno- histochemistry: Initial observations. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 1065–1071. [Google Scholar] [CrossRef]
- Kim, S.; Loevner, L.; Quon, H.; Sherman, E.; Weinstein, G.; Kilger, A.; Poptani, H. Diffusion-weighted magnetic resonance imaging for predicting and detecting early response to chemoradiation therapy of squamous cell carcinomas of the head and neck. Clin. Cancer Res. 2009, 15, 986–994. [Google Scholar] [CrossRef] [PubMed]
- King, A.D.; Chow, K.K.; Yu, K.H.; Mo, F.K.; Yeung, D.K.; Yuan, J.; Ahuja, A.T. Head and neck squamous cell carcinoma: Diagnostic performance of diffusion-weighted MR imaging for the prediction of treatment response. Radiology 2013, 266, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Nakajo, M.; Nakajo, M.; Kajiya, Y.; Tani, A.; Kamiyama, T.; Yonekura, R.; Fukukura, Y.; Matsuzaki, T.; Nishimoto, K.; Nomoto, M.; et al. FDG PET/CT and diffusion-weighted imaging of head and neck squamous cell carcinoma: Comparison of prognostic significance between primary tumor standardized uptake value and apparent diffusion coefficient. Clin. Nucl. Med. 2012, 37, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Kolff-Gart, A.S.; Pouwels, P.J.; Noij, D.P.; Ljumanovic, R.; Vandecaveye, V.; de Keyzer, F.; Castelijns, J.A. Diffusion-weighted imaging of the head and neck in healthy subjects: Reproducibility of ADC values in different MRI systems and repeat sessions. AJNR Am. J. Neuroradiol. 2015, 36, 384–390. [Google Scholar] [CrossRef]
- Kimura, H.; Braun, R.D.; Ong, E.T.; Hsu, R.; Secomb, T.W.; Papahadjopoulos, D.; Hong, K.; Dewhirst, M.W. Fluctuations in red cell flux in tumor microvessels can lead to transient hypoxia and reoxygenation in tumor parenchyma. Cancer Res. 1996, 56, 5522–5528. [Google Scholar]
| Maintained Complete Response at 2 Years | Relapse of Disease Within 2 Years | ||||||
|---|---|---|---|---|---|---|---|
| Age/ Gender | Primary Site and Stage | p16 | Treatment | Age/ Gender | Primary Site Stage | p16 | Treatment |
| 67 M | Hypopharynx T3N2bM0 | n/a | Neo: cisplatin/5FU Chemo: cisplatin Radiation: IMRT | 70 M | Oropharynx T4N2M0 | n/a | Neo: nil Chemo: cetuximab Radiation: IMRT |
| 55 F | Soft palate T2N2cM0 | n/a | Neo: cisplatin/5FU Chemo: cetuximab Radiation: IMRT | 60 M | Pyriform fossa T3N2bM0 | n/a | Neo: cisplatin/5FU Chemo: cetuximab Radiation: IMRT |
| 74 F | Tonsillar (fauc) T1N3M0 | + | Neo: cisplatin/5FU Chemo: cisplatin Radiation: IMRT | 64 F | Soft palate T1N2bM0 | − | Neo: cisplatin/5FU Chemo: cetuximab Radiation: IMRT |
| 56 M | Tonsil T1N2M0 | n/a | Neo: cisplatin/5FU Chemo: cisplatin Radiation: IMRT | 48 M | Pyriform fossa T2N2bM0 | n/a | Neo: cisplatin/5FU Chemo: cisplatin Radiation: IMRT |
| 56 M | Tonsil (fauc) T2N2cM0 | + | Neo: nil Chemo: cisplatin Radiation: IMRT | 57 M | Tonsil (fauc) T2N2bM0 | − | Neo: cisplatin/5FU Chemo: cetuximab Radiation: IMRT |
| 67 M | Tonsil T1N2bM0 | + | Neo: cisplatin/5FU Chemo: cisplatin Radiation: IMRT | 56 M | Tonsil T3N2bM0 | + | Neo: cisplatin/5FU Chemo: cisplatin Radiation: IMRT |
| 56 M | Tongue base T4N2cM0 | + | Neo: cisplatin/5FU Chemo: cisplatin Radiation: IMRT | 43 M | Tonsil T3N2bM0 | + | Neo: cisplatin/5FU Chemo: cisplatin Radiation: IMRT |
| 63 M | Tonsil (fauc) T2N2bM0 | + | Neo: cisplatin/5FU Chemo: cisplatin Radiation: IMRT | 52 M | Glossotonsillar sulcus T4N2cM0 | n/a | Neo: cisplatin/5FU Chemo: cetuximab Radiation: IMRT |
| 52 M | Unknown TxN2M0 | + | Neo: cisplatin/5FU (single) Chemo: cisplatin Radiation: IMRT | 54 M | Tongue base T3N2cM0 | n/a | Neo: cisplatin/5FU Chemo: cisplatin Radiation: IMRT |
| 57 M | Tonsil T2N2bM0 | + | Neo: cisplatin/5FU Chemo: cisplatin Radiation: IMRT | 64 F | Oropharynx T4N2cM0 | n/a | Neo: cisplatin/5FU Chemo: cisplatin Radiation: IMRT |
| 49 M | Tonsil T4N2bM0 | + | Neo: cisplatin/5FU Chemo: cisplatin Radiation: IMRT | 74 F | Tongue base T2N2bM0 | n/a | Neo: nil Chemo: cisplatin Radiation: IMRT |
| 33 M | Tongue base (presumed) TxN2cM0 | + | Neo: nil Chemo: cisplatin Radiation: IMRT | 49 M | Tongue base T3N2bM0 | n/a | Neo: nil Chemo: cisplatin Radiation: IMRT |
| 60 M | Tonsil T4N2bM0 | n/a | Neo: cisplatin/5FU Chemo: cisplatin Radiation: IMRT | 59 M | Epiglottis T2N2cM0 | n/a | Neo: cisplatin/5FU Chemo: cisplatin Radiation: IMRT |
| 47 F | Tonsil T2N2bM0 | + | Neo: nil Chemo: cisplatin Radiation: IMRT | 44 M | Tonsil T1N2bM0 | + | Neo: nil Chemo: cisplatin Radiation: IMRT |
| 46 M | Tonsil T3N2bM0 | − | Neo: nil Chemo: cisplatin Radiation: IMRT | 63 M | Tongue base T4N3M0 | + | Neo: cisplatin/5FU Chemo: cisplatin Radiation: IMRT |
| 62 M | Tonsil T2N2bM0 | + | Neo: nil Chemo: cisplatin Radiation: IMRT | 49 F | Pyriform fossa T2N2bM0 | n/a | Neo: nil Chemo: cisplatin Radiation: IMRT |
| 63 M | Pyriform fossa T3N2bM0 | n/a | Neo: nil Chemo: cisplatin Radiation: IMRT | 25 F | Tonsil T2N2bM0 | n/a | Neo: nil Chemo: cisplatin Radiation: IMRT |
| 67 M | Tonsil T4N2cM0 | n/a | Neo: cisplatin/5FU Chemo: cisplatin Radiation: IMRT | 75 M | Tongue base T4aN2bM0 | − | Neo: cisplatin/5FU Chemo: cisplatin Radiation: IMRT |
| 62 M | Tongue base T4N2cM0 | + | Neo: cisplatin/5FU Chemo: cisplatin Radiation: IMRT | 64 F | Supraglottic T3N2cM0 | n/a | Neo: cisplatin/5FU Chemo: cisplatin Radiation: IMRT |
| 75 F | Tonsil T3N2bM0 | n/a | Neo: cisplatin/5FU Chemo: cisplatin Radiation: IMRT | 52 M | Pyriform fossa T3N2bM0 | n/a | Neo: nil (ALD) Chemo: cisplatin Radiation: IMRT |
| 69 M | Pyriform fossa T2N2cM0 | − | Neo: cisplatin/5FU Chemo: cisplatin Radiation: IMRT | 68 M | Hypopharnyx T4N2cM0 | n/a | Neo: carbopl/5FU Chemo: cetuximab Radiation: IMRT |
| 73 M | Tonsil T3N2bM0 | + | Neo: carboplat/5FU Chemo: carboplatin Radiation: IMRT | 71 M | Tonsil T4N2bM0 | − | Neo: nil Chemo: cisplatin Radiation: IMRT |
| 54 M | Nasopharynx T4N1M0 | − | Neo: carboplat/5FU Chemo: carboplatin Radiation: IMRT | ||||
| 76 M | Hypopharynx T3N2cM0 | − | Neo: nil Chemo: nil Radiation: IMRT | ||||
| 67 M | Tonsil T3N2cM0 | + | Neo: carboplat/5FU Chemo: carboplatin Radiation: IMRT | ||||
| 79 M | Tonsil T1N2bM0 | + | Neo: cisplatin/5FU Chemo: cetuximab Radiation: IMRT | ||||
| 62 M | Tongue base T2 N2c M0 | − | Neo: cisplatin/5FU Chemo: cisplatin Radiation: IMRT | ||||
| 69 M | Tonsil T3N2bM0 | + | Neo: cisplatin/5FU Chemo: cisplatin Radiation: IMRT | ||||
| 51 F | Unknown TxN2bM | − | Neo: cisplatin/5FU Chemo: cisplatin Radiation: IMRT | ||||
| 72 F | Tonsil T2N2aM0 | + | Neo: cisplatin/5FU Chemo: cisplatin Radiation: IMRT | ||||
| 59 M | Hypopharnyx T4N2bM0 | n/a | Neo: cisplatin/5FU Chemo: cetuximab Radiation: IMRT | ||||
| 41 M | Post-pharynx T4N2cM0 | − | Neo: cisplatin/5FU Chemo: cisplatin Radiation: IMRT | ||||
| T2w TSE | T2* GRE | STIR-EPI DWI | |
|---|---|---|---|
| Orientation | Axial | Axial | Axial |
| Repetition time (ms) | 6670 | 1450 | 9400 |
| Echo time (ms) | 93 | 12, 24, 36, 48 | 93 |
| Flip angle (degrees) | 120 | 25 | 90 |
| Bandwidth (Hz/Px) | 250 | 140 | 1502 |
| Field of view (mm) | 180 × 180 | 195 × 250 | 206 × 206 |
| Acquired matrix | 256 × 256 | 512 × 400 | 128 × 128 |
| Slice thickness (mm) | 3 | 5 | 4 |
| Slice gap (mm) | 0.3 | 2.5 | 0.4 |
| Averages | 1 | 1 | 4 |
| Phase encoding direction | Anteroposterior | Anteroposterior | Anteroposterior |
| Fat suppression | No | No | STIR |
| Base matrix | 256 | 256 × 100 | 128 |
| Number of acquisitions | 1 | 1 | 1 |
| b-values (s.mm−2) | n/a | n/a | 0, 50, 100, 300, 600, 1000 |
| Total acquisition time (min) | 2 m 54 s | 2 min 53 s | 2 m 31 s |
| PARAMETER | TREATMENT OUTCOME GROUP | p-Value/Odds Ratio | |||
|---|---|---|---|---|---|
| Complete Response (CR) | Residual/Recurrent Nodal Disease (RD) | ||||
| Number of patients (percentage) | 32 (59%) | 22 (41%) | |||
| Qualitative Descriptors | |||||
| Fisher’s exact | Univariate Odds Ratio (95% CI) | ||||
| Contour | Round | 16 nodes | 9 nodes | 0.55 | 1 |
| Ovoid | 16 nodes | 13 nodes | 1.65 (0.53, 5.18) | ||
| Margins | Irregular | 20 nodes | 10 nodes | 0.17 | 1 |
| Regular | 12 nodes | 12 nodes | 1.40 (0.37, 5.24) | ||
| Enhancement | Heterogenous | 20 nodes | 14 nodes | 0.39 | n/a |
| Diffuse | 11 nodes | 8 nodes | |||
| Necrosis | Present | 19 nodes | 17 nodes | 0.32 | 1 |
| Absent | 13 nodes | 5 nodes | 0.32 (0.08, 1.21) | ||
| Quantitative Parameters | |||||
| Mann–Whitney | Univariate Odds Ratio (95% CI) | ||||
| Mean short axis diameter (mm) | 18.5 | 22.6 | 0.21 | 0.83 (0.57, 1.21) * | |
| Mean of ADC median values (10−3 mm2/s) | 0.91 | 0.89 | 0.99 | 0.88 (0.60, 1.29) ** | |
| Mean of ADC skewness | 0.59 | 0.67 | 0.63 | 0.78 (0.27, 2.29) | |
| Mean of ADC kurtosis | 1.04 | 1.2 | 0.72 | 0.77 (0.29, 2.24) | |
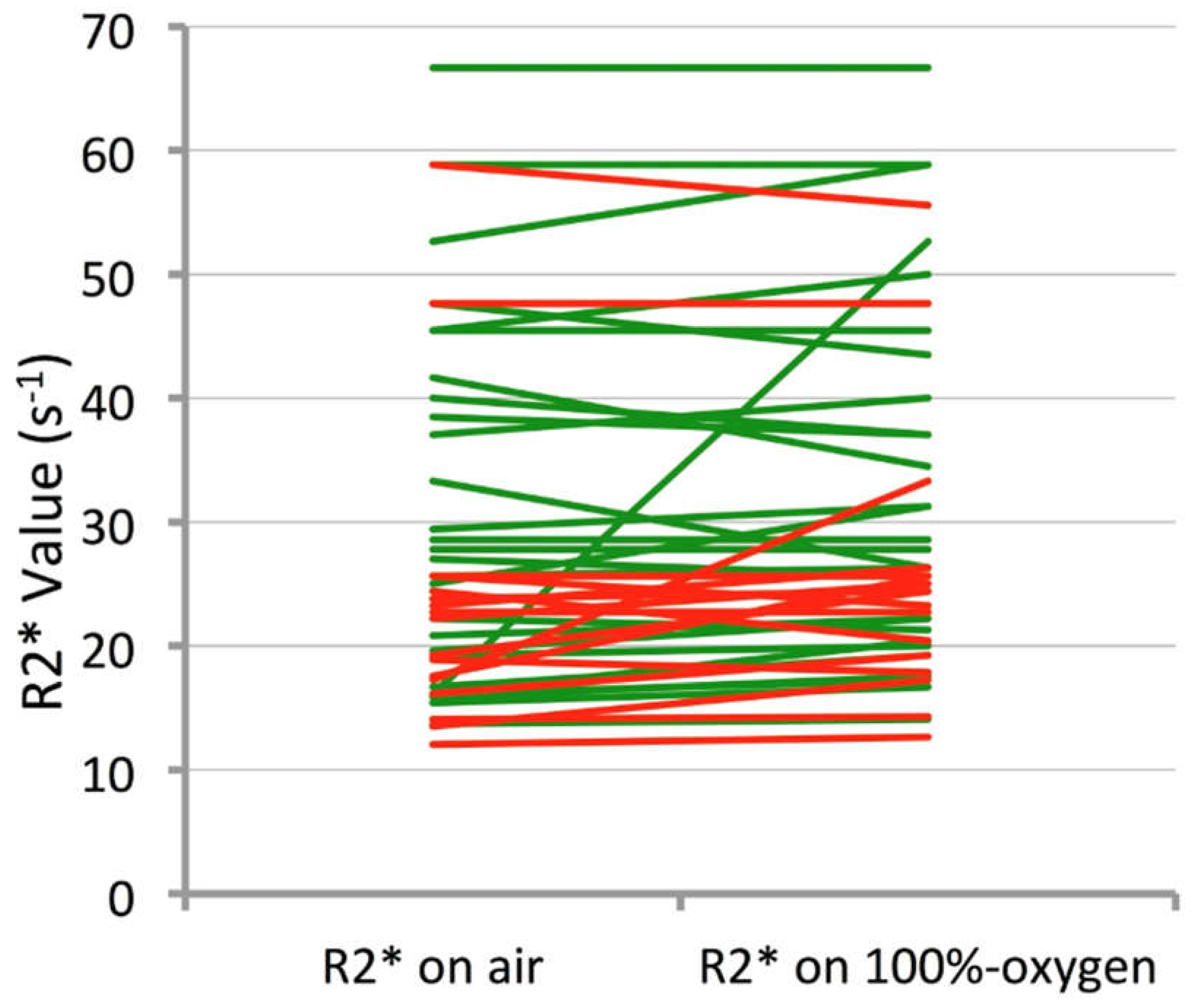
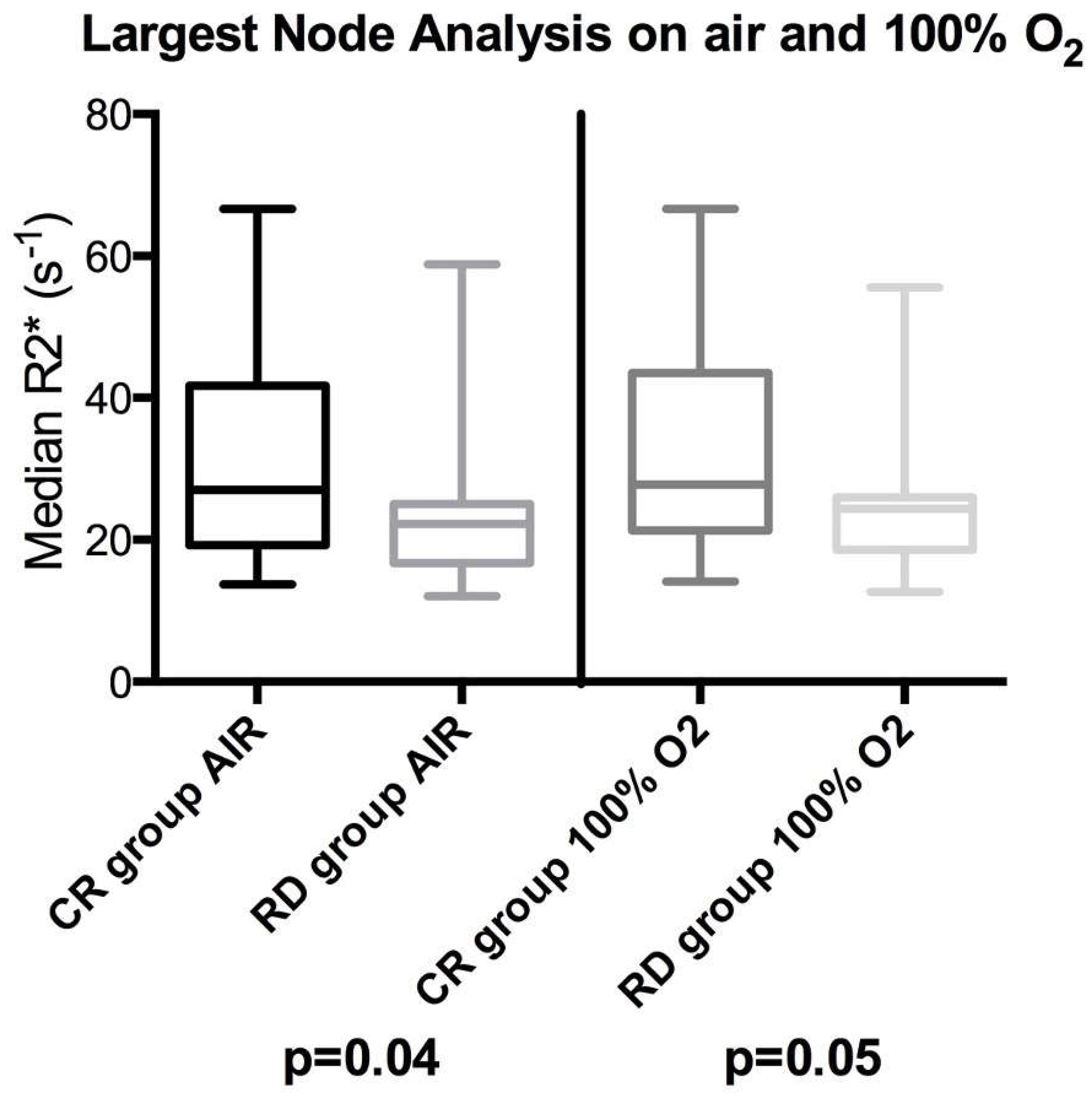
| Mean of R2* median values (s−1) | Air | 25.6 | 20.2 | 0.04 | 1.4 (0.98, 2.04) ** p = 0.05 |
| 100% O2 | 27.6 | 22.5 | 0.05 | 1.4 (0.94, 2.12) ** p = 0.06 | |
| Wilcoxon p-value | 0.012 | 0.055 | |||
| Univariate Odds Ratio (95% CI) | 0.74 (0.55, 1.56) p = 0.15 | ||||
| Mean of R2* skewness | Air | 0.757 | 0.483 | 0.39 | 1.60 (0.69, 3.70) |
| 100% O2 | 0.582 | 0.777 | 0.32 | 0.79 (0.28, 2.26) | |
| Wilcoxon p-value | 0.597 | 0.356 | |||
| Mean of R2* kurtosis | Air | 1.57 | 0.489 | 0.64 | 1.12 (0.82, 1.56) |
| 100% O2 | 0.667 | 1.37 | 0.44 | 0.89 (0.64, 1.22) | |
| Wilcoxon p-value | 0.36 | 0.33 | |||
| PARAMETER | TREATMENT OUTCOME GROUP | p-Value | ||
|---|---|---|---|---|
| Complete Response (CR; n = 32 pts/59%) | Residual/Recurrent Nodal Disease (RD; n = 22 pts/41%) | |||
| Number of nodes (percentage) | 104 (61.2%) | 66 (38.9%) | ||
| Qualitative Descriptors | ||||
| Fisher’s exact | ||||
| Contour | Round | 50 nodes | 23 nodes | 0.11 |
| Ovoid | 54 nodes | 43 nodes | ||
| Margins | Irregular | 50 nodes | 38 nodes | 0.27 |
| Regular | 54 nodes | 28 nodes | ||
| Enhancement | Heterogenous | 52 nodes | 35 nodes | 0.64 |
| Diffuse | 52 nodes | 31 nodes | ||
| Necrosis | Present | 48 nodes | 38 nodes | 0.16 |
| Absent | 56 nodes | 28 nodes | ||
| Quantitative Parameters | ||||
| Linear Mixed | ||||
| Mean short axis diameter (mm) | 12.0 (11.0; 13.1) | 13.5 (12.1; 15.0) | 0.21 | |
| Mean of ADC median values a (10−3 mm2/s) | 0.86 (0.81; 0.93) | 0.88 (0.83; 0.96) | 0.91 | |
| Mean of ADC skewness | 0.72 (0.55; 0.93) | 0.58 (0.38; 0.79) | 0.26 | |
| Mean of ADC kurtosis a | 3.74 (3.29; 4.13) | 3.71 (3.25; 4.23) | 0.97 | |
| Mean of R2* median values (s−1) | Air | 26.4 (23.6; 29.9) | 22.1 (19.5; 25.4) | 0.049 |
| 100% O2 | 28.1 (25.1; 31.9) | 23.6 (20.8; 27.4) | 0.07 | |
| Pairwise p-value | 0.0006 | 0.14 | ||
| Mean of R2* skewness | Air b | 0.5 (0.4; 0.7) | 0.4 (0.3; 0.6) | 0.15 |
| 100% O2 b | 0.5 (0.4; 0.7) | 0.6 (0.4; 0.7) | 0.91 | |
| pairwise p-value | >0.99 | 0.05 | ||
| Mean of R2* kurtosis | Air c | 0.2 (−0.1; 0.6) | 0.1 (−0.3; 0.5) | 0.51 |
| 100% O2 d | 0.5 (0.2; 1.0) | 0.2 (−0.2; 0.7) | 0.26 | |
| Pairwise p-value | 0.36 | 0.68 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sidhu, H.S.; Price, D.; Beale, T.; Morley, S.; Adeleke, S.; Papoutsaki, M.-V.; Forster, M.; Carnell, D.; Mendes, R.; Taylor, S.A.; et al. Oxygen-Enhanced R2* Weighted MRI and Diffusion Weighted MRI of Head and Neck Squamous Cell Cancer Lymph Nodes in Prediction of 2-Year Outcome Following Chemoradiotherapy. Cancers 2025, 17, 2333. https://doi.org/10.3390/cancers17142333
Sidhu HS, Price D, Beale T, Morley S, Adeleke S, Papoutsaki M-V, Forster M, Carnell D, Mendes R, Taylor SA, et al. Oxygen-Enhanced R2* Weighted MRI and Diffusion Weighted MRI of Head and Neck Squamous Cell Cancer Lymph Nodes in Prediction of 2-Year Outcome Following Chemoradiotherapy. Cancers. 2025; 17(14):2333. https://doi.org/10.3390/cancers17142333
Chicago/Turabian StyleSidhu, Harbir Singh, David Price, Tim Beale, Simon Morley, Sola Adeleke, Marianthi-Vasiliki Papoutsaki, Martin Forster, Dawn Carnell, Ruheena Mendes, Stuart Andrew Taylor, and et al. 2025. "Oxygen-Enhanced R2* Weighted MRI and Diffusion Weighted MRI of Head and Neck Squamous Cell Cancer Lymph Nodes in Prediction of 2-Year Outcome Following Chemoradiotherapy" Cancers 17, no. 14: 2333. https://doi.org/10.3390/cancers17142333
APA StyleSidhu, H. S., Price, D., Beale, T., Morley, S., Adeleke, S., Papoutsaki, M.-V., Forster, M., Carnell, D., Mendes, R., Taylor, S. A., & Punwani, S. (2025). Oxygen-Enhanced R2* Weighted MRI and Diffusion Weighted MRI of Head and Neck Squamous Cell Cancer Lymph Nodes in Prediction of 2-Year Outcome Following Chemoradiotherapy. Cancers, 17(14), 2333. https://doi.org/10.3390/cancers17142333







