ROR1 as an Immunotherapeutic Target for Inducing Antitumor Helper T Cell Responses Against Head and Neck Squamous Cell Carcinoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Immunohistochemistry
2.2. Cell Lines
2.3. Flow Cytometry
2.4. Synthetic Peptides
2.5. In Vitro Induction of ROR1-Reactive CD4+ Helper T Cells
2.6. Analysis of ROR1-Specific Responses with Established CD4+ Helper T Cell Lines
2.7. Cytotoxicity Assay
2.8. ROR1403–417 Peptide-Reactive Responses in Patients with HNSCC and Healthy Individuals
2.9. Statistical Analysis
3. Results
3.1. ROR1 Expression in HNSCC Tissues and Cell Lines
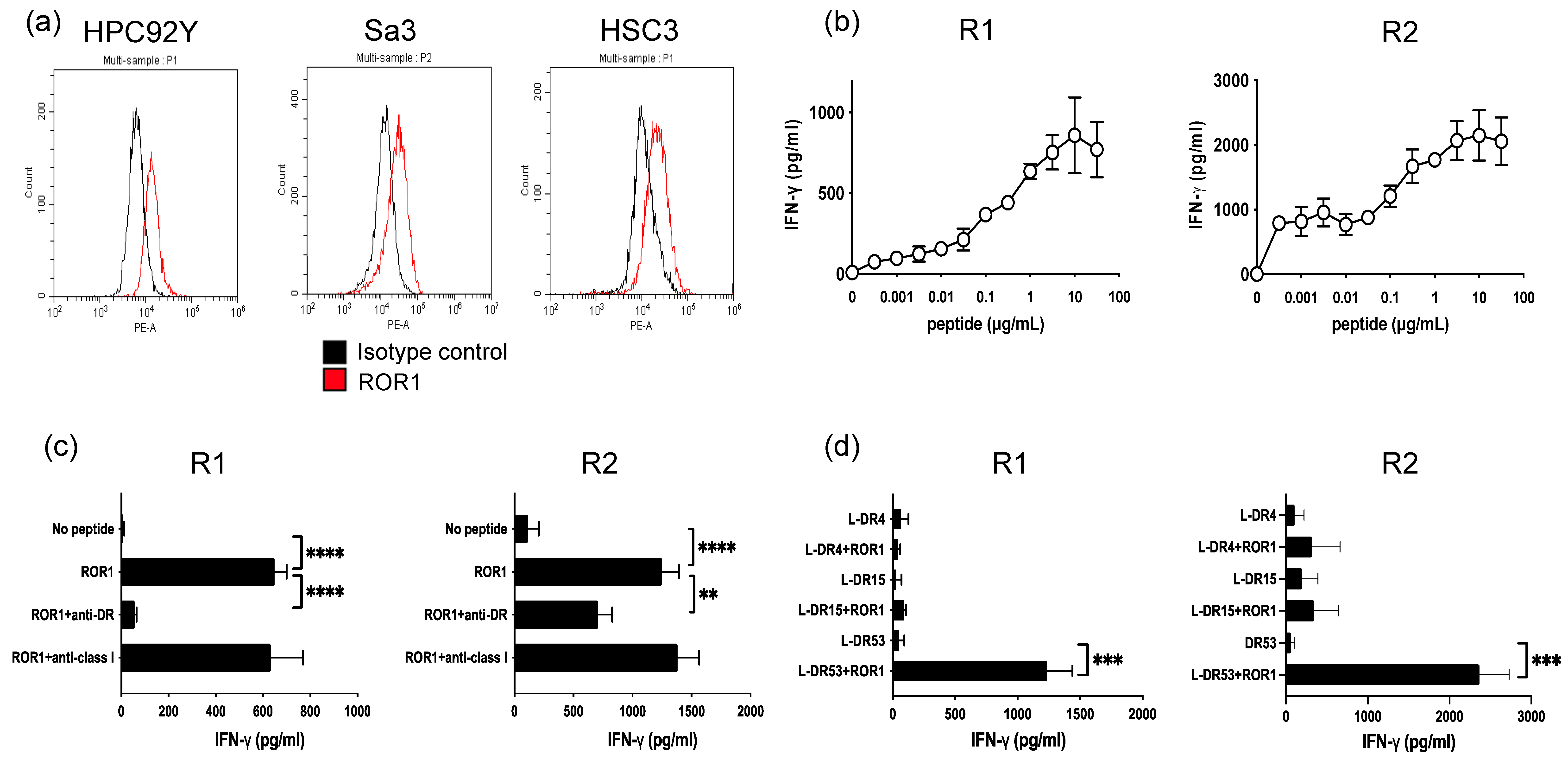
3.2. Generation of ROR1403–417—Reactive HTLs
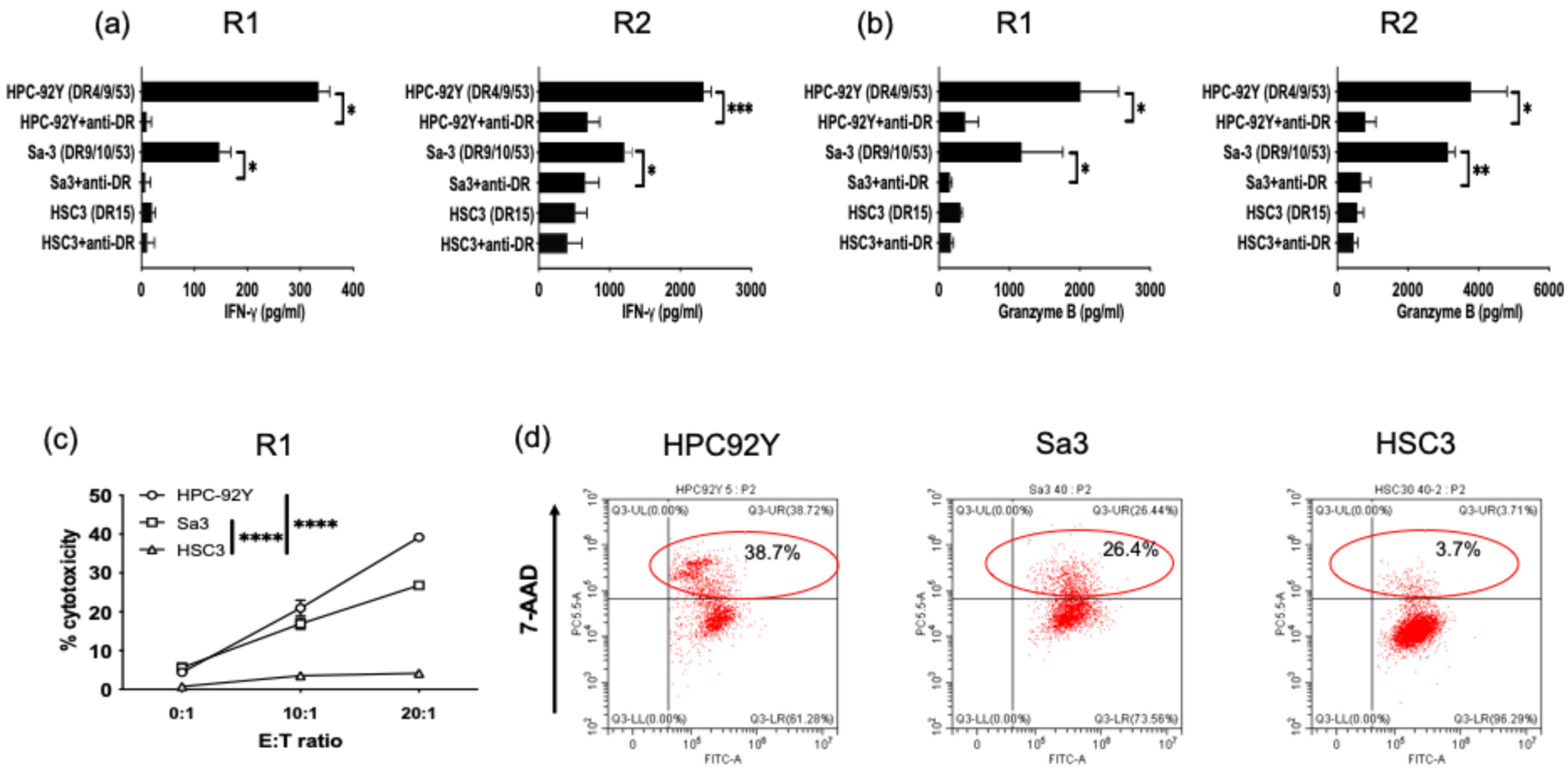
3.3. Direct Tumor Recognition and Cytokine Production by ROR1-Reactive HTLs
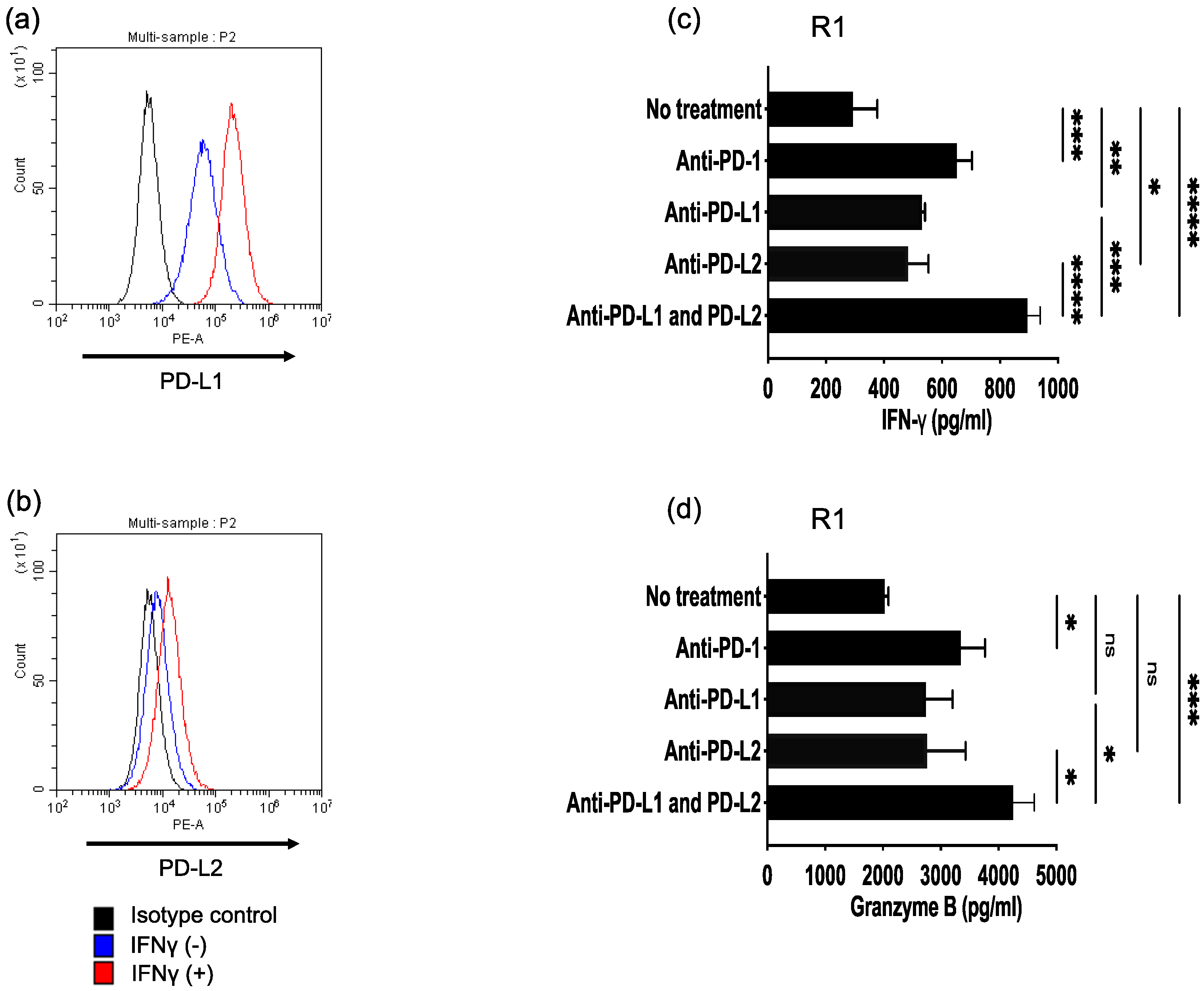
3.4. Antitumor Activity of ROR1-Reactive HTLs Enhanced by PD-L1/PD-1 and PD-L2/PD-1 Axes Blockade
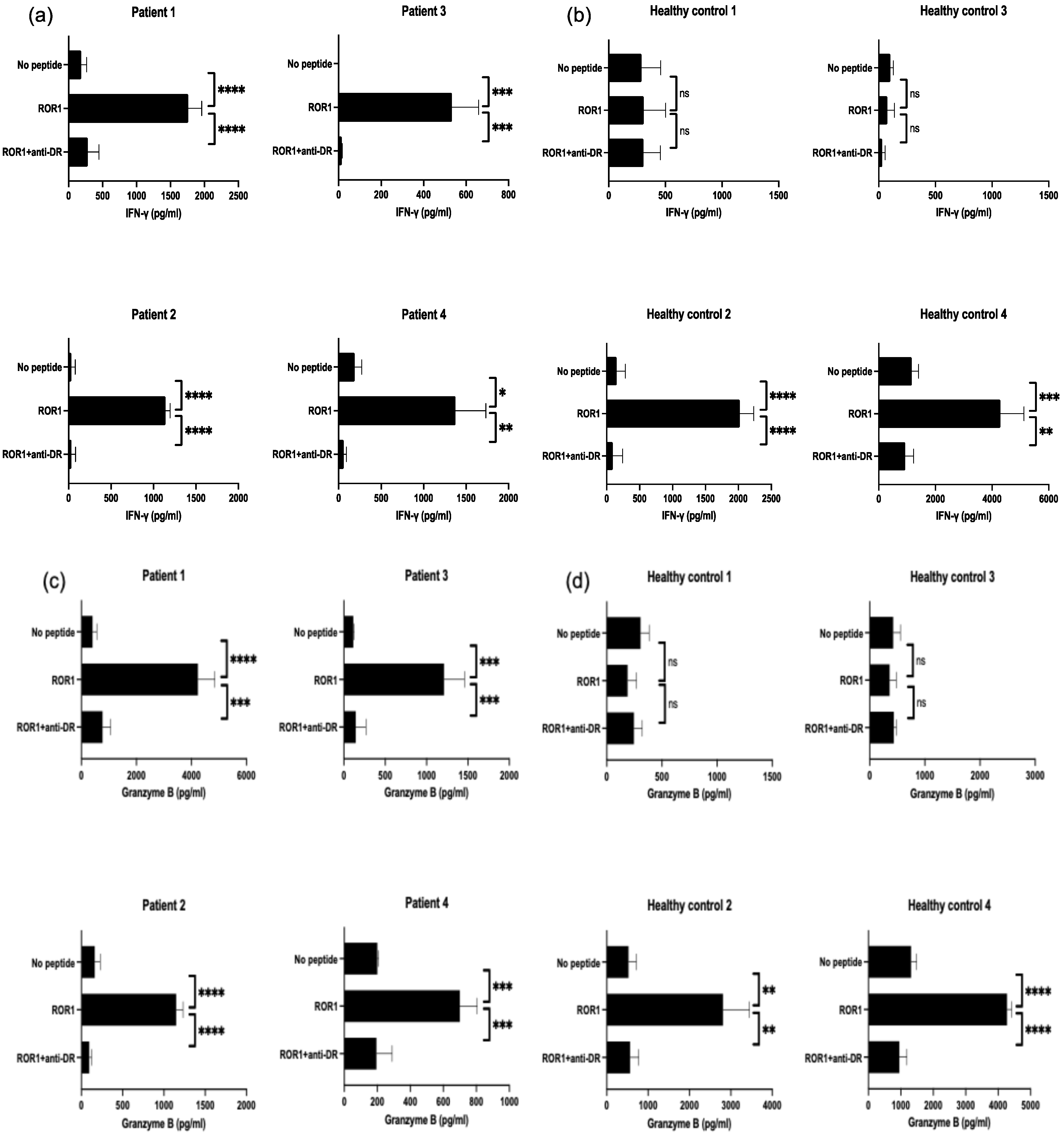
3.5. Detection of ROR1403–417—Reactive T Cells in PBMCs from Patients with HNSCC and Healthy Individuals
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADC | Antibody–drug conjugate |
| APCs | Antigen-presenting cells |
| CTLs | Cytotoxic T lymphocytes |
| γPBMCs | γ-irradiated autologous PBMCs |
| HLA | Human leukocyte antigen |
| HNSCC | Head and neck squamous cell carcinoma |
| HTL | Helper T cell |
| ICIs | Immune checkpoint inhibitors |
| IHC | Immunohistochemistry |
| OS | Overall survival |
| PBMCs | Peripheral blood mononuclear cells |
| PFS | Progression-free survival |
| ROR1 | Receptor tyrosine kinase-like orphan receptor 1 |
| TAA | Tumor-associated antigen |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Chow, L.Q.M. Head and Neck Cancer. N. Engl. J. Med. 2020, 382, 60–72. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G., Jr.; Psyrri, A.; Basté, N.; Neupane, P.; Bratland, Å.; et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef]
- Kumai, T.; Yamaki, H.; Kono, M.; Hayashi, R.; Wakisaka, R.; Komatsuda, H. Antitumor Peptide-Based Vaccine in the Limelight. Vaccines 2022, 10, 70. [Google Scholar] [CrossRef]
- Zhou, Y.; Wei, Y.; Tian, X.; Wei, X. Cancer vaccines: Current status and future directions. J. Hematol. Oncol. 2025, 18, 18. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Goodpaster, T.; Randolph-Habecker, J.; Hoffstrom, B.G.; Jalikis, F.G.; Koch, L.K.; Berger, C.; Kosasih, P.L.; Rajan, A.; Sommermeyer, D.; et al. Analysis of ROR1 Protein Expression in Human Cancer and Normal Tissues. Clin. Cancer Res. 2017, 23, 3061–3071. [Google Scholar] [CrossRef]
- Berger, C.; Sommermeyer, D.; Hudecek, M.; Berger, M.; Balakrishnan, A.; Paszkiewicz, P.J.; Kosasih, P.L.; Rader, C.; Riddell, S.R. Safety of targeting ROR1 in primates with chimeric antigen receptor-modified T cells. Cancer Immunol. Res. 2015, 3, 206–216. [Google Scholar] [CrossRef]
- Jaeger-Ruckstuhl, C.A.; Specht, J.M.; Voutsinas, J.M.; MacMillan, H.R.; Wu, Q.V.; Muhunthan, V.; Berger, C.; Pullarkat, S.; Wright, J.H.; Yeung, C.C.S.; et al. Phase I Study of ROR1-Specific CAR-T Cells in Advanced Hematopoietic and Epithelial Malignancies. Clin. Cancer Res. 2025, 31, 503–514. [Google Scholar] [CrossRef]
- Kumai, T.; Ohkuri, T.; Nagato, T.; Matsuda, Y.; Oikawa, K.; Aoki, N.; Kimura, S.; Celis, E.; Harabuchi, Y.; Kobayashi, H. Targeting HER-3 to elicit antitumor helper T cells against head and neck squamous cell carcinoma. Sci. Rep. 2015, 5, 16280. [Google Scholar] [CrossRef] [PubMed]
- Nakae, S.; Suto, H.; Iikura, M.; Kakurai, M.; Sedgwick, J.D.; Tsai, M.; Galli, S.J. Mast cells enhance T cell activation: Importance of mast cell costimulatory molecules and secreted TNF. J. Immunol. 2006, 176, 2238–2248. [Google Scholar] [CrossRef]
- Castle, J.C.; Kreiter, S.; Diekmann, J.; Lower, M.; van de Roemer, N.; de Graaf, J.; Selmi, A.; Diken, M.; Boegel, S.; Paret, C.; et al. Exploiting the mutanome for tumor vaccination. Cancer Res. 2012, 72, 1081–1091. [Google Scholar] [CrossRef]
- Braun, D.A.; Moranzoni, G.; Chea, V.; McGregor, B.A.; Blass, E.; Tu, C.R.; Vanasse, A.P.; Forman, C.; Forman, J.; Afeyan, A.B.; et al. A neoantigen vaccine generates antitumour immunity in renal cell carcinoma. Nature 2025, 639, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Deng, L.; Jackson, K.R.; Talukder, A.H.; Katailiha, A.S.; Bradley, S.D.; Zou, Q.; Chen, C.; Huo, C.; Chiu, Y.; et al. Neoantigen vaccination induces clinical and immunologic responses in non-small cell lung cancer patients harboring EGFR mutations. J. Immunother. Cancer 2021, 9, e002531. [Google Scholar] [CrossRef]
- Zacharakis, N.; Chinnasamy, H.; Black, M.; Xu, H.; Lu, Y.C.; Zheng, Z.; Pasetto, A.; Langhan, M.; Shelton, T.; Prickett, T.; et al. Immune recognition of somatic mutations leading to complete durable regression in metastatic breast cancer. Nat. Med. 2018, 24, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Zhao, H.; Hu, S.; Dong, L.; Dou, Y.; Li, J.; Gao, Q.; Zhang, B. Tumor-associated antigen prediction using a single-sample gene expression state inference algorithm. Cell Rep. Methods 2024, 4, 100906. [Google Scholar] [CrossRef]
- Shabani, M.; Naseri, J.; Shokri, F. Receptor tyrosine kinase-like orphan receptor 1: A novel target for cancer immunotherapy. Expert. Opin. Ther. Targets 2015, 19, 941–955. [Google Scholar] [CrossRef]
- Baskar, S.; Kwong, K.Y.; Hofer, T.; Levy, J.M.; Kennedy, M.G.; Lee, E.; Staudt, L.M.; Wilson, W.H.; Wiestner, A.; Rader, C. Unique cell surface expression of receptor tyrosine kinase ROR1 in human B-cell chronic lymphocytic leukemia. Clin. Cancer Res. 2008, 14, 396–404. [Google Scholar] [CrossRef]
- Hudecek, M.; Schmitt, T.M.; Baskar, S.; Lupo-Stanghellini, M.T.; Nishida, T.; Yamamoto, T.N.; Bleakley, M.; Turtle, C.J.; Chang, W.C.; Greisman, H.A.; et al. The B-cell tumor-associated antigen ROR1 can be targeted with T cells modified to express a ROR1-specific chimeric antigen receptor. Blood 2010, 116, 4532–4541. [Google Scholar] [CrossRef] [PubMed]
- Dave, H.; Anver, M.R.; Butcher, D.O.; Brown, P.; Khan, J.; Wayne, A.S.; Baskar, S.; Rader, C. Restricted cell surface expression of receptor tyrosine kinase ROR1 in pediatric B-lineage acute lymphoblastic leukemia suggests targetability with therapeutic monoclonal antibodies. PLoS ONE 2012, 7, e52655. [Google Scholar] [CrossRef]
- Fukuda, T.; Chen, L.; Endo, T.; Tang, L.; Lu, D.; Castro, J.E.; Widhopf, G.F., 2nd; Rassenti, L.Z.; Cantwell, M.J.; Prussak, C.E.; et al. Antisera induced by infusions of autologous Ad-CD154-leukemia B cells identify ROR1 as an oncofetal antigen and receptor for Wnt5a. Proc. Natl. Acad. Sci. USA 2008, 105, 3047–3052. [Google Scholar] [CrossRef] [PubMed]
- Tigu, A.B.; Munteanu, R.; Moldovan, C.; Rares, D.; Kegyes, D.; Tomai, R.; Moisoiu, V.; Ghiaur, G.; Tomuleasa, C.; Einsele, H.; et al. Therapeutic advances in the targeting of ROR1 in hematological cancers. Cell Death Discov. 2024, 10, 471. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Ghia, E.M.; Chen, L.; Rassenti, L.Z.; DeBoever, C.; Widhopf, G.F., 2nd; Yu, J.; Neuberg, D.S.; Wierda, W.G.; Rai, K.R.; et al. High-level ROR1 associates with accelerated disease progression in chronic lymphocytic leukemia. Blood 2016, 128, 2931–2940. [Google Scholar] [CrossRef] [PubMed]
- Barna, G.; Mihalik, R.; Timar, B.; Tombol, J.; Csende, Z.; Sebestyen, A.; Bodor, C.; Csernus, B.; Reiniger, L.; Petak, I.; et al. ROR1 expression is not a unique marker of CLL. Hematol. Oncol. 2011, 29, 17–21. [Google Scholar] [CrossRef]
- Meng, L.; Du, Y.; Deng, B.; Duan, Y. miR-379-5p regulates the proliferation, cell cycle, and cisplatin resistance of oral squamous cell carcinoma cells by targeting ROR1. Am. J. Transl. Res. 2023, 15, 1626–1639. [Google Scholar]
- Jeong, S.Y.; Lee, K.J.; Cha, J.; Park, S.Y.; Kim, H.S.; Kim, J.H.; Lee, J.J.; Kim, N.; Park, S.T. Meta-Analysis of Survival Effects of Receptor Tyrosine Kinase-like Orphan Receptor 1 (ROR1). Medicina (Kaunas) 2022, 58, 1867. [Google Scholar] [CrossRef]
- Shin, J.H.; Yoon, H.J.; Kim, S.M.; Lee, J.H.; Myoung, H. Analyzing the factors that influence occult metastasis in oral tongue cancer. J. Korean Assoc. Oral. Maxillofac. Surg. 2020, 46, 99–107. [Google Scholar] [CrossRef]
- Wang, M.L.; Barrientos, J.C.; Furman, R.R.; Mei, M.; Barr, P.M.; Choi, M.Y.; de Vos, S.; Kallam, A.; Patel, K.; Kipps, T.J.; et al. Zilovertamab Vedotin Targeting of ROR1 as Therapy for Lymphoid Cancers. NEJM Evid. 2022, 1, EVIDoa2100001. [Google Scholar] [CrossRef]
- Lemech, C.R.; Zuniga, R.; Barve, M.A.; Song, Y.; Zhang, J.; Zhou, K.; Zhang, L.; Shen, L.; Bishnoi, S.; Cherng, H.-J.J.; et al. A phase 1a/b, multi-regional, first-in-human study of CS5001, a novel anti-ROR1 ADC, in patients with advanced solid tumors and lymphomas. J. Clin. Oncol. 2024, 42, 3023. [Google Scholar] [CrossRef]
- Giuntoli, R.L., 2nd; Lu, J.; Kobayashi, H.; Kennedy, R.; Celis, E. Direct costimulation of tumor-reactive CTL by helper T cells potentiate their proliferation, survival, and effector function. Clin. Cancer Res. 2002, 8, 922–931. [Google Scholar]
- Perez-Diez, A.; Joncker, N.T.; Choi, K.; Chan, W.F.; Anderson, C.C.; Lantz, O.; Matzinger, P. CD4 cells can be more efficient at tumor rejection than CD8 cells. Blood 2007, 109, 5346–5354. [Google Scholar] [CrossRef] [PubMed]
- Castro, F.; Cardoso, A.P.; Goncalves, R.M.; Serre, K.; Oliveira, M.J. Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion. Front. Immunol. 2018, 9, 847. [Google Scholar] [CrossRef]
- Cullen, S.P.; Brunet, M.; Martin, S.J. Granzymes in cancer and immunity. Cell Death Differ. 2010, 17, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Sousa, L.G.; Rajapakshe, K.; Rodriguez Canales, J.; Chin, R.L.; Feng, L.; Wang, Q.; Barrese, T.Z.; Massarelli, E.; William, W.; Johnson, F.M.; et al. ISA101 and nivolumab for HPV-16(+) cancer: Updated clinical efficacy and immune correlates of response. J. Immunother. Cancer 2022, 10, e004232. [Google Scholar] [CrossRef]
- Aggarwal, C.; Saba, N.F.; Algazi, A.; Sukari, A.; Seiwert, T.Y.; Haigentz, M.; Porosnicu, M.; Bonomi, M.; Boyer, J.; Esser, M.T.; et al. Safety and Efficacy of MEDI0457 plus Durvalumab in Patients with Human Papillomavirus-Associated Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. 2023, 29, 560–570. [Google Scholar] [CrossRef]
- O’Day, S.; Hodi, F.S.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Zhu, X.; Yellin, M.J.; Hoos, A.; Urba, W.J. A phase III, randomized, double-blind, multicenter study comparing monotherapy with ipilimumab or gp100 peptide vaccine and the combination in patients with previously treated, unresectable stage III or IV melanoma. J. Clin. Oncol. 2010, 28, 4. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Weber, J.S.; Carlino, M.S.; Khattak, A.; Meniawy, T.; Ansstas, G.; Taylor, M.H.; Kim, K.B.; McKean, M.; Long, G.V.; Sullivan, R.J.; et al. Individualised neoantigen therapy mRNA-4157 (V940) plus pembrolizumab versus pembrolizumab monotherapy in resected melanoma (KEYNOTE-942): A randomised, phase 2b study. Lancet 2024, 403, 632–644. [Google Scholar] [CrossRef]
- Riaz, N.; Morris, L.; Havel, J.J.; Makarov, V.; Desrichard, A.; Chan, T.A. The role of neoantigens in response to immune checkpoint blockade. Int. Immunol. 2016, 28, 411–419. [Google Scholar] [CrossRef]
- Chang, E.; Pelosof, L.; Lemery, S.; Gong, Y.; Goldberg, K.B.; Farrell, A.T.; Keegan, P.; Veeraraghavan, J.; Wei, G.; Blumenthal, G.M.; et al. Systematic Review of PD-1/PD-L1 Inhibitors in Oncology: From Personalized Medicine to Public Health. Oncologist 2021, 26, e1786–e1799. [Google Scholar] [CrossRef]
- Wang, Y.; Du, J.; Gao, Z.; Sun, H.; Mei, M.; Wang, Y.; Ren, Y.; Zhou, X. Evolving landscape of PD-L2: Bring new light to checkpoint immunotherapy. Br. J. Cancer 2022, 128, 1196–1207. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Liu, C.; Zhang, X.; Zhou, Q.; Li, Y.; Xu, Y.; Gao, Z.; Xu, Y.; Kong, L.; Yang, A.; et al. PD-L2 based immune signature confers poor prognosis in HNSCC. Oncoimmunology 2021, 10, 1947569. [Google Scholar] [CrossRef] [PubMed]
- Sato, R.; Komatsuda, H.; Inoue, T.; Wakisaka, R.; Kono, M.; Yamaki, H.; Ohara, K.; Kumai, T.; Kishibe, K.; Hayashi, T.; et al. Combined approach for predicting the efficacy of nivolumab in head and neck carcinoma by tissue and soluble expressions of PD-L1 and PD-L2. Head. Neck 2024, 46, 2233–2243. [Google Scholar] [CrossRef] [PubMed]
- Yearley, J.H.; Gibson, C.; Yu, N.; Moon, C.; Murphy, E.; Juco, J.; Lunceford, J.; Cheng, J.; Chow, L.Q.M.; Seiwert, T.Y.; et al. PD-L2 Expression in Human Tumors: Relevance to Anti-PD-1 Therapy in Cancer. Clin. Cancer Res. 2017, 23, 3158–3167. [Google Scholar] [CrossRef]
- Yoshitake, Y.; Fukuma, D.; Yuno, A.; Hirayama, M.; Nakayama, H.; Tanaka, T.; Nagata, M.; Takamune, Y.; Kawahara, K.; Nakagawa, Y.; et al. Phase II clinical trial of multiple peptide vaccination for advanced head and neck cancer patients revealed induction of immune responses and improved OS. Clin. Cancer Res. 2015, 21, 312–321. [Google Scholar] [CrossRef]
- Kono, M.; Wakisaka, R.; Komatsuda, H.; Hayashi, R.; Kumai, T.; Yamaki, H.; Sato, R.; Nagato, T.; Ohkuri, T.; Kosaka, A.; et al. Immunotherapy targeting tumor-associated antigen in a mouse model of head and neck cancer. Head. Neck 2024, 46, 2056–2067. [Google Scholar] [CrossRef]
- Cachot, A.; Bilous, M.; Liu, Y.C.; Li, X.; Saillard, M.; Cenerenti, M.; Rockinger, G.A.; Wyss, T.; Guillaume, P.; Schmidt, J.; et al. Tumor-specific cytolytic CD4 T cells mediate immunity against human cancer. Sci. Adv. 2021, 7, eabe3348. [Google Scholar] [CrossRef]
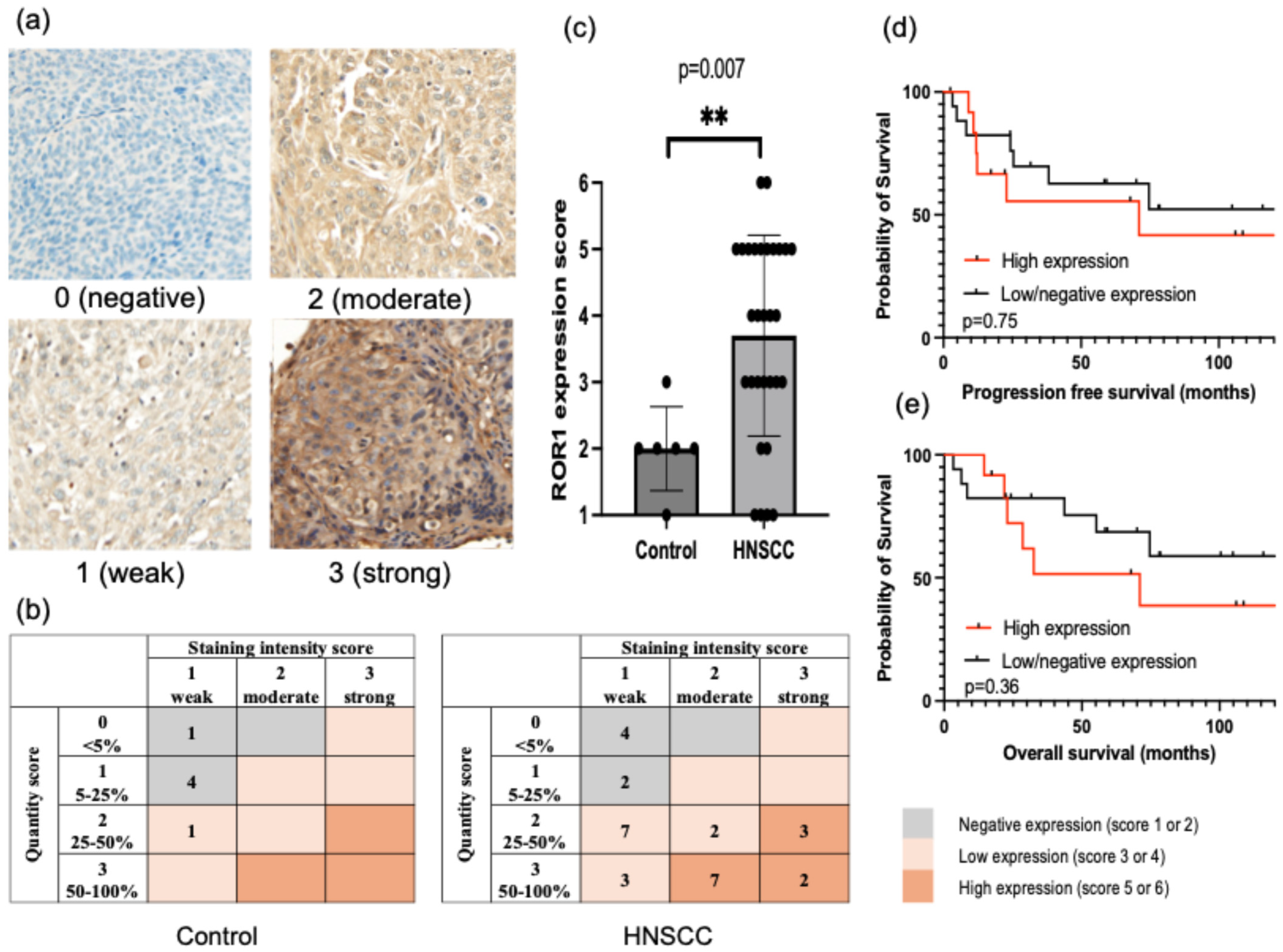
| Low/Negative Expression (n = 18) | High Expression (n = 12) | p Value | |
|---|---|---|---|
| Age | |||
| <70 | 11 | 6 | 0.71 |
| ≧70 | 7 | 6 | |
| Sex | |||
| Male | 16 | 12 | 0.5 |
| Female | 2 | 0 | |
| HPV | |||
| positive | 10 | 4 | 0.28 |
| negative | 8 | 8 | |
| T classification | |||
| T1–2 | 13 | 3 | 0.02 * |
| T3–4 | 5 | 9 | |
| N classification | |||
| N0–1 | 12 | 2 | 0.01 * |
| N2–3 | 6 | 10 | |
| M classification | |||
| M0 | 18 | 12 | NA |
| M1 | 0 | 0 | |
| Clinical stage | |||
| I–II | 14 | 4 | 0.02 * |
| III–IV | 4 | 8 |
| Sex | Age (Years) | TNM Classification | Clinical Stage | Primary Site | HLA-DR Alleles | IFN-γ (pg/mL) | |||
|---|---|---|---|---|---|---|---|---|---|
| Granzyme B (pg/mL) | |||||||||
| No Peptide | ROR1 | ROR1+ anti-DR | |||||||
| Patient 1 | Male | 80 | T4aN2bM0 | IVA | Hypopharyngeal | NA | 183 ± 81 | 1756 ± 201 | 281 ± 166 |
| 406 ± 156 | 4234 ± 617 | 768 ± 293 | |||||||
| Patient 2 | Female | 69 | T4aN3bM1 | IVC | Hypopharyngeal | NA | 31 ± 48 | 1138 ± 56 | 32 ± 50 |
| 161 ± 67 | 1148 ± 84 | 95 ± 28 | |||||||
| Patient 3 | Male | 53 | T2N2M0 | III | Nasopharyngeal | NA | < | 533 ± 126 | 14 ± 3 |
| 114 ± 9 | 1211 ± 248 | 144 ± 123 | |||||||
| Patient 4 | Male | 69 | T4aN0M0 | IVA | Laryngeal | NA | 186 ± 86 | 1373 ± 360 | 59 ± 32 |
| 201 ± 7 | 700 ± 105 | 195 ± 95 | |||||||
| Healthy control 1 | Male | 32 | NA | NA | NA | 4/15/53 | 288 ± 171 | 308 ± 195 | 305 ± 151 |
| 306 ± 78 | 188 ± 81 | 247 ± 75 | |||||||
| Healthy control 2 | Male | 31 | NA | NA | NA | 9/11/53 | 150 ± 134 | 2019 ± 216 | 90 ± 151 |
| 525 ± 190 | 2810 ± 640 | 564 ± 207 | |||||||
| Healthy control 3 | Female | 33 | NA | NA | NA | 12/14 | 101 ± 27 | 75 ± 62 | 29 ± 27 |
| 426 ± 131 | 360 ± 122 | 437 ± 48 | |||||||
| Healthy control 4 | Male | 40 | NA | NA | NA | 9/12/53 | 1160 ± 246 | 4284 ± 842 | 925 ± 308 |
| 1315 ± 169 | 4274 ± 143 | 953 ± 230 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, R.; Yamaki, H.; Inoue, T.; Sakaue, S.; Ominato, H.; Wakisaka, R.; Komatsuda, H.; Kono, M.; Ohara, K.; Kosaka, A.; et al. ROR1 as an Immunotherapeutic Target for Inducing Antitumor Helper T Cell Responses Against Head and Neck Squamous Cell Carcinoma. Cancers 2025, 17, 2326. https://doi.org/10.3390/cancers17142326
Sato R, Yamaki H, Inoue T, Sakaue S, Ominato H, Wakisaka R, Komatsuda H, Kono M, Ohara K, Kosaka A, et al. ROR1 as an Immunotherapeutic Target for Inducing Antitumor Helper T Cell Responses Against Head and Neck Squamous Cell Carcinoma. Cancers. 2025; 17(14):2326. https://doi.org/10.3390/cancers17142326
Chicago/Turabian StyleSato, Ryosuke, Hidekiyo Yamaki, Takahiro Inoue, Shota Sakaue, Hisataka Ominato, Risa Wakisaka, Hiroki Komatsuda, Michihisa Kono, Kenzo Ohara, Akemi Kosaka, and et al. 2025. "ROR1 as an Immunotherapeutic Target for Inducing Antitumor Helper T Cell Responses Against Head and Neck Squamous Cell Carcinoma" Cancers 17, no. 14: 2326. https://doi.org/10.3390/cancers17142326
APA StyleSato, R., Yamaki, H., Inoue, T., Sakaue, S., Ominato, H., Wakisaka, R., Komatsuda, H., Kono, M., Ohara, K., Kosaka, A., Ohkuri, T., Nagato, T., Kumai, T., Kishibe, K., Kobayashi, H., & Takahara, M. (2025). ROR1 as an Immunotherapeutic Target for Inducing Antitumor Helper T Cell Responses Against Head and Neck Squamous Cell Carcinoma. Cancers, 17(14), 2326. https://doi.org/10.3390/cancers17142326





