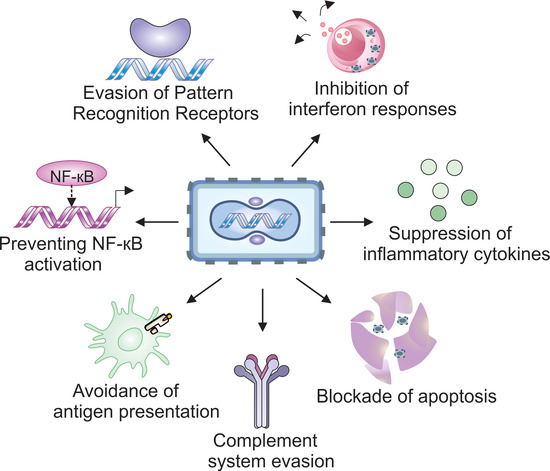
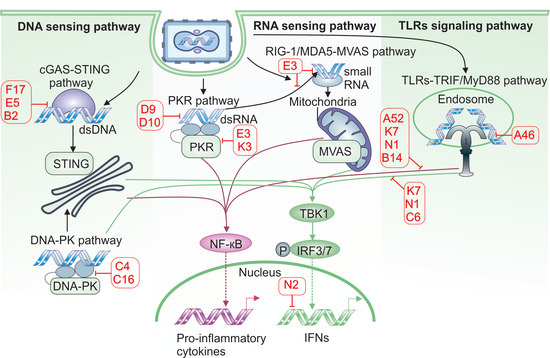
Simple Summary
This review explores the potential of the vaccinia virus (VACV) as a powerful tool in oncolytic cancer treatment. While current therapies often fall short for patients with advanced cancer, VACV offers a promising approach by directly killing tumor cells and activating the body’s immune system—particularly T cells—to fight cancer. The review highlights the virus’s unique ability to evade immune defenses, its interactions with the immune system, and ongoing research into its use as an oncolytic agent against various cancer types. Overall, VACV is presented as a versatile and impactful candidate for future cancer therapies.
Abstract
Despite significant advances in cancer therapy, the prognosis for patients with advanced, disseminated disease remains poor. This underscores the urgent need for novel treatments that not only eliminate tumor cells effectively but also stimulate a strong, durable anti-cancer immune response. Among emerging strategies, oncolytic viruses have shown exceptional promise due to their selective cytotoxicity and their ability to activate T cell-mediated immune responses. In this review, we focus on the vaccinia virus (VACV), a member of the Poxviridae family, which has emerged as a leading candidate in modern oncolytic immunotherapy. We examine the virus’s properties that enable it to evade antiviral defenses and serve as a versatile, potent oncolytic agent. Furthermore, we explore its interactions with various components of the immune system and how these contribute to the induction of a robust T cell-driven response. Finally, we assess current efforts to harness VACV for the treatment of various cancer types and highlight future directions where its application is most likely to succeed. Overall, our goal is to present VACV as a powerful and broadly applicable platform with the potential to transform the landscape of oncology.
1. Introduction
Vaccinia virus (VACV) is an Orthopoxvirus, and a prototypic member of large Poxviridae family. Due to its genomic and antigenic similarities to variola virus, along with high immunogenicity and low virulence, VACV was extensively used as a smallpox vaccine, ultimately contributing to the successful eradication of the disease. Like other Orthopoxviruses, it replicates exclusively in the cytoplasm and does not integrate into the host genome [1,2,3]. The exact origin of VACV remains unclear: One theory suggests that it evolved from either smallpox or cowpox virus; another posits that all three viruses share a common ancestor; a third proposes that it may have been derived from horsepox virus [4]. In the wild, VACV has been reported to cause sporadic infections in cattle and water buffalos, but its natural host and reservoir remain unknown [5].
VACV is an enveloped, double-stranded DNA virus with a relatively large genome size of approximately 190 to 200 kb. Both DNA strands are connected at the termini by partially complementary AT-rich loops, forming one continuous polynucleotide chain [6]. Interestingly, despite many years of genomic and transcriptomic research, the exact number of genes and proteins encoded by the VACV genome remains unknown. Most sources estimate the number of VACV genes to be between 200 and 250 [7,8,9].
Over the years, several strains of VACV have been developed. The most studied strain is the Western Reserve (WR) strain, which was created in the United States through repeated passages of the New York City Board of Health (NYCBH) strain in rabbits, mice, and various mammalian cell cultures. Due to its adaptation to multiple hosts, it produces high titer in vitro and causes neuropathogenic effects in vivo, making it unsuitable for use as a vaccine [10].
In contrast to the WR strain, the Lister strain was developed at the Lister Institute in the United Kingdom, and the NYCBH strain is much less virulent while still retaining the ability to replicate in humans and other mammals. The Lister and Wyeth strains (with Wyeth being the commercial name for the NYCBH strain) were most commonly used for vaccinations during the smallpox eradication campaign [11]. They were known to induce strong immunity accompanied by high antibody titers, although they occasionally caused undesired side effects, including severe eczema and encephalitis [12].
Other VACV strains include Copenhagen and IHD (International Health Department), the latter being particularly notable for high production of extracellular enveloped viruses (EEVs) [13]. Finally, the modified vaccinia virus Ankara (MVA) strain represents a more attenuated version of the VACV Ankara strain. MVA cannot replicate in vivo and exhibits only limited replication in mammalian cells in vitro. It was developed through over 570 serial passages of the original virus in primary chicken embryo fibroblasts, resulting in the deletion of over 30 kb fragment of its genome [11].
Today, most oncolytic VACV strains developed for therapy are based on various modifications of strains such as Lister, Wyeth, Copenhagen, WR, or IHD.
2. Infection and Replication
2.1. Viral Entry
A schematic illustration of the VACV’s life cycle is shown in Figure 1. In contrast to the majority of viruses such as adenoviruses or herpesviruses, VACV does not rely on binding to a specific cellular receptor for entry. Instead, it attaches to host cells using glycosaminoglycans (GAGs), specifically heparan sulfate and chondroitin sulfate for attachment [14,15]. Since GAGs are widely expressed on a variety of cell types, this broadens VACV’s host range, allowing it to infect multiple cell types across different species.
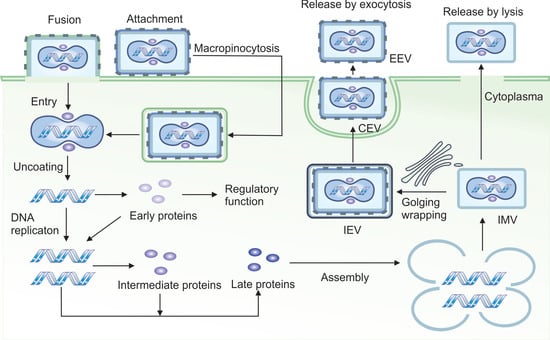
Figure 1.
Life cycle of vaccinia virus. VACV enters host cells either through direct membrane fusion (primary route) or via macropinocytosis (secondary route) after binding to the cell surface. Following entry, the viral core is released into the cytoplasm, where core-associated enzymes initiate transcription of early genes. These early genes promote viral DNA replication and suppress host antiviral responses. DNA replication then triggers the expression of intermediate genes, which in turn activates the transcription of late genes responsible for virion assembly. VACV produces four distinct forms of virions: (1) intracellular mature virions (IMVs) that accumulate within the cytoplasm and are released only upon cell lysis; (2) intracellular enveloped virions (IEVs) that acquire additional membranes from the trans-Golgi network or endosomes and are transported the cell surface via microtubules; (3) cell-associated enveloped virions (CEVs) that are formed when IEVs fuse with the plasma membrane, enabling direct cell-to-cell spread; (4) extracellular enveloped virions (EEVs), a subset of CEVs that are released from the cell and facilitate long-range viral dissemination to distant cells.
Additionally, a scavenger receptor MARCO (Macrophage receptor with collagenous structure) has been reported to facilitate VACV binding and enhance its interaction with GAGs [16].
The primary and most common mechanism of VACV entry involves direct fusion of the viral and cell membranes. This multistep process includes close apposition of both membranes, lipid mixing of the outer membrane leaflets to form a hemifusion intermediate, followed by the formation and expansion of a fusion pore, which allows the viral core or nucleoprotein to enter the cytoplasm [17]. Notably, this fusion can occur at neutral pH, allowing VACV to bypass the endocytic pathway. As a result, the virus avoids pathogen recognition receptors (PRRs) localized in endosomes and can initiate replication rapidly.
Alternatively, as a secondary route, VACV can enter cells via macropinocytosis, wherein membrane fusion occurs within endosomal vesicles and is triggered by lower pH [18]. This pathway is complex and involves Rho GTPases and the tyrosine kinase activity of the epidermal growth factor receptor (EGFR) in the host cell [19]. Depending on the strain, VACV may induce the formation of blebs or filopodia in the target cell to facilitate the internalization of viral particles.
Overall, at least 16 different viral proteins are involved in VACV entry: 4 in attachment and 12 in penetration. However, the precise molecular details of these processes remain incompletely understood [17].
2.2. Replication
The VACV virion consists of a compact protein core tightly wrapped around the viral DNA, along with two lateral bodies that contain proteins involved in counteracting host antiviral defenses [20]. Almost immediately after VACV enters a host cell, the lateral bodies dissociate from the core, leading to the release of H1 phosphatase, which inactivates the signal transducer and activates transcription 1 (STAT1) protein, thereby blocking interferon signaling [21].
Within 20 min of entry, components of the viral core initiate transcription of approximately 50% of the viral genome, producing a set of early mRNAs [22]. These early transcripts encode proteins required for DNA replication, which begins roughly 2 h post-infection [23]. DNA replication then triggers the expression of intermediate genes, followed by late genes essential for the assembly of mature virions [22]. The first complete virions are typically produced around 6 h after initial infection, and cell lysis generally begins within 15–48 h post-entry [24].
A hallmark of VACV-infected cells is the presence of cytoplasmic structures known as virosomes or virus factories. These complexes, composed of viral proteins and protrusions of endoplasmic reticulum (ER) and Golgi-derived membranes, are the sites of viral DNA replication, transcription, and virion assembly [22,25].
To carry out replication independently of the host cell nucleus, VACV encodes a broad array of viral replication machinery, including enzymes that substitute for nuclear counterparts. These include DNA polymerase (E9), uracil DNA glycosylase (D4), and D5 protein, which possesses helicase and primase activity [26]. Additional proteins such as single-strand DNA-binding protein (I3) [27], Holliday junction resolvase (A22) [28], and ligase (A50) [29] further support autonomous replication, thereby minimizing the risk of non-specific integration into the host genome.
VACV also synthesizes its own nucleotide precursors via enzymes such as thymidine kinase (TK; encoded by J2R) [30], thymidylate kinase (encoded by A48R) [31], and ribonucleotide reductase (RR; encoded by F4L and I4L) [32].
During its replication cycle, VACV produces four distinct types of virions: intracellular mature virus (IMV), intracellular enveloped virus (IEV), cell-associated enveloped virus (CEV), and extracellular enveloped virus (EEV) [33]. Different VACV strains produce varying proportions of virion types, which affects their spread, infectivity, and ability to evade the immune system [34]. IMV is the most abundant and stable form of the virus, remaining within the cell until released by lysis. It is well-suited for host-to-host transmission. IEV is formed when IMV is wrapped in a secondary membrane, typically derived from the Golgi apparatus, and is transported to the cell surface along microtubules for dissemination. After fusing with the plasma membrane, the IEV loses two of its envelope proteins and becomes a CEV, a form that remains attached to the cell surface [35]. CEV induces the formation of actin tails, which propel the virus away from the cell, facilitating cell-to-cell spread. EEV is the fully released form, enabling long-range dissemination of the virus [35].
It is estimated that approximately 2500–5000 new viral particles are produced per infected cell [36].
4. Strategies to Avoid Cell Death Mechanisms
Programmed cell death is a fundamental defense mechanism that helps prevent viral spread within the host. If an infected cell undergoes death before the virus can complete replication, it fails to produce progeny virions and is eliminated through phagocytosis by immune cells such as dendritic cells and macrophages [83,84]. Consequently, VACV evolved multiple tools to control mechanisms of programmed cell death and utilize them to its advantage (Figure 4).
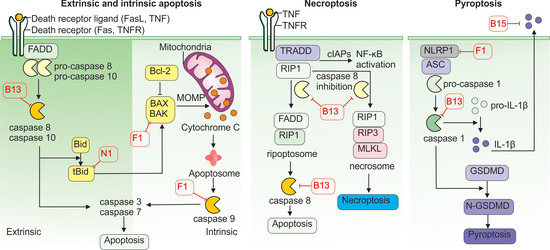
Figure 4.
Inhibition of cell death pathways by vaccinia virus proteins. VACV employs multiple strategies to inhibit host cell death pathways and promote viral replication. The VACV F1 disrupts the intrinsic apoptosis pathway by blocking caspase-9 activation and suppressing pro-apoptotic factors BAX and BAK. Additionally, B13 functions as a pan-caspase inhibitor, blocking the death receptor-mediated extrinsic apoptosis pathway, whereas N1 blocks this signaling cascade by targeting the pro-apoptotic protein tBid. B13 also inhibits caspase-8 activation, and in its inactive form, caspase-8 allows the stabilization of RIP1 and RIP3, which interact via the RIP homotypic interaction motif (RHIM). This complex promotes the phosphorylation and oligomerization of MLKL (mixed lineage kinase domain-like protein), ultimately leading to plasma membrane rupture and necrotic cell death. VACV can also counteract pyroptosis using F1, which blocks the NLRP1 inflammasome. In addition, B13 inhibits caspase-1 intracellularly, and B15 acts extracellularly as a soluble IL-1β receptor.
Apoptosis is the most common form of programmed cell death. It can be triggered by internal or external signals and relies on caspases—a family of cysteine proteases that cleave target proteins at aspartic acid residues. Two major apoptotic pathways exist: extrinsic and intrinsic.
The extrinsic pathway is initiated by the activation of specific cell surface receptors, such as the TNFR and the Fas-ligand (FASL) receptor. Activated receptors induce the formation of a protein complex composed of death receptor adaptor proteins—TNFR-associated death domain (TRADD) and/or Fas-associated death domain (FADD)—which in turn activate the intracellular protease caspase-8. The activated caspase-8 then triggers downstream caspases-3 and -7, which cleave several cellular substrates, such as nuclear lamins and gelsolin, leading to DNA fragmentation and cell death [83].
The intrinsic pathway is activated by cellular stress such as DNA damage, oxidative stress, or nutrient deprivation, resulting in the permeabilization of the outer mitochondrial membrane. This process is mediated by Bax and Bak proteins, which form pores in the membrane, allowing cytochrome c to escape into the cytosol. Cytochrome c binds to apoptotic protease-activating factor-1 (APAF-1), forming a complex that activates caspase-9. Caspase-9, in turn, activates caspases-3 and -7, culminating in apoptosis [83].
Both apoptotic pathways can be triggered during viral infection, and VACV has evolved mechanisms to counteract them. The VACV protein B13 binds and inhibits both caspase-8 and caspase-9, effectively blocking the extrinsic and intrinsic pathways simultaneously [85]. Another viral protein, F1, inhibits both the precursor and active forms of caspase-9 and prevents the activation of caspases-3 and -7. F1 also interferes with Bak’s ability to bind Bax and form pores in the mitochondrial membrane. In addition, F1 blocks the pro-apoptotic protein Bim, a key antagonist of the anti-apoptotic factor Bcl-2. These actions are reinforced by the N1 protein, which neutralizes other Bcl-2 antagonists, Bid and Bad [86]. As a result, the mitochondrial pathway of apoptosis is effectively dismantled in VACV-infected cells. Other VACV proteins, such as B22, Golgi anti-apoptotic protein (GAAP), and E3, have also been implicated in blocking various stages of apoptosis [85].
In addition to apoptosis, VACV can inhibit pyroptosis, another form of programmed cell death that is dependent on the activation of caspase-1 via the inflammasome—a multiprotein complex. Pyroptosis is a highly pro-inflammatory process, typically triggered when intracellular PRRs such as NOD-like receptors (NLRs), especially NLRP1 (nucleotide-binding oligomerization domain, leucine-rich repeat and pyrin domain-containing 1), detect PAMPs. This recognition initiates the assembly of the adaptor protein ASC (apoptosis-associated, speck-like protein containing a CARD), which activates caspase-1. Activated caspase-1 processes pro-interleukin-1β (pro-IL-1β) into its active form and also activates gasdermin D (GSDMD), which forms pores in the cellular membrane, releasing pro-inflammatory contents [87].
Although commonly associated with bacterial infections, pyroptosis also plays a role in VACV infection [88]. VACV’s F1 protein binds the NLRP1 subunit, preventing inflammasome assembly and caspase-1 activation [88]. Additionally, B13 can directly inhibit caspase-1 activity [85], while B15 acts as a soluble decoy receptor for IL-1β, neutralizing pyroptosis-induced inflammatory signals and suppressing immune cell activation [78].
Interestingly, when apoptosis is inhibited in VACV-infected cells, necroptosis, another form of programmed cell death, may be triggered. Necroptosis occurs when caspase-8 is inhibited and cannot cleave two of its downstream targets, receptor-interacting serine/threonine kinase 1 (RIP1) and its relative RIP3. In this context, RIP1 and RIP3, along with inactive caspase-8, form the ripoptosome complex, which activates mixed lineage kinase domain-like pseudokinase (MLKL), a protein that oligomerizes and forms pores in the cell membrane, leading to cell lysis and the release of intracellular contents [89]. Because B13 strongly inhibits caspase-8, it can induce inadvertently promote ripoptosome formation and necroptosis [90].
This strategy offers dual benefits to VACV: by delaying apoptosis, VACV gains time to complete replication and produce progeny virions; and by inducing necroptosis, it facilitates viral release and dissemination to neighboring cells.
5. Interactions with Immune Cells
5.1. Dendritic Cells
As a pathogen, VACV interacts with various immune cell types, either directly by infecting them or indirectly by altering immune system function. Studies have shown that VACV exhibits a preference for infecting myeloid cells such as monocytes and dendritic cells (DCs), rather than B or T lymphocytes, although both lymphocyte types can still be infected [91].
Dendritic cells play a central role in the immune response by presenting viral antigens and initiating T cell-mediated immunity. In one study, VACV infection of immature DCs derived from peripheral blood mononuclear cells (PBMCs) impaired their maturation, as evidenced by reduced expression of key maturation markers including CD83, CD86, Human Leukocyte Antigen–DR isotype, (HLA-DR), and CD25 [92]. These infected DCs also showed a diminished capacity to stimulate T cell proliferation and exhibited increased levels of apoptosis. Notably, immature DCs were found to be significantly more susceptible to VACV infection than their mature counterparts [92].
Similar findings were observed in murine models: bone marrow-derived DCs failed to mature following VACV infection, as indicated by reduced expression of CD40, CD80, and CD86, diminished production of pro-inflammatory cytokines, and impaired activation of CD8+ T lymphocytes [93]. In contrast, mature DCs displayed greater resistance to VACV infection, with a lower proportion of infected cells and preserved antigen presentation and CD8+ T cell activation capabilities [93].
Interestingly, studies in mice infected with VACV reported that viral infection may actually promote DC maturation. Ex vivo analysis of splenic dendritic cells from VAVCV-infected mice showed increased expression of MHC I and co-stimulatory molecules CD40 and CD86 on their cell surface [94]. These DCs also exhibited elevated levels of IFN-β and demonstrated enhanced capacity to produce IL-10 and IL-12 upon LPS stimulation [94]. While these findings appear to contradict earlier results, they may be reconciled by the observation that fewer than 1% of splenic DCs were directly infected by VACV. This suggests that increased maturation may have occurred in uninfected DCs in response to cytokine signaling, particularly type I IFNs.
Conversely, the same study found that VACV infection reduced expression of MHCII on DCs and impaired their ability to present antigens to CD4+ T lymphocytes [94], a disruption potentially attributable to the viral A35 protein. Collectively, these findings suggest that DCs respond to VACV infection by preferentially activating CD8+ cell responses over CD4+ T cell responses, which aligns with the immune system’s typical strategy for combating viral infections.
5.2. Macrophages
Macrophages are another type of immune cell that can be infected by VACV. Studies have demonstrated that VACV is capable of infecting and replicating in both human M1 and M2 macrophages in vitro, with the M2 subset producing more than twice the viral titer per cell compared to M1 macrophages [95]. Infected macrophages exhibited cytoplasmic viral factories, while levels of apoptosis and necrosis remained low even 48 h post-infection. Notably, activation of macrophages with LPS and IFN-γ did not affect viral replication, whereas stimulation with IL-10 or a combination of LPS and IL-1β significantly inhibited viral production.
Interestingly, the majority of virions generated in macrophages were EEVs, which are primarily involved in long-range viral dissemination. Given the migratory capacity of macrophages between tissues, these findings suggest that macrophages may play a critical role in the systemic spread of the virus within the host [95]. In contrast, monocytes examined in the same study supported only minimal VACV replication and exhibited high levels of apoptosis, indicating a less permissive environment for viral propagation [95].
5.3. Neutrophils and NK Cells
While the role of neutrophils in VACV infection has not been extensively characterized, studies suggest they contribute significantly to early virus clearance and the initiation of adaptive T cell responses. Experiments using oncolytic VACV demonstrated that neutrophils internalize more virus than any other cell subset, accounting for over 80% of viral uptake and subsequently degrade the virus within phagocytic vesicles [96]. Interestingly, depletion of neutrophils using anti-Ly6G antibodies significantly enhanced the efficacy of oncolytic VACV in treating B16F10 tumors [96]. Additionally, neutrophils have been shown to transport VACV antigens to the bone marrow, where they deliver them to antigen-presenting cells, thereby promoting the development of CD8+ T cell responses [97].
Natural killer (NK) cells also play an important role in the immune response to VACV. Although VACV can directly infect NK cells, the functional consequences of this infection remain poorly understood [96]. Nonetheless, studies have shown that NK cells are recruited to sites of VACV infection, where they become activated, proliferate, and lyse infected cells [98,99,100]. Notably, VACV infection does not reduce MHC I expression or the expression of ligands for the NK Group 2 Member D (NKG2D) receptor on infected cells. Instead, it induces the expression of ligands for other activating NK cell receptors, including NKp46, NKp44, and NKp30 [100]. In a mouse model of pulmonary VACV infection, NK cells were found to produce high levels of IFN-γ prior to the infiltration of CD8+ T cells, helping to limit viral replication during the early stages of infection [98]. Furthermore, NK cells were observed to form a memory-like Thy+ subset capable of protecting naïve immunodeficient mice from lethal VACV challenge [101].
5.4. B and T Lymphocytes
VACV also interacts with components of the adaptive immune system. B cells, which produce virus-neutralizing antibodies, can also function as APCs to stimulate T cell responses. Although VACV can bind to multiple B cell subpopulations, it primarily infects and replicates in memory B cells [102]. However, successful viral replication requires B cell activation; in the absence of stimulation, infection is abortive and late gene expression is not induced [102].
A murine model of respiratory VACV infection revealed that virus-specific antibodies begin to appear around day 15 post-infection, peak at day 148, and play only a minor role in controlling primary infection [103]. In contrast, serum from mice with a fully developed B cell response was highly effective in mitigating the effects of subsequent VACV exposure [103]. Although the B cell response to VACV develops slowly, it results in durable immune memory. In humans, memory B cells and protective antibodies specific to the VACV-based smallpox vaccine have been detected up to 50 and 59 years post-vaccination, respectively [104]. Similarly, in mice, VACV induces a robust B cell memory response, though its protective role appears secondary to that of memory T cells [105].
T cells, while only weakly susceptible to VACV infection [106], play a central role in viral clearance. Experimental data show that CD8+ T lymphocytes are both necessary and sufficient to protect mice against VACV respiratory infection [103]. Depletion of CD8+ cells renders immunocompetent mice susceptible to VACV-induced mortality, whereas adoptive transfer of naïve CD8+ T cells rescues immunodeficient RAG-/- mice from lethal infection [103]. Although CD4+ T cells are essential for the development of a functional antibody response, their depletion does not impair survival or virus clearance in the respiratory model [103]. Interestingly, in an intraperitoneal model of VACV infection, CD4+ T cells played a more substantial role by supporting proper CD8+ T cell priming [107].
Additional evidence points to a role for γδ T cells in CD8+ T cell activation. These cells can present VACV antigens via MHC I and secrete cytokines such as IL-1 and IFN-α, thereby contributing to antiviral defense [108].
Like B cells, T cells also generate robust and long-lasting memory. In humans, VACV-specific CD8+ memory T cells have been detected up to 50 years post-vaccination, maintaining cytotoxic activity against infected cells [109]. Remarkably, CD4+ memory T cells isolated form these individuals also exhibited cytotoxic, rather than regulatory, properties [109].
6. VACV as a Tool for Oncolytic Cancer Therapy
6.1. Advantages of VACV as an Anti-Cancer Agent
VACV possesses several characteristics that make it an exceptionally valuable tool for oncolytic cancer therapy. First, it can infect a wide range of cell types. While many viruses, such as adenoviruses, are restricted to infecting cells that express a specific receptor, VACV can infect tumors originating from various tissues [1,19,110,111]. This gives it advantage over, for instance, Herpes Simplex Virus 1 (HSV-1), which is also used as oncolytic vector but displays preferential neurotropism and, in the case of non-neural cancers, can only be administered intratumorally [112]. Moreover, VACV binds to MARCO, a protein commonly found on tumor-associated macrophages and myeloid-derived suppressor cells (MDSCs). This property makes it a promising candidate for modifying the tumor microenvironment, for example, by delivering genes that reverse the immunosuppressive phenotype of myeloid cells.
The ability of VACV to attach to a variety of cell types is particularly advantageous because tumors, especially at advanced stages, are highly heterogenous, with multiple subpopulations of cells expressing different receptors and surface markers. As a result, oncolytic agents that rely on a single receptor type have limited capacity to infect diverse tumor cells and produce significant therapeutic effects. In contrast, VACV can enter multiple types of tumor cells and destroy them without depending on a specific receptor. Therefore, as long as VACV replication remains confined to cancer cells and does not significantly affect healthy tissues, its broad, receptor-dependent tropism is not a limitation but rather a benefit.
Second, the large genome of VACV allows for the insertion of sizable therapeutic transgene constructs of at least 25 kb [113,114]. This gives VACV an advantage over other types of vectors, such as adenoviruses, which, in order to remain replication-competent, have a more limited capacity, typical of constructs smaller than 10 kb [115]. Additionally, because VACV replicates entirely in the cytosol, there is no risk of transgene integration into the host’s genome, thereby minimizing the chance of transformation if normal cells are infected.
Third, VACV has a rapid replication cycle. It produces new virions within 6–8 h and causes lysis of infected cells within 48 h, enabling it to quickly infect and destroy tumor cells before the immune system can neutralize the virus. Notably, VACV replicates at comparable rates under both normoxic and hypoxic conditions, making it particularly effective against tumors in low oxygen environments such as pancreatic cancer [116].
Additionally, VACV suppresses proteins involved in apoptosis, allowing it to replicate efficiently in cancer cells with defective apoptosis pathways. While resistance to apoptosis undermines many conventional therapies, it actually enhances VACV replication by prolonging host cell survival. Instead of apoptosis, VACV induces necroptosis and cell lysis, resulting in the release of damage-associated molecular pattern (DAMP) molecules such as high-mobility group box 1 protein (HMGB1), adenosine triphosphate (ATP), calreticulin (CRT), and heat shock protein 90 (HSP90) [117]. The release of these DAMPs characterizes immunogenic cell death (ICD), which promotes leukocyte recruitment, dendritic cell maturation, and priming CD8+ T lymphocytes against tumor antigens [118].
Consequently, VACV is especially promising for treating “cold” tumors—those with poor immune cell infiltration—by converting them into “hot” tumors actively infiltrated by NK and T cells. Finally, VACV induces a predominantly CD8+ cytotoxic T cell response, which is particularly beneficial in cancer therapy, as CD4+ T cells and B cells can sometimes exert immunosuppressive effects.
6.2. Chimeric VACV: CF33
To further enhance the oncolytic properties of VACV, researchers have employed several strategies that are summarized in Figure 5. One such approach is chimerization, which involves combining genetic elements from different, closely related poxviruses to generate chimeric viruses with optimal oncolytic potential. In a study conducted at City of Hope, researchers co-infected cells with cowpox, raccoonpox, rabbitpox, and six different strains of VACV to generate a diverse pool of chimeric orthopoxviruses. From this pool, 100 chimeric viruses were isolated and screened using high-throughput methods for their efficacy against the NCI-60 panel of human cancer cell lines [119,120]. However, it should be noted that 2D cell cultures do not capture the complexity of real tumors, including tumor microenvironment interactions, the effects of the host immune system, and the heterogeneity of tumor architecture. As a result, findings from 2D cultures may overestimate therapeutic efficacy while underestimating potential toxicity or immune interactions.
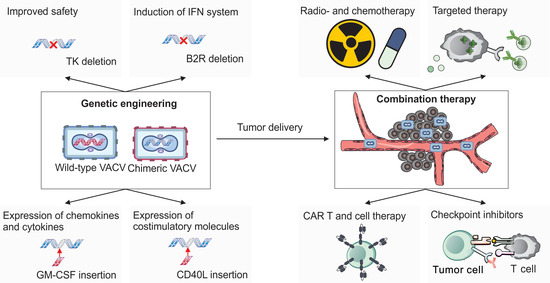
Figure 5.
Vaccinia virus in cancer therapy. Wild-type or chimeric strains of VACV can be genetically engineered in multiple ways to improve both safety and therapeutic efficacy for cancer treatment. Deletion of the thymidine kinase (TK) gene restricts viral replication to rapidly dividing tumor cells, improving tumor selectivity. Removal of the B2R gene enhances host interferon responses, further supporting anti-tumor immunity. Therapeutic transgenes can be inserted into the VACV genome to modulate the tumor microenvironment. For example, expression of cytokines and chemokines such as GM-CSF (granulocyte–macrophage colony-stimulating factor) or CCL5 (RANTES) promotes the recruitment and activation of immune cells within tumors. Additionally, the expression of co-stimulatory molecules such as CD40L or OX40L enhances T cell activation and survival. As an oncolytic agent, VACV can be employed either as a monotherapy or in combination with other treatments to improve therapeutic outcomes. Combination strategies include conventional approaches such as radio- and chemotherapy, as well as emerging modalities such as CAR-T cells and other adoptive cell therapies, immune checkpoint inhibitors, and targeted therapies involving antibody–drug conjugates and small-molecule inhibitors.
Among these candidates, the chimeric virus CF33 demonstrated superior oncolytic activity compared to its parental strains, including robust cytotoxicity against six different human pancreatic cancer cell lines. It also exhibited a strong ability to induce immunogenic cell death and significantly inhibited the growth of pancreatic and triple-negative breast cancer xenografts in mouse models. Interestingly, although CF33 was generated through gain-of-function chimera screening and lacks deletions of viral virulent genes such as TK, it nonetheless showed impressive safety profiles in animal models [119,120]. Sequence analysis of the CF33 genome revealed that it is primarily derived from three VACV strains—IHD, Lister, and WR—which together account for approximately 60% of its genome. No genetic material from raccoonpox or cowpox viruses was detected, indicating that CF33 is in fact a chimeric VACV, rather than a broader orthopoxvirus hybrid [121].
Building on CF33, several derivative viruses have been developed. One such derivative, CF33-hNIS (also known as VAXINIA), incorporates the human sodium iodide symporter (hNIS) gene, enabling non-invasive imaging of viral distribution via using PET scans. This modification allows researchers to track the virus’s localization and replication within the body [122].
Another derivative, CF33-hNIS-antiPDL1, expresses both hNIS and a single-chain variable fragment targeting PD-L1, an immune checkpoint protein. Preclinical studies in models of triple-negative breast cancer and gastric cancer peritoneal metastases have shown that this dual-function virus not only enhances direct tumor cell killing but also reprograms the tumor microenvironment to improve immune recognition and activation [121,123].
A third variant, CF33-CD19, is engineered to express a truncated CD19 on the surface of infected cancer cells. This strategy enables targeting of solid tumors with CD19-specific CAR-T cells, which typically show limited efficacy against solid tumors due to the lack of appropriate surface antigens [124].
All three CF33-based viruses are currently under clinical evaluation:
CF33-hNIS is being tested as a monotherapy or in combination with pembrolizumab in adults with metastatic or advanced solid tumors (NCT05346484),
CF33-hNIS-antiPDL1 is in clinical trials for the treatment of metastatic triple-negative breast cancer (NCT05081492),
CF33-CD19 is being evaluated as a monotherapy or in combination with blinatumomab for metastatic solid tumors (NCT06063317).
6.3. Genetic Engineering of VACV
Creating novel oncolytic variants of VACV typically involves precise insertions and deletions within the viral genome. However, due to its large genome size (~195 kb), VACV is not amenable to standard molecular cloning techniques commonly used for smaller viral vectors, such as lentiviral systems. Consequently, genetic engineering approaches are required.
The most traditional and widely used method is based on homologous recombination. In this approach, a plasmid carrying the desired genetic construct is introduced into VACV-infected cells. Recombination occurs between homologous sequences on the plasmid and the viral genome, enabling the exchange of genetic material [125]. A typical construct includes a desired transgene (e.g., a cytokine) accompanied by a selection marker (e.g., GFP or β-galactosidase), each driven by separate promoters. The expression cassette is flanked by sequences homologous to regions of the viral genome—commonly the termini of the TK gene—to allow for targeted insertion. Recombinant viruses are isolated using marker-based selection or screening, followed by multiple rounds of plaque purification.
While conceptually straightforward and technically accessible, this method is labor-intensive and inefficient: recombination events occur at low frequency, yielding approximately one recombinant per 1000 wild-type virions [9].
To enhance efficiency, several alternative strategies have been developed, including the use of bacterial artificial chromosomes (BACs) [126] and genome-editing techniques based on CRISPR-Cas9 [127]. A method, known as MAVERICC (marker-free vaccinia virus engineering of recombinants through in vitro CRISPR/Cas9 cleavage) integrates the strengths of previous approaches [9].
In the MAVERICC method, the VACV genome is first cleaved in vitro at specific sites using Cas9 guided by sequence-specific RNAs. This cleaved genome is then co-transfected with an amplicon containing the desired transgene flanked by sequences homologous to the targeted genomic region. Recombination occurs within the host cell, which is also infected with a replication-defective helper poxvirus. The helper virus supplies the necessary proteins to facilitate homologous recombination but cannot replicate itself. As a result, only recombinant VACV that have repaired the Cas9-induced cleavage through homologous recombination with the amplicon can be propagated. This method enables the generation of engineered viruses with >90% efficiency, without the need for selection markers, serial passaging, or extensive screening [9].
Alternative approaches involve the use of an antibiotic resistance gene inserted into the construct that recombines with the viral genome. In this strategy, after recombination, viruses are seeded onto cells treated with an antibiotic that blocks protein synthesis. As a result, only viruses that have successfully recombined and contain the antibiotic resistance gene can carry out protein synthesis and produce a new generation of virions.
The latest iteration of this method combines antibiotic selection with the use of transposons and long-read nanopore sequencing. In this approach, transposons containing both the antibiotic resistance gene and the desired construct are transfected into cells infected with VACV. After selection, viral genomes are analyzed using long-read nanopore sequencing, which enables precise characterization of the introduced mutations. With this strategy, the desired modification of VACV can be achieved in as quickly as 5 days [128].
6.4. Commonly Targeted Viral Genes for Modification in VACV-Based Oncolytic Viruses
Over the years, numerous strategies have been developed to enhance the suitability of VACV for cancer therapy [1]. Cancer cells possess several characteristics that facilitate enhanced replication of VACV, ultimately leading to cell lysis. These include deficient apoptotic pathways [129], reduced expression of tumor suppressor genes (such as p53), and impaired antiviral and interferon signaling mechanisms [130]. In addition, due to their high proliferative rates and intense DNA synthesis demands, cancer cells typically exhibit elevated levels of cytosolic TK and RR [131,132].
To exploit this metabolic environment, researchers have engineered VACV strains with deletions in the viral genes encoding TK and/or RR. These modified viruses rely on host cell-derived enzymes to synthesize DNA precursors, thereby restricting viral replication to cancer cells where these enzymes are abundant [133,134]. Interestingly, endothelial cells in tumor-associated vasculature also exhibit elevated TK expression, largely due to stimulation by vascular endothelial growth factor (VEGF). This makes VACV a potential anti-angiogenic agent. For example, the oncolytic strain JX-594—engineered to express human granulocyte–monocyte colony-stimulating factor (hGM-CSF) and β-galactosidase (β-gal)—has been shown to selectively replicate within tumor-associated blood vessels, causing their collapse while sparing normal vasculature [135].
Another modification involves deletion of the C11R, which encodes the viral growth factor (VGF). VGF stimulates the EGFR-Ras signaling pathway to promote cell proliferation. In the absence of VGF, VACV replication is impaired unless the host cell has an active EGFR pathway—a common feature of many cancer cells [136].
Additional deletions used to enhance oncolytic specificity and safety include A56R and F14.5L. The A56R gene encodes a hemagglutinin-like protein (A56) that prevents infected cells from forming syncytia, protects against superinfection, and inhibits complement-mediated lysis [137]. Despite these roles, A56 is not essential for viral replication and is often replaced with therapeutic transgenes. The F14.5L gene is involved in regulating cell adhesion and contributes to viral virulence in vivo, though it is not required for replication [138]. Its deletion is commonly employed as a safety measure, especially important for treating potentially immunocompromised cancer patients [139]. Other deletions introduced to increase safety and tumor specificity include genes such as SPI-1 (B22) and -2 (B13), B18R, N1L, A41L, A49L, and F1L. A list of these deletions and their associated viruses is provided in Table 1.

Table 1.
Examples of viral genes deleted from vaccinia virus to enhance tumor selectivity and promote immune system activation.
Table 1.
Examples of viral genes deleted from vaccinia virus to enhance tumor selectivity and promote immune system activation.
| Deleted Viral Genes | Functions of Deleted Viral Genes | Example of Virus | References |
|---|---|---|---|
| TK (Thymidine kinase) | Provides material for viral DNA replication | vCB2 (vvLuc) | [134] |
| TK and VGF (Viral growth factor) | Supports viral replication, accelerates cell growth and metabolism | vvDD-GFP | [140] |
| SPI-1 (B22) and -2 (B13) | Inhibit apoptosis | vSP | [141] |
| TK, SPI-1 and -2 | Supports viral replication, inhibit apoptosis | vSPT | [142] |
| Soluble type I IFN receptor (B18R) | Inhibits mobilization of the immune cells | WR-delB18 | [143] |
| Soluble type I IFN receptor and TK | Inhibits mobilization of the immune cell, supports viral replication | ΔB18RΔTK | [143] |
| F14.5L, TK, and HA | Promotes virulence in vivo, supports viral replication, inhibits complement-mediated lysis of infected cell | GLV-1h68 | [144] |
| TK, RR (ribonucleotide reductase) | Support viral replication by providing material for DNA synthesis | TG6002 | [145] |
| TK, N1L, and A41L | Supports viral replication, inhibits apoptosis, interferes with chemokine signaling | VVLΔTKΔN1LΔA41L | [146] |
| A49L | Inhibits NF-κB activation | vΔA49L | [147] |
| TK, F1L | Supports viral replication, inhibits apoptosis | ΔTK/F1L | [148] |
| TK, B2R | Supports viral replication, inhibits the cGAS/STING pathway | WR/TK−/ΔB2 | [149] |
6.5. Expression of Chemokines and Cytokines
Many VACV-based oncolytic viruses are engineered to express immunostimulatory genes, primarily chemokines and cytokines, to enhance anti-tumor immunity. These modifications generally pursue two main objectives: (1) to recruit antigen-presenting cells, particularly DCs, promote their maturation, and improve their capacity to present antigens to T cells; (2) to modulate existing T cell responses by promoting T cell survival, sustaining activation, and preventing exhaustion.
One of the most commonly used transgenes is GM-CSF, which is known to promote tumor infiltration by NK and DCs and accelerates DC maturation [150]. GM-CSF has been used as an immunostimulant in a variety of oncolytic viruses, including adenovirus [151], Sindbis [152], and the only FDA-approved oncolytic virus in the U.S., the HSV-based T-VEC [153]. Multiple VACV vectors engineered to express GM-CSF have shown promising results in preclinical studies [135,154,155].
An alternative approach strategy involves incorporating genes encoding chemokines such as CXCL11 and CCL5 (RANTES). A VACV strain expressing CCL5 induced significant infiltration of DCs, CD4+ T cells, and NK cells, effectively suppressing tumor growth in the MC38 murine model [156]. Similarly, another VACV expressing CXCL11 was highly effective in the same model, attracting large numbers of CD8+ lymphocytes and inducing strong IFN-γ expression [157].
To further enhance T cell-mediated responses, pro-inflammatory cytokine genes such as IL-12 [158], IL-15 [159], and IL-23 [160] have been inserted into the VACV genome. These modifications led to improved anti-tumor effects with increased CD8+ T cell infiltration, consistent with the roles of these cytokines in supporting cytotoxic T lymphocyte (CTL) proliferation, survival, and effector function.
Interestingly, the anti-inflammatory cytokine IL-10 also enhanced anti-tumor effects when expressed by oncolytic VACV in a mouse model of pancreatic cancer [161]. This finding is counterintuitive, given that IL-10 is generally associated with the suppression of immune responses, including inhibition of IL-12 production. The study revealed that IL-10 expressing VACV persisted longer within tumors, led to fewer VACV-specific CD8+ T cells, and resulted in reduced infiltration by macrophages [161]. This suggests that IL-10 may prolong viral replication within tumor tissue, thereby enhancing direct oncolysis and potentially facilitating a more robust anti-tumor immune response. Additionally, IL10 is known to impair macrophage antigen presentation to CD4+ cells [162], potentially skewing the immune response toward a CD8+ T cell-driven cytotoxic pathway.
6.6. Induction of the IFN System
Robust activation of the type I IFN response is critical for the development of effective cytotoxic immunity, which is a key objective in cancer immunotherapy [163]. However, VACV encodes multiple genes that inhibit the host IFN response, posing a challenge for its use as an oncolytic agent. To address this, researchers have developed strategies to prevent VACV from suppressing IFN production. One such strategy involves deletion of the B2R gene, which encodes a nuclease that degrades cGAMP—a key activator of the cGAS-STING pathway [149]. Another targeted gene is E5R, a gene that promotes degradation of cGAS itself [50]. VACV strains lacking B2R exhibit elevated IFN expression and enhanced anti-tumor activity in vivo [149]. Notably, B2R deletion also reduces viral virulence, making the modified virus potentially safer for use in immunocompromised patients.
An alternative strategy involves the insertion of the gene-encoding, DNA-dependent activator of IFN-regulatory factors (DAI), which can activate IRF3 through a cGAS-independent pathway [164]. In one study, DAI-expressing VACV demonstrated significantly improved inhibition of melanoma tumor growth in both syngeneic mouse models and humanized mice, even when the B2R gene remained intact [164].
A more novel approach utilized the gene-encoding, white-spotted charr lectin (WCL), a plant-derived protein previously linked to strong anti-tumor effects. VACV engineered to express WCL gene was shown to robustly activate IRF3 and induce high levels of type I IFNs, leading to the effective suppression of hepatocellular carcinoma in a mouse model [165].
6.7. Expression of Co-Stimulatory Molecules
To boost the activity of tumor-primed T cells, various research groups have developed VACVs encoding co-stimulatory molecules such as CD40L, 4-1BBL, and OX40L. Signals derived from these molecules are known to enhance T cell activity, reduce apoptosis, and increase the production of pro-inflammatory cytokines. When delivered intratumorally, these engineered viruses successfully inhibited the growth of B16 melanoma tumors in immunocompetent mice and extended the survival of the treated animals [166,167,168].
Additionally, a CD40L-expressing VACV effectively slowed the progression of bladder cancer in a mouse xenograft model by activating the NF-κB pathway in T cells and promoting the secretion of TNF-α, IL-1α, and RANTES [167]. In another study, a VACV encoding a tandem of Fms-related tyrosine kinase 3 ligand (Flt3l) and OX40L genes completely eliminated A20 lymphoma tumors and significantly delayed the development of spontaneous tumors in the MMTV-PyMT mouse model of triple-negative breast cancer (TNBC) [168]. The use of the OX40L/FLt3l-expressing virus also led to a marked depletion of regulatory T cells (Tregs), reprogramming them into a more cytotoxic-like phenotype [168].
6.8. Other Strategies
Researchers have also explored several innovative strategies to enhance the anti-tumor properties of VACV. In one notable study, a VACV engineered to express a secretory, bispecific T cell engager composed of single-chain variable antibody fragments specific for CD3 and the tumor antigen EphA. This virus effectively recruited T lymphocytes to EphA-expressing cancer cells and induced complete clearance of A549 tumors in SCID mice infused with human PBMCs [169].
Another approach targeted the transforming growth factor beta (TGF-β) pathway, which is frequently upregulated in immune-resistant tumors. A VACV expressing a soluble TGF-β inhibitor was able to eliminate head and neck squamous cell carcinoma (HNSCC) tumors that were resistant to treatment with a control VACV containing B2R and TK deletions. The engineered virus reduced the number of Tregs and increased their sensitivity to IFN-γ signaling [170].
Finally, to target tumor vasculature, scientists engineered a VACV to express the anti-VEGF single-chain antibody GLAF1. Treatment with this virus dramatically decreased blood vessel density within tumors and inhibited disease progression in xenograft models [171].
Overall, as our understanding of immune stimulation continues to grow, we can expect an increasing number of innovative genetic constructs to be tested as transgenes in the new generation of oncolytic VACVs.
7. Vaccinia Virus in Combination with Other Therapeutic Strategies
7.1. Combination with CAR-T Therapies
Although various forms of oncolytic VACV have demonstrated promising results as monotherapies in cancer treatment, many researchers are now exploring their use in combination with other therapeutic approaches. One notable strategy involves combining VACV with chimeric antigen receptor T cell (CAR-T) therapy.
CAR-T-based therapies are highly effective against CD19-expressing lymphomas; however, their efficacy in treating solid tumors remains limited due to the lack of tumor-specific antigens [172]. To overcome this challenge, researchers engineered VACV to express the CD19 gene. Because VACV preferentially replicates in tumor tissues, it can induce CD19 expression on the surface of cancer cells, rendering them susceptible to CAR-T cell-mediated cytotoxicity. This combination demonstrated promising results in the B16 melanoma model [173], as well as in the MC-38 colorectal cancer model and human tumor xenograft models [174].
In another approach, a CXCL11-expressing VACV was used to attract mesothelin-specific CAR-T cells toward mesothelin-positive TC-1 tumors. While both the virus and CAR-T cells individually inhibited tumor progression following intravenous injections, their combination produced a significantly more potent effect [175].
These findings suggest that VACV can serve as a valuable adjunct to enhance the efficacy of CAR-T therapies, particularly in the treatment of solid tumors.
7.2. Combination with Checkpoint Inhibitors
Since many oncolytic VACVs can activate immune responses in immunologically “cold” tumors, a logical strategy is to combine them with immune checkpoint inhibitors, which can sustain and amplify T cell activity once it has been initiated [176,177]. For example, the combination of IL-21-expressing VACV with an anti-PD-1 antibody produced a significantly stronger therapeutic effect in a mouse glioma model than either treatment alone [178]. Similar synergistic effects were observed in A20 and EL4 lymphoma models when combining a VACV expressing manganese superoxide dismutase (MnSOD) with anti-PD-L1 antibodies [179]. In the MC-38 colon cancer model, the efficacy of a CXCL11-expressing VACV was also substantially enhanced by co-administration of anti-PD-L1 therapy [180].
Because systemic administration of checkpoint inhibitors is often associated with immune-related toxicities, researchers have also explored engineering VACVs to express checkpoint-inhibitory molecules directly within tumors. Leveraging the virus’s tumor-specific replication, this approach allows for localized delivery of checkpoint inhibitors, potentially reducing systemic side effects. A VACV co-expressing a soluble PD-L1 inhibitor and GM-CSF demonstrated high efficacy in treating B16 melanoma tumors [181]. Another engineered virus encoding a cell-depleting anti-CTLA4 antibody and GM-CSF successfully eradicated tumors in multiple models, including breast (EMT6), colon (MC-38), and melanoma (B16) models, following intratumoral administration. Tumor regression in these models was associated with a marked reduction in CD4+ Treg cells and exhausted CD8+ T cells, alongside expansion of activated cytotoxic CD8+ T cells. Combining this therapy with anti-PD-1 antibodies further improved its therapeutic efficacy [182].
Another checkpoint target explored with VACV is TIGIT (T cell immunoreceptor with Ig and ITIM domains), a common marker of exhausted and regulatory T cells. Researchers engineered a VACV to express the variable domains of heavy and light chains of an anti-TIGIT antibody. This virus effectively slowed the progression of several subcutaneously implanted tumor models, including EMT6 (breast), CT26, MC-38 (colon), and H22 (liver) tumors. Once again, combining the treatment with anti-PD-1 therapy further enhanced the anti-tumor effects [183].
Collectively, these findings support the idea that immune checkpoint inhibitors, whether co-administered or encoded directly within the virus, are likely to play a critical role in the future development of VACV-based cancer therapies.
7.3. Combination with Radio- and Chemotherapy
Chemotherapy and radiotherapy remain among the most widely used cancer treatments, and the potential of combining these with VACV has been explored in several studies. Radiotherapy has been shown to enhance the efficacy of oncolytic VACV in preclinical models of glioblastoma [184,185] and pancreatic cancer [186]. In the TC-1 lung cancer model, combining VACV with radiotherapy increased tumor cell necroptosis and stimulated the release of DAMP molecules. This, in turn, activated T cells and reduced the populations of Tregs and M2 macrophages [187]. Owing to its tumor selectivity, VACV is a promising agent for sensitizing tumors to radiation.
One commonly employed strategy involved engineering VACV to express the sodium iodide symporter (NIS) [188,189,190]. Infection with this virus significantly increased the uptake of radioactive Iodine-131 (131I) in prostate cancer xenografts, leading to tumor growth inhibition and prolonged survival in treated mice [191]. In another strategy, researchers developed a radiotherapy system using VACV expressing the somatotropin receptor in combination with a radioisotope-labeled somatotropin analog. In both subcutaneous and disseminated mouse models of colorectal cancer, intraperitoneal administration of the virus resulted in tumor-specific localization and targeted uptake of the radiolabeled compound. This specific accumulation of radioisotopes within the tumor tissue significantly inhibited tumor growth, improved survival, and did not cause systemic toxicity [192].
VACV has also been studied in combination with conventional chemotherapy. Paclitaxel, for example, significantly enhanced the anti-tumor efficacy of VACV in HCT116 tumors grown in athymic mice. This effect was associated with type I IFN production and the release of HMGB1, a key DAMP molecule [193]. Furthermore, both cisplatin and gemcitabine improved the efficacy of oncolytic VACV in pancreatic tumor xenografts in nude mice [194]. Similarly, treatment with cyclophosphamide (CPA) or rapamycin increased VACV’s ability to inhibit the growth of malignant gliomas in rat models [195]. CPA also enhanced the efficacy of intravenously administered VACV in lung cancer xenografts by increasing viral spread within the tumor and reducing tumor vasculature [196].
Another commonly employed strategy involves arming VACV with genes that convert inactive prodrugs into active chemotherapeutic agents. Given VACV’s selectivity for tumor tissue, this approach allows high local concentrations of the active drug while minimizing systemic toxicity. For example, a VACV engineered to express super cytosine deaminase (SCD) effectively converted the prodrug 5-fluorocytosine (5-FC) into the chemotherapeutic compound 5-fluorouracil (5-FU). In the presence of prodrug, the virus induced death in cancer cell lines that were otherwise resistant to VACV-mediated lysis [197]. Another engineered VACV expressed β-galactosidase to activate a prodrug containing a β-galactosidase-specific cleavage site. In a breast cancer xenograft model, this virus–prodrug system led to accelerated tumor shrinkage and induced apoptosis in multiple cancer cell lines when the prodrug was present [198].
7.4. Combination with Small-Molecule Inhibitors
The oncolytic potential of VACV has also been evaluated in combination with various small-molecule inhibitors used in cancer therapy. Trametinib, a clinically approved inhibitor of mitogen-activated protein kinase MEK, was found to enhance VACV replication in vitro and improve its ability to inhibit the growth of ovarian cancer xenografts [199]. Idelalisib, an FDA-approved selective inhibitor of phosphoinositide 3-kinase delta (PI3Kδ), significantly increased viral delivery to tumors following intravenous injection and substantially enhanced the therapeutic effect [200]. Other notable inhibitors that have been shown to improve the anti-cancer activity of oncolytic VACVs include trichostatin A, a histone deacetylase inhibitor, and sunitinib, a multitargeted receptor tyrosine kinase inhibitor. Trichostatin A markedly increased viral replication and spread within tumor tissues [201], while sunitinib robustly enhanced CD8+ T cell infiltration, suppressed Tregs, and increased tumor cell apoptosis by more than threefold [202]. Inhibitors targeting the VEGF pathway have also been found to potentiate the oncolytic effects of VACV [171,203]. As the number of novel inhibitors targeting tumor-specific pathways continues to grow, combining them with VACV holds great promise for achieving even more effective cancer therapies.
8. Clinical Trials and Potential Obstacles
The specificity of VACV for cancer cells and the promising results from preclinical models have spurred the development of multiple VACV-based oncolytic viruses, many of which have progressed to clinical trials. A key consideration in patient treatment is the choice of viral delivery route. Systemic administration, such as intravenous or intraperitoneal injection, is the most convenient; however, it requires the virus to have high tumor-targeting specificity. Moreover, repeated systemic dosing can be challenging due to the development of neutralizing antibodies, which reduce the efficacy of subsequent treatments.
In contrast, intratumoral injection allows for direct delivery of the virus into the tumor, enabling the use of lower doses and minimizing the impact of circulating neutralizing antibodies. However, this method has its limitations—it is not feasible when tumors are inaccessible or numerous, and it often requires complex medical procedures. These procedures can be burdensome for patients and may affect their daily lives.
Current clinical trials involving VACV utilize both systemic and intratumoral delivery approaches. Table 2 summarizes clinical studies published between 2009 and 2025, while a comprehensive list of earlier clinical trials has been published elsewhere [204]. An up-to-date list of ongoing clinical trials involving VACV can be found at https://clinicaltrials.gov/(accessed on 3 July 2025). We also provide a summary of these ongoing trials in Table 3.

Table 2.
Clinical studies of vaccinia virus as an oncolytic agent published between 2009 and 2025.

Table 3.
List of currently (as of July 2025) running clinical trials involving different modifications of vaccinia virus according to https://clinicaltrials.gov/.
As of April 2025, only two trials involving oncolytic VACV have reached phase III. The first is a study evaluating intraperitoneal delivery of Genelux’s Olvi-Vec (GL-ONC1) in combination with a platinum-based chemotherapy regimen for the treatment of platinum-resistant refractory ovarian cancer (NCT05281471). Olvi-Vec was engineered by inserting three expression cassettes—encoding a Renilla luciferase–Aequorea green fluorescent protein fusion, β-galactosidase, and β-glucuronidase—into the F14.5L, J2R (encoding TK), and A56R (encoding hemagglutinin) loci of the parental VACV genome. In addition to this phase III trial, Olvi-Vec is currently in phase I trials for the treatment of small-cell and non-small lung cancer. The results of the phase III trial are expected to be announced in the first half of 2026.
The second oncolytic VACV to reach a phase III trial (NCT02562755) is Pexa-Vac (JX-594), developed by SillaJen. JX-594 is engineered with a deletion of the TK gene and insertion of transgenes encoding human GM-CSF and β-galactosidase. It is administered via intratumoral injection. Pexa-Vac has been evaluated in clinical trials for renal, colorectal, and liver cancers, and advanced to a phase III trial for hepatocellular carcinoma in combination with the targeted therapy sorafenib. Unfortunately, this trial failed to demonstrate a survival benefit compared to the control group [1].
Nevertheless, additional trials exploring JX-594 in combination with other therapeutic agents and across different cancer types are still ongoing.
The fact that no VACV-based oncolytic therapies have yet received FDA approval does not mean that they hold less potential than similar approaches based on HSV or adenoviral vectors. First, because of its ability to replicate selectively in cancer cells, VACV is often administered intravenously—a more challenging delivery route compared to the intratumoral injections used for other viruses. Second, it has been tested against highly aggressive and immune-resistant tumors such as liver cancer, pancreatic cancer, and lung cancer, which are generally less responsive to immunotherapy than, for example, melanoma. Finally, the viruses used in earlier unsuccessful trials were first-generation vectors that did not fully exploit VACV’s potential to induce a robust anti-cancer response. As newer generations of oncolytic VACV continue to be developed—with novel inserts and highly effective transgene combinations—more candidates are expected to enter clinical trials, potentially yielding more promising results.
Despite its many advantages, VACV also presents certain challenges that may limit its therapeutic potential. As a replicating virus, it poses a theoretical risk to immunocompromised patients, particularly when administered systemically (e.g., via intravenous injection). However, this risk is significantly mitigated by the presence of multiple attenuating mutations engineered into oncolytic VACV strains, many of which are based on the Lister strain, which has a long history of safe use as a smallpox vaccine.
Another major challenge is the strong immune memory elicited by VACV at both the B and T cell levels. This response can limit the efficacy of repeated administrations, especially through intravenous delivery. High titers of neutralizing antibodies may block the virus from binding to target cells, while cytotoxic memory T cells can destroy infected cancer cells before sufficient viral replication and spread occurs. Because VACV was used as the smallpox vaccine, which was administered globally until 1981, a significant proportion of patients over the age of 50 are likely to retain some level of anti-VACV immunity. In theory, this pre-existing immunity could reduce the efficacy of VACV-based oncolytic therapies in older patients compared to younger individuals who have never been exposed to the smallpox vaccine. For patients with particularly high levels of anti-VACV antibodies, intratumoral injection may be the preferred delivery route. Alternatively, pre-treatment with pharmaceutical agents prior to intravenous injection can help minimize the effects of pre-existing immunity.
Strategies to overcome anti-VACV immune memory have already been developed. For example, treatment with the cyclooxygenase-2 (COX-2) inhibitor celecoxib prior to administering a second viral dose significantly reduced the generation of neutralization antibodies and restored viral titers in pre-immunized models [226]. Similarly, inhibiting the complement system with the synthetic compound CP40 or depleting it with cobra venom factor (CVF) markedly increased viral titers in both blood and tumor tissue of pre-immunized animals [227].
To further protect VACV from complement-mediated neutralization, researchers have engineered a modified virus that expresses the N-terminal fragment of the complement regulatory protein CD55 fused to six membrane proteins found on IMVs. This engineered virus maintained its replication efficiency and infectivity while successfully evading neutralization by VACV-specific antibodies generated after multiple systemic administrations [228].
In conclusion, although pre-existing immunity to VACV poses a significant challenge for repeated dosing, recent advances offer practical strategies to overcome these challenges and enhance the clinical efficacy of oncolytic VACV.
9. Conclusions
The limited efficacy of current treatments for disseminated cancer underscores the urgent need for more innovative and potent treatment strategies. VACV has emerged as a highly promising platform for viro-immunotherapy due to its safety, genetic flexibility, and ability to deliver multiple therapeutic genes directly to tumor tissue. It has shown significant potential both as monotherapy and in combination with other treatment modalities.
Although the phase III clinical trial of JX-594 did not yield the desired outcome, it is important to note that this virus represents an earlier generation construct, far less advanced than the more sophisticated candidates currently under development. As our understanding of tumor biology, immunology, and viral engineering continues to evolve, we are poised to design increasingly sophisticated VACV derivatives with improved efficacy and tumor specificity. Moreover, the strategic integration of VACV into combination therapies is expected to become more refined and effective.
With ongoing research and technological advancements, the future of cancer treatment involving VACV appears highly promising and is likely to play a pivotal role in the next generation of oncologic therapies.
Author Contributions
Conceptualization, M.S. and N.G.C.; writing—original draft preparation, M.S.; writing—review and editing, M.S. and N.G.C.; visualization, Y.Y.; supervision, N.G.C.; funding acquisition, N.G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
All authors are employees of ViroMissile, Inc.
Conflicts of Interest
M.S., Y.Y., and N.G.C. are all employees of ViroMissile, Inc.
References
- Xu, L.; Sun, H.; Lemoine, N.R.; Xuan, Y.; Wang, P. Oncolytic Vaccinia Virus and Cancer Immunotherapy. Front. Immunol. 2024, 14, 1324744. [Google Scholar] [CrossRef] [PubMed]
- Kaynarcalidan, O.; Moreno Mascaraque, S.; Drexler, I. Vaccinia Virus: From Crude Smallpox Vaccines to Elaborate Viral Vector Vaccine Design. Biomedicines 2021, 9, 1780. [Google Scholar] [CrossRef] [PubMed]
- Greseth, M.D.; Czarnecki, M.W.; Bluma, M.S.; Traktman, P. Isolation and Characterization of vΔI3 Confirm That Vaccinia Virus SSB Plays an Essential Role in Viral Replication. J. Virol. 2018, 92, e01719-17. [Google Scholar] [CrossRef] [PubMed]
- Wittek, R. Vaccinia Virus (Poxviridae). In Encyclopedia of Virology, 2nd ed.; Granoff, A., Webster, R.G., Eds.; Elsevier: Oxford, UK, 1999; pp. 1865–1872. ISBN 978-0-12-227030-7. [Google Scholar]
- Molteni, C.; Forni, D.; Cagliani, R.; Clerici, M.; Sironi, M. Genetic Ancestry and Population Structure of Vaccinia Virus. NPJ Vaccines 2022, 7, 92. [Google Scholar] [CrossRef]
- Moss, B. Poxvirus DNA Replication. Cold Spring Harb. Perspect. Biol. 2013, 5, a010199. [Google Scholar] [CrossRef]
- Yang, Z.; Maruri-Avidal, L.; Sisler, J.; Stuart, C.A.; Moss, B. Cascade Regulation of Vaccinia Virus Gene Expression Is Modulated by Multistage Promoters. Virology 2013, 447, 213–220. [Google Scholar] [CrossRef]
- Chou, W.; Ngo, T.; Gershon, P.D. An Overview of the Vaccinia Virus Infectome: A Survey of the Proteins of the Poxvirus-Infected Cell. J. Virol. 2012, 86, 1487–1499. [Google Scholar] [CrossRef]
- Laudermilch, E.; Chandran, K. MAVERICC: Marker-Free Vaccinia Virus Engineering of Recombinants through in Vitro CRISPR/Cas9 Cleavage. J. Mol. Biol. 2021, 433, 166896. [Google Scholar] [CrossRef]
- Jacobs, B.L.; Langland, J.O.; Kibler, K.V.; Denzler, K.L.; White, S.D.; Holechek, S.A.; Wong, S.; Huynh, T.; Baskin, C.R. Vaccinia Virus Vaccines: Past, Present and Future. Antivir. Res. 2009, 84, 1–13. [Google Scholar] [CrossRef]
- de Freitas, L.F.D.; Oliveira, R.P.; Miranda, M.C.G.; Rocha, R.P.; Barbosa-Stancioli, E.F.; Faria, A.M.C.; da Fonseca, F.G. The Virulence of Different Vaccinia Virus Strains Is Directly Proportional to Their Ability To Downmodulate Specific Cell-Mediated Immune Compartments In Vivo. J. Virol. 2019, 93, e02191-18. [Google Scholar] [CrossRef]
- Belongia, E.A.; Naleway, A.L. Smallpox Vaccine: The Good, the Bad, and the Ugly. Clin. Med. Res. 2003, 1, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Meiser, A.; Boulanger, D.; Sutter, G.; Krijnse Locker, J. Comparison of Virus Production in Chicken Embryo Fibroblasts Infected with the WR, IHD-J and MVA Strains of Vaccinia Virus: IHD-J Is Most Efficient in Trans-Golgi Network Wrapping and Extracellular Enveloped Virus Release. J. Gen. Virol. 2003, 84, 1383–1392. [Google Scholar] [CrossRef]
- Bengali, Z.; Townsley, A.C.; Moss, B. Vaccinia Virus Strain Differences in Cell Attachment and Entry. Virology 2009, 389, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Carter, G.C.; Law, M.; Hollinshead, M.; Smith, G.L. Entry of the Vaccinia Virus Intracellular Mature Virion and Its Interactions with Glycosaminoglycans. J. Gen. Virol. 2005, 86, 1279–1290. [Google Scholar] [CrossRef]
- MacLeod, D.T.; Nakatsuji, T.; Wang, Z.; di Nardo, A.; Gallo, R.L. Vaccinia Virus Binds to the Scavenger Receptor MARCO on the Surface of Keratinocytes. J. Investig. Dermatol. 2015, 135, 142–150. [Google Scholar] [CrossRef]
- Laliberte, J.P.; Weisberg, A.S.; Moss, B. The Membrane Fusion Step of Vaccinia Virus Entry Is Cooperatively Mediated by Multiple Viral Proteins and Host Cell Components. PLoS Pathog. 2011, 7, e1002446. [Google Scholar] [CrossRef] [PubMed]
- Townsley, A.C.; Weisberg, A.S.; Wagenaar, T.R.; Moss, B. Vaccinia Virus Entry into Cells via a Low-pH-Dependent Endosomal Pathway. J. Virol. 2006, 80, 8899–8908. [Google Scholar] [CrossRef]
- Mercer, J.; Knébel, S.; Schmidt, F.I.; Crouse, J.; Burkard, C.; Helenius, A. Vaccinia Virus Strains Use Distinct Forms of Macropinocytosis for Host-Cell Entry. Proc. Natl. Acad. Sci. USA 2010, 107, 9346–9351. [Google Scholar] [CrossRef]
- Greseth, M.D.; Traktman, P. The Life Cycle of the Vaccinia Virus Genome. Annu. Rev. Virol. 2022, 9, 239–259. [Google Scholar] [CrossRef]
- Schmidt, F.I.; Bleck, C.K.E.; Reh, L.; Novy, K.; Wollscheid, B.; Helenius, A.; Stahlberg, H.; Mercer, J. Vaccinia Virus Entry Is Followed by Core Activation and Proteasome-Mediated Release of the Immunomodulatory Effector VH1 from Lateral Bodies. Cell Rep. 2013, 4, 464–476. [Google Scholar] [CrossRef]
- Tolonen, N.; Doglio, L.; Schleich, S.; Locker, J.K. Vaccinia Virus DNA Replication Occurs in Endoplasmic Reticulum-Enclosed Cytoplasmic Mini-Nuclei. MBoC 2001, 12, 2031–2046. [Google Scholar] [CrossRef] [PubMed]
- Moss, B. Cascade Regulation of Vaccinia Virus Gene Expression. In Regulation of Gene Expression in Animal Viruses; Carrasco, L., Sonenberg, N., Wimmer, E., Eds.; Springer: Boston, MA, USA, 1993; pp. 13–24. ISBN 978-1-4615-2928-6. [Google Scholar]
- Howell, L.M.; Gracie, N.P.; Newsome, T.P. Single-Cell Analysis of VACV Infection Reveals Pathogen-Driven Timing of Early and Late Phases and Host-Limited Dynamics of Virus Production. PLoS Pathog. 2024, 20, e1012423. [Google Scholar] [CrossRef]
- Liu, L.; Cooper, T.; Howley, P.M.; Hayball, J.D. From Crescent to Mature Virion: Vaccinia Virus Assembly and Maturation. Viruses 2014, 6, 3787–3808. [Google Scholar] [CrossRef] [PubMed]
- Boyle, K.A.; Stanitsa, E.S.; Greseth, M.D.; Lindgren, J.K.; Traktman, P. Evaluation of the Role of the Vaccinia Virus Uracil DNA Glycosylase and A20 Proteins as Intrinsic Components of the DNA Polymerase Holoenzyme. J. Biol. Chem. 2011, 286, 24702–24713. [Google Scholar] [CrossRef]
- Rochester, S.C.; Traktman, P. Characterization of the Single-Stranded DNA Binding Protein Encoded by the Vaccinia Virus I3 Gene. J. Virol. 1998, 72, 2917–2926. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.D.; Moss, B. Repression of Vaccinia Virus Holliday Junction Resolvase Inhibits Processing of Viral DNA into Unit-Length Genomes. J. Virol. 2001, 75, 6460–6471. [Google Scholar] [CrossRef] [PubMed]
- Paran, N.; De Silva, F.S.; Senkevich, T.G.; Moss, B. Cellular DNA Ligase I Is Recruited to Cytoplasmic Vaccinia Virus Factories and Masks the Role of the Vaccinia Ligase in Viral DNA Replication. Cell Host Microbe 2009, 6, 563–569. [Google Scholar] [CrossRef]
- El Omari, K.; Solaroli, N.; Karlsson, A.; Balzarini, J.; Stammers, D.K. Structure of Vaccinia Virus Thymidine Kinase in Complex with dTTP: Insights for Drug Design. BMC Struct. Biol. 2006, 6, 22. [Google Scholar] [CrossRef]
- Topalis, D.; Collinet, B.; Gasse, C.; Dugué, L.; Balzarini, J.; Pochet, S.; Deville-Bonne, D. Substrate Specificity of Vaccinia Virus Thymidylate Kinase. FEBS J. 2005, 272, 6254–6265. [Google Scholar] [CrossRef]
- Gammon, D.B.; Gowrishankar, B.; Duraffour, S.; Andrei, G.; Upton, C.; Evans, D.H. Vaccinia Virus–Encoded Ribonucleotide Reductase Subunits Are Differentially Required for Replication and Pathogenesis. PLoS Pathog. 2010, 6, e1000984. [Google Scholar] [CrossRef]
- Smith, G.L.; Vanderplasschen, A.; Law, M. The Formation and Function of Extracellular Enveloped Vaccinia Virus. J. Gen. Virol. 2002, 83, 2915–2931. [Google Scholar] [CrossRef] [PubMed]
- Roberts, K.L.; Smith, G.L. Vaccinia Virus Morphogenesis and Dissemination. Trends Microbiol. 2008, 16, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.L.; Law, M. The Exit of Vaccinia Virus from Infected Cells. Virus Res. 2004, 106, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Zeh, H.J.; Downs-Canner, S.; McCart, J.A.; Guo, Z.S.; Rao, U.N.M.; Ramalingam, L.; Thorne, S.H.; Jones, H.L.; Kalinski, P.; Wieckowski, E.; et al. First-in-Man Study of Western Reserve Strain Oncolytic Vaccinia Virus: Safety, Systemic Spread, and Antitumor Activity. Mol. Ther. 2015, 23, 202–214. [Google Scholar] [CrossRef]
- Schwanke, H.; Stempel, M.; Brinkmann, M.M. Of Keeping and Tipping the Balance: Host Regulation and Viral Modulation of IRF3-Dependent IFNB1 Expression. Viruses 2020, 12, 733. [Google Scholar] [CrossRef]
- Cell Fate in Antiviral Response Arises in the Crosstalk of IRF, NF-κB and JAK/STAT Pathways|Nature Communications. Available online: https://www.nature.com/articles/s41467-017-02640-8 (accessed on 18 April 2025).
- El-Jesr, M.; Teir, M.; Maluquer de Motes, C. Vaccinia Virus Activation and Antagonism of Cytosolic DNA Sensing. Front. Immunol. 2020, 11, 568412. [Google Scholar] [CrossRef]
- Peters, N.E.; Ferguson, B.J.; Mazzon, M.; Fahy, A.S.; Krysztofinska, E.; Arribas-Bosacoma, R.; Pearl, L.H.; Ren, H.; Smith, G.L. A Mechanism for the Inhibition of DNA-PK-Mediated DNA Sensing by a Virus. PLoS Pathog. 2013, 9, e1003649. [Google Scholar] [CrossRef]
- Scutts, S.R.; Ember, S.W.; Ren, H.; Ye, C.; Lovejoy, C.A.; Mazzon, M.; Veyer, D.L.; Sumner, R.P.; Smith, G.L. DNA-PK Is Targeted by Multiple Vaccinia Virus Proteins to Inhibit DNA Sensing. Cell Rep. 2018, 25, 1953–1965.e4. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.-H.; MacMillan, J.B.; Chen, Z.J. RNA Polymerase III Detects Cytosolic DNA and Induces Type I Interferons through the RIG-I Pathway. Cell 2009, 138, 576–591. [Google Scholar] [CrossRef]
- Valentine, R.; Smith, G.L. Inhibition of the RNA Polymerase III-Mediated dsDNA-Sensing Pathway of Innate Immunity by Vaccinia Virus Protein E3. J. Gen. Virol. 2010, 91, 2221–2229. [Google Scholar] [CrossRef]
- Unterholzner, L.; Keating, S.E.; Baran, M.; Horan, K.A.; Jensen, S.B.; Sharma, S.; Sirois, C.M.; Jin, T.; Latz, E.; Xiao, T.S.; et al. IFI16 Is an Innate Immune Sensor for Intracellular DNA. Nat. Immunol. 2010, 11, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Almine, J.F.; O’Hare, C.A.J.; Dunphy, G.; Haga, I.R.; Naik, R.J.; Atrih, A.; Connolly, D.J.; Taylor, J.; Kelsall, I.R.; Bowie, A.G.; et al. IFI16 and cGAS Cooperate in the Activation of STING during DNA Sensing in Human Keratinocytes. Nat. Commun. 2017, 8, 14392. [Google Scholar] [CrossRef] [PubMed]
- Ablasser, A.; Schmid-Burgk, J.L.; Hemmerling, I.; Horvath, G.L.; Schmidt, T.; Latz, E.; Hornung, V. Cell Intrinsic Immunity Spreads to Bystander Cells via the Intercellular Transfer of cGAMP. Nature 2013, 503, 530–534. [Google Scholar] [CrossRef]
- Luteijn, R.D.; Zaver, S.A.; Gowen, B.G.; Wyman, S.K.; Garelis, N.E.; Onia, L.; McWhirter, S.M.; Katibah, G.E.; Corn, J.E.; Woodward, J.J.; et al. SLC19A1 Transports Immunoreactive Cyclic Dinucleotides. Nature 2019, 573, 434–438. [Google Scholar] [CrossRef]
- Seo, G.J.; Yang, A.; Tan, B.; Kim, S.; Liang, Q.; Choi, Y.; Yuan, W.; Feng, P.; Park, H.-S.; Jung, J.U. Akt Kinase-Mediated Checkpoint of cGAS DNA Sensing Pathway. Cell Rep. 2015, 13, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Meade, N.; Furey, C.; Li, H.; Verma, R.; Chai, Q.; Rollins, M.G.; DiGiuseppe, S.; Naghavi, M.H.; Walsh, D. Poxviruses Evade Cytosolic Sensing through Disruption of an mTORC1-mTORC2 Regulatory Circuit. Cell 2018, 174, 1143–1157.e17. [Google Scholar] [CrossRef]
- Yang, N.; Wang, Y.; Dai, P.; Li, T.; Zierhut, C.; Tan, A.; Zhang, T.; Xiang, J.Z.; Ordureau, A.; Funabiki, H.; et al. Vaccinia E5 Is a Major Inhibitor of the DNA Sensor cGAS. Nat. Commun. 2023, 14, 2898. [Google Scholar] [CrossRef]
- Eaglesham, J.B.; Pan, Y.; Kupper, T.S.; Kranzusch, P.J. Viral and Metazoan Poxins Are cGAMP-Specific Nucleases That Restrict cGAS-STING Signaling. Nature 2019, 566, 259–263. [Google Scholar] [CrossRef]
- Unterholzner, L.; Sumner, R.P.; Baran, M.; Ren, H.; Mansur, D.S.; Bourke, N.M.; Randow, F.; Smith, G.L.; Bowie, A.G. Vaccinia Virus Protein C6 Is a Virulence Factor That Binds TBK-1 Adaptor Proteins and Inhibits Activation of IRF3 and IRF7. PLoS Pathog. 2011, 7, e1002247. [Google Scholar] [CrossRef]
- Schröder, M.; Baran, M.; Bowie, A.G. Viral Targeting of DEAD Box Protein 3 Reveals Its Role in TBK1/IKKε-Mediated IRF Activation. EMBO J. 2008, 27, 2147–2157. [Google Scholar] [CrossRef]
- Ferguson, B.J.; Benfield, C.T.O.; Ren, H.; Lee, V.H.; Frazer, G.L.; Strnadova, P.; Sumner, R.P.; Smith, G.L. Vaccinia Virus Protein N2 Is a Nuclear IRF3 Inhibitor That Promotes Virulence. J. Gen. Virol. 2013, 94, 2070–2081. [Google Scholar] [CrossRef]
- Willis, K.L.; Langland, J.O.; Shisler, J.L. Viral Double-Stranded RNAs from Vaccinia Virus Early or Intermediate Gene Transcripts Possess PKR Activating Function, Resulting in NF-κB Activation, When the K1 Protein Is Absent or Mutated. J. Biol. Chem. 2011, 286, 7765–7778. [Google Scholar] [CrossRef] [PubMed]
- García, M.A.; Gil, J.; Ventoso, I.; Guerra, S.; Domingo, E.; Rivas, C.; Esteban, M. Impact of Protein Kinase PKR in Cell Biology: From Antiviral to Antiproliferative Action. Microbiol. Mol. Biol. Rev. 2006, 70, 1032–1060. [Google Scholar] [CrossRef]
- Szczerba, M.; Subramanian, S.; Trainor, K.; McCaughan, M.; Kibler, K.V.; Jacobs, B.L. Small Hero with Great Powers: Vaccinia Virus E3 Protein and Evasion of the Type I IFN Response. Biomedicines 2022, 10, 235. [Google Scholar] [CrossRef]
- Cao, J.; Varga, J.; Deschambault, Y. Poxvirus Encoded eIF2α Homolog, K3 Family Proteins, Is a Key Determinant of Poxvirus Host Species Specificity. Virology 2020, 541, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Bruneau, R.C.; Brennan, G.; Rothenburg, S. Battle Royale: Innate Recognition of Poxviruses and Viral Immune Evasion. Biomedicines 2021, 9, 765. [Google Scholar] [CrossRef] [PubMed]
- Rivas, C.; Gil, J.; Mělková, Z.; Esteban, M.; Díaz-Guerra, M. Vaccinia Virus E3L Protein Is an Inhibitor of the Interferon (IFN)-Induced 2-5A Synthetase Enzyme. Virology 1998, 243, 406–414. [Google Scholar] [CrossRef]
- Chan, Y.K.; Gack, M.U. RIG-I-like Receptor Regulation in Virus Infection and Immunity. Curr. Opin. Virol. 2015, 12, 7–14. [Google Scholar] [CrossRef]
- Deng, L.; Dai, P.; Parikh, T.; Cao, H.; Bhoj, V.; Sun, Q.; Chen, Z.; Merghoub, T.; Houghton, A.; Shuman, S. Vaccinia Virus Subverts a Mitochondrial Antiviral Signaling Protein-Dependent Innate Immune Response in Keratinocytes through Its Double-Stranded RNA Binding Protein, E3. J. Virol. 2008, 82, 10735–10746. [Google Scholar] [CrossRef]
- Botos, I.; Segal, D.M.; Davies, D.R. The Structural Biology of Toll-like Receptors. Structure 2011, 19, 447–459. [Google Scholar] [CrossRef] [PubMed]
- El-Zayat, S.R.; Sibaii, H.; Mannaa, F.A. Toll-like Receptors Activation, Signaling, and Targeting: An Overview. Bull. Natl. Res. Cent. 2019, 43, 187. [Google Scholar] [CrossRef]
- Harte, M.T.; Haga, I.R.; Maloney, G.; Gray, P.; Reading, P.C.; Bartlett, N.W.; Smith, G.L.; Bowie, A.; O’Neill, L.A.J. The Poxvirus Protein A52R Targets Toll-like Receptor Signaling Complexes to Suppress Host Defense. J. Exp. Med. 2003, 197, 343–351. [Google Scholar] [CrossRef]
- Stack, J.; Haga, I.R.; Schröder, M.; Bartlett, N.W.; Maloney, G.; Reading, P.C.; Fitzgerald, K.A.; Smith, G.L.; Bowie, A.G. Vaccinia Virus Protein A46R Targets Multiple Toll-like–Interleukin-1 Receptor Adaptors and Contributes to Virulence. J. Exp. Med. 2005, 201, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, A.; Keating, S.E.; Carty, M.; Bowie, A.G. Poxviral Protein E3–Altered Cytokine Production Reveals That DExD/H-Box Helicase 9 Controls Toll-like Receptor–Stimulated Immune Responses. J. Biol. Chem. 2018, 293, 14989–15001. [Google Scholar] [CrossRef]
- Dai, P.; Cao, H.; Merghoub, T.; Avogadri, F.; Wang, W.; Parikh, T.; Fang, C.-M.; Pitha, P.M.; Fitzgerald, K.A.; Rahman, M.M.; et al. Myxoma Virus Induces Type I Interferon Production in Murine Plasmacytoid Dendritic Cells via a TLR9/MyD88-, IRF5/IRF7-, and IFNAR-Dependent Pathway. J. Virol. 2011, 85, 10814–10825. [Google Scholar] [CrossRef] [PubMed]
- DiPerna, G.; Stack, J.; Bowie, A.G.; Boyd, A.; Kotwal, G.; Zhang, Z.; Arvikar, S.; Latz, E.; Fitzgerald, K.A.; Marshall, W.L. Poxvirus Protein N1L Targets the I-κB Kinase Complex, Inhibits Signaling to NF-κB by the Tumor Necrosis Factor Superfamily of Receptors, and Inhibits NF-κB and IRF3 Signaling by Toll-like Receptors. J. Biol. Chem. 2004, 279, 36570–36578. [Google Scholar] [CrossRef]
- Albarnaz, J.D.; Ren, H.; Torres, A.A.; Shmeleva, E.V.; Melo, C.A.; Bannister, A.J.; Brember, M.P.; Chung, B.Y.-W.; Smith, G.L. Molecular Mimicry of NF-κB by Vaccinia Virus Protein Enables Selective Inhibition of Antiviral Responses. Nat. Microbiol. 2022, 7, 154–168. [Google Scholar] [CrossRef]
- Mansur, D.S.; de Motes, C.M.; Unterholzner, L.; Sumner, R.P.; Ferguson, B.J.; Ren, H.; Strnadova, P.; Bowie, A.G.; Smith, G.L. Poxvirus Targeting of E3 Ligase β-TrCP by Molecular Mimicry: A Mechanism to Inhibit NF-κB Activation and Promote Immune Evasion and Virulence. PLoS Pathog. 2013, 9, e1003183. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.L.; Benfield, C.T.O.; Maluquer de Motes, C.; Mazzon, M.; Ember, S.W.J.; Ferguson, B.J.; Sumner, R.P. Vaccinia Virus Immune Evasion: Mechanisms, Virulence and Immunogenicity. J. Gen. Virol. 2013, 94, 2367–2392. [Google Scholar] [CrossRef]
- Symons, J.A.; Alcamí, A.; Smith, G.L. Vaccinia Virus Encodes a Soluble Type I Interferon Receptor of Novel Structure and Broad Species Specificity. Cell 1995, 81, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Mann, B.A.; Huang, J.H.; Li, P.; Chang, H.-C.; Slee, R.B.; O’Sullivan, A.; Mathur, A.; Yeh, N.; Klemsz, M.J.; Brutkiewicz, R.R.; et al. Vaccinia Virus Blocks Stat1-Dependent and Stat1-Independent Gene Expression Induced by Type I and Type II Interferons. J. Interferon Cytokine Res. 2008, 28, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-de Miranda, F.J.; Alonso-Sánchez, I.; Alcamí, A.; Hernaez, B. TNF Decoy Receptors Encoded by Poxviruses. Pathogens 2021, 10, 1065. [Google Scholar] [CrossRef] [PubMed]
- Symons, J.A.; Tscharke, D.C.; Price, N.; Smith, G.L. A Study of the Vaccinia Virus Interferon-Gamma Receptor and Its Contribution to Virus Virulence. J. Gen. Virol. 2002, 83, 1953–1964. [Google Scholar] [CrossRef]
- Reading, P.C.; Smith, G.L. Vaccinia Virus Interleukin-18-Binding Protein Promotes Virulence by Reducing Gamma Interferon Production and Natural Killer and T-Cell Activity. J. Virol. 2003, 77, 9960–9968. [Google Scholar] [CrossRef]
- Alcami, A.; Smith, G.L. A Soluble Receptor for Interleukin-1β Encoded by Vaccinia Virus: A Novel Mechanism of Virus Modulation of the Host Response to Infection. Cell 1992, 71, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Charles, A.; Janeway, J.; Travers, P.; Walport, M.; Shlomchik, M.J. The Complement System and Innate Immunity. In Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Science: New York, NY, USA, 2001. [Google Scholar]
- Bernet, J.; Mullick, J.; Panse, Y.; Parab, P.B.; Sahu, A. Kinetic Analysis of the Interactions between Vaccinia Virus Complement Control Protein and Human Complement Proteins C3b and C4b. J. Virol. 2004, 78, 9446–9457. [Google Scholar] [CrossRef]
- Li, P.; Wang, N.; Zhou, D.; Yee, C.S.K.; Chang, C.-H.; Brutkiewicz, R.R.; Blum, J.S. Disruption of MHC Class II-Restricted Antigen Presentation by Vaccinia Virus1. J. Immunol. 2005, 175, 6481–6488. [Google Scholar] [CrossRef]
- Rehm, K.E.; Connor, R.F.; Jones, G.J.B.; Yimbu, K.; Roper, R.L. Vaccinia Virus A35R Inhibits MHC Class II Antigen Presentation. Virology 2010, 397, 176. [Google Scholar] [CrossRef]
- Orzalli, M.H.; Kagan, J.C. Apoptosis and Necroptosis as Host Defense Strategies to Prevent Viral Infection. Trends Cell Biol. 2017, 27, 800–809. [Google Scholar] [CrossRef]
- Gregory, C.D.; Devitt, A. The Macrophage and the Apoptotic Cell: An Innate Immune Interaction Viewed Simplistically? Immunology 2004, 113, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Veyer, D.L.; Carrara, G.; Maluquer de Motes, C.; Smith, G.L. Vaccinia Virus Evasion of Regulated Cell Death. Immunol. Lett. 2017, 186, 68–80. [Google Scholar] [CrossRef]
- Maluquer de Motes, C.; Cooray, S.; Ren, H.; Almeida, G.M.F.; McGourty, K.; Bahar, M.W.; Stuart, D.I.; Grimes, J.M.; Graham, S.C.; Smith, G.L. Inhibition of Apoptosis and NF-κB Activation by Vaccinia Protein N1 Occur via Distinct Binding Surfaces and Make Different Contributions to Virulence. PLoS Pathog. 2011, 7, e1002430. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Pan, R.; Ouyang, Y.; Gu, W.; Xiao, T.; Yang, H.; Tang, L.; Wang, H.; Xiang, B.; Chen, P. Pyroptosis in Health and Disease: Mechanisms, Regulation and Clinical Perspective. Sig. Transduct. Target. Ther. 2024, 9, 245. [Google Scholar] [CrossRef] [PubMed]
- Gerlic, M.; Faustin, B.; Postigo, A.; Yu, E.C.-W.; Proell, M.; Gombosuren, N.; Krajewska, M.; Flynn, R.; Croft, M.; Way, M.; et al. Vaccinia Virus F1L Protein Promotes Virulence by Inhibiting Inflammasome Activation. Proc. Natl. Acad. Sci. USA 2013, 110, 7808–7813. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, T.; Lei, T.; Zhang, D.; Du, S.; Girani, L.; Qi, D.; Lin, C.; Tong, R.; Wang, Y. RIP1/RIP3-Regulated Necroptosis as a Target for Multifaceted Disease Therapy (Review). Int. J. Mol. Med. 2019, 44, 771–786. [Google Scholar] [CrossRef]
- Koehler, H.S.; Jacobs, B.L. Subversion of Programed Cell Death by Poxviruses. Curr. Top. Microbiol. Immunol. 2023, 442, 105–131. [Google Scholar] [CrossRef]
- Chahroudi, A.; Chavan, R.; Koyzr, N.; Waller, E.K.; Silvestri, G.; Feinberg, M.B. Vaccinia Virus Tropism for Primary Hematolymphoid Cells Is Determined by Restricted Expression of a Unique Virus Receptor. J. Virol. 2005, 79, 10397–10407. [Google Scholar] [CrossRef]
- Engelmayer, J.; Larsson, M.; Subklewe, M.; Chahroudi, A.; Cox, W.I.; Steinman, R.M.; Bhardwaj, N. Vaccinia Virus Inhibits the Maturation of Human Dendritic Cells: A Novel Mechanism of Immune Evasion1. J. Immunol. 1999, 163, 6762–6768. [Google Scholar] [CrossRef]
- Yates, N.L.; Alexander-Miller, M.A. Vaccinia Virus Infection of Mature Dendritic Cells Results in Activation of Virus-Specific Naïve CD8+ T Cells: A Potential Mechanism for Direct Presentation. Virology 2007, 359, 349–361. [Google Scholar] [CrossRef]
- Yao, Y.; Li, P.; Singh, P.; Thiele, A.T.; Wilkes, D.S.; Renukaradhya, G.J.; Brutkiewicz, R.R.; Travers, J.B.; Luker, G.D.; Hong, S.-C.; et al. Vaccinia Virus Infection Induces Dendritic Cell Maturation but Inhibits Antigen Presentation by MHC Class II. Cell Immunol. 2007, 246, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Byrd, D.; Shepherd, N.; Lan, J.; Hu, N.; Amet, T.; Yang, K.; Desai, M.; Yu, Q. Primary Human Macrophages Serve as Vehicles for Vaccinia Virus Replication and Dissemination. J. Virol. 2014, 88, 6819–6831. [Google Scholar] [CrossRef]
- Zhou, D.; Xu, W.; Ding, X.; Guo, H.; Wang, J.; Zhao, G.; Zhang, C.; Zhang, Z.; Wang, Z.; Wang, P.; et al. Transient Inhibition of Neutrophil Functions Enhances the Antitumor Effect of Intravenously Delivered Oncolytic Vaccinia Virus. Cancer Sci. 2024, 115, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Duffy, D.; Perrin, H.; Abadie, V.; Benhabiles, N.; Boissonnas, A.; Liard, C.; Descours, B.; Reboulleau, D.; Bonduelle, O.; Verrier, B.; et al. Neutrophils Transport Antigen from the Dermis to the Bone Marrow, Initiating a Source of Memory CD8+ T Cells. Immunity 2012, 37, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Abboud, G.; Tahiliani, V.; Desai, P.; Varkoly, K.; Driver, J.; Hutchinson, T.E.; Salek-Ardakani, S. Natural Killer Cells and Innate Interferon Gamma Participate in the Host Defense against Respiratory Vaccinia Virus Infection. J. Virol. 2015, 90, 129–141. [Google Scholar] [CrossRef]
- Dokun, A.O.; Kim, S.; Smith, H.R.; Kang, H.S.; Chu, D.T.; Yokoyama, W.M. Specific and Nonspecific NK Cell Activation during Virus Infection. Nat. Immunol. 2001, 2, 951–956. [Google Scholar] [CrossRef]
- Chisholm, S.E.; Reyburn, H.T. Recognition of Vaccinia Virus-Infected Cells by Human Natural Killer Cells Depends on Natural Cytotoxicity Receptors. J. Virol. 2006, 80, 2225–2233. [Google Scholar] [CrossRef]
- Gillard, G.O.; Bivas-Benita, M.; Hovav, A.-H.; Grandpre, L.E.; Panas, M.W.; Seaman, M.S.; Haynes, B.F.; Letvin, N.L. Thy1+ Nk Cells from Vaccinia Virus-Primed Mice Confer Protection against Vaccinia Virus Challenge in the Absence of Adaptive Lymphocytes. PLoS Pathog. 2011, 7, e1002141. [Google Scholar] [CrossRef]
- Shepherd, N.; Lan, J.; Li, W.; Rane, S.; Yu, Q. Primary Human B Cells at Different Differentiation and Maturation Stages Exhibit Distinct Susceptibilities to Vaccinia Virus Binding and Infection. J. Virol. 2019, 93, e00973-19. [Google Scholar] [CrossRef]
- Goulding, J.; Bouge, R.; Tahiliani, V.; Croft, M.; Salek-Ardakani, S. CD8 T Cells Are Essential for Recovery from a Respiratory Vaccinia Virus Infection. J. Immunol. 2012, 189, 2432–2440. [Google Scholar] [CrossRef] [PubMed]
- Bernasconi, N.L.; Traggiai, E.; Lanzavecchia, A. Maintenance of Serological Memory by Polyclonal Activation of Human Memory B Cells. Science 2002, 298, 2199–2202. [Google Scholar] [CrossRef]
- Liu, L.; Zhong, Q.; Tian, T.; Dubin, K.; Athale, S.K.; Kupper, T.S. Epidermal Injury and Infection during Poxvirus Immunization Is Crucial for the Generation of Highly Protective T Cell-Mediated Immunity. Nat. Med. 2010, 16, 224–227. [Google Scholar] [CrossRef]
- Sánchez-Puig, J.M.; Sánchez, L.; Roy, G.; Blasco, R. Susceptibility of Different Leukocyte Cell Types to Vaccinia Virus Infection. Virol. J. 2004, 1, 10. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Molloy, M.J.; Usherwood, E.J. CD4+ T-Cell Dependence of Primary CD8+ T-Cell Response against Vaccinia Virus Depends upon Route of Infection and Viral Dose. Cell Mol. Immunol. 2016, 13, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Dai, R.; Huang, X.; Yang, Y. γδT Cells Are Required for CD8+ T Cell Response to Vaccinia Viral Infection. Front. Immunol. 2021, 12, 727046. [Google Scholar] [CrossRef]
- Demkowicz, W.E.; Littaua, R.A.; Wang, J.; Ennis, F.A. Human Cytotoxic T-Cell Memory: Long-Lived Responses to Vaccinia Virus. J. Virol. 1996, 70, 2627–2631. [Google Scholar] [CrossRef]
- Yaghchi, C.A.; Zhang, Z.; Alusi, G.; Lemoine, N.R.; Wang, Y. Vaccinia Virus, a Promising New Therapeutic Agent for Pancreatic Cancer. Immunotherapy 2015, 7, 1249–1258. [Google Scholar] [CrossRef]
- Lei, W.; Ye, Q.; Hao, Y.; Chen, J.; Huang, Y.; Yang, L.; Wang, S.; Qian, W. CD19-Targeted BiTE Expression by an Oncolytic Vaccinia Virus Significantly Augments Therapeutic Efficacy against B-Cell Lymphoma. Blood Cancer J. 2022, 12, 35. [Google Scholar] [CrossRef]
- Totsch, S.K.; Schlappi, C.; Kang, K.-D.; Ishizuka, A.S.; Lynn, G.M.; Fox, B.; Beierle, E.A.; Whitley, R.J.; Markert, J.M.; Gillespie, G.Y.; et al. Oncolytic Herpes Simplex Virus Immunotherapy for Brain Tumors: Current Pitfalls and Emerging Strategies to Overcome Therapeutic Resistance. Oncogene 2019, 38, 6159–6171. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.L.; Moss, B. Infectious Poxvirus Vectors Have Capacity for at Least 25 000 Base Pairs of Foreign DNA. Gene 1983, 25, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, F.; Schmitt, D.; Brandely, R.; Brua, C.; Silvestre, N.; Findeli, A.; Foloppe, J.; Top, S.; Kappler-Gratias, S.; Quentin-Froignant, C.; et al. Fluorescent Tagged Vaccinia Virus Genome Allows Rapid and Efficient Measurement of Oncolytic Potential and Discovery of Oncolytic Modulators. Biomedicines 2020, 8, 543. [Google Scholar] [CrossRef] [PubMed]
- Salauddin, M.; Saha, S.; Hossain, M.G.; Okuda, K.; Shimada, M. Clinical Application of Adenovirus (AdV): A Comprehensive Review. Viruses 2024, 16, 1094. [Google Scholar] [CrossRef]
- Hiley, C.T.; Yuan, M.; Lemoine, N.R.; Wang, Y. Lister Strain Vaccinia Virus, a Potential Therapeutic Vector Targeting Hypoxic Tumours. Gene Ther. 2010, 17, 281–287. [Google Scholar] [CrossRef]
- Ma, J.; Ramachandran, M.; Jin, C.; Quijano-Rubio, C.; Martikainen, M.; Yu, D.; Essand, M. Characterization of Virus-Mediated Immunogenic Cancer Cell Death and the Consequences for Oncolytic Virus-Based Immunotherapy of Cancer. Cell Death Dis. 2020, 11, 48. [Google Scholar] [CrossRef]
- Fucikova, J.; Kepp, O.; Kasikova, L.; Petroni, G.; Yamazaki, T.; Liu, P.; Zhao, L.; Spisek, R.; Kroemer, G.; Galluzzi, L. Detection of Immunogenic Cell Death and Its Relevance for Cancer Therapy. Cell Death Dis. 2020, 11, 1013. [Google Scholar] [CrossRef]
- O’Leary, M.P.; Choi, A.H.; Kim, S.-I.; Chaurasiya, S.; Lu, J.; Park, A.K.; Woo, Y.; Warner, S.G.; Fong, Y.; Chen, N.G. Novel Oncolytic Chimeric Orthopoxvirus Causes Regression of Pancreatic Cancer Xenografts and Exhibits Abscopal Effect at a Single Low Dose. J. Transl. Med. 2018, 16, 110. [Google Scholar] [CrossRef]
- Choi, A.H.; O’Leary, M.P.; Lu, J.; Kim, S.-I.; Fong, Y.; Chen, N.G. Endogenous Akt Activity Promotes Virus Entry and Predicts Efficacy of Novel Chimeric Orthopoxvirus in Triple-Negative Breast Cancer. Mol. Ther. Oncolytics 2018, 9, 22–29. [Google Scholar] [CrossRef]
- Chaurasiya, S.; Yang, A.; Zhang, Z.; Lu, J.; Valencia, H.; Kim, S.-I.; Woo, Y.; Warner, S.G.; Olafsen, T.; Zhao, Y.; et al. A Comprehensive Preclinical Study Supporting Clinical Trial of Oncolytic Chimeric Poxvirus CF33-hNIS-Anti-PD-L1 to Treat Breast Cancer. Mol. Ther. Methods Clin. Dev. 2021, 24, 102–116. [Google Scholar] [CrossRef]
- Warner, S.G.; Kim, S.-I.; Chaurasiya, S.; O’Leary, M.P.; Lu, J.; Sivanandam, V.; Woo, Y.; Chen, N.G.; Fong, Y. A Novel Chimeric Poxvirus Encoding hNIS Is Tumor-Tropic, Imageable, and Synergistic with Radioiodine to Sustain Colon Cancer Regression. Mol. Ther. Oncolytics 2019, 13, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Zhang, Z.; Chaurasiya, S.; Park, A.K.; Jung, A.; Lu, J.; Kim, S.-I.; Priceman, S.; Fong, Y.; Woo, Y. Development of the Oncolytic Virus, CF33, and Its Derivatives for Peritoneal-Directed Treatment of Gastric Cancer Peritoneal Metastases. J. Immunother. Cancer 2023, 11, e006280. [Google Scholar] [CrossRef]
- Chen, C.; Park, A.K.; Monroy, I.; Ren, Y.; Kim, S.-I.; Chaurasiya, S.; Priceman, S.J.; Fong, Y. Using Oncolytic Virus to Retask CD19-Chimeric Antigen Receptor T Cells for Treatment of Pancreatic Cancer: Toward a Universal Chimeric Antigen Receptor T-Cell Strategy for Solid Tumor. J. Am. Coll. Surg. 2024, 238, 436–447. [Google Scholar] [CrossRef]
- Earl, P.L.; Moss, B.; Wyatt, L.S. Generation of Recombinant Vaccinia Viruses. Curr. Protoc. Protein Sci. 2017, 89, 5.13.1–5.13.18. [Google Scholar] [CrossRef]
- Domi, A.; Moss, B. Cloning the Vaccinia Virus Genome as a Bacterial Artificial Chromosome in Escherichia Coli and Recovery of Infectious Virus in Mammalian Cells. Proc. Natl. Acad. Sci. USA 2002, 99, 12415–12420. [Google Scholar] [CrossRef]
- Yuan, M.; Zhang, W.; Wang, J.; Al Yaghchi, C.; Ahmed, J.; Chard, L.; Lemoine, N.R.; Wang, Y. Efficiently Editing the Vaccinia Virus Genome by Using the CRISPR-Cas9 System. J. Virol. 2015, 89, 5176–5179. [Google Scholar] [CrossRef]
- Rezaei, R.; Boulton, S.; Ahmadi, M.; Petryk, J.; Da Silva, M.; Kooshki Zamani, N.; Singaravelu, R.; St-Laurent, G.; Daniel, L.; Sadeghipour, A.; et al. Antibiotic-Mediated Selection of Randomly Mutagenized and Cytokine-Expressing Oncolytic Viruses. Nat. Biomed. Eng. 2025, 9, 822–835. [Google Scholar] [CrossRef]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef]
- de Queiroz, N.M.G.P.; Xia, T.; Konno, H.; Barber, G.N. Ovarian Cancer Cells Commonly Exhibit Defective STING Signaling Which Affects Sensitivity to Viral Oncolysis. Mol. Cancer Res. 2019, 17, 974–986. [Google Scholar] [CrossRef]
- Aye, Y.; Li, M.; Long, M.J.C.; Weiss, R.S. Ribonucleotide Reductase and Cancer: Biological Mechanisms and Targeted Therapies. Oncogene 2015, 34, 2011–2021. [Google Scholar] [CrossRef]
- Bitter, E.E.; Townsend, M.H.; Erickson, R.; Allen, C.; O’Neill, K.L. Thymidine Kinase 1 through the Ages: A Comprehensive Review. Cell Biosci. 2020, 10, 138. [Google Scholar] [CrossRef]
- Potts, K.G.; Irwin, C.R.; Favis, N.A.; Pink, D.B.; Vincent, K.M.; Lewis, J.D.; Moore, R.B.; Hitt, M.M.; Evans, D.H. Deletion of F4L (Ribonucleotide reductase) in Vaccinia Virus Produces a Selective Oncolytic Virus and Promotes Anti-Tumor Immunity with Superior Safety in Bladder Cancer Models. EMBO Mol. Med. 2017, 9, 638–654. [Google Scholar] [CrossRef]
- Puhlmann, M.; Brown, C.K.; Gnant, M.; Huang, J.; Libutti, S.K.; Alexander, H.R.; Bartlett, D.L. Vaccinia as a Vector for Tumor-Directed Gene Therapy: Biodistribution of a Thymidine Kinase-Deleted Mutant. Cancer Gene Ther. 2000, 7, 66–73. [Google Scholar] [CrossRef]
- Breitbach, C.J.; Arulanandam, R.; De Silva, N.; Thorne, S.H.; Patt, R.; Daneshmand, M.; Moon, A.; Ilkow, C.; Burke, J.; Hwang, T.-H.; et al. Oncolytic Vaccinia Virus Disrupts Tumor-Associated Vasculature in Humans. Cancer Res. 2013, 73, 1265–1275. [Google Scholar] [CrossRef]
- Lai, A.C.-K.; Pogo, B.G.-T. Attenuated Deletion Mutants of Vaccinia Virus Lacking the Vaccinia Growth Factor Are Defective in Replication in Vivo. Microb. Pathog. 1989, 6, 219–226. [Google Scholar] [CrossRef]
- DeHaven, B.C.; Gupta, K.; Isaacs, S.N. The Vaccinia Virus A56 Protein: A Multifunctional Transmembrane Glycoprotein That Anchors Two Secreted Viral Proteins. J. Gen. Virol. 2011, 92, 1971–1980. [Google Scholar] [CrossRef]
- Izmailyan, R.; Chang, W. Vaccinia Virus WR53.5/F14.5 Protein Is a New Component of Intracellular Mature Virus and Is Important for Calcium-Independent Cell Adhesion and Vaccinia Virus Virulence in Mice. J. Virol. 2008, 82, 10079–10087. [Google Scholar] [CrossRef]
- Zhang, Q.; Liang, C.; Yu, Y.A.; Chen, N.; Dandekar, T.; Szalay, A.A. The Highly Attenuated Oncolytic Recombinant Vaccinia Virus GLV-1h68: Comparative Genomic Features and the Contribution of F14.5L Inactivation. Mol. Genet. Genom. 2009, 282, 417–435. [Google Scholar] [CrossRef]
- McCart, J.A.; Ward, J.M.; Lee, J.; Hu, Y.; Alexander, H.R.; Libutti, S.K.; Moss, B.; Bartlett, D.L. Systemic Cancer Therapy with a Tumor-Selective Vaccinia Virus Mutant Lacking Thymidine Kinase and Vaccinia Growth Factor Genes. Cancer Res. 2001, 61, 8751–8757. [Google Scholar]
- Guo, Z.S.; Naik, A.; O’Malley, M.E.; Popovic, P.; Demarco, R.; Hu, Y.; Yin, X.; Yang, S.; Zeh, H.J.; Moss, B.; et al. The Enhanced Tumor Selectivity of an Oncolytic Vaccinia Lacking the Host Range and Antiapoptosis Genes SPI-1 and SPI-2. Cancer Res. 2005, 65, 9991–9998. [Google Scholar] [CrossRef]
- Yang, S.; Guo, Z.S.; O’Malley, M.E.; Yin, X.; Zeh, H.J.; Bartlett, D.L. A New Recombinant Vaccinia with Targeted Deletion of Three Viral Genes: Its Safety and Efficacy as an Oncolytic Virus. Gene Ther. 2007, 14, 638–647. [Google Scholar] [CrossRef]
- Kirn, D.H.; Wang, Y.; Le Boeuf, F.; Bell, J.; Thorne, S.H. Targeting of Interferon-Beta to Produce a Specific, Multi-Mechanistic Oncolytic Vaccinia Virus. PLoS Med. 2007, 4, e353. [Google Scholar] [CrossRef]
- Eradication of Solid Human Breast Tumors in Nude Mice with an Intravenously Injected Light-Emitting Oncolytic Vaccinia Virus|Cancer Research|American Association for Cancer Research. Available online: https://aacrjournals.org/cancerres/article/67/20/10038/533730/Eradication-of-Solid-Human-Breast-Tumors-in-Nude (accessed on 28 May 2025).
- Foloppe, J.; Kempf, J.; Futin, N.; Kintz, J.; Cordier, P.; Pichon, C.; Findeli, A.; Vorburger, F.; Quemeneur, E.; Erbs, P. The Enhanced Tumor Specificity of TG6002, an Armed Oncolytic Vaccinia Virus Deleted in Two Genes Involved in Nucleotide Metabolism. Mol. Ther. Oncolytics 2019, 14, 1–14. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, Y.; Zhao, G.; Yang, Y.; Yan, W.; Wang, R.; Han, B.; Wang, L.; Zhang, Z.; Chen, L.; et al. Novel Oncolytic Vaccinia Virus Armed with Interleukin-27 Is a Potential Therapeutic Agent for the Treatment of Murine Pancreatic Cancer. J. Immunother. Cancer 2025, 13, e010341. [Google Scholar] [CrossRef]
- Neidel, S.; Torres, A.A.; Ren, H.; Smith, G.L. Leaky Scanning Translation Generates a Second A49 Protein That Contributes to Vaccinia Virus Virulence. J. Gen. Virol. 2020, 101, 533–541. [Google Scholar] [CrossRef]
- Pelin, A.; Foloppe, J.; Petryk, J.; Singaravelu, R.; Hussein, M.; Gossart, F.; Jennings, V.A.; Stubbert, L.J.; Foster, M.; Storbeck, C.; et al. Deletion of Apoptosis Inhibitor F1L in Vaccinia Virus Increases Safety and Oncolysis for Cancer Therapy. Mol. Ther. Oncolytics 2019, 14, 246–252. [Google Scholar] [CrossRef]
- Riederer, S.; del Canizo, A.; Navas, J.; Peter, M.G.; Link, E.K.; Sutter, G.; Rojas, J.J. Improving Poxvirus-Mediated Antitumor Immune Responses by Deleting Viral cGAMP-Specific Nuclease. Cancer Gene Ther. 2023, 30, 1029–1039. [Google Scholar] [CrossRef]
- Papatriantafyllou, M. GM-CSF in Focus. Nat. Rev. Immunol. 2011, 11, 370–371. [Google Scholar] [CrossRef]
- 87. Preclinical and Clinical Evaluation of Oncolytic Immunotherapy with Ad5/3-E2F1-Δ24-GMCSF (CGTG-602), a GM-CSF Producing Adenovirus Targeted to Tumors on Four Levels. Mol. Ther. 2012, 20, S36. [CrossRef]
- Shi, X.; Sun, K.; Li, L.; Xian, J.; Wang, P.; Jia, F.; Xu, F. Oncolytic Activity of Sindbis Virus with the Help of GM-CSF in Hepatocellular Carcinoma. Int. J. Mol. Sci. 2024, 25, 7195. [Google Scholar] [CrossRef]
- Zhang, T.; Jou, T.H.-T.; Hsin, J.; Wang, Z.; Huang, K.; Ye, J.; Yin, H.; Xing, Y. Talimogene Laherparepvec (T-VEC): A Review of the Recent Advances in Cancer Therapy. J. Clin. Med. 2023, 12, 1098. [Google Scholar] [CrossRef]
- Deng, L.; Fan, J.; Guo, M.; Huang, B. Oncolytic and Immunologic Cancer Therapy with GM-CSF-Armed Vaccinia Virus of Tian Tan Strain Guang9. Cancer Lett. 2016, 372, 251–257. [Google Scholar] [CrossRef]
- Xuan, Y.; Yan, W.; Wang, R.; Wang, X.; Guo, Y.; Dun, H.; Huan, Z.; Xu, L.; Han, R.; Sun, X.; et al. GM-CSF and IL-21-Armed Oncolytic Vaccinia Virus Significantly Enhances Anti-Tumor Activity and Synergizes with Anti-PD1 Immunotherapy in Pancreatic Cancer. Front. Immunol. 2025, 15, 1506632. [Google Scholar] [CrossRef]
- Li, J.; O’Malley, M.; Urban, J.; Sampath, P.; Guo, Z.S.; Kalinski, P.; Thorne, S.H.; Bartlett, D.L. Chemokine Expression From Oncolytic Vaccinia Virus Enhances Vaccine Therapies of Cancer. Mol. Ther. 2011, 19, 650–657. [Google Scholar] [CrossRef]
- Liu, Z.; Ravindranathan, R.; Li, J.; Kalinski, P.; Guo, Z.S.; Bartlett, D.L. CXCL11-Armed Oncolytic Poxvirus Elicits Potent Antitumor Immunity and Shows Enhanced Therapeutic Efficacy. OncoImmunology 2016, 5, e1091554. [Google Scholar] [CrossRef]
- Ge, Y.; Wang, H.; Ren, J.; Liu, W.; Chen, L.; Chen, H.; Ye, J.; Dai, E.; Ma, C.; Ju, S.; et al. Oncolytic Vaccinia Virus Delivering Tethered IL-12 Enhances Antitumor Effects with Improved Safety. J. Immunother. Cancer 2020, 8, e000710. [Google Scholar] [CrossRef]
- Shakiba, Y.; Vorobyev, P.O.; Yusubalieva, G.M.; Kochetkov, D.V.; Zajtseva, K.V.; Valikhov, M.P.; Kalsin, V.A.; Zabozlaev, F.G.; Semkina, A.S.; Troitskiy, A.V.; et al. Oncolytic Therapy with Recombinant Vaccinia Viruses Targeting the Interleukin-15 Pathway Elicits a Synergistic Response. Mol. Ther.-Oncolytics 2023, 29, 158–168. [Google Scholar] [CrossRef]
- Chen, L.; Chen, H.; Ye, J.; Ge, Y.; Wang, H.; Dai, E.; Ren, J.; Liu, W.; Ma, C.; Ju, S.; et al. Intratumoral Expression of Interleukin 23 Variants Using Oncolytic Vaccinia Virus Elicit Potent Antitumor Effects on Multiple Tumor Models via Tumor Microenvironment Modulation. Theranostics 2021, 11, 6668–6681. [Google Scholar] [CrossRef] [PubMed]
- Chard, L.S.; Maniati, E.; Wang, P.; Zhang, Z.; Gao, D.; Wang, J.; Cao, F.; Ahmed, J.; El Khouri, M.; Hughes, J.; et al. A Vaccinia Virus Armed with Interleukin-10 Is a Promising Therapeutic Agent for Treatment of Murine Pancreatic Cancer. Clin. Cancer Res. 2015, 21, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.K.; Cho, K.-J.; Ishido, S.; Roche, P.A. Interleukin 10 (IL-10)-Mediated Immunosuppression. J. Biol. Chem. 2015, 290, 27158–27167. [Google Scholar] [CrossRef]
- Holicek, P.; Guilbaud, E.; Klapp, V.; Truxova, I.; Spisek, R.; Galluzzi, L.; Fucikova, J. Type I Interferon and Cancer. Immunol. Rev. 2024, 321, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Hirvinen, M.; Capasso, C.; Guse, K.; Garofalo, M.; Vitale, A.; Ahonen, M.; Kuryk, L.; Vähä-Koskela, M.; Hemminki, A.; Fortino, V.; et al. Expression of DAI by an Oncolytic Vaccinia Virus Boosts the Immunogenicity of the Virus and Enhances Antitumor Immunity. Mol. Ther.-Oncolytics 2016, 3, 16002. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, N.; Liu, T.; Jia, X.; Ye, T.; Chen, K.; Li, G. Oncolytic Vaccinia Virus Expressing White-Spotted Charr Lectin Regulates Antiviral Response in Tumor Cells and Inhibits Tumor Growth In Vitro and In Vivo. Mar. Drugs 2021, 19, 292. [Google Scholar] [CrossRef]
- Hinterberger, M.; Giessel, R.; Fiore, G.; Graebnitz, F.; Bathke, B.; Wennier, S.; Chaplin, P.; Melero, I.; Suter, M.; Lauterbach, H.; et al. Intratumoral Virotherapy with 4-1BBL Armed Modified Vaccinia Ankara Eradicates Solid Tumors and Promotes Protective Immune Memory. J. Immunother. Cancer 2021, 9, e001586. [Google Scholar] [CrossRef]
- Parviainen, S.; Ahonen, M.; Diaconu, I.; Hirvinen, M.; Karttunen, Å.; Vähä-Koskela, M.; Hemminki, A.; Cerullo, V. CD40 Ligand and tdTomato-Armed Vaccinia Virus for Induction of Antitumor Immune Response and Tumor Imaging. Gene Ther. 2014, 21, 195–204. [Google Scholar] [CrossRef]
- Yang, N.; Wang, Y.; Liu, S.; Tariq, S.B.; Luna, J.M.; Mazo, G.; Tan, A.; Zhang, T.; Wang, J.; Yan, W.; et al. OX40L-Expressing Recombinant Modified Vaccinia Virus Ankara Induces Potent Antitumor Immunity via Reprogramming Tregs. J. Exp. Med. 2023, 220, e20221166. [Google Scholar] [CrossRef]
- Yu, F.; Wang, X.; Guo, Z.S.; Bartlett, D.L.; Gottschalk, S.M.; Song, X.-T. T-Cell Engager-Armed Oncolytic Vaccinia Virus Significantly Enhances Antitumor Therapy. Mol. Ther. 2014, 22, 102–111. [Google Scholar] [CrossRef]
- DePeaux, K.; Rivadeneira, D.B.; Lontos, K.; Dean, V.G.; Gunn, W.G.; Watson, M.J.; Yao, T.; Wilfahrt, D.; Hinck, C.; Wieteska, L.; et al. An Oncolytic Virus–Delivered TGFβ Inhibitor Overcomes the Immunosuppressive Tumor Microenvironment. J. Exp. Med. 2023, 220, e20230053. [Google Scholar] [CrossRef]
- Frentzen, A.; Yu, Y.A.; Chen, N.; Zhang, Q.; Weibel, S.; Raab, V.; Szalay, A.A. Anti-VEGF Single-Chain Antibody GLAF-1 Encoded by Oncolytic Vaccinia Virus Significantly Enhances Antitumor Therapy. Proc. Natl. Acad. Sci. USA 2009, 106, 12915–12920. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.E.; Mackall, C.L. CAR T Cell Therapy: Inroads to Response and Resistance. Nat. Rev. Immunol. 2019, 19, 73–74. [Google Scholar] [CrossRef]
- Aalipour, A.; Boeuf, F.L.; Tang, M.; Murty, S.; Simonetta, F.; Lozano, A.X.; Shaffer, T.M.; Bell, J.C.; Gambhir, S.S. Viral Delivery of CAR Targets to Solid Tumors Enables Effective Cell Therapy. Mol. Ther.-Oncolytics 2020, 17, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Park, A.K.; Fong, Y.; Kim, S.-I.; Yang, J.; Murad, J.P.; Lu, J.; Jeang, B.; Chang, W.-C.; Chen, N.G.; Thomas, S.H.; et al. Effective Combination Immunotherapy Using Oncolytic Viruses to Deliver CAR Targets to Solid Tumors. Sci. Transl. Med. 2020, 12, eaaz1863. [Google Scholar] [CrossRef] [PubMed]
- Moon, E.K.; Wang, L.-C.S.; Bekdache, K.; Lynn, R.C.; Lo, A.; Thorne, S.H.; Albelda, S.M. Intra-Tumoral Delivery of CXCL11 via a Vaccinia Virus, but Not by Modified T Cells, Enhances the Efficacy of Adoptive T Cell Therapy and Vaccines. OncoImmunology 2018, 7, e1395997. [Google Scholar] [CrossRef]
- Sivanandam, V.; LaRocca, C.J.; Chen, N.G.; Fong, Y.; Warner, S.G. Oncolytic Viruses and Immune Checkpoint Inhibition: The Best of Both Worlds. Mol. Ther. Oncolytics 2019, 13, 93–106. [Google Scholar] [CrossRef]
- Chaurasiya, S.; Chen, N.G.; Fong, Y. Oncolytic Viruses and Immunity. Curr. Opin. Immunol. 2018, 51, 83–90. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Z.; Zhang, C.; Zhang, N.; Wang, P.; Chu, Y.; Dunmall, L.S.C.; Lemoine, N.R.; Wang, Y. An Effective Therapeutic Regime for Treatment of Glioma Using Oncolytic Vaccinia Virus Expressing IL-21 in Combination with Immune Checkpoint Inhibition. Mol. Ther.-Oncolytics 2022, 26, 105–119. [Google Scholar] [CrossRef]
- Lou, J.; Dong, J.; Xu, R.; Zeng, H.; Fang, L.; Wu, Y.; Liu, Y.; Wang, S. Remodeling of the Tumor Microenvironment Using an Engineered Oncolytic Vaccinia Virus Improves PD-L1 Inhibition Outcomes. Biosci. Rep. 2021, 41, BSR20204186. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ravindranathan, R.; Kalinski, P.; Guo, Z.S.; Bartlett, D.L. Rational Combination of Oncolytic Vaccinia Virus and PD-L1 Blockade Works Synergistically to Enhance Therapeutic Efficacy. Nat. Commun. 2017, 8, 14754. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Kang, X.; Chen, K.S.; Jehng, T.; Jones, L.; Chen, J.; Huang, X.F.; Chen, S.-Y. An Engineered Oncolytic Virus Expressing PD-L1 Inhibitors Activates Tumor Neoantigen-Specific T Cell Responses. Nat. Commun. 2020, 11, 1395. [Google Scholar] [CrossRef] [PubMed]
- Semmrich, M.; Marchand, J.-B.; Fend, L.; Rehn, M.; Remy, C.; Holmkvist, P.; Silvestre, N.; Svensson, C.; Kleinpeter, P.; Deforges, J.; et al. Vectorized Treg-Depleting αCTLA-4 Elicits Antigen Cross-Presentation and CD8+ T Cell Immunity to Reject ‘Cold’ Tumors. J. Immunother. Cancer 2022, 10, e003488. [Google Scholar] [CrossRef]
- Zuo, S.; Wei, M.; Xu, T.; Kong, L.; He, B.; Wang, S.; Wang, S.; Wu, J.; Dong, J.; Wei, J. An Engineered Oncolytic Vaccinia Virus Encoding a Single-Chain Variable Fragment against TIGIT Induces Effective Antitumor Immunity and Synergizes with PD-1 or LAG-3 Blockade. J. Immunother. Cancer 2021, 9, e002843. [Google Scholar] [CrossRef]
- Advani, S.J.; Buckel, L.; Chen, N.G.; Scanderbeg, D.J.; Geissinger, U.; Zhang, Q.; Yu, Y.A.; Aguilar, R.J.; Mundt, A.J.; Szalay, A.A. Preferential Replication of Systemically Delivered Oncolytic Vaccinia Virus in Focally Irradiated Glioma Xenografts. Clin. Cancer Res. 2012, 18, 2579–2590. [Google Scholar] [CrossRef]
- Storozynsky, Q.T.; Agopsowicz, K.C.; Noyce, R.S.; Bukhari, A.B.; Han, X.; Snyder, N.; Umer, B.A.; Gamper, A.M.; Godbout, R.; Evans, D.H.; et al. Radiation Combined with Oncolytic Vaccinia Virus Provides Pronounced Antitumor Efficacy and Induces Immune Protection in an Aggressive Glioblastoma Model. Cancer Lett. 2023, 562, 216169. [Google Scholar] [CrossRef]
- Dai, M.H.; Liu, S.L.; Chen, N.G.; Zhang, T.P.; You, L.; Zhang, F.Q.; Chou, T.C.; Szalay, A.A.; Fong, Y.; Zhao, Y.P. Oncolytic Vaccinia Virus in Combination with Radiation Shows Synergistic Antitumor Efficacy in Pancreatic Cancer. Cancer Lett. 2014, 344, 282–290. [Google Scholar] [CrossRef]
- Chen, W.-Y.; Chen, Y.-L.; Lin, H.-W.; Chang, C.-F.; Huang, B.-S.; Sun, W.-Z.; Cheng, W.-F. Stereotactic Body Radiation Combined with Oncolytic Vaccinia Virus Induces Potent Anti-Tumor Effect by Triggering Tumor Cell Necroptosis and DAMPs. Cancer Lett. 2021, 523, 149–161. [Google Scholar] [CrossRef]
- Gholami, S.; Haddad, D.; Chen, C.-H.; Chen, N.G.; Zhang, Q.; Zanzonico, P.B.; Szalay, A.A.; Fong, Y. Novel Therapy for Anaplastic Thyroid Carcinoma Cells Using an Oncolytic Vaccinia Virus Carrying the Human Sodium Iodide Symporter. Surgery 2011, 150, 1040–1047. [Google Scholar] [CrossRef]
- Gholami, S.; Chen, C.-H.; Lou, E.; De Brot, M.; Fujisawa, S.; Chen, N.G.; Szalay, A.A.; Fong, Y. Vaccinia Virus GLV-1h153 Is Effective in Treating and Preventing Metastatic Triple-Negative Breast Cancer. Ann. Surg. 2012, 256, 437. [Google Scholar] [CrossRef]
- Haddad, D.; Chen, N.G.; Zhang, Q.; Chen, C.-H.; Yu, Y.A.; Gonzalez, L.; Carpenter, S.G.; Carson, J.; Au, J.; Mittra, A.; et al. Insertion of the Human Sodium Iodide Symporter to Facilitate Deep Tissue Imaging Does Not Alter Oncolytic or Replication Capability of a Novel Vaccinia Virus. J. Transl. Med. 2011, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, D.C.; Kyula, J.N.; Rosenfelder, N.; Chao-Chu, J.; Kramer-Marek, G.; Khan, A.A.; Roulstone, V.; McLaughlin, M.; Melcher, A.A.; Vile, R.G.; et al. Oncolytic Vaccinia Virus as a Vector for Therapeutic Sodium Iodide Symporter Gene Therapy in Prostate Cancer. Gene Ther. 2016, 23, 357–368. [Google Scholar] [CrossRef]
- Ottolino-Perry, K.; Mealiea, D.; Sellers, C.; Acuna, S.A.; Angarita, F.A.; Okamoto, L.; Scollard, D.; Ginj, M.; Reilly, R.; McCart, J.A. Vaccinia Virus and Peptide-Receptor Radiotherapy Synergize to Improve Treatment of Peritoneal Carcinomatosis. Mol. Ther.-Oncolytics 2023, 29, 44–58. [Google Scholar] [CrossRef]
- Huang, B.; Sikorski, R.; Kirn, D.H.; Thorne, S.H. Synergistic Anti-Tumor Effects between Oncolytic Vaccinia Virus and Paclitaxel Are Mediated by the IFN Response and HMGB1. Gene Ther. 2011, 18, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.A.; Galanis, C.; Woo, Y.; Chen, N.; Zhang, Q.; Fong, Y.; Szalay, A.A. Regression of Human Pancreatic Tumor Xenografts in Mice after a Single Systemic Injection of Recombinant Vaccinia Virus GLV-1h68. Mol. Cancer Ther. 2009, 8, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Lun, X.Q.; Jang, J.-H.; Tang, N.; Deng, H.; Head, R.; Bell, J.C.; Stojdl, D.F.; Nutt, C.L.; Senger, D.L.; Forsyth, P.A.; et al. Efficacy of Systemically Administered Oncolytic Vaccinia Virotherapy for Malignant Gliomas Is Enhanced by Combination Therapy with Rapamycin or Cyclophosphamide. Clin. Cancer Res. 2009, 15, 2777–2788. [Google Scholar] [CrossRef]
- Hofmann, E.; Weibel, S.; Szalay, A.A. Combination Treatment with Oncolytic Vaccinia Virus and Cyclophosphamide Results in Synergistic Antitumor Effects in Human Lung Adenocarcinoma Bearing Mice. J. Transl. Med. 2014, 12, 197. [Google Scholar] [CrossRef]
- Berchtold, S.; Beil, J.; Raff, C.; Smirnow, I.; Schell, M.; D’Alvise, J.; Gross, S.; Lauer, U.M. Assessing and Overcoming Resistance Phenomena against a Genetically Modified Vaccinia Virus in Selected Cancer Cell Lines. Int. J. Mol. Sci. 2020, 21, 7618. [Google Scholar] [CrossRef]
- Seubert, C.M.; Stritzker, J.; Hess, M.; Donat, U.; Sturm, J.B.; Chen, N.; von Hof, J.M.; Krewer, B.; Tietze, L.F.; Gentschev, I.; et al. Enhanced Tumor Therapy Using Vaccinia Virus Strain GLV-1h68 in Combination with a β-Galactosidase-Activatable Prodrug Seco-Analog of Duocarmycin SA. Cancer Gene Ther. 2011, 18, 42–52. [Google Scholar] [CrossRef]
- Lee, S.; Yang, W.; Kim, D.K.; Kim, H.; Shin, M.; Choi, K.U.; Suh, D.S.; Kim, Y.H.; Hwang, T.-H.; Kim, J.H. Inhibition of MEK-ERK Pathway Enhances Oncolytic Vaccinia Virus Replication in Doxorubicin-Resistant Ovarian Cancer. Mol. Ther.-Oncolytics 2022, 25, 211–224. [Google Scholar] [CrossRef]
- Ferguson, M.S.; Dunmall, L.S.C.; Gangeswaran, R.; Marelli, G.; Tysome, J.R.; Burns, E.; Whitehead, M.A.; Aksoy, E.; Alusi, G.; Hiley, C.; et al. Transient Inhibition of PI3Kδ Enhances the Therapeutic Effect of Intravenous Delivery of Oncolytic Vaccinia Virus. Mol. Ther. 2020, 28, 1263–1275. [Google Scholar] [CrossRef]
- MacTavish, H.; Diallo, J.-S.; Huang, B.; Stanford, M.; Boeuf, F.L.; Silva, N.D.; Cox, J.; Simmons, J.G.; Guimond, T.; Falls, T.; et al. Enhancement of Vaccinia Virus Based Oncolysis with Histone Deacetylase Inhibitors. PLoS ONE 2010, 5, e14462. [Google Scholar] [CrossRef]
- Kim, M.; Nitschké, M.; Sennino, B.; Murer, P.; Schriver, B.J.; Bell, A.; Subramanian, A.; McDonald, C.E.; Wang, J.; Cha, H.; et al. Amplification of Oncolytic Vaccinia Virus Widespread Tumor Cell Killing by Sunitinib through Multiple Mechanisms. Cancer Res. 2018, 78, 922–937. [Google Scholar] [CrossRef]
- Heo, J.; Breitbach, C.J.; Moon, A.; Kim, C.W.; Patt, R.; Kim, M.K.; Lee, Y.K.; Oh, S.Y.; Woo, H.Y.; Parato, K.; et al. Sequential Therapy With JX-594, A Targeted Oncolytic Poxvirus, Followed by Sorafenib in Hepatocellular Carcinoma: Preclinical and Clinical Demonstration of Combination Efficacy. Mol. Ther. 2011, 19, 1170–1179. [Google Scholar] [CrossRef]
- Chen, N.G.; and Szalay, A.A. Oncolytic Vaccinia Virus: A Theranostic Agent for Cancer. Future Virol. 2010, 5, 763–784. [Google Scholar] [CrossRef]
- Heo, J.; Reid, T.; Ruo, L.; Breitbach, C.J.; Rose, S.; Bloomston, M.; Cho, M.; Lim, H.Y.; Chung, H.C.; Kim, C.W.; et al. Randomized Dose-Finding Clinical Trial of Oncolytic Immunotherapeutic Vaccinia JX-594 in Liver Cancer. Nat. Med. 2013, 19, 329–336. [Google Scholar] [CrossRef]
- Moehler, M.; Heo, J.; Lee, H.C.; Tak, W.Y.; Chao, Y.; Paik, S.W.; Yim, H.J.; Byun, K.S.; Baron, A.; Ungerechts, G.; et al. Vaccinia-Based Oncolytic Immunotherapy Pexastimogene Devacirepvec in Patients with Advanced Hepatocellular Carcinoma after Sorafenib Failure: A Randomized Multicenter Phase IIb Trial (TRAVERSE). Oncoimmunology 2019, 8, 1615817. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Galle, P.R.; Chao, Y.; Erinjeri, J.; Heo, J.; Borad, M.J.; Luca, A.; Burke, J.; Pelusio, A.; Agathon, D.; et al. PHOCUS: A Phase 3, Randomized, Open-Label Study of Sequential Treatment with Pexa-Vec (JX-594) and Sorafenib in Patients with Advanced Hepatocellular Carcinoma. Liver Cancer 2023, 13, 256–272. [Google Scholar] [CrossRef]
- Toulmonde, M.; Cousin, S.; Kind, M.; Guegan, J.-P.; Bessede, A.; Le Loarer, F.; Perret, R.; Cantarel, C.; Bellera, C.; Italiano, A. Randomized Phase 2 Trial of Intravenous Oncolytic Virus JX-594 Combined with Low-Dose Cyclophosphamide in Patients with Advanced Soft-Tissue Sarcoma. J. Hematol. Oncol. 2022, 15, 149. [Google Scholar] [CrossRef]
- Heo, J.; Breitbach, C.; Cho, M.; Hwang, T.-H.; Kim, C.W.; Jeon, U.B.; Woo, H.Y.; Yoon, K.T.; Lee, J.W.; Burke, J.; et al. Phase II Trial of Pexa-Vec (Pexastimogene Devacirepvec; JX-594), an Oncolytic and Immunotherapeutic Vaccinia Virus, Followed by Sorafenib in Patients with Advanced Hepatocellular Carcinoma (HCC). JCO 2013, 31, 4122. [Google Scholar] [CrossRef]
- Kim, S.-G.; Ha, H.K.; Lim, S.; De Silva, N.S.; Pelusio, A.; Mun, J.H.; Patt, R.H.; Breitbach, C.J.; Burke, J.M. Phase II Trial of Pexa-Vec (Pexastimogene Devacirepvec; JX-594), an Oncolytic and Immunotherapeutic Vaccinia Virus, in Patients with Metastatic, Refractory Renal Cell Carcinoma (RCC). JCO 2018, 36, 671. [Google Scholar] [CrossRef]
- Immunization Strategy with Intra-Tumoral Injections of Pexa-Vec with Ipilimumab in Metastatic/Advanced Solid Tumors. (ISI-JX). Available online: https://clinicaltrials.gov/study/NCT02977156 (accessed on 11 July 2025).
- Monge, C.; Xie, C.; Myojin, Y.; Coffman, K.; Hrones, D.M.; Wang, S.; Hernandez, J.M.; Wood, B.J.; Levy, E.B.; Juburi, I.; et al. Phase I/II Study of PexaVec in Combination with Immune Checkpoint Inhibition in Refractory Metastatic Colorectal Cancer. J. Immunother. Cancer 2023, 11, e005640. [Google Scholar] [CrossRef]
- Lauer, U.M.; Schell, M.; Beil, J.; Berchtold, S.; Koppenhöfer, U.; Glatzle, J.; Königsrainer, A.; Möhle, R.; Nann, D.; Fend, F.; et al. Phase I Study of Oncolytic Vaccinia Virus GL-ONC1 in Patients with Peritoneal Carcinomatosis. Clin. Cancer Res. 2018, 24, 4388–4398. [Google Scholar] [CrossRef]
- Pedersen, J.V.; Karapanagiotou, E.M.; Biondo, A.; Tunariu, N.; Puglisi, M.; Denholm, K.A.; Sassi, S.; Mansfield, D.; Yap, T.A.; De Bono, J.S.; et al. A Phase I Clinical Trial of a Genetically Modified and Imageable Oncolytic Vaccinia Virus GL-ONC1 with Clinical Green Fluorescent Protein (GFP) Imaging. JCO 2011, 29, 2577. [Google Scholar] [CrossRef]
- Mell, L.K.; Brumund, K.T.; Daniels, G.A.; Advani, S.J.; Zakeri, K.; Wright, M.E.; Onyeama, S.-J.; Weisman, R.A.; Sanghvi, P.R.; Martin, P.J.; et al. Phase I Trial of Intravenous Oncolytic Vaccinia Virus (GL-ONC1) with Cisplatin and Radiotherapy in Patients with Locoregionally Advanced Head and Neck Carcinoma. Clin. Cancer Res. 2017, 23, 5696–5702. [Google Scholar] [CrossRef]
- Krug, L.M.; Zauderer, M.G.; Adusumili, P.S.; McGee, E.; Sepkowitz, K.; Klang, M.; Yu, Y.A.; Scigalla, P.; Rusch, V.W. Phase I Study of Intra-Pleural Administration of GL-ONC1, an Oncolytic Vaccinia Virus, in Patients with Malignant Pleural Effusion. JCO 2015, 33, 7559. [Google Scholar] [CrossRef]
- Chintala, N.K.; Choe, J.K.; McGee, E.; Bellis, R.; Saini, J.K.; Banerjee, S.; Moreira, A.L.; Zauderer, M.G.; Adusumilli, P.S.; Rusch, V.W. Correlative Analysis from a Phase I Clinical Trial of Intrapleural Administration of Oncolytic Vaccinia Virus (Olvi-Vec) in Patients with Malignant Pleural Mesothelioma. Front. Immunol. 2023, 14, 1112960. [Google Scholar] [CrossRef] [PubMed]
- Holloway, R.W.; Mendivil, A.A.; Kendrick, J.E.; Abaid, L.N.; Brown, J.V.; LeBlanc, J.; McKenzie, N.D.; Mori, K.M.; Ahmad, S. Clinical Activity of Olvimulogene Nanivacirepvec–Primed Immunochemotherapy in Heavily Pretreated Patients With Platinum-Resistant or Platinum-Refractory Ovarian Cancer. JAMA Oncol. 2023, 9, 903–908. [Google Scholar] [CrossRef]
- Genelux Clinical Trial Summary. Available online: https://Genelux.Com/Clinical-Trials-Summary/ (accessed on 8 May 2025).
- GL-ONC1 Administered Intravenously Prior to Surgery to Patients with Solid Organ Cancers. Available online: https://clinicaltrials.gov/study/NCT02714374 (accessed on 11 July 2025).
- Expanded Access to Provide GL-ONC1 for the Treatment of Advanced Cancers with No Standard of Care. Available online: https://clinicaltrials.gov/study/NCT03420430 (accessed on 11 July 2025).
- Safety and Efficacy of the ONCOlytic VIRus Armed for Local Chemotherapy, TG6002/5-FC, in Recurrent Glioblastoma Patients (ONCOVIRAC). Available online: https://www.clinicaltrials.gov/study/NCT03294486 (accessed on 11 July 2025).
- Bendjama, K.; Cassier, P.; Moreno, V.; Doger, B.; Calvo, E.; de Miguel, M.; Jungels, C.; Erbs, P.; Carpentier, D.; Sadoun, A. Abstract LB179: Oncolytic Virus TG6002 Locates to Tumors after Intravenous Infusion and Induces Tumor-Specific Expression of a Functional pro-Drug Activating Enzyme in Patients with Advanced Gastrointestinal Carcinomas. Cancer Res. 2021, 81, LB179. [Google Scholar] [CrossRef]
- West, E.J.; Sadoun, A.; Bendjama, K.; Erbs, P.; Smolenschi, C.; Cassier, P.A.; de Baere, T.; Sainte-Croix, S.; Brandely, M.; Melcher, A.A.; et al. A Phase I Clinical Trial of Intrahepatic Artery Delivery of TG6002 in Combination with Oral 5-Fluorocytosine in Patients with Liver-Dominant Metastatic Colorectal Cancer. Clin. Cancer Res. 2025, 31, 1243–1256. [Google Scholar] [CrossRef] [PubMed]
- Study of TBio-6517 Given Alone or in Combination with Pembrolizumab in Solid Tumors (RAPTOR). Available online: https://www.clinicaltrials.gov/study/NCT04301011 (accessed on 11 July 2025).
- Chang, C.-L.; Ma, B.; Pang, X.; Wu, T.-C.; Hung, C.-F. Treatment With Cyclooxygenase-2 Inhibitors Enables Repeated Administration of Vaccinia Virus for Control of Ovarian Cancer. Mol. Ther. 2009, 17, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Evgin, L.; Acuna, S.A.; de Souza, C.T.; Marguerie, M.; Lemay, C.G.; Ilkow, C.S.; Findlay, C.S.; Falls, T.; Parato, K.A.; Hanwell, D.; et al. Complement Inhibition Prevents Oncolytic Vaccinia Virus Neutralization in Immune Humans and Cynomolgus Macaques. Mol. Ther. 2015, 23, 1066–1076. [Google Scholar] [CrossRef]
- Lee, N.; Jeon, Y.-H.; Yoo, J.; Shin, S.; Lee, S.; Park, M.-J.; Jung, B.-J.; Hong, Y.-K.; Lee, D.-S.; Oh, K. Generation of Novel Oncolytic Vaccinia Virus with Improved Intravenous Efficacy through Protection against Complement-Mediated Lysis and Evasion of Neutralization by Vaccinia Virus-Specific Antibodies. J. Immunother. Cancer 2023, 11, e006024. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).