Stathmin Serine 16 Phosphorylation Is a Key Regulator of Cell Cycle Progression Without Activating Migration and Invasion In Vitro
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Prostate and Breast Cancer Clinicogenomics
2.2. Cells and Reagents
2.3. qPCR
2.4. Cell Viability Assays
2.5. Transfection Assays
2.6. Doubling Time Assays
2.7. Migration and Invasion Assays
2.8. Western Blot Analysis
2.9. Flow Cytometry
2.10. Quantification and Statistical Analysis
3. Results
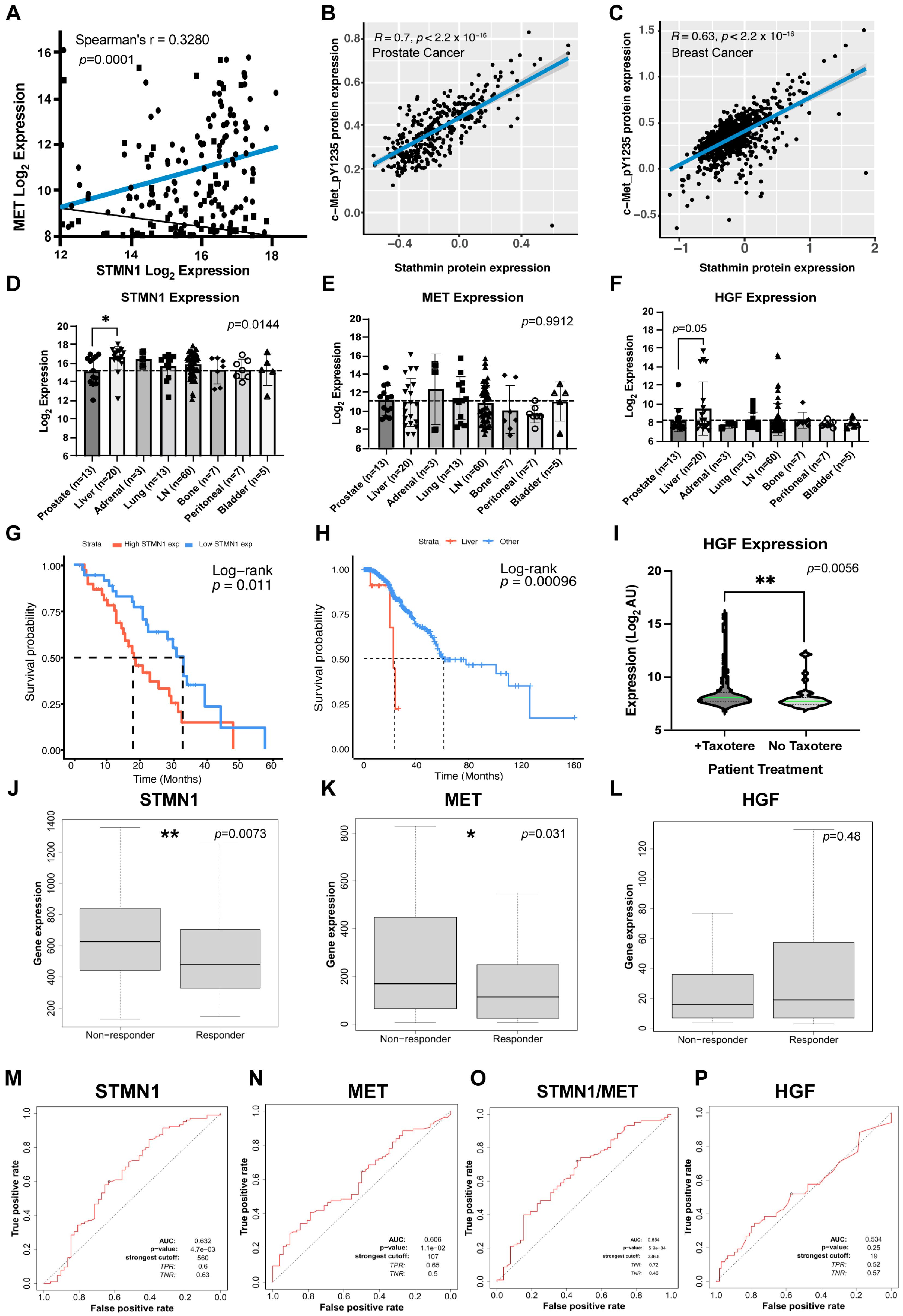
3.1. Increased STMN1 Is Associated with Increased MET and HGF in mCRPC
3.2. Increased STMN1 and HGF Expression Is Associated with Liver Metastasis and Decreased Overall Survival
3.3. STMN1 and MET Predict Response to Taxane Chemotherapy in Node-Positive Bc Patients
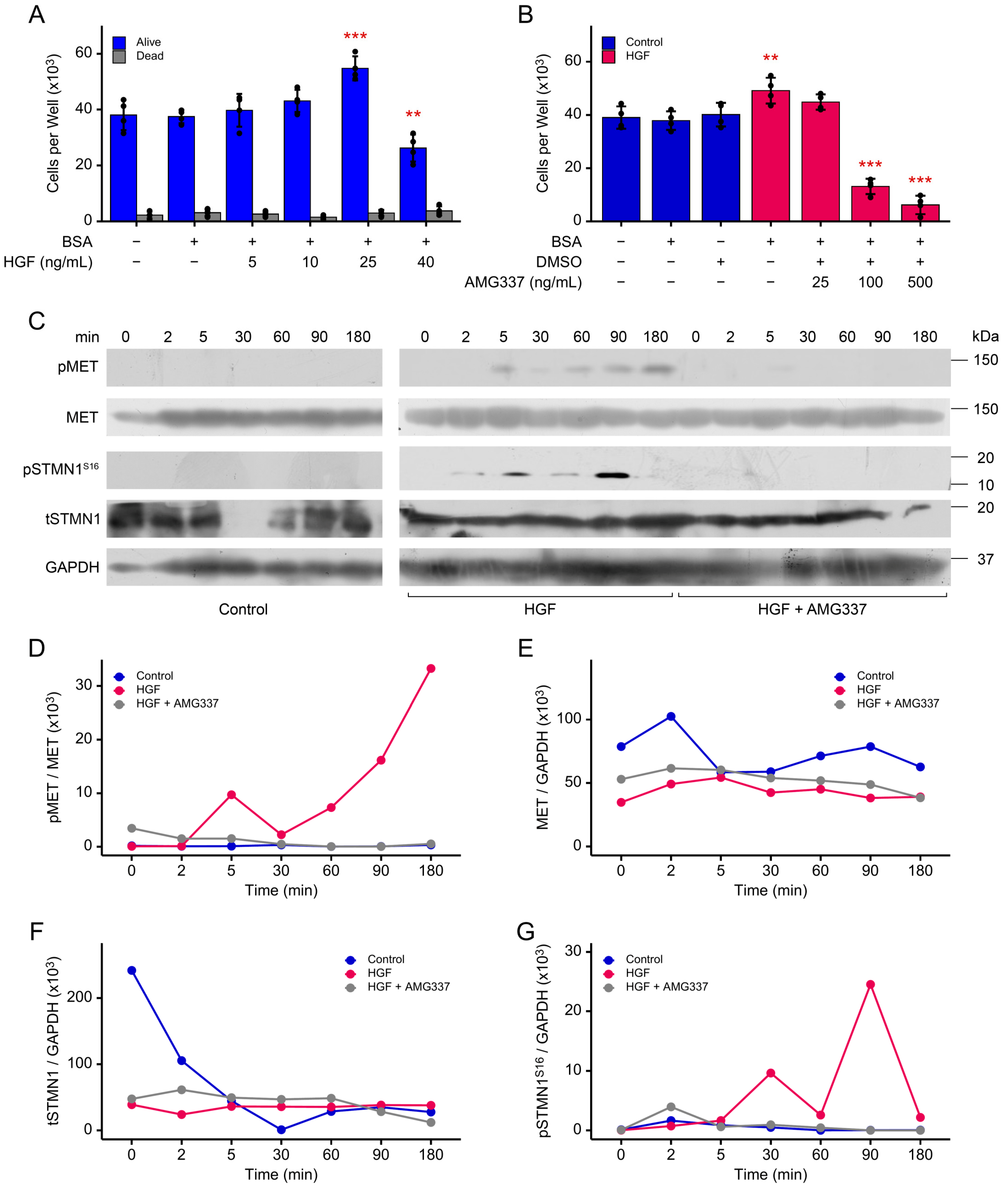
3.4. STMN1S16 Phosphorylation and Cell Proliferation Are Regulated by HGF/MET
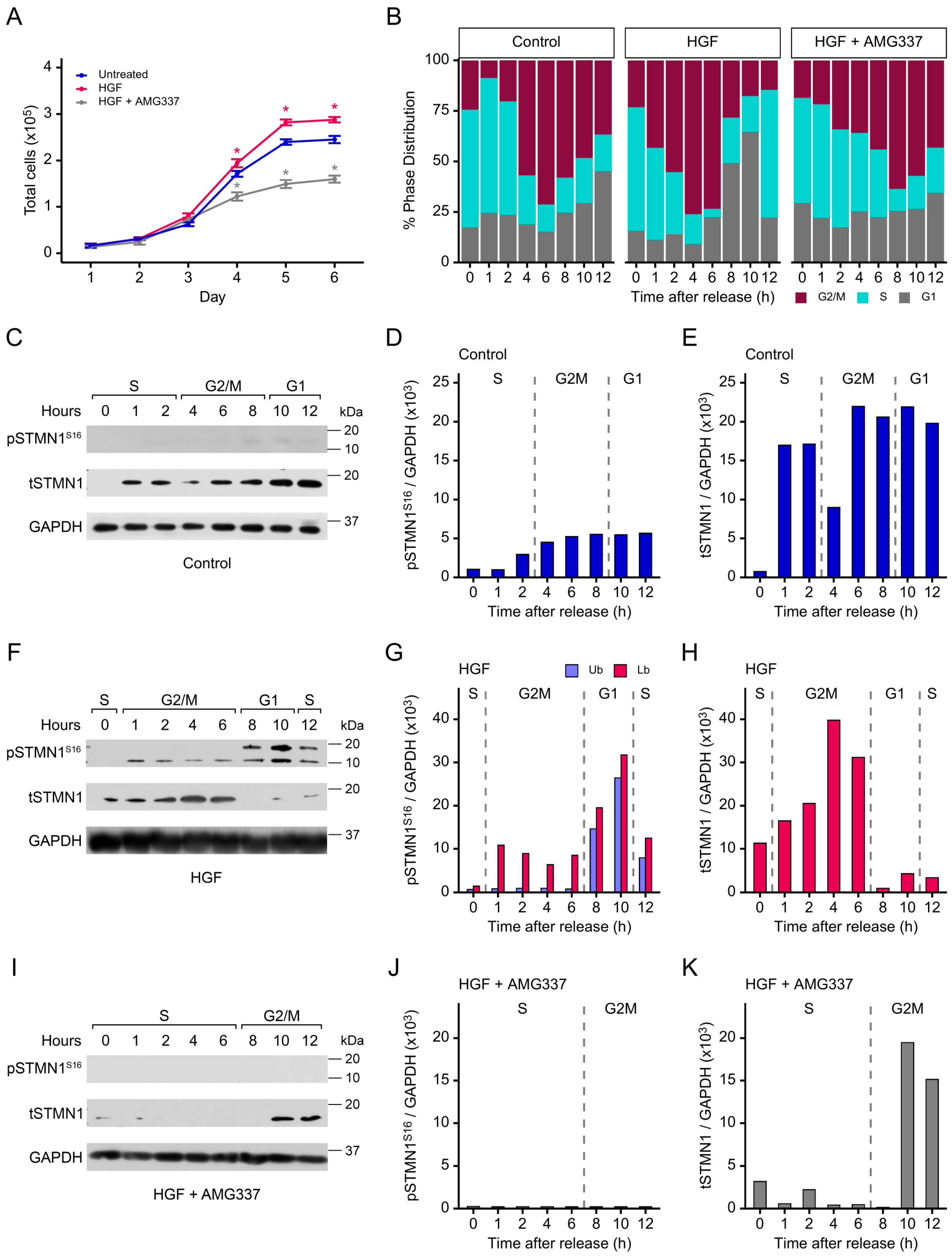
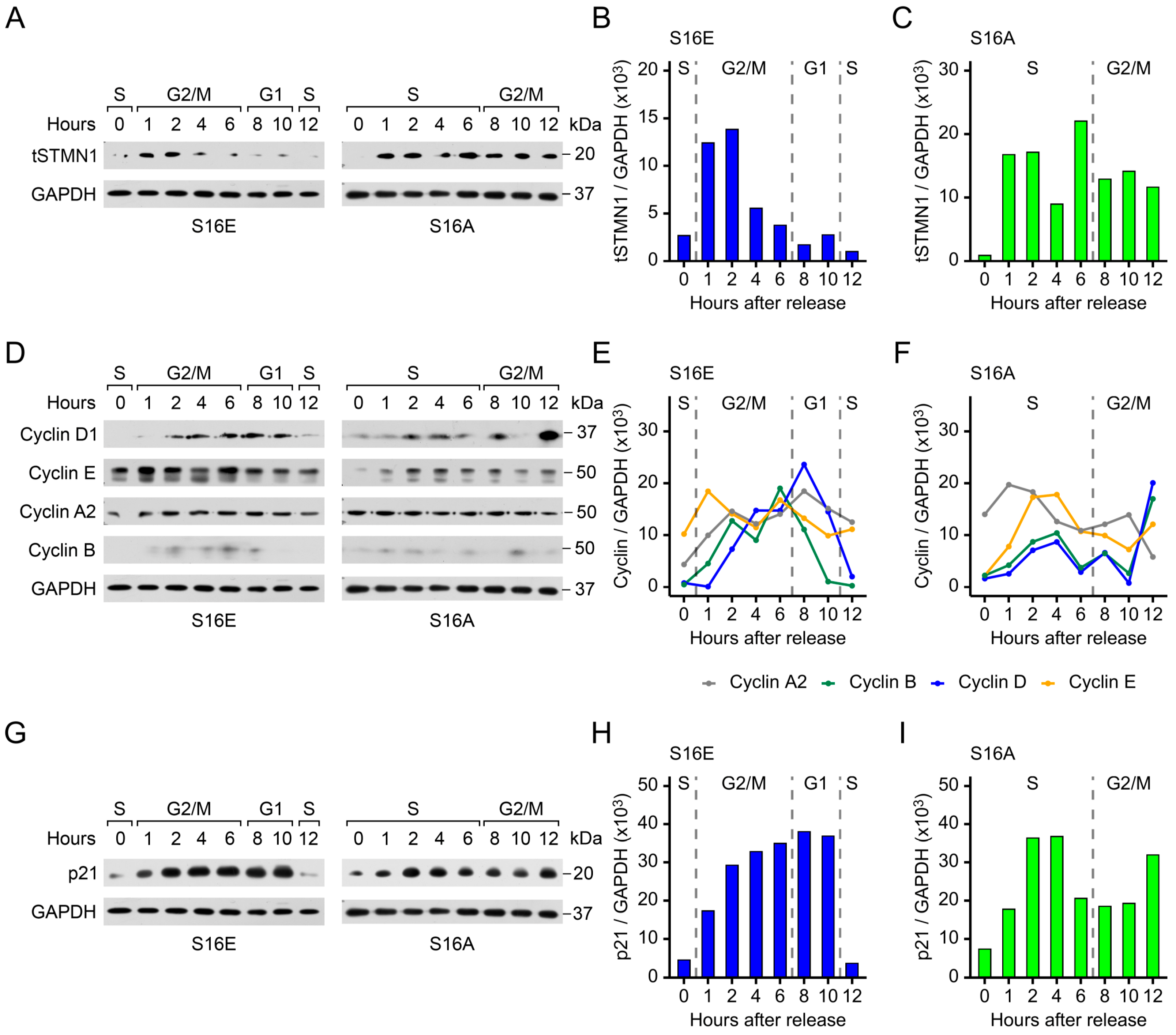
3.5. Both pSTMN1S16 and tSTMN1 Levels Are Modulated by HGF/MET During Cell Cycle Progression
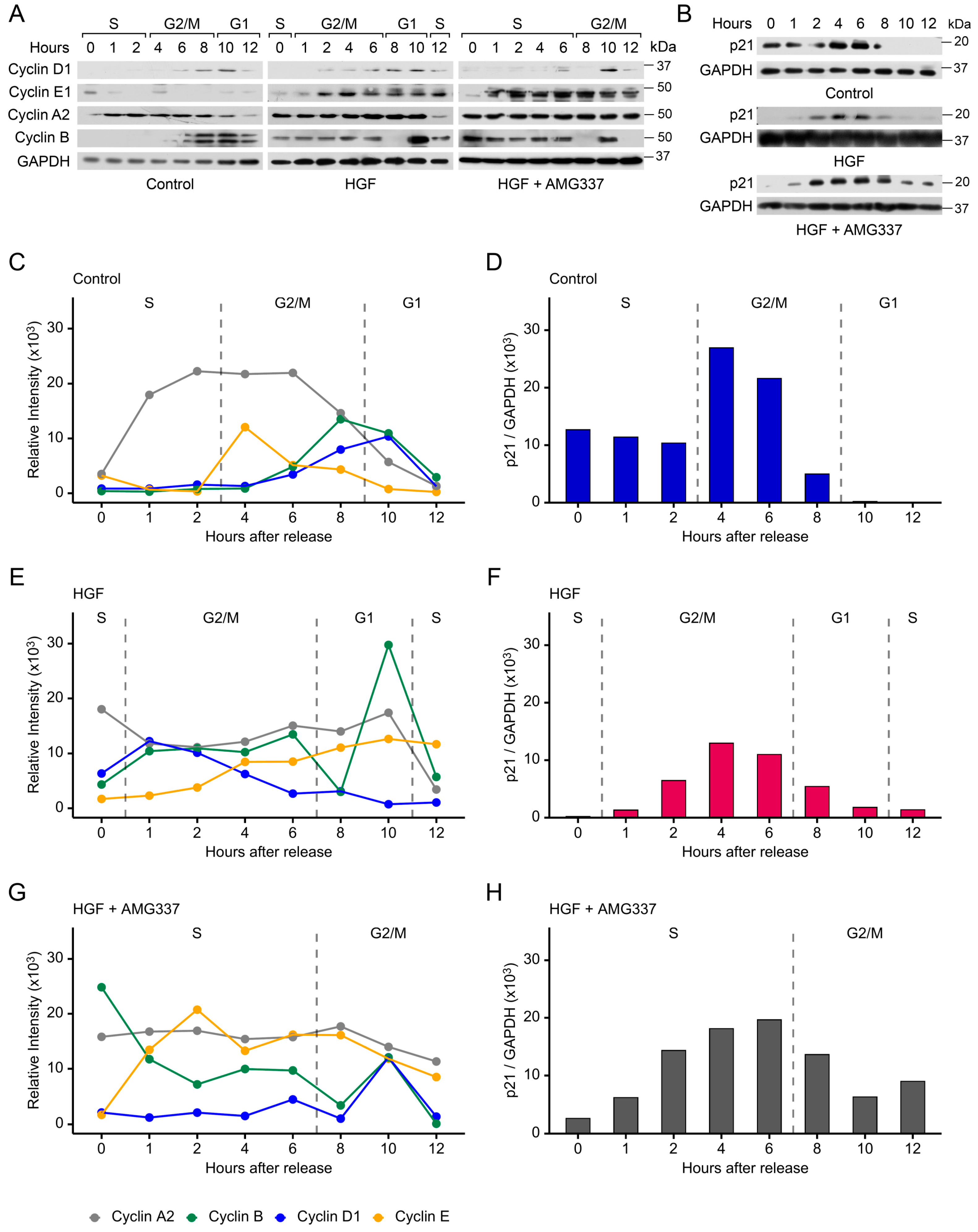
3.6. Cyclins and p21 Are Concomitantly Regulated by pSTMN1S16
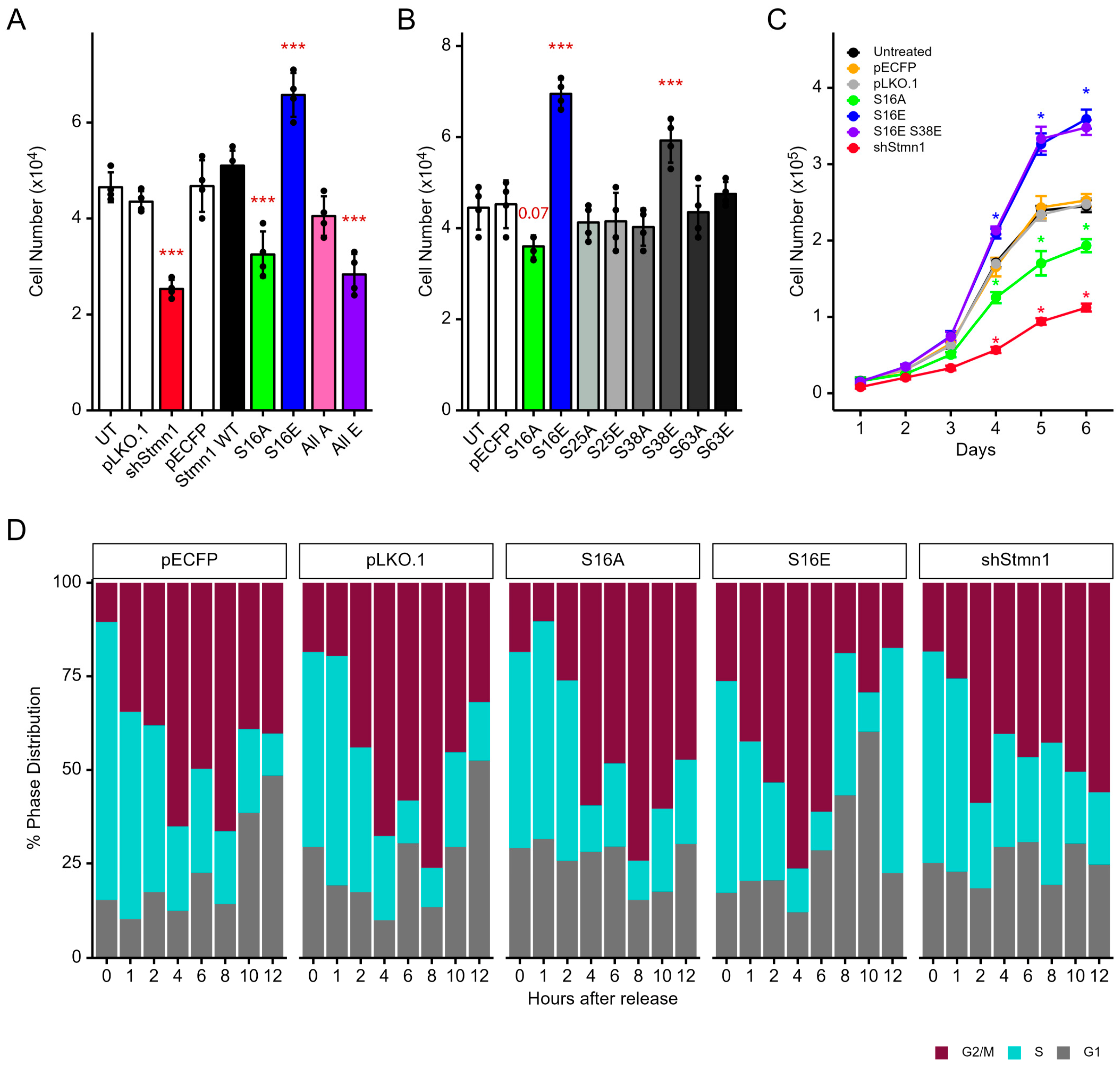
3.7. pStathminS16 Alone Is Sufficient for Regulating Cell Cycle Progression and Growth
3.8. STMN1S16E and STMN1S16A Modulate tSTMN1, Cyclins, and p21 Levels
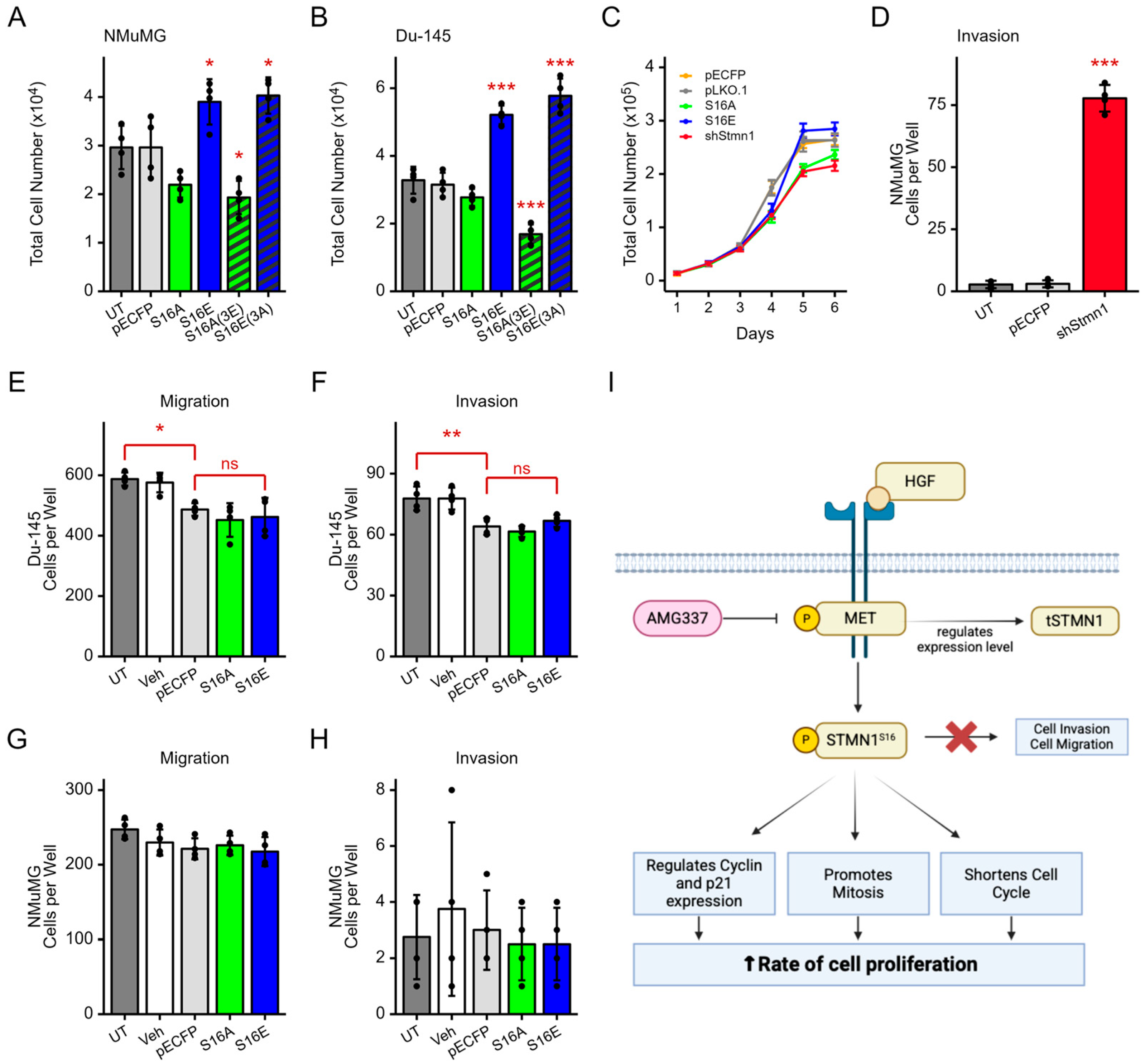
3.9. pSTMN1S16 Regulates Cell Proliferation but Not Metastatic Potential
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A | alanine |
| ADT | androgen deprivation therapy |
| a.k.a. | also known as |
| ANOVA | analysis of variance |
| AR | androgen receptor |
| BC | breast cancer |
| BPH | benign prostate hyperplasia |
| CAMKII | Calcium/Calmodulin-dependent Protein Kinase II |
| CDK1 | cyclin dependent kinase 1 |
| cDNA | copy deoxynucleic acid |
| CTX | C-terminal telopeptide |
| E | glutamic acid |
| ECL | Enhanced Chemiluminescence |
| EMT | epithelial mesenchymal transition |
| Fig | figure |
| FoxM1 | Forkhead Box M1 |
| G1/S/G0/M | phases of cell cycle |
| HGF | hepatocyte growth factor |
| m | metastatic |
| mCRPC | metastatic castration-resistant prostate cancer |
| MET | MET proto-oncogene, Receptor Tyrosine Kinase |
| p | phospho |
| p21 | Cyclin Dependent Kinase Inhibitor 1A |
| P53 | tumor protein 53 |
| PCa | prostate cancer |
| pECFP | plasmid enhanced cyan fluorescent protein |
| pLKO.1/shSTMN1 | MISSION® pLKO.1-puro Non-Target shRNA control |
| RNA | ribonucleic acid |
| STMN1 | Stathmin 1 |
| S | serine |
| sh | Short hairpin |
| Sp1 | Sp1 transcription factor |
| t | total |
| VEGFR-2 | vascular endothelial growth factor receptor 2 |
References
- Roy, S.; Saad, F. Metastatic castrate-resistant prostate cancer: A new horizon beyond the androgen receptors. Curr. Opin. Support. Palliat. Care 2022, 16, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Muralidhar, A.; Potluri, H.K.; Jaiswal, T.; McNeel, D.G. Targeted Radiation and Immune Therapies-Advances and Opportunities for the Treatment of Prostate Cancer. Pharmaceutics 2023, 15, 252. [Google Scholar] [CrossRef] [PubMed]
- Matthews, H.K.; Bertoli, C.; de Bruin, R.A.M. Cell cycle control in cancer. Nat. Rev. Mol. Cell Biol. 2022, 23, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Sumanasuriya, S.; De Bono, J. Treatment of Advanced Prostate Cancer-A Review of Current Therapies and Future Promise. Cold Spring Harb. Perspect. Med. 2018, 8, a030635. [Google Scholar] [CrossRef]
- Iacopetta, D.; Ceramella, J.; Baldino, N.; Sinicropi, M.S.; Catalano, A. Targeting Breast Cancer: An Overlook on Current Strategies. Int. J. Mol. Sci. 2023, 24, 3643. [Google Scholar] [CrossRef]
- Herbst, R.S.; Khuri, F.R. Mode of action of docetaxel—A basis for combination with novel anticancer agents. Cancer Treat. Rev. 2003, 29, 407–415. [Google Scholar] [CrossRef]
- Martin, S.K.; Kyprianou, N. Exploitation of the Androgen Receptor to Overcome Taxane Resistance in Advanced Prostate Cancer. Adv. Cancer Res. 2015, 127, 123–158. [Google Scholar] [CrossRef]
- Curmi, P.A.; Gavet, O.; Charbaut, E.; Ozon, S.; Lachkar-Colmerauer, S.; Manceau, V.; Siavoshian, S.; Maucuer, A.; Sobel, A. Stathmin and its phosphoprotein family: General properties, biochemical and functional interaction with tubulin. Cell Struct. Funct. 1999, 24, 345–357. [Google Scholar] [CrossRef]
- Sobel, A. Stathmin: A relay phosphoprotein for multiple signal transduction? Trends Biochem. Sci. 1991, 16, 301–305. [Google Scholar] [CrossRef]
- Cassimeris, L. The oncoprotein 18/stathmin family of microtubule destabilizers. Curr. Opin. Cell Biol. 2002, 14, 18–24. [Google Scholar] [CrossRef]
- Beretta, L.; Dobransky, T.; Sobel, A. Multiple phosphorylation of stathmin. Identification of four sites phosphorylated in intact cells and in vitro by cyclic AMP-dependent protein kinase and p34cdc2. J. Biol. Chem. 1993, 268, 20076–20084. [Google Scholar] [CrossRef] [PubMed]
- Rubin, C.I.; Atweh, G.F. The role of stathmin in the regulation of the cell cycle. J. Cell Biochem. 2004, 93, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yeh, Y.A.; Cheng, S.; Gu, X.; Yang, S.; Li, L.; Khater, N.P.; Kasper, S.; Yu, X. Stathmin 1 expression in neuroendocrine and proliferating prostate cancer. Discov. Oncol. 2025, 16, 19. [Google Scholar] [CrossRef] [PubMed]
- Yang, H. Tau and stathmin proteins in breast cancer: A potential therapeutic target. Clin. Exp. Pharmacol. Physiol. 2022, 49, 445–452. [Google Scholar] [CrossRef]
- Jurmeister, S.; Ramos-Montoya, A.; Sandi, C.; Pertega-Gomes, N.; Wadhwa, K.; Lamb, A.D.; Dunning, M.J.; Attig, J.; Carroll, J.S.; Fryer, L.G.; et al. Identification of potential therapeutic targets in prostate cancer through a cross-species approach. EMBO Mol. Med. 2018, 10, e8274. [Google Scholar] [CrossRef]
- Xu, J.; Wu, W.; Tang, Y.; Lin, Y.; Xue, Y.; Hu, J.; Lin, D. PRL-3 exerts oncogenic functions in myeloid leukemia cells via aberrant dephosphorylation of stathmin and activation of STAT3 signaling. Aging 2019, 11, 7817–7829. [Google Scholar] [CrossRef]
- Chen, X.; Liao, Y.; Long, D.; Yu, T.; Shen, F.; Lin, X. The Cdc2/Cdk1 inhibitor, purvalanol A, enhances the cytotoxic effects of taxol through Op18/stathmin in non-small cell lung cancer cells in vitro. Int. J. Mol. Med. 2017, 40, 235–242. [Google Scholar] [CrossRef]
- Takahashi, K.; Suzuki, K. Membrane transport of WAVE2 and lamellipodia formation require Pak1 that mediates phosphorylation and recruitment of stathmin/Op18 to Pak1-WAVE2-kinesin complex. Cell Signal. 2009, 21, 695–703. [Google Scholar] [CrossRef]
- Machado-Neto, J.A.; Rodrigues Alves, A.P.N.; Fernandes, J.C.; Coelho-Silva, J.L.; Scopim-Ribeiro, R.; Fenerich, B.A.; da Silva, F.B.; Scheucher, P.S.; Simoes, B.P.; Rego, E.M.; et al. Paclitaxel induces Stathmin 1 phosphorylation, microtubule stability and apoptosis in acute lymphoblastic leukemia cells. Heliyon 2017, 3, e00405. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, T.; Song, X.; Tian, Y.; Wei, Y.; Wang, J.; Li, X.; Yang, X. Siva 1 inhibits proliferation, migration and invasion by phosphorylating Stathmin in ovarian cancer cells. Oncol. Lett. 2017, 14, 1512–1518. [Google Scholar] [CrossRef]
- Wu, H.; Deng, W.W.; Yang, L.L.; Zhang, W.F.; Sun, Z.J. Expression and phosphorylation of Stathmin 1 indicate poor survival in head and neck squamous cell carcinoma and associate with immune suppression. Biomark. Med. 2018, 12, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Wik, E.; Birkeland, E.; Trovik, J.; Werner, H.M.; Hoivik, E.A.; Mjos, S.; Krakstad, C.; Kusonmano, K.; Mauland, K.; Stefansson, I.M.; et al. High phospho-Stathmin(Serine38) expression identifies aggressive endometrial cancer and suggests an association with PI3K inhibition. Clin. Cancer Res. 2013, 19, 2331–2341. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Jiang, P.; Du, W.; Wu, Z.; Li, C.; Qiao, M.; Yang, X.; Wu, M. Siva1 suppresses epithelial-mesenchymal transition and metastasis of tumor cells by inhibiting stathmin and stabilizing microtubules. Proc. Natl. Acad. Sci. USA 2011, 108, 12851–12856. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, S.; Shen, F.; Long, D.; Yu, T.; Wu, M.; Lin, X. In Vitro neutralization of autocrine IL-10 affects Op18/stathmin signaling in non-small cell lung cancer cells. Oncol. Rep. 2019, 41, 501–511. [Google Scholar] [CrossRef]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef]
- Humphrey, P.A.; Zhu, X.; Zarnegar, R.; Swanson, P.E.; Ratliff, T.L.; Vollmer, R.T.; Day, M.L. Hepatocyte growth factor and its receptor (c-MET) in prostatic carcinoma. Am. J. Pathol. 1995, 147, 386–396. [Google Scholar]
- Hass, R.; Jennek, S.; Yang, Y.; Friedrich, K. c-Met expression and activity in urogenital cancers—Novel aspects of signal transduction and medical implications. Cell Commun. Signal. CCS 2017, 15, 10. [Google Scholar] [CrossRef]
- Knudsen, B.S.; Edlund, M. Prostate cancer and the met hepatocyte growth factor receptor. Adv. Cancer Res. 2004, 91, 31–67. [Google Scholar] [CrossRef]
- Knudsen, B.S.; Gmyrek, G.A.; Inra, J.; Scherr, D.S.; Vaughan, E.D.; Nanus, D.M.; Kattan, M.W.; Gerald, W.L.; Vande Woude, G.F. High expression of the Met receptor in prostate cancer metastasis to bone. Urology 2002, 60, 1113–1117. [Google Scholar] [CrossRef]
- Varkaris, A.; Corn, P.G.; Gaur, S.; Dayyani, F.; Logothetis, C.J.; Gallick, G.E. The role of HGF/c-Met signaling in prostate cancer progression and c-Met inhibitors in clinical trials. Expert Opin. Investig. Drugs 2011, 20, 1677–1684. [Google Scholar] [CrossRef]
- Xie, Y.; Lu, W.; Liu, S.; Yang, Q.; Carver, B.S.; Li, E.; Wang, Y.; Fazli, L.; Gleave, M.; Chen, Z. Crosstalk between nuclear MET and SOX9/beta-catenin correlates with castration-resistant prostate cancer. Mol. Endocrinol. 2014, 28, 1629–1639. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, P.A.; Halabi, S.; Picus, J.; Sanford, B.; Vogelzang, N.J.; Small, E.J.; Kantoff, P.W. Prognostic significance of plasma scatter factor/hepatocyte growth factor levels in patients with metastatic hormone- refractory prostate cancer: Results from cancer and leukemia group B 150005/9480. Clin. Genitourin. Cancer 2006, 4, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Aftab, D.T.; McDonald, D.M. MET and VEGF: Synergistic targets in castration-resistant prostate cancer. Clin. Transl. Oncol. 2011, 13, 703–709. [Google Scholar] [CrossRef]
- Raghav, K.P.; Wang, W.; Liu, S.; Chavez-MacGregor, M.; Meng, X.; Hortobagyi, G.N.; Mills, G.B.; Meric-Bernstam, F.; Blumenschein, G.R., Jr.; Gonzalez-Angulo, A.M. cMET and phospho-cMET protein levels in breast cancers and survival outcomes. Clin. Cancer Res. 2012, 18, 2269–2277. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, C.; Cui, S. Expression of hepatocyte growth factor in breast cancer and its effect on prognosis and sensitivity to chemotherapy. Mol. Med. Rep. 2015, 11, 1037–1042. [Google Scholar] [CrossRef][Green Version]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef]
- Lan, H.; Wu, B.; Jin, K.; Chen, Y. Beyond boundaries: Unraveling innovative approaches to combat bone-metastatic cancers. Front. Endocrinol. 2023, 14, 1260491. [Google Scholar] [CrossRef]
- Kumar, A.; Coleman, I.; Morrissey, C.; Zhang, X.; True, L.D.; Gulati, R.; Etzioni, R.; Bolouri, H.; Montgomery, B.; White, T.; et al. Substantial interindividual and limited intraindividual genomic diversity among tumors from men with metastatic prostate cancer. Nat. Med. 2016, 22, 369–378. [Google Scholar] [CrossRef]
- Abida, W.; Cyrta, J.; Heller, G.; Prandi, D.; Armenia, J.; Coleman, I.; Cieslik, M.; Benelli, M.; Robinson, D.; Van Allen, E.M.; et al. Genomic correlates of clinical outcome in advanced prostate cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 11428–11436. [Google Scholar] [CrossRef]
- Stopsack, K.H.; Nandakumar, S.; Wibmer, A.G.; Haywood, S.; Weg, E.S.; Barnett, E.S.; Kim, C.J.; Carbone, E.A.; Vasselman, S.E.; Nguyen, B.; et al. Oncogenic Genomic Alterations, Clinical Phenotypes, and Outcomes in Metastatic Castration-Sensitive Prostate Cancer. Clin. Cancer Res. 2020, 26, 3230–3238. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2010. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Fekete, J.T.; Gyorffy, B. ROCplot.org: Validating predictive biomarkers of chemotherapy/hormonal therapy/anti-HER2 therapy using transcriptomic data of 3104 breast cancer patients. Int. J. Cancer 2019, 145, 3140–3151. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.; Ghosh, R.; Vummidi Giridhar, P.; Gu, G.; Case, T.; SM, B.; Kasper, S. Inhibition of Stathmin1 Accelerates the Metastatic Process. Cancer Res. 2012, 72, 5407–5417. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Gu, G.; Tillman, E.; Yuan, J.; Wang, Y.; Fazli, L.; Rennie, P.S.; Kasper, S. Increased expression and differential phosphorylation of stathmin may promote prostate cancer progression. Prostate 2007, 67, 1038–1052. [Google Scholar] [CrossRef]
- Wang, R.C.; Wang, Z. Synchronization of Cultured Cells to G1, S, G2, and M Phases by Double Thymidine Block. Methods Mol. Biol. 2022, 2579, 61–71. [Google Scholar] [CrossRef]
- Wee, P.; Wang, Z. Cell Cycle Synchronization of HeLa Cells to Assay EGFR Pathway Activation. Methods Mol. Biol. 2017, 1652, 167–181. [Google Scholar] [CrossRef]
- Li, J.; Lu, Y.; Akbani, R.; Ju, Z.; Roebuck, P.L.; Liu, W.; Yang, J.Y.; Broom, B.M.; Verhaak, R.G.; Kane, D.W.; et al. TCPA: A resource for cancer functional proteomics data. Nat. Methods 2013, 10, 1046–1047. [Google Scholar] [CrossRef]
- Li, J.; Akbani, R.; Zhao, W.; Lu, Y.; Weinstein, J.N.; Mills, G.B.; Liang, H. Explore, Visualize, and Analyze Functional Cancer Proteomic Data Using the Cancer Proteome Atlas. Cancer Res. 2017, 77, e51–e54. [Google Scholar] [CrossRef]
- Ulm, M.; Ramesh, A.V.; McNamara, K.M.; Ponnusamy, S.; Sasano, H.; Narayanan, R. Therapeutic advances in hormone-dependent cancers: Focus on prostate, breast and ovarian cancers. Endocr. Connect. 2019, 8, R10–R26. [Google Scholar] [CrossRef]
- Mueller, K.L.; Yang, Z.Q.; Haddad, R.; Ethier, S.P.; Boerner, J.L. EGFR/Met association regulates EGFR TKI resistance in breast cancer. J. Mol. Signal. 2010, 5, 8. [Google Scholar] [CrossRef]
- Tuong, Z.K.; Loudon, K.W.; Berry, B.; Richoz, N.; Jones, J.; Tan, X.; Nguyen, Q.; George, A.; Hori, S.; Field, S.; et al. Resolving the immune landscape of human prostate at a single-cell level in health and cancer. Cell Rep. 2021, 37, 110132. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Karaszewska, B.; Kang, Y.K.; Chung, H.C.; Shankaran, V.; Siena, S.; Go, N.F.; Yang, H.; Schupp, M.; Cunningham, D. A Multicenter Phase II Study of AMG 337 in Patients with MET-Amplified Gastric/Gastroesophageal Junction/Esophageal Adenocarcinoma and Other MET-Amplified Solid Tumors. Clin. Cancer Res. 2019, 25, 2414–2423. [Google Scholar] [CrossRef] [PubMed]
- le Gouvello, S.; Manceau, V.; Sobel, A. Serine 16 of stathmin as a cytosolic target for Ca2+/calmodulin-dependent kinase II after CD2 triggering of human T lymphocytes. J. Immunol. 1998, 161, 1113–1122. [Google Scholar] [CrossRef]
- Gradin, H.M.; Marklund, U.; Larsson, N.; Chatila, T.A.; Gullberg, M. Regulation of microtubule dynamics by Ca2+/calmodulin-dependent kinase IV Gr-dependent phosphorylation of oncoprotein 18. Mol. Cell Biol. 1997, 17, 3459–3467. [Google Scholar] [CrossRef]
- Chen, G.; Deng, X. Cell Synchronization by Double Thymidine Block. Bio-Protocol 2018, 8, e2994. [Google Scholar] [CrossRef]
- Belletti, B.; Baldassarre, G. Stathmin: A protein with many tasks. New biomarker and potential target in cancer. Expert Opin. Ther. Targets 2011, 15, 1249–1266. [Google Scholar] [CrossRef]
- Chen, P.W.; Lin, S.J.; Tsai, S.C.; Lin, J.H.; Chen, M.R.; Wang, J.T.; Lee, C.P.; Tsai, C.H. Regulation of microtubule dynamics through phosphorylation on stathmin by Epstein-Barr virus kinase BGLF4. J. Biol. Chem. 2010, 285, 10053–10063. [Google Scholar] [CrossRef]
- Abbas, T.; Dutta, A. p21 in cancer: Intricate networks and multiple activities. Nat. Rev. Cancer 2009, 9, 400–414. [Google Scholar] [CrossRef]
- Tseng, Y.H.; Huang, Y.H.; Lin, T.K.; Wu, S.M.; Chi, H.C.; Tsai, C.Y.; Tsai, M.M.; Lin, Y.H.; Chang, W.C.; Chang, Y.T.; et al. Thyroid hormone suppresses expression of stathmin and associated tumor growth in hepatocellular carcinoma. Sci. Rep. 2016, 6, 38756. [Google Scholar] [CrossRef]
- Wang, R.; Wang, Z.; Yang, J.; Liu, X.; Wang, L.; Guo, X.; Zeng, F.; Wu, M.; Li, G. LRRC4 inhibits the proliferation of human glioma cells by modulating the expression of STMN1 and microtubule polymerization. J. Cell Biochem. 2011, 112, 3621–3629. [Google Scholar] [CrossRef]
- Ghule, P.N.; Seward, D.J.; Fritz, A.J.; Boyd, J.R.; van Wijnen, A.J.; Lian, J.B.; Stein, J.L.; Stein, G.S. Higher order genomic organization and regulatory compartmentalization for cell cycle control at the G1/S-phase transition. J. Cell Physiol. 2018, 233, 6406–6413. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Hitomi, M.; Stacey, D.W. Variations in cyclin D1 levels through the cell cycle determine the proliferative fate of a cell. Cell Div. 2006, 1, 32. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.A.; Aakre, M.E.; Gorska, A.E.; Price, J.O.; Eltom, S.E.; Pietenpol, J.A.; Moses, H.L. Induction by transforming growth factor-beta1 of epithelial to mesenchymal transition is a rare event in vitro. Breast Cancer Res. 2004, 6, R215–R231. [Google Scholar] [CrossRef] [PubMed]
- Accornero, P.; Miretti, S.; Bersani, F.; Quaglino, E.; Martignani, E.; Baratta, M. Met receptor acts uniquely for survival and morphogenesis of EGFR-dependent normal mammary epithelial and cancer cells. PLoS ONE 2012, 7, e44982. [Google Scholar] [CrossRef]
- Xue, J.; Pu, Y.; Smith, J.; Gao, X.; Wang, C.; Wu, B. Identifying metastatic ability of prostate cancer cell lines using native fluorescence spectroscopy and machine learning methods. Sci. Rep. 2021, 11, 2282. [Google Scholar] [CrossRef]
- Nafissi, N.N.; Kosiorek, H.E.; Butterfield, R.J.; Moore, C.; Ho, T.; Singh, P.; Bryce, A.H. Evolving Natural History of Metastatic Prostate Cancer. Cureus 2020, 12, e11484. [Google Scholar] [CrossRef]
- Gandaglia, G.; Abdollah, F.; Schiffmann, J.; Trudeau, V.; Shariat, S.F.; Kim, S.P.; Perrotte, P.; Montorsi, F.; Briganti, A.; Trinh, Q.D.; et al. Distribution of metastatic sites in patients with prostate cancer: A population-based analysis. Prostate 2014, 74, 210–216. [Google Scholar] [CrossRef]
- Ma, B.; Wheeler, S.E.; Clark, A.M.; Whaley, D.L.; Yang, M.; Wells, A. Liver protects metastatic prostate cancer from induced death by activating E-cadherin signaling. Hepatology 2016, 64, 1725–1742. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Shore, N.D.; Szmulewitz, R.Z.; Petrylak, D.P.; Holzbeierlein, J.; Villers, A.; Azad, A.; Alcaraz, A.; Alekseev, B.; Iguchi, T.; et al. Efficacy of Enzalutamide plus Androgen Deprivation Therapy in Metastatic Hormone-Sensitive Prostate Cancer by Pattern of Metastatic Spread: ARCHES Post Hoc Analyses. J. Urol. 2021, 205, 1361–1371. [Google Scholar] [CrossRef]
- Hsieh, S.Y.; Huang, S.F.; Yu, M.C.; Yeh, T.S.; Chen, T.C.; Lin, Y.J.; Chang, C.J.; Sung, C.M.; Lee, Y.L.; Hsu, C.Y. Stathmin1 overexpression associated with polyploidy, tumor-cell invasion, early recurrence, and poor prognosis in human hepatoma. Mol. Carcinog. 2010, 49, 476–487. [Google Scholar] [CrossRef]
- Nie, W.; Xu, M.D.; Gan, L.; Huang, H.; Xiu, Q.; Li, B. Overexpression of stathmin 1 is a poor prognostic biomarker in non-small cell lung cancer. Lab. Investig. A J. Tech. Methods Pathol. 2015, 95, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Kuang, X.Y.; Chen, L.; Zhang, Z.J.; Liu, Y.R.; Zheng, Y.Z.; Ling, H.; Qiao, F.; Li, S.; Hu, X.; Shao, Z.M. Stathmin and phospho-stathmin protein signature is associated with survival outcomes of breast cancer patients. Oncotarget 2015, 6, 22227–22238. [Google Scholar] [CrossRef] [PubMed]
- Mercier, I.; Casimiro, M.C.; Wang, C.; Rosenberg, A.L.; Quong, J.; Minkeu, A.; Allen, K.G.; Danilo, C.; Sotgia, F.; Bonuccelli, G.; et al. Human breast cancer-associated fibroblasts (CAFs) show caveolin-1 downregulation and RB tumor suppressor functional inactivation: Implications for the response to hormonal therapy. Cancer Biol. Ther. 2008, 7, 1212–1225. [Google Scholar] [CrossRef] [PubMed]
- Askeland, C.; Wik, E.; Finne, K.; Birkeland, E.; Arnes, J.B.; Collett, K.; Knutsvik, G.; Kruger, K.; Davidsen, B.; Aas, T.; et al. Stathmin expression associates with vascular and immune responses in aggressive breast cancer subgroups. Sci. Rep. 2020, 10, 2914. [Google Scholar] [CrossRef]
- Bhalla, K.N. Microtubule-targeted anticancer agents and apoptosis. Oncogene 2003, 22, 9075–9086. [Google Scholar] [CrossRef]
- Kaposi-Novak, P.; Lee, J.S.; Gomez-Quiroz, L.; Coulouarn, C.; Factor, V.M.; Thorgeirsson, S.S. Met-regulated expression signature defines a subset of human hepatocellular carcinomas with poor prognosis and aggressive phenotype. J. Clin. Investig. 2006, 116, 1582–1595. [Google Scholar] [CrossRef]
- Carr, J.R.; Park, H.J.; Wang, Z.; Kiefer, M.M.; Raychaudhuri, P. FoxM1 mediates resistance to herceptin and paclitaxel. Cancer Res. 2010, 70, 5054–5063. [Google Scholar] [CrossRef]
- Singer, S.; Ehemann, V.; Brauckhoff, A.; Keith, M.; Vreden, S.; Schirmacher, P.; Breuhahn, K. Protumorigenic overexpression of stathmin/Op18 by gain-of-function mutation in p53 in human hepatocarcinogenesis. Hepatology 2007, 46, 759–768. [Google Scholar] [CrossRef]
- Chen, Y.L.; Uen, Y.H.; Li, C.F.; Horng, K.C.; Chen, L.R.; Wu, W.R.; Tseng, H.Y.; Huang, H.Y.; Wu, L.C.; Shiue, Y.L. The E2F transcription factor 1 transactives stathmin 1 in hepatocellular carcinoma. Ann. Surg. Oncol. 2013, 20, 4041–4054. [Google Scholar] [CrossRef]
- Lee, J.M.; Hammaren, H.M.; Savitski, M.M.; Baek, S.H. Control of protein stability by post-translational modifications. Nat. Commun. 2023, 14, 201. [Google Scholar] [CrossRef]
- Larsson, N.; Marklund, U.; Gradin, H.M.; Brattsand, G.; Gullberg, M. Control of microtubule dynamics by oncoprotein 18: Dissection of the regulatory role of multisite phosphorylation during mitosis. Mol. Cell Biol. 1997, 17, 5530–5539. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Tian, Y.; Moldobaeva, N.; Sarich, N.; Birukova, A.A. Microtubule dynamics control HGF-induced lung endothelial barrier enhancement. PLoS ONE 2014, 9, e105912. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, S.; Safferling, K.; Westphal, K.; Hrabowski, M.; Muller, U.; Angel, P.; Wiechert, L.; Ehemann, V.; Muller, B.; Holland-Cunz, S.; et al. Stathmin regulates keratinocyte proliferation and migration during cutaneous regeneration. PLoS ONE 2013, 8, e75075. [Google Scholar] [CrossRef]
- Alesi, G.N.; Jin, L.; Li, D.; Magliocca, K.R.; Kang, Y.; Chen, Z.G.; Shin, D.M.; Khuri, F.R.; Kang, S. RSK2 signals through stathmin to promote microtubule dynamics and tumor metastasis. Oncogene 2016, 35, 5412–5421. [Google Scholar] [CrossRef]
- Smith, D.C.; Smith, M.R.; Sweeney, C.; Elfiky, A.A.; Logothetis, C.; Corn, P.G.; Vogelzang, N.J.; Small, E.J.; Harzstark, A.L.; Gordon, M.S.; et al. Cabozantinib in patients with advanced prostate cancer: Results of a phase II randomized discontinuation trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013, 31, 412–419. [Google Scholar] [CrossRef]
- Smith, M.; De Bono, J.; Sternberg, C.; Le Moulec, S.; Oudard, S.; De Giorgi, U.; Krainer, M.; Bergman, A.; Hoelzer, W.; De Wit, R.; et al. Phase III Study of Cabozantinib in Previously Treated Metastatic Castration-Resistant Prostate Cancer: COMET-1. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2016, 34, 3005–3013. [Google Scholar] [CrossRef]
- Verras, M.; Lee, J.; Xue, H.; Li, T.H.; Wang, Y.; Sun, Z. The androgen receptor negatively regulates the expression of c-Met: Implications for a novel mechanism of prostate cancer progression. Cancer Res. 2007, 67, 967–975. [Google Scholar] [CrossRef]
- Qiao, Y.; Feng, F.Y.; Wang, Y.; Cao, X.; Han, S.; Wilder-Romans, K.; Navone, N.M.; Logothetis, C.; Taichman, R.S.; Keller, E.T.; et al. Mechanistic Support for Combined MET and AR Blockade in Castration-Resistant Prostate Cancer. Neoplasia 2016, 18, 1–9. [Google Scholar] [CrossRef]
- Tripathi, A.; Supko, J.G.; Gray, K.P.; Melnick, Z.J.; Regan, M.M.; Taplin, M.E.; Choudhury, A.D.; Pomerantz, M.M.; Bellmunt, J.; Yu, C.; et al. Dual Blockade of c-MET and the Androgen Receptor in Metastatic Castration-resistant Prostate Cancer: A Phase I Study of Concurrent Enzalutamide and Crizotinib. Clin. Cancer Res. 2020, 26, 6122–6131. [Google Scholar] [CrossRef]
- Hu, G.; Cun, X.; Ruan, S.; Shi, K.; Wang, Y.; Kuang, Q.; Hu, C.; Xiao, W.; He, Q.; Gao, H. Utilizing G2/M retention effect to enhance tumor accumulation of active targeting nanoparticles. Sci. Rep. 2016, 6, 27669. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deford, P.L.; VonHandorf, A.P.; Hunt, B.G.; Venkatraman, S.; Waltz, S.E.; Burns, K.A.; Kasper, S. Stathmin Serine 16 Phosphorylation Is a Key Regulator of Cell Cycle Progression Without Activating Migration and Invasion In Vitro. Cancers 2025, 17, 2322. https://doi.org/10.3390/cancers17142322
Deford PL, VonHandorf AP, Hunt BG, Venkatraman S, Waltz SE, Burns KA, Kasper S. Stathmin Serine 16 Phosphorylation Is a Key Regulator of Cell Cycle Progression Without Activating Migration and Invasion In Vitro. Cancers. 2025; 17(14):2322. https://doi.org/10.3390/cancers17142322
Chicago/Turabian StyleDeford, Paul L., Andrew P. VonHandorf, Brian G. Hunt, Simran Venkatraman, Susan E. Waltz, Katherine A. Burns, and Susan Kasper. 2025. "Stathmin Serine 16 Phosphorylation Is a Key Regulator of Cell Cycle Progression Without Activating Migration and Invasion In Vitro" Cancers 17, no. 14: 2322. https://doi.org/10.3390/cancers17142322
APA StyleDeford, P. L., VonHandorf, A. P., Hunt, B. G., Venkatraman, S., Waltz, S. E., Burns, K. A., & Kasper, S. (2025). Stathmin Serine 16 Phosphorylation Is a Key Regulator of Cell Cycle Progression Without Activating Migration and Invasion In Vitro. Cancers, 17(14), 2322. https://doi.org/10.3390/cancers17142322







