Imaging Evaluation of Ovarian Masses in a Pediatric Population: A Comprehensive Overview
Simple Summary
Abstract
1. Introduction
2. Search Strategy and Study Selection
3. Non-Neoplastic Ovarian Lesions
3.1. Functional Cysts
3.2. Hemorrhagic Cysts
3.3. Ovarian Torsion
3.4. Pelvic Inflammatory Disease/Tubo-Ovarian Abscess
4. Neoplastic Ovarian Lesions
4.1. Germ Cell Tumors
4.1.1. Mature Teratoma
4.1.2. Immature Teratoma
4.1.3. Dysgerminoma
4.1.4. Yolk Sac Tumor
4.1.5. Other Germ Cell Tumors
Non-Gestational Choriocarcinoma
Embryonal Carcinoma
Polyembryoma
Mixed Germ Cell Tumors
Gonadoblastoma
4.2. Sex Cord–Stromal Tumors
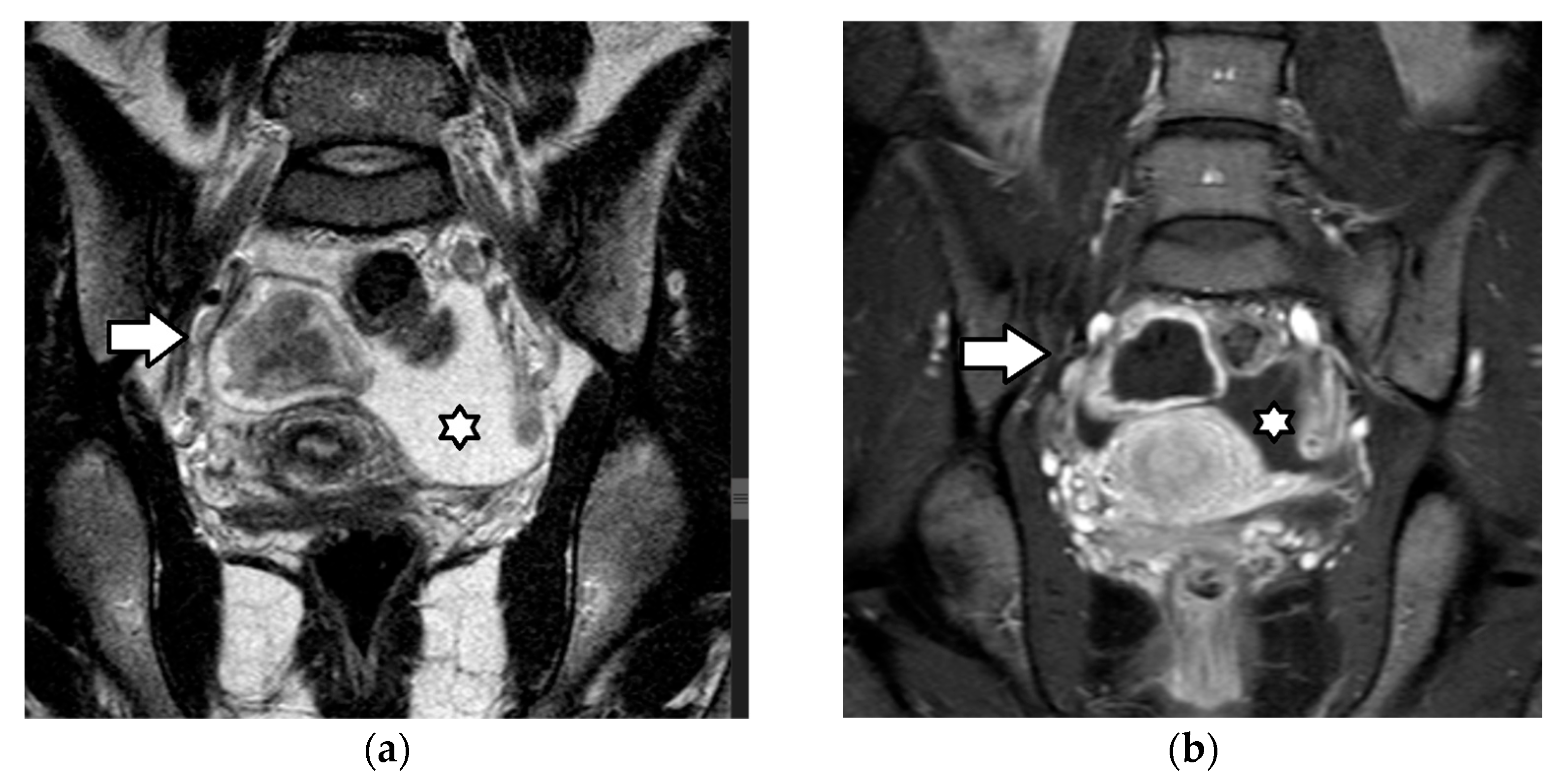
4.2.1. Juvenile Granulosa Cell Tumors
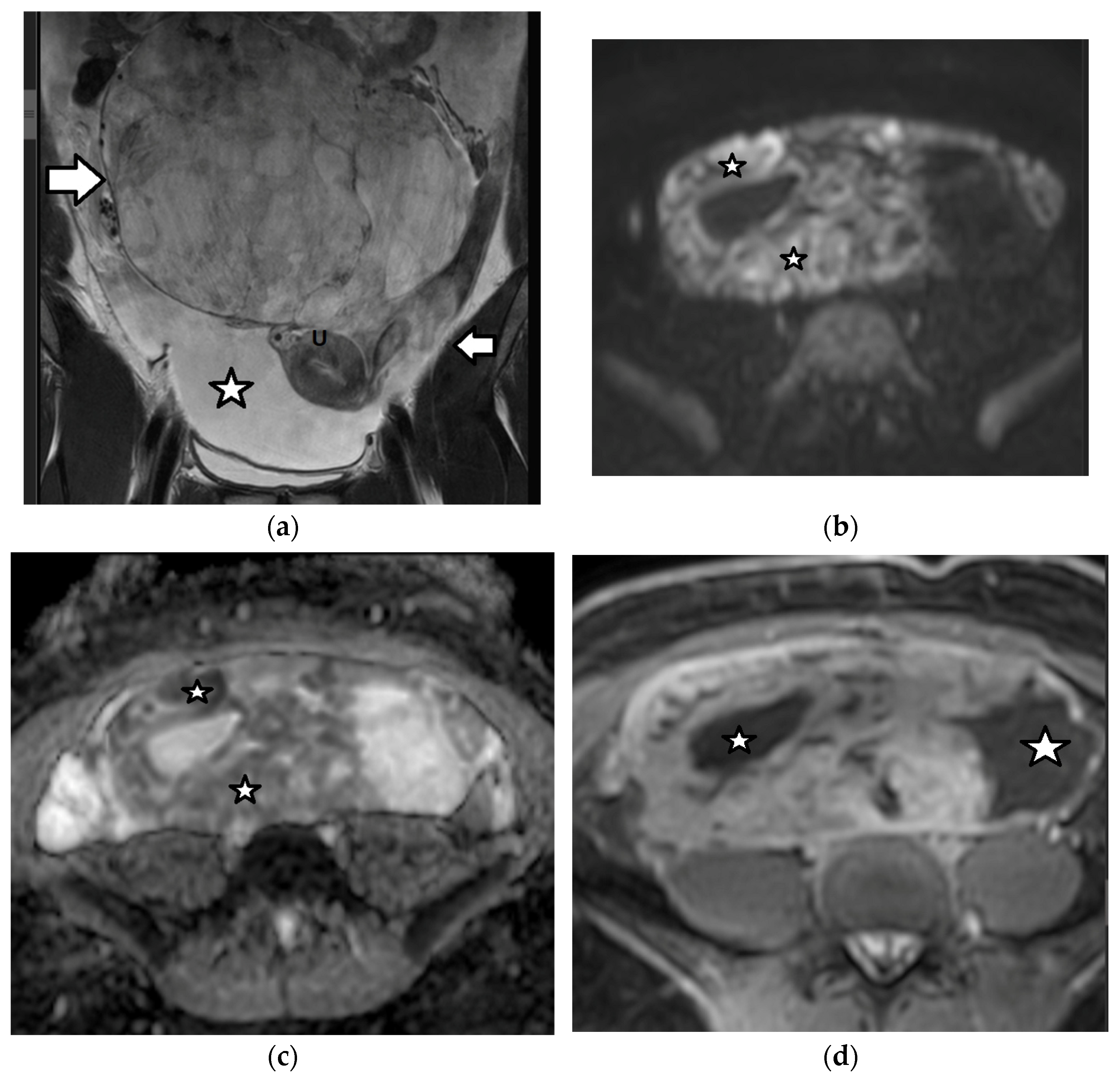
4.2.2. Sertoli–Leydig Cell Tumor
4.3. Epithelial Tumors
4.3.1. Benign Epithelial Ovarian Tumors
4.3.2. Borderline and Malignant Epithelial Ovarian Tumors
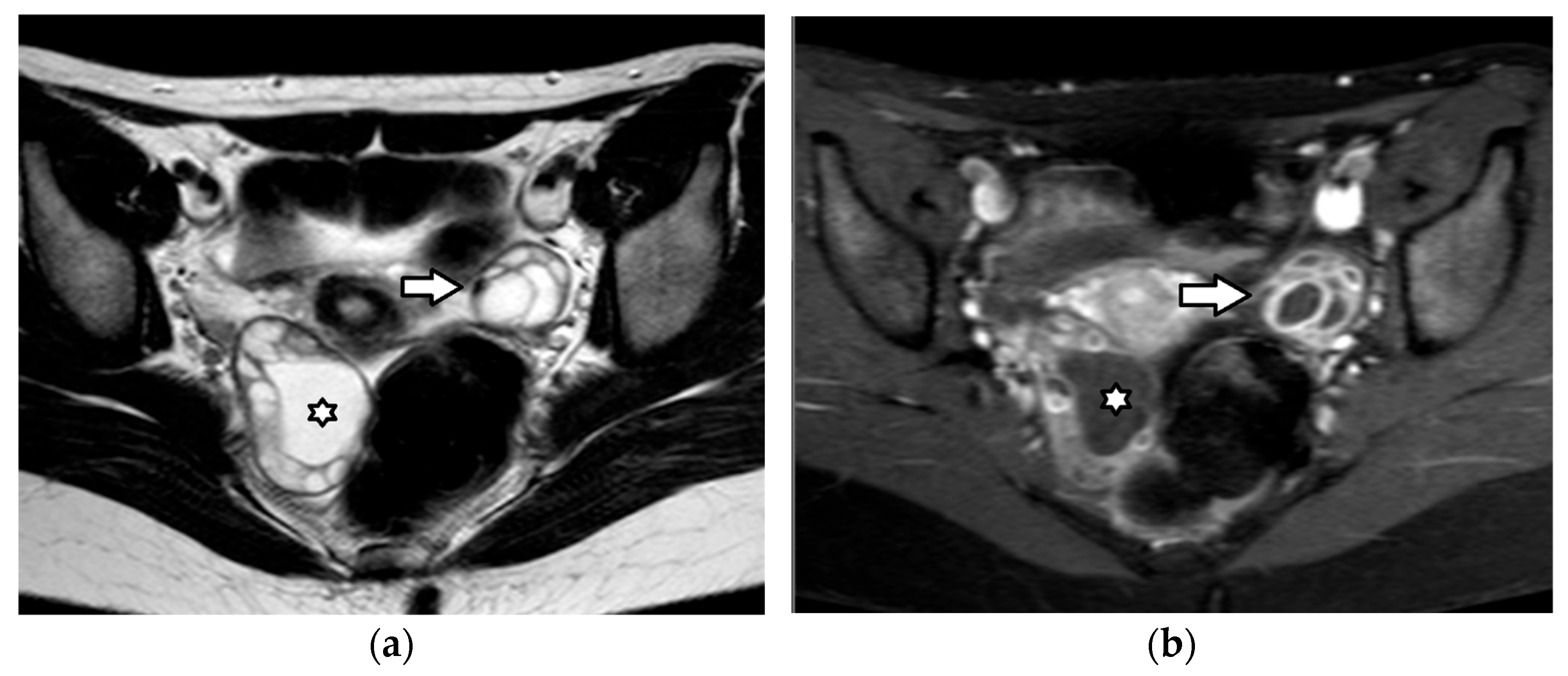
4.3.3. Endometriomas
5. Limitations
6. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PID | pelvic inflammatory disease |
| GCTs | germ cell tumors |
| SCSTs | sex cord–stromal tumors |
| AFP | alpha fetoprotein |
| β-hCG | beta-human chorionic gonadotropin |
| LDH | lactate dehydrogenase |
| CA 125 | cancer antigen 125 |
| FIGO | International Federation of Gynecology and Obstetrics |
| COG | Children’s Oncology Group |
| RMI | Risk of Malignancy Index |
| ROMA | Risk of Ovarian Malignancy Algorithm |
| IOTA | International Ovarian Tumor Analysis |
| O-RADS | Ovarian–Adnexal Reporting and Data System |
| ADNEX | Assessment of Different Neoplasias in the Adnexa |
| ACR | American College of Radiology |
| CEUS | contrast-enhanced ultrasound |
| T1WI | T1-weighted imaging |
| T2WI | T2-weighted imaging |
| TOA | tubo-ovarian abscess |
| MOGCT | malignant ovarian germ cell tumor |
| MCT | mature cystic teratoma |
| JGCT | juvenile granulosa cell tumor |
| SLCT | Sertoli–Leydig cell tumor |
| AI | artificial intelligence |
References
- Hanafy, A.K.; Mujtaba, B.; Yedururi, S.; Jensen, C.T.; Sanchez, R.; Austin, M.T.; Morani, A.C. Imaging in pediatric ovarian tumors. Abdom. Radiol. 2020, 45, 520–536. [Google Scholar] [CrossRef] [PubMed]
- Behr, G.G.; Morani, A.C.; Artunduaga, M.; Desoky, S.M.; Epelman, M.; Friedman, J.; Lala, S.V.; Seekins, J.; Towbin, A.J.; Back, S.J. Imaging of pediatric ovarian tumors: A COG Diagnostic Imaging Committee/SPR Oncology Committee White Paper. Pediatr. Blood Cancer 2023, 70, e29995. [Google Scholar] [CrossRef]
- Birbas, E.; Kanavos, T.; Gkrozou, F.; Skentou, C.; Daniilidis, A.; Vatopoulou, A. Ovarian masses in children and adolescents: A review of the literature with emphasis on the diagnostic approach. Children 2023, 10, 1114. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.H.; Kim, J.W.; Shin, S.S.; Jeong, S.I.; Lim, H.S.; Choi, Y.D.; Lee, K.H.; Kang, W.D.; Jeong, Y.Y.; Kang, H.K. Review of ovarian tumors in children and adolescents: Radiologic-pathologic correlation. RadioGraphics 2014, 34, 2039–2055. [Google Scholar] [CrossRef]
- Zhang, M.; Jiang, W.; Li, G.; Xu, C. Ovarian masses in children and adolescents—An analysis of 521 clinical cases. J. Pediatr. Adolesc. Gynecol. 2014, 27, e73–e77. [Google Scholar] [CrossRef]
- Lala, S.V.; Strubel, N. Ovarian neoplasms of childhood. Pediatr. Radiol. 2019, 49, 1463–1475. [Google Scholar] [CrossRef]
- Bourgioti, C.; Konidari, M.; Moulopoulos, L.A. Manifestations of ovarian cancer in relation to other pelvic diseases by MRI. Cancers 2023, 15, 2106. [Google Scholar] [CrossRef] [PubMed]
- Epelman, M.; Chikwava, K.R.; Chauvin, N.; Servaes, S. Imaging of pediatric ovarian neoplasms. Pediatr. Radiol. 2011, 41, 1085–1099. [Google Scholar] [CrossRef]
- Surratt, J.T.; Siegel, M.J. Imaging of pediatric ovarian masses. RadioGraphics 1991, 11, 533–548. [Google Scholar] [CrossRef]
- Wohlmuth, C.; Wohlmuth-Wieser, I. Gynecologic malignancies in children and adolescents: How common is the uncommon? J. Clin. Med. 2021, 10, 722. [Google Scholar] [CrossRef]
- You, W.; Dainty, L.A.; Rose, G.S.; Krivak, T.; McHale, M.T.; Olsen, C.H.; Elkas, J.C. Gynecologic malignancies in women aged less than 25 years. Obstet. Gynecol. 2005, 105, 1405–1409. [Google Scholar] [CrossRef] [PubMed]
- Cooper, N.; Meehan, H.; Linton-Reid, K.; Barcroft, J.; Danin, J.; Seah, M.; Sadigh, S.; Bharwani, N.; Sur, S.; Fotopoulou, C.; et al. Clinical utility of ultrasonography in pediatric and adolescent gynecology: Retrospective review of 1313 ultrasound examinations. Ultrasound Obstet. Gynecol. 2025, 65, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Oltmann, S.C.; Garcia, N.M.; Barber, R.; Hicks, B.; Fischer, A.C. Pediatric ovarian malignancies: How efficacious are current staging practices? J. Pediatr. Surg. 2010, 45, 1096–1102. [Google Scholar] [CrossRef]
- Margioula-Siarkou, C.; Petousis, S.; Margioula-Siarkou, G.; Mavromatidis, G.; Chatzinikolaou, F.; Hatzipantelis, E.; Guyon, F.; Dinas, K. Therapeutic management and prognostic factors for ovarian malignant tumours in adolescents: A comprehensive review of current guidelines. Diagnostics 2023, 13, 1080. [Google Scholar] [CrossRef]
- Sessa, C.; Schneider, D.T.; Planchamp, F.; Baust, K.; Braicu, E.I.; Concin, N.; Godzinski, J.; McCluggage, W.G.; Orbach, D.; Pautier, P.; et al. ESGO-SIOPE guidelines for the management of adolescents and young adults with non-epithelial ovarian cancers. Lancet Oncol. 2020, 21, e360–e368. [Google Scholar] [CrossRef]
- Spinelli, C.; Strambi, S.; Masoni, B.; Ghionzoli, M.; Bertocchini, A.; Sanna, B.; Morganti, R.; Messina, M.; Molinaro, F.; Tursini, S.; et al. Surgical management of ovarian teratomas in childhood: A multicentric study on 110 cases and a literature review. Gynecol. Endocrinol. 2021, 37, 950–954. [Google Scholar] [CrossRef]
- Stankovic, Z.B.; Bjelica, A.; Djukic, M.K.; Savic, D. Value of ultrasonographic detection of normal ovarian tissue in the differential diagnosis of adnexal masses in pediatric patients. Ultrasound Obstet. Gynecol. 2010, 36, 88–92. [Google Scholar] [CrossRef]
- Servaes, S.; Victoria, T.; Lovrenski, J.; Epelman, M. Contemporary pediatric gynecologic imaging. Semin. Ultrasound CT MR 2010, 31, 116–140. [Google Scholar] [CrossRef] [PubMed]
- Farràs Roca, L.; Alshehri, E.D.; Goldberg, H.R.; Amirabadi, A.; Kives, S.; Allen, L.; Navarro, O.M.; Lam, C.Z. Diagnostic Performance of a Sonographic Volume and Solid Vascular Tissue Score (VSVTS) for Preoperative Risk Assessment of Pediatric and Adolescent Adnexal Masses. J. Pediatr. Adolesc. Gynecol. 2021, 34, 377–382. [Google Scholar] [CrossRef]
- Goldberg, H.R.; Kives, S.; Allen, L.; Navarro, O.M.; Lam, C.Z. Preoperative Risk Stratification of Adnexal Masses in the Pediatric and Adolescent Population: Evaluating the Decision Tree System. J. Pediatr. Adolesc. Gynecol. 2019, 32, 633–638. [Google Scholar] [CrossRef]
- Terzic, M.; Aimagambetova, G.; Norton, M.; Della Corte, L.; Marín-Buck, A.; Lisón, J.F.; Amer-Cuenca, J.J.; Zito, G.; Garzon, S.; Caruso, S.; et al. Scoring systems for the evaluation of adnexal masses nature: Current knowledge and clinical applications. J. Obstet. Gynaecol. 2021, 41, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Mina, M.; Kosmas, I.; Tsakiridis, I.; Mamopoulos, A.; Kalogiannidis, I.; Athanasiadis, A.; Dagklis, T. Prediction Models of Adnexal Masses: State-of-the-Art Review. Obstet. Gynecol. Surv. 2021, 76, 211–222. [Google Scholar] [CrossRef]
- Andreotti, R.F.; Timmerman, D.; Strachowski, L.M.; Froyman, W.; Benacerraf, B.R.; Bennett, G.L.; Bourne, T.; Brown, D.L.; Coleman, B.G.; Frates, M.C.; et al. O-RADS US risk stratification and management system: A consensus guideline from the ACR Ovarian-Adnexal Reporting and Data System Committee. Radiology 2020, 294, 168–185. [Google Scholar] [CrossRef] [PubMed]
- Strachowski, L.M.; Jha, P.; Chawla, T.P.; Davis, K.M.; Dove, C.K.; Glanc, P.; Morgan, T.A.; Andreotti, R.F. O-RADS for ultrasound: A user’s guide, from the AJR special series on radiology reporting and data systems. Am. J. Roentgenol. 2021, 216, 1150–1165. [Google Scholar] [CrossRef] [PubMed]
- Peces Rama, A.; Llanos Llanos, M.C.; Sanchez Ferrer, M.L.; Alcazar Zambrano, J.L.; Martinez Mendoza, A.; Nieto Diaz, A. Simple descriptors and simple rules of the International Ovarian Tumor Analysis (IOTA) Group: A prospective study of combined use for the description of adnexal masses. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 195, 7–11. [Google Scholar] [CrossRef]
- Ueland, F.; DePriest, P.; Pavlik, E.; Kryscio, R.; van Nagell, J., Jr. Preoperative differentiation of malignant from benign ovarian tumors: The efficacy of morphology indexing and Doppler flow sonography. Gynecol. Oncol. 2003, 91, 46–50. [Google Scholar] [CrossRef]
- Stankovic, Z.B.; Sedlecky, K.; Savic, D.; Lukac, B.J.; Maz Ibrada, I.; Perovic, S. Ovarian preservation from tumors and torsions in girls: Prospective diagnostic study. J. Pediatr. Adolesc. Gynecol. 2017, 30, 405–412. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; An, S.; Ma, Q.; Tu, Y.; Shang, N.; Pan, Y. American college of radiology ovarian-adnexal reporting and data system ultrasound (O-RADS): Diagnostic performance and inter-reviewer agreement for ovarian masses in children. Front. Pediatr. 2023, 8, 1091735. [Google Scholar] [CrossRef]
- Xun, L.; Zhai, L.; Xu, H. Comparison of conventional, doppler and contrast-enhanced ultrasonography in differential diagnosis of ovarian masses: A systematic review and meta-analysis. BMJ Open 2021, 11, e052830. [Google Scholar] [CrossRef]
- Yi, Y.Y.; Li, C.; Zhu, W.J.; Hou, Y.L. Diagnostic performance of contrast-enhanced ultrasound (CEUS) combined with Ovarian-Adnexal Reporting and Data System (O-RADS) ultrasound risk stratification for adnexal masses: A systematic review and meta-analysis. Clin. Radiol. 2024, 79, e1167–e1175. [Google Scholar] [CrossRef]
- Trinci, M.; Danti, G.; Di Maurizio, M.; Tursini, S.; Briganti, V.; Galluzzo, M.; Miele, V. Can contrast enhanced ultrasound (CEUS) be useful in the diagnosis of ovarian torsion in pediatric females? A preliminary monocentric experience. J. Ultrasound 2021, 24, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Fan, S.; Feng, X.; Liu, L.; Zhou, J.; Wu, Z.; Zhou, L. Performance of grayscale combined with contrast-enhanced ultrasound in differentiating benign and malignant pediatric ovarian masses. Eur. Radiol. 2025, 35, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Van Nimwegen, L.W.E.; Mavinkurve-Groothuis, A.M.C.; de Krijger, R.R.; Hulsker, C.C.C.; Goverde, A.J.; Zsiros, J.; Littooij, A.S. MR imaging in discriminating between benign and malignant paediatric ovarian masses: A systematic review. Eur. Radiol. 2020, 30, 1166–1181. [Google Scholar] [CrossRef] [PubMed]
- Thomassin-Naggara, I.; Poncelet, E.; Jalaguier-Coudray, A.; Guerra, A.; Fournier, L.S.; Stojanovic, S.; Millet, I.; Bharwani, N.; Juhan, V.; Cunha, T.M.; et al. Ovarian-Adnexal Reporting Data System Magnetic Resonance Imaging (O-RADS MRI) Score for risk stratification of sonographically indeterminate adnexal masses. JAMA Netw. Open 2020, 3, e1919896. [Google Scholar] [CrossRef]
- Sadowski, E.A.; Thomassin-Naggara, I.; Rockall, A.; Maturen, K.E.; Forstner, R.; Jha, P.; Nougaret, S.; Siegelman, E.S.; Reinhold, C. O-RADS MRI risk stratification system: Guide for assessing adnexal lesions from the ACR O-RADS Committee. Radiology 2022, 303, 35–47. [Google Scholar] [CrossRef]
- Munari, A.M.; Monti, C.B.; Viglio, C.; Folco, G.; Rizzetto, F.; Zirpoli, S. MRI of pediatric ovarian masses: Validation of the O-RADS framework. Eur. Radiol. 2025, 35, 5073–5080. [Google Scholar] [CrossRef]
- Bergus, K.C.; Knaus, M.E.; Onwuka, A.J.; Afrazi, A.; Breech, L.; Corkum, K.S.; Dillon, P.A.; Ehrlich, P.F.; Fallat, M.E.; Fraser, J.D.; et al. Diagnostic performance of magnetic resonance imaging for pediatric ovarian neoplasms: A multi-institutional review. J. Pediatr. Adolesc. Gynecol. 2024, 37, 192–197. [Google Scholar] [CrossRef]
- Emil, S.; Youssef, F.; Arbash, G.; Baird, R.; Laberge, J.M.; Puligandla, P.; Albuquerque, P. The utility of magnetic resonance imaging in the diagnosis and management of pediatric benign ovarian lesions. J. Pediatr. Surg. 2018, 53, 2013–2018. [Google Scholar] [CrossRef]
- Janssen, C.L.; Littooij, A.S.; Fiocco, M.; Huige, J.C.B.; de Krijger, R.R.; Hulsker, C.C.C.; Goverde, A.J.; Zsiros, J.; Mavinkurve-Groothuis, A.M.C. The diagnostic value of magnetic resonance imaging in differentiating benign and malignant pediatric ovarian tumors. Pediatr. Radiol. 2021, 51, 427–434. [Google Scholar] [CrossRef]
- Foti, P.V.; Attina, G.; Spadola, S.; Caltabiano, R.; Farina, R.; Palmucci, S.; Zarbo, G.; Zarbo, R.; D’Arrigo, M.; Milone, P.; et al. MR imaging of ovarian masses: Classification and differential diagnosis. Insights Imaging 2016, 7, 21–41. [Google Scholar] [CrossRef]
- Marro, A.; Allen, L.M.; Kives, S.L.; Moineddin, R.; Chavhan, G.B. Simulated impact of pelvic MRI in treatment planning for pediatric adnexal masses. Pediatr. Radiol. 2016, 46, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Renaud, E.J.; Sømme, S.; Islam, S.; Cameron, D.B.; Gates, R.L.; Williams, R.F.; Jancelewicz, T.; Oyetunji, T.A.; Grabowski, J.; Diefenbach, K.A.; et al. Ovarian masses in the child and adolescent: An American Pediatric Surgical Association Outcomes and Evidence-Based Practice Committee systematic review. J. Pediatr. Surg. 2019, 54, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, A.E.; Fallat, M.E.; Hewitt, G.; Hertweck, P.; Onwuka, A.; Afrazi, A.; Aldrink, J.H.; Bence, C.; Burns, R.C.; Corkum, K.S.; et al. Factors Associated with Torsion in Pediatric Patients with Ovarian Masses. J. Surg. Res. 2021, 263, 110–115. [Google Scholar] [CrossRef]
- Shah, R.U.; Lawrence, C.; Fickenscher, K.A.; Shao, L.; Lowe, L.H. Imaging of Pediatric Pelvic Neoplasms. Radiol. Clin. N. Am. 2011, 49, 729–748. [Google Scholar] [CrossRef]
- Caprio, M.G.; Di Serafino, M.; De Feo, A.; Guerriero, E.; Perillo, T.; Barbuto, L.; Vezzali, N.; Rossi, E.; Ferro, F.; Vallone, G.; et al. Ultrasonographic and multimodal imaging of pediatric genital female diseases. J. Ultrasound 2019, 22, 273–289. [Google Scholar] [CrossRef]
- Mărginean, C.O.; Mărginean, C.; Chinceşan, M.; Mărginean, M.O.; Meliţ, L.E.; Săsăran, V.; Mărginean, C.D. Pediatric ovarian tumors, a challenge for pediatrician and gynecologist: Three case reports (CARE compliant). Medicine 2019, 98, e15242. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, R.J. Abdominal masses. Surg. Clin. N. Am. 1985, 65, 1481–1504. [Google Scholar] [CrossRef]
- Banlı-Cesur, I.; Tanrıdan-Okcu, N.; Özçelik, Z. Ovarian masses in children and adolescents: Analysis on 146 patients. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 101901. [Google Scholar] [CrossRef]
- Cecchetto, G. Gonadal germ cell tumors in children and adolescents. J. Indian. Assoc. Pediatr. Surg. 2014, 19, 189–194. [Google Scholar] [CrossRef]
- Kirks, D.R.; Merten, D.F.; Grossman, H.; Bowie, J.D. Diagnostic imaging of pediatric abdominal masses: An overview. Radiol. Clin. N. Am. 1981, 19, 527–545. [Google Scholar] [CrossRef]
- Kapoor, R.; Mandelia, A.; Kumar, B.; Upadhyaya, V.D.; Verma, A.; Kanneganti, P.; Kumar, T.; Agarwal, N.; Goel, R.; Prajapati, P. Ovarian Masses in Children: Surgical Experience and Outcomes. J. Indian Assoc. Pediatr. Surg. 2024, 29, 617–622. [Google Scholar] [CrossRef]
- Xac, M.C.; Jetelina, K.K.; Jarin, J.; Wilson, E. Benign, Borderline, and Malignant Pediatric Adnexal Masses: A 10-Year Review. J. Pediatr. Adolesc. Gynecol. 2021, 34, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, C.M.; Goldstein, A.M. Adnexal masses in children and adolescents. Clin. Obstet. Gynecol. 2015, 58, 76–92. [Google Scholar] [CrossRef] [PubMed]
- Luczak, J.; Gorecki, W.; Patkowski, D.; Baglaj, M.; Drosdzol-Cop, A.; Adamkiewicz-Drozynska, E.; Zaleska-Dorobisz, U.; Patyk, M.; Hirnle, L. Recommendations of procedures to follow in the case of ovarian lesions in girls. Ginekol. Pol. 2022, 93, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Madenci, A.L.; Vandewalle, R.J.; Dieffenbach, B.V.; Laufer, M.R.; Boyd, T.K.; Voss, S.D.; Frazier, A.L.; Billmire, D.F.; Rescorla, F.J.; Weil, B.R.; et al. Multicenter pre-operative assessment of pediatric ovarian malignancy. J. Pediatr. Surg. 2019, 54, 1921–1925. [Google Scholar] [CrossRef]
- Knaus, M.E.; Onwuka, A.J.; Afrazi, A.; Breech, L.; Corkum, K.S.; Dillon, P.A.; Ehrlich, P.F.; Fallat, M.E.; Fraser, J.D.; Gadepalli, S.K.; et al. Multi-Institutional Review of the Preoperative Diagnostic Accuracy for Pediatric Ovarian Mature Cystic Teratomas. J. Pediatr. Adolesc. Gynecol. 2022, 35, 478–485. [Google Scholar] [CrossRef]
- Pomeranz, A.J.; Sabnis, S. Misdiagnoses of ovarian masses in children and adolescents. Pediatr. Emerg. Care 2004, 20, 172–174. [Google Scholar] [CrossRef]
- How, J.A.; Marino, J.L.; Grover, S.R.; Heloury, Y.; Sullivan, M.; Mellor, A.; McNally, O.; Jayasinghe, Y. Surgically Managed Ovarian Masses at the Royal Children’s Hospital, Melbourne—19 Year Experience. J. Pediatr. Surg. 2019, 54, 1913–1920. [Google Scholar] [CrossRef]
- Li, D.; Zhang, J.; Kiryu, S.; Zhang, X.; Wang, F. Clinical and CT Features of Ovarian Torsion in Infants, Children, and Adolescents. Int. J. Gynaecol. Obstet. 2022, 156, 444–449. [Google Scholar] [CrossRef]
- Anthony, E.Y.; Caserta, M.P.; Singh, J.; Chen, M.Y. Adnexal masses in female pediatric patients. AJR Am. J. Roentgenol. 2012, 198, W426–W431. [Google Scholar] [CrossRef]
- Garel, L.; Dubois, J.; Grignon, A.; Filiatrault, D.; Van Vliet, G. US of the Pediatric Female Pelvis: A Clinical Perspective. RadioGraphics 2001, 21, 1393–1407. [Google Scholar] [CrossRef]
- Kitami, M.; Aoki, H.; Saito, M. “Follow the Fallopian tube”: A technique to improve sonographic identification of ovaries in children. J. Clin. Ultrasound 2021, 49, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Hayes-Jordan, A. Surgical management of the incidentally identified ovarian mass. Semin. Pediatr. Surg. 2005, 14, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, Y.A.; Kives, S. Ovarian cysts in adolescents: Medical and surgical management. Adolesc. Med. State Art Rev. 2012, 23, 178–191. [Google Scholar] [PubMed]
- Bašković, M.; Habek, D.; Zaninović, L.; Milas, I.; Pogorelić, Z. The Evaluation, Diagnosis, and Management of Ovarian Cysts, Masses, and Their Complications in Fetuses, Infants, Children, and Adolescents. Healthcare 2025, 13, 775. [Google Scholar] [CrossRef]
- Papic, J.C.; Finnell, S.M.; Slaven, J.E.; Billmire, D.F.; Rescorla, F.J.; Leys, C.M. Predictors of ovarian malignancy in children: Overcoming clinical barriers of ovarian preservation. J. Pediatr. Surg. 2014, 49, 144–147. [Google Scholar] [CrossRef]
- Bai, R.; Bonanni, J.R.; Guo, J.; Li, Z.; Yu, X.; Zhao, J.; Zeng, J.; Garvin, G.; Yan, Y. Opting for conservative management over surgery for neonatal ovarian cysts. J. Clin. Ultrasound 2024, 52, 705–716. [Google Scholar] [CrossRef]
- Łuczak, J.; Bagłaj, M. Selecting treatment method for ovarian masses in children—24 years of experience. J. Ovarian Res. 2017, 10, 59. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Lu, D.; Liu, Z.; Shi, G. Ovarian masses in children and adolescents in China: Analysis of 203 cases. J. Ovarian Res. 2013, 6, 47. [Google Scholar] [CrossRef]
- Otjen, J.P.; Stanescu, A.L.; Alessio, A.M.; Parisi, M.T. Ovarian torsion: Developing a machine-learned algorithm for diagnosis. Pediatr. Radiol. 2020, 50, 706–714. [Google Scholar] [CrossRef]
- Özcan, R.; Kuruoğlu, S.; Dervişoğlu, S.; Eliçevik, M.; Emir, H.; Büyükünal, C. Ovary-sparing surgery for teratomas in children. Pediatr. Surg. Int. 2013, 29, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Haller, J.O.; Schneider, M.; Kassner, E.G.; Staiano, S.J.; Noyes, M.B.; Campos, E.M.; McPherson, H. Ultrasonography in pediatric gynecology and obstetrics. AJR Am. J. Roentgenol. 1977, 128, 423–429. [Google Scholar] [CrossRef]
- Tarca, E.; Trandafir, L.M.; Cojocaru, E.; Costea, C.F.; Rosu, S.T.; Butnariu, L.I.; Iordache, A.C.; Munteanu, V.; Luca, A.C. Diagnosis Difficulties and Minimally Invasive Treatment for Ovarian Masses in Adolescents. Int. J. Womens Health 2022, 14, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Rogers, E.M.; Casadiego Cubides, G.; Lacy, J.; Gerstle, J.T.; Kives, S.; Allen, L. Preoperative risk stratification of adnexal masses: Can we predict the optimal surgical management? J. Pediatr. Adolesc. Gynecol. 2014, 27, 125–128. [Google Scholar] [CrossRef]
- Lee, W.; Lee, M.Y.; Teo, H. Ultrasound and alternative multimodality imaging of intra-abdominal and pelvic cystic masses in the newborn. Ultrasound 2021, 29, 241–251. [Google Scholar] [CrossRef]
- Bergeron, L.M.; Bishop, K.C.; Hoefgen, H.R.; Abraham, M.S.; Tutlam, N.T.; Merritt, D.F.; Peipert, J.F. Surgical Management of Benign Adnexal Masses in the Pediatric/Adolescent Population: An 11-Year Review. J. Pediatr. Adolesc. Gynecol. 2017, 30, 123–127. [Google Scholar] [CrossRef][Green Version]
- Spinelli, C.; Pucci, V.; Strambi, S.; Piccolo, R.L.; Martin, A.; Messineo, A. Treatment of ovarian lesions in children and adolescents: A retrospective study of 130 cases. Pediatr. Hematol. Oncol. 2015, 32, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, Y.A.; Lacy, J.A.; Kives, S.; Allen, L. Characteristics and management of adnexal masses in a canadian pediatric and adolescent population. J. Obstet. Gynaecol. Can. 2011, 33, 935–943. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Z.; Zhou, H.; Cao, D.; Yang, J.; Shen, K.; Lang, J. Clinicopathological features and surgical procedures of adnexal masses with abdominal pain in pediatric and adolescent patients. Orphanet J. Rare Dis. 2024, 19, 132. [Google Scholar] [CrossRef]
- Anders, J.F.; Powell, E.C. Urgency of evaluation and outcome of acute ovarian torsion in pediatric patients. Arch. Pediatr. Adolesc. Med. 2005, 159, 532–535. [Google Scholar] [CrossRef]
- Mayer, J.P.; Bettolli, M.; Kolberg-Schwerdt, A.; Lempe, M.; Schlesinger, F.; Hayek, I.; Schaarschmidt, K. Laparoscopic approach to ovarian mass in children and adolescents: Already a standard in therapy. J. Laparoendosc. Adv. Surg. Tech. A 2009, 19, S111–S115. [Google Scholar] [CrossRef]
- Braungart, S.; Craigie, R.J.; Losty, P.D. Controversies in the management of ovarian tumours in prepubertal children—A BAPS and UK CCLG Surgeons Cancer Group National Survey. J. Pediatr. Surg. 2018, 53, 2231–2234. [Google Scholar] [CrossRef]
- Grabowski, A.; Korlacki, W.; Pasierbek, M. Laparoscopy in elective and emergency management of ovarian pathology in children and adolescents. Wideochir Inne Tech. Maloinwazyjne 2014, 9, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Yamout, S.Z.; Gosche, J.R. Management and outcomes of ovarian masses in children and adolescents. Am. Surg. 2008, 74, 1062–1065. [Google Scholar] [CrossRef] [PubMed]
- Moss, E.H.; Carty, H.; Sprigg, A. A retrospective study of large ovarian masses in paediatric practice. Eur. J. Radiol. 1993, 17, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.L.; Bober, S.E.; Bow, S.N. Imaging the pediatric pelvis: The normal and abnormal genital tract and simulators of its diseases. Urol. Radiol. 1992, 14, 273–283. [Google Scholar] [CrossRef]
- Hermans, A.J.; Kluivers, K.B.; Wijnen, M.H.; Bulten, J.; Massuger, L.F.; Coppus, S.F. Diagnosis and treatment of adnexal masses in children and adolescents. Obstet. Gynecol. 2015, 125, 611–615. [Google Scholar] [CrossRef]
- Ziereisen, F.; Guissard, G.; Damry, N.; Avni, E.F. Sonographic imaging of the paediatric female pelvis. Eur. Radiol. 2005, 15, 1296–1309. [Google Scholar] [CrossRef]
- Haller, J.O.; Bass, I.S.; Friedman, A.P. Pelvic masses in girls: An 8-year retrospective analysis stressing ultrasound as the prime imaging modality. Pediatr. Radiol. 1984, 14, 363–368. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Xu, C.; Huang, X.; Zhong, L.; Kang, X.; Yang, Z.; Liu, Y. Childhood ovarian juvenile granulosa cell tumor: A retrospective study with 3 cases including clinical features, pathologic results, and therapies. J. Pediatr. Hematol. Oncol. 2011, 33, 241–245. [Google Scholar] [CrossRef]
- Quillin, S.P.; Siegel, M.J. CT features of benign and malignant teratomas in children. J. Comput. Assist. Tomogr. 1992, 16, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Willems, R.P.; Slangen, B.; Busari, J.O. Abdominal swelling in two teenage girls: Two case reports of massive ovarian tumours in puberty. BMJ Case Rep. 2012, 2012, bcr1120115143. [Google Scholar] [CrossRef] [PubMed]
- Horta, M.; Cunha, T.M. Sex Cord-Stromal Tumors of the Ovary: A Comprehensive Review and Update for Radiologists. Diagn. Interv. Radiol. 2015, 21, 277–286. [Google Scholar] [CrossRef]
- Zhao, S.; Sun, F.; Bao, L.; Chu, C.; Li, H.; Yin, Q.; Guan, W.; Wang, D. Pure dysgerminoma of the ovary: CT and MRI features with pathological correlation in 13 tumors. J. Ovarian Res. 2020, 13, 71. [Google Scholar] [CrossRef]
- Alotaibi, M.O.; Navarro, O.M. Imaging of ovarian teratomas in children: A 9-year review. Can. Assoc. Radiol. J. 2010, 61, 23–28. [Google Scholar] [CrossRef]
- Golladay, E.S.; Mollitt, D.L. Ovarian masses in the child and adolescent. South. Med. J. 1983, 76, 954–957. [Google Scholar] [CrossRef]
- Wu, A.; Siegel, M.J. Sonography of pelvic masses in children: Diagnostic predictability. AJR Am. J. Roentgenol. 1987, 148, 1199–1202. [Google Scholar] [CrossRef]
- Quillin, S.P.; Siegel, M.J. Transabdominal color Doppler ultrasonography of the painful adolescent ovary. J. Ultrasound Med. 1994, 13, 549–555. [Google Scholar] [CrossRef]
- Fiegel, H.C.; Gfroerer, S.; Theilen, T.M.; Friedmacher, F.; Rolle, U. Ovarian lesions and tumors in infants and older children. Innov. Surg. Sci. 2021, 6, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Guleria, K.; Suneja, A.; Vaid, N.B.; Rajaram, S.; Wadhwa, N. Adolescent ovarian masses: A retrospective analysis. J. Obstet. Gynaecol. 2016, 36, 515–517. [Google Scholar] [CrossRef] [PubMed]
- Skiadas, V.T.; Koutoulidis, V.; Eleytheriades, M.; Gouliamos, A.; Moulopoulos, L.A.; Deligeoroglou, E.; Vlachos, L.; Kreatsas, G. Ovarian masses in young adolescents: Imaging findings with surgical confirmation. Eur. J. Gynaecol. Oncol. 2004, 25, 201–206. [Google Scholar] [PubMed]
- Depoers, C.; Martin, F.A.; Nyangoh Timoh, K.; Morcet, J.; Proisy, M.; Henno, S.; Lavoue, V.; Arnaud, A.P. A Preoperative Scoring System for Adnexal Mass in Children and Adolescents to Preserve Their Future Fertility. J. Pediatr. Adolesc. Gynecol. 2019, 32, 57–63. [Google Scholar] [CrossRef]
- Terzic, M.; Rapisarda, A.M.C.; Della Corte, L.; Manchanda, R.; Aimagambetova, G.; Norton, M.; Garzon, S.; Riemma, G.; King, C.R.; Chiofalo, B.; et al. Diagnostic work-up in paediatric and adolescent patients with adnexal masses: An evidence-based approach. J. Obstet. Gynaecol. 2021, 41, 503–515. [Google Scholar] [CrossRef]
- AlDakhil, L.; Aljuhaimi, A.; AlKhattabi, M.; Alobaid, S.; Mattar, R.E.; Alobaid, A. Ovarian neoplasia in adolescence: A retrospective chart review of girls with neoplastic ovarian tumors in Saudi Arabia. J. Ovarian Res. 2022, 15, 105. [Google Scholar] [CrossRef]
- Łuczak, J.; Bagłaj, M.; Dryjański, P.; Kalcowska, A.; Banaszyk-Pucała, N.; Boczar, M.; Dymek, K.; Fryczek, M.; Giżewska-Kacprzak, K.; Górecki, W.; et al. What Should Be the Topics of a Prospective Study on Ovarian Masses in Children?—Results of a Multicenter Retrospective Study and a Scoping Literature Review. Curr. Oncol. 2022, 29, 1488–1500. [Google Scholar] [CrossRef]
- Oltmann, S.C.; Fischer, A.; Barber, R.; Huang, R.; Hicks, B.; Garcia, N. Pediatric ovarian malignancy presenting as ovarian torsion: Incidence and relevance. J. Pediatr. Surg. 2010, 45, 135–139. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, D.; Wang, F. Clinical and Computed Tomographic Features of Ovarian Lesions in Infants, Children, and Adolescents: A Series of 222 Cases. J. Pediatr. Adolesc. Gynecol. 2021, 34, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Sintim-Damoa, A.; Majmudar, A.S.; Cohen, H.L.; Parvey, L.S. Pediatric Ovarian Torsion: Spectrum of Imaging Findings. RadioGraphics 2017, 37, 1892–1908. [Google Scholar] [CrossRef]
- Loh, A.H.; Ong, C.L.; Lam, S.L.; Chua, J.H.; Chui, C.H. Pediatric risk of malignancy index for preoperative evaluation of childhood ovarian tumors. Pediatr. Surg. Int. 2012, 28, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.G.; So, K.A.; Hwang, J.Y.; Kim, N.R.; Yang, E.J.; Shim, S.H.; Lee, S.J.; Kim, T.J. Ultrasonographic diagnosis and surgical outcomes of adnexal masses in children and adolescents. Sci. Rep. 2022, 12, 3949. [Google Scholar] [CrossRef]
- Ye, G.; Xu, T.; Liu, J.; Xu, W.; Lv, Z. The role of preoperative imaging and tumor markers in predicting malignant ovarian masses in children. Pediatr. Surg. Int. 2020, 36, 333–339. [Google Scholar] [CrossRef]
- Naffaa, L.; Deshmukh, T.; Tumu, S.; Johnson, C.; Boyd, K.P.; Meyers, A.B. Imaging of acute pelvic pain in girls: Ovarian torsion and beyond. Curr. Probl. Diagn. Radiol. 2017, 46, 317–329. [Google Scholar] [CrossRef]
- Kokoska, E.R.; Keller, M.S.; Weber, T.R. Acute ovarian torsion in children. Am. J. Surg. 2000, 180, 462–465. [Google Scholar] [CrossRef]
- Cass, D.L. Ovarian Torsion. Semin. Pediatr. Surg. 2005, 14, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Ngo, A.-V.; Otjen, J.P.; Parisi, M.T.; Ferguson, M.R.; Otto, R.K.; Stanescu, A.L. Pediatric Ovarian Torsion: A Pictorial Review. Pediatr. Radiol. 2015, 45, 1845–1855. [Google Scholar] [CrossRef]
- Servaes, S.; Zurakowski, D.; Laufer, M.R.; Feins, N.; Chow, J.S. Sonographic Findings of Ovarian Torsion in Children. Pediatr. Radiol. 2007, 37, 446–451. [Google Scholar] [CrossRef]
- Linam, L.E.; Darolia, R.; Naffaa, L.N.; Breech, L.L.; O’Hara, S.M.; Hillard, P.J.; Huppert, J.S. US Findings of Adnexal Torsion in Children and Adolescents: Size Really Does Matter. Pediatr. Radiol. 2007, 37, 1013–1019. [Google Scholar] [CrossRef]
- Rougier, E.; Mar, W.; Della Valle, V.; Morel, B.; Irtan, S.; Audureau, E.; Coulomb-L’Hermine, A.; Ducou Le Pointe, H.; Blondiaux, E. Added Value of MRI for the Diagnosis of Adnexal Torsion in Children and Adolescents after Inconclusive Ultrasound Examination. Diagn. Interv. Imaging 2020, 101, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Gkrozou, F.; Tsonis, O.; Vatopoulou, A.; Galaziou, G.; Paschopoulos, M. Ovarian Teratomas in Children and Adolescents: Our Own Experience and Review of Literature. Children 2022, 9, 1571. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, K.E.; Cooper, A.R. The Approach to Ovarian Dermoids in Adolescents and Young Women. J. Pediatr. Adolesc. Gynecol. 2011, 24, 176–180. [Google Scholar] [CrossRef]
- Billmire, D.; Dicken, B.; Rescorla, F.; Ross, J.; Piao, J.; Huang, L.; Krailo, M.; Pashankar, F.; Lindsay Frazier, L.; Children’s Oncology Group. Imaging Appearance of Nongerminoma Pediatric Ovarian Germ Cell Tumors Does Not Discriminate Benign from Malignant Histology. J. Pediatr. Adolesc. Gynecol. 2021, 34, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Jezierska, M.; Gawrychowska, A.; Stefanowicz, J. Diagnostic, Prognostic and Predictive Markers in Pediatric Germ Cell Tumors—Past, Present and Future. Diagnostics 2022, 12, 278. [Google Scholar] [CrossRef]
- Morowitz, M.; Huff, D.; von Allmen, D. Epithelial Ovarian Tumors in Children: A Retrospective Analysis. J. Pediatr. Surg. 2003, 38, 331–335. [Google Scholar] [CrossRef]
- Sri Paran, T.; Mortell, A.; Devaney, D.; Pinter, A.; Puri, P. Mucinous Cystadenoma of the Ovary in Perimenarchal Girls. Pediatr. Surg. Int. 2006, 22, 224–227. [Google Scholar] [CrossRef]
- Taskinen, S.; Fagerholm, R.; Lohi, J.; Taskinen, M. Pediatric Ovarian Neoplastic Tumors: Incidence, Age at Presentation, Tumor Markers and Outcome. Acta Obstet. Gynecol. Scand. 2015, 94, 425–429. [Google Scholar] [CrossRef]
- Asăvoaie, C.; Fufezan, O.; Coşarcă, M. Ovarian and uterine ultrasonography in pediatric patients: Pictorial essay. Med. Ultrason. 2014, 16, 160–167. [Google Scholar] [CrossRef][Green Version]
- Sayasneh, A.; Ekechi, C.; Ferrara, L.; Kaijser, J.; Stalder, C.; Sur, S.; Timmerman, D.; Bourne, T. The characteristic ultrasound features of specific types of ovarian pathology. Int. J. Oncol. 2015, 46, 445–458. [Google Scholar] [CrossRef]
- Tamai, K.; Koyama, T.; Saga, T.; Kido, A.; Kataoka, M.; Umeoka, S.; Fujii, S.; Togashi, K. MR Features of Physiologic and Benign Conditions of the Ovary. Eur. Radiol. 2006, 16, 2700–2711. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, S.; Irshad, A.; Lewis, M.; Anis, M. Ovarian Cystic Lesions. Radiol. Clin. N. Am. 2013, 51, 1067–1085. [Google Scholar] [CrossRef]
- Park, S.B.; Lee, J.B. MRI Features of Ovarian Cystic Lesions. J. Magn. Reson. Imaging 2014, 40, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.T.; Naik, M.; Bharwani, N.; Sudderuddin, S.A.; Rockall, A.G.; Stewart, V.R. Adnexal Torsion: Review of Radiologic Appearances. RadioGraphics 2021, 41, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.C.; Bhatt, S.; Dogra, V.S. Pearls and Pitfalls in Diagnosis of Ovarian Torsion. RadioGraphics 2008, 28, 1355–1368. [Google Scholar] [CrossRef]
- Duigenan, S.; Oliva, E.; Lee, S.I. Ovarian Torsion: Diagnostic Features on CT and MRI with Pathologic Correlation. AJR Am. J. Roentgenol. 2012, 198, W122–W131. [Google Scholar] [CrossRef]
- Rha, S.E.; Byun, J.Y.; Jung, S.E.; Jung, J.I.; Choi, B.G.; Kim, B.S.; Kim, H.; Lee, J.M. CT and MR Imaging Features of Adnexal Torsion. RadioGraphics 2002, 22, 283–294. [Google Scholar] [CrossRef]
- Haque, T.L.; Togashi, K.; Kabayashi, H.; Fujii, S.; Konishi, J. Adnexal Torsion: MR Imaging Findings of Viable Ovary. Eur. Radiol. 2000, 10, 1954–1957. [Google Scholar] [CrossRef]
- Fujii, S.; Kaneda, S.; Kakite, S.; Kanasaki, Y.; Matsusue, E.; Harada, T.; Kaminou, T.; Ogawa, T. Diffusion-weighted Imaging Findings of Adnexal Torsion: Initial Results. Eur. J. Radiol. 2009, 77, 330–334. [Google Scholar] [CrossRef]
- Kato, H.; Kanematsu, M.; Uchiyama, M.; Yano, R.; Furui, T.; Morishige, K. Diffusion-Weighted Imaging of Ovarian Torsion: Usefulness of Apparent Diffusion Coefficient (ADC) Values for the Detection of Hemorrhagic Infarction. Magn. Reson. Med. Sci. 2014, 13, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Thomassin-Naggara, I.; Darai, E.; Bazot, M. Gynecological Pelvic Infection: What Is the Role of Imaging? Diagn. Interv. Imaging 2012, 93, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Tukeva, T.A.; Aronen, H.J.; Karjalainen, P.T.; Molander, P.; Paavonen, T.; Paavonen, J. MR Imaging in Pelvic Inflammatory Disease: Comparison with Laparoscopy and US. Radiology 1999, 210, 209–216. [Google Scholar] [CrossRef]
- Revzin, M.V.; Mathur, M.; Dave, H.B.; Macer, M.L.; Spektor, M. Pelvic Inflammatory Disease: Multimodality Imaging Approach with Clinical-Pathologic Correlation. RadioGraphics 2016, 36, 1579–1596. [Google Scholar] [CrossRef] [PubMed]
- Bonde, A.; Andreazza Dal Lago, E.; Foster, B.; Javadi, S.; Palmquist, S.; Bhosale, P. Utility of the Diffusion Weighted Sequence in Gynecological Imaging: Review Article. Cancers 2022, 14, 4468. [Google Scholar] [CrossRef]
- Koulouris, C.R.; Penson, R.T. Ovarian Stromal and Germ Cell Tumors. Semin. Oncol. 2009, 36, 126–136. [Google Scholar] [CrossRef]
- Park, S.B.; Kim, J.K.; Kim, K.R.; Cho, K.S. Imaging Findings of Complications and Unusual Manifestations of Ovarian Teratomas. RadioGraphics 2008, 28, 969–983. [Google Scholar] [CrossRef]
- Saleh, M.; Bhosale, P.; Menias, C.O.; Ramalingam, P.; Jensen, C.; Iyer, R.; Ganeshan, D. Ovarian Teratomas: Clinical Features, Imaging Findings and Management. Abdom. Radiol. 2021, 46, 2293–2307. [Google Scholar] [CrossRef]
- Outwater, E.K.; Siegelman, E.S.; Hunt, J.L. Ovarian Teratomas: Tumor Types and Imaging Characteristics. RadioGraphics 2001, 21, 475–490. [Google Scholar] [CrossRef]
- Choudhary, S.; Fasih, N.; McInnes, M.; Marginean, C. Imaging of Ovarian Teratomas: Appearances and Complications. J. Med. Imaging Radiat. Oncol. 2009, 53, 480–488. [Google Scholar] [CrossRef]
- Sachs, J.R.; Dyer, R.B. The “Tip of the Iceberg” Sign. Abdom. Imaging 2015, 40, 934–935. [Google Scholar] [CrossRef]
- Sahin, H.; Abdullazade, S.; Sanci, M. Mature Cystic Teratoma of the Ovary: A Cutting-Edge Overview on Imaging Features. Insights Imaging 2017, 8, 227–241. [Google Scholar] [CrossRef]
- Tsili, A.C.; Dalkalitsis, N.; Paraskevaidis, E.; Tsampoulas, K. Multi-detector CT Features of Benign Adnexal Lesions. Acad. Radiol. 2010, 17, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Szymon, O.; Kiszka-Wiłkojć, A.; Fryczek, M.; Taczanowska-Niemczuk, A.; Wyrobek, Ł.; Górecki, W. “Floating Ball Sign” in the Diagnostic Imaging of Mature Ovarian Teratomas in Children. Pediatr. Surg. Int. 2023, 39, 215. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Yoshimitsu, K.; Irie, H.; Aibe, H.; Tajima, T.; Nishie, A.; Asayama, Y.; Matake, K.; Kakihara, D.; Matsuura, S.; et al. Diffusion-Weighted Echo-Planar MR Imaging and ADC Mapping in the Differential Diagnosis of Ovarian Cystic Masses: Usefulness of Detecting Keratinoid Substances in Mature Cystic Teratomas. J. Magn. Reason. Imaging 2005, 22, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Poncelet, E.; Delpierre, C.; Kerdraon, O.; Lucot, J.P.; Collinet, P.; Bazot, M. Value of Dynamic Contrast-Enhanced MRI for Tissue Characterization of Ovarian Teratomas: Correlation with Histopathology. Clin. Radiol. 2013, 68, 909–916. [Google Scholar] [CrossRef]
- Brammer, H.M., III; Buck, J.L.; Hayes, W.S.; Sheth, S.; Tavassoli, F.A. From the archives of the AFIP. Malignant germ cell tumors of the ovary: Radiologic-pathologic correlation. RadioGraphics 1990, 10, 715–724. [Google Scholar] [CrossRef]
- Saba, L.; Guerriero, S.; Sulcis, R.; Virgilio, B.; Melis, G.; Mallarini, G. Mature and immature ovarian teratomas: CT, US and MR imaging characteristics. Eur. J. Radiol. 2009, 72, 454–463. [Google Scholar] [CrossRef]
- Yamaoka, T.; Togashi, K.; Koyama, T.; Fujiwara, T.; Higuchi, T.; Iwasa, Y.; Fujii, S.; Konishi, J. Immature Teratoma of the Ovary: Correlation of MR Imaging and Pathologic Findings. Eur. Radiol. 2003, 13, 313–319. [Google Scholar] [CrossRef]
- Wang, D.; Jia, C.W.; Feng, R.E.; Shi, H.H.; Sun, J. Gliomatosis Peritonei: A Series of Eight Cases and Review of the Literature. J. Ovarian Res. 2016, 9, 45. [Google Scholar] [CrossRef]
- Levy, A.D.; Shaw, J.C.; Sobin, L.H. Secondary Tumors and Tumorlike Lesions of the Peritoneal Cavity: Imaging Features with Pathologic Correlation. RadioGraphics 2009, 29, 347–373. [Google Scholar] [CrossRef]
- Shaaban, A.M.; Rezvani, M.; Elsayes, K.M.; Baskin Jr, H.; Mourad, A.; Foster, B.R.; Jarboe, E.A.; Menias, C.O. Ovarian Malignant Germ Cell Tumors: Cellular Classification and Clinical and Imaging Features. RadioGraphics 2014, 34, 777–801. [Google Scholar] [CrossRef] [PubMed]
- Goudie, C.; Witkowski, L.; Vairy, S.; McCluggage, W.G.; Foulkes, W.D. Paediatric Ovarian Tumours and Their Associated Cancer Susceptibility Syndromes. J. Med. Genet. 2018, 55, 1–10. [Google Scholar] [CrossRef]
- Kitajima, K.; Hayashi, M.; Kuwata, Y.; Imanaka, K.; Sugimura, K. MRI Appearances of Ovarian Dysgerminoma. Eur. J. Radiol. Extra 2007, 61, 23–25. [Google Scholar] [CrossRef]
- Anfelter, P.; Testa, A.; Chiappa, V.; Froyman, W.; Fruscio, R.; Guerriero, S.; Alcazar, J.L.; Mascillini, F.; Pascual, M.A.; Sibal, M.; et al. Imaging in gynecological disease (17): Ultrasound features of malignant ovarian yolk sac tumors (endodermal sinus tumors). Ultrasound Obstet. Gynecol. 2020, 56, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.K.; Zheng, Y.; Lin, J.B.; Xu, G.X.; Cai, A.Q.; Zhou, X.G.; Zhang, G.J. CT Imaging of Ovarian Yolk Sac Tumor with Emphasis on Differential Diagnosis. Sci. Rep. 2015, 5, 11000. [Google Scholar] [CrossRef]
- Yamaoka, T.; Togashi, K.; Koyama, T.; Ueda, H.; Nakai, A.; Fujii, S.; Yamabe, H.; Konishi, J. Yolk Sac Tumor of the Ovary: Radiologic-Pathologic Correlation in Four Cases. J. Comput. Assist. Tomogr. 2000, 24, 605–609. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, S.; Schwartz, P.E. CT Scan Detects Linear Tear in Ovarian Yolk Sac Tumor. Eur. J. Radiol. Extra 2010, 73, e73–e75. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, C.; Zou, L.; Wang, Y.; Song, X.; Ma, Y.; Liu, A. Clinicopathological Analysis of Non-Gestational Ovarian Choriocarcinoma: Report of Two Cases and Review of the Literature. Oncol. Lett. 2016, 11, 2599–2604. [Google Scholar] [CrossRef]
- Moro, F.; Castellano, L.M.; Franchi, D.; Epstein, E.; Fischerova, D.; Froyman, W.; Timmerman, D.; Zannoni, G.F.; Scambia, G.; Valentin, L.; et al. Imaging in gynecological disease (22): Clinical and ultrasound characteristics of ovarian embryonal carcinomas, non-gestational choriocarcinomas and malignant mixed germ cell tumors. Ultrasound Obstet. Gynecol. 2021, 57, 987–994. [Google Scholar] [CrossRef]
- Youssef, A.T. Rare occurrence of ovarian choriocarcinoma: Ultrasound evaluation. J. Ultrasound 2025, 28, 213–216. [Google Scholar] [CrossRef]
- Seymour, E.Q.; Hood, J.B.; Underwood, P.B., Jr.; Williamson, H.O. Gonadoblastoma: An ovarian tumor with characteristic pelvic calcifications. AJR Am. J. Roentgenol. 1976, 127, 1001–1002. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roth, L.M.; Cheng, L. Gonadoblastoma: Origin and Outcome. Hum. Pathol. 2020, 100, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, G.; Sebire, N.J.; McHugh, K. Imaging of the Unusual Pediatric “Blastomas”. Cancer Imaging 2009, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.O.; Tsunoda, H.; Kitagawa, Y.; Ueno, T.; Yoshikawa, H.; Saida, Y. Functioning Ovarian Tumors: Direct and Indirect Findings at MR Imaging. RadioGraphics 2004, 24, S147–S166. [Google Scholar] [CrossRef]
- Jung, S.E.; Rha, S.E.; Lee, J.M.; Park, S.Y.; Oh, S.N.; Cho, K.S.; Lee, E.J.; Byun, J.Y.; Hahn, S.T. CT and MRI Findings of Sex Cord Stromal Tumor of the Ovary. AJR Am. J. Roentgenol. 2005, 185, 207–215. [Google Scholar] [CrossRef]
- Van Holsbeke, C.; Domali, E.; Holland, T.K.; Achten, R.; Testa, A.C.; Valentin, L.; Jurkovic, D.; Moerman, P.; Timmerman, D. Imaging of gynecological disease (3): Clinical and ultrasound characteristics of granulosa cell tumors of the ovary. Ultrasound Obstet. Gynecol. 2008, 31, 450–456. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, S.H. Granulosa Cell Tumor of the Ovary: Common Findings and Unusual Appearances on CT and MR. J. Comput. Assist. Tomogr. 2002, 26, 756–761. [Google Scholar] [CrossRef]
- Kitamura, Y.; Kanegawa, K.; Muraji, T.; Sugimura, K. MR imaging of juvenile granulosa cell tumour of the ovary: A case report. Pediatr. Radiol. 2000, 30, 360. [Google Scholar] [CrossRef]
- Jung, S.E.; Lee, J.M.; Rha, S.E.; Byun, J.Y.; Jung, J.I.; Hahn, S.T. CT and MR Imaging of Ovarian Tumors with Emphasis on Differential Diagnosis. RadioGraphics 2002, 22, 1305–1325. [Google Scholar] [CrossRef]
- Buy, J.N.; Ghossain, M.A.; Sciot, C.; Bazot, M.; Guinet, C.; Prévot, S.; Hugol, D.; Laromiguiere, M.; Truc, J.B.; Poitout, P.; et al. Epithelial tumors of the ovary: CT findings and correlation with US. Radiology 1991, 178, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Mascilini, F.; Moro, F.; Pasciuto, T.; Sladkevicius, P.; Froyman, W.; Jokubkiene, L.; Van Holsbeke, C.; Franchi, D.; Epstein, E.; Guerriero, S.; et al. Imaging in gynecological disease (28): Clinical and ultrasound characteristics of serous and mucinous cystadenomas in the adnexa. Ultrasound Obstet. Gynecol. 2025. [Google Scholar] [CrossRef]
- Moro, F.; Zannoni, G.F.; Arciuolo, D.; Pasciuto, T.; Amoroso, S.; Mascilini, F.; Mainenti, S.; Scambia, G.; Testa, A.C. Imaging in gynecological disease (11): Clinical and ultrasound features of mucinous ovarian tumors. Ultrasound Obstet. Gynecol. 2017, 50, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Moro, F.; Baima Poma, C.; Zannoni, G.F.; Vidal Urbinati, A.; Pasciuto, T.; Ludovisi, M.; Moruzzi, M.C.; Carinelli, S.; Franchi, D.; Scambia, G.; et al. Imaging in gynecological disease (12): Clinical and ultrasound features of invasive and non-invasive malignant serous ovarian tumors. Ultrasound Obstet. Gynecol. 2017, 50, 788–799. [Google Scholar] [CrossRef]
- Flicek, K.T.; VanBuren, W.; Dudiak, K.; Lahkman, Y.; Chen, L.W.; Butler, K.; Menias, C.O. Borderline epithelial ovarian tumors: What the radiologist should know. Abdom. Radiol. 2021, 46, 2350–2366. [Google Scholar] [CrossRef] [PubMed]
- Outwater, E.K.; Huang, A.B.; Dunton, C.J.; Talerman, A.; Capuzzi, D.M. Papillary Projections in Ovarian Neoplasms: Appearance on MRI. J. Magn. Reson. Imaging 1997, 7, 689–695. [Google Scholar] [CrossRef]
- Parasar, P.; Ozcan, P.; Terry, K.L. Endometriosis: Epidemiology, Diagnosis and Clinical Management. Curr. Obstet. Gynecol. Rep. 2017, 6, 34–41. [Google Scholar] [CrossRef]
- Millischer, A.E.; Santulli, P.; Da Costa, S.; Bordonne, C.; Cazaubon, E.; Marcellin, L.; Chapron, C. Adolescent endometriosis: Prevalence increases with age on magnetic resonance imaging scan. Fertil. Steril. 2023, 119, 626–633. [Google Scholar] [CrossRef]
- Martire, F.G.; Russo, C.; Selntigia, A.; Nocita, E.; Soreca, G.; Lazzeri, L.; Zupi, E.; Exacoustos, C. Early noninvasive diagnosis of endometriosis: Dysmenorrhea and specific ultrasound findings are important indicators in young women. Fertil. Steril. 2023, 119, 455–464. [Google Scholar] [CrossRef]
- Van Holsbeke, C.; Van Calster, B.; Guerriero, S.; Savelli, L.; Paladini, D.; Lissoni, A.A.; Czekierdowski, A.; Fischerova, D.; Zhang, J.; Mestdagh, G.; et al. Endometriomas: Their Ultrasound Characteristics. Ultrasound Obstet. Gynecol. 2010, 35, 730–740. [Google Scholar] [CrossRef]
- Woodward, P.J.; Sohaey, R.; Mezzetti, T.P., Jr. Endometriosis: Radiologic-Pathologic Correlation. RadioGraphics 2001, 21, 193–216. [Google Scholar] [CrossRef] [PubMed]
- Bourgioti, C.; Preza, O.; Panourgias, E.; Chatoupis, K.; Antoniou, A.; Nikolaidou, M.E.; Moulopoulos, L.A. MR Imaging of Endometriosis: Spectrum of Disease. Diagn. Interv. Imaging 2017, 98, 751–767. [Google Scholar] [CrossRef] [PubMed]
- Kido, A.; Himoto, Y.; Moribata, Y.; Kurata, Y.; Nakamoto, Y. MRI in the Diagnosis of Endometriosis and Related Diseases. Korean J. Radiol. 2022, 23, 426. [Google Scholar] [CrossRef] [PubMed]
- Corwin, M.T.; Gerscovich, E.O.; Lamba, R.; Wilson, M.; McGahan, J.P. Differentiation of Ovarian Endometriomas from Hemorrhagic Cysts at MR Imaging: Utility of the T2 Dark Spot Sign. Radiology 2014, 271, 126–132. [Google Scholar] [CrossRef]



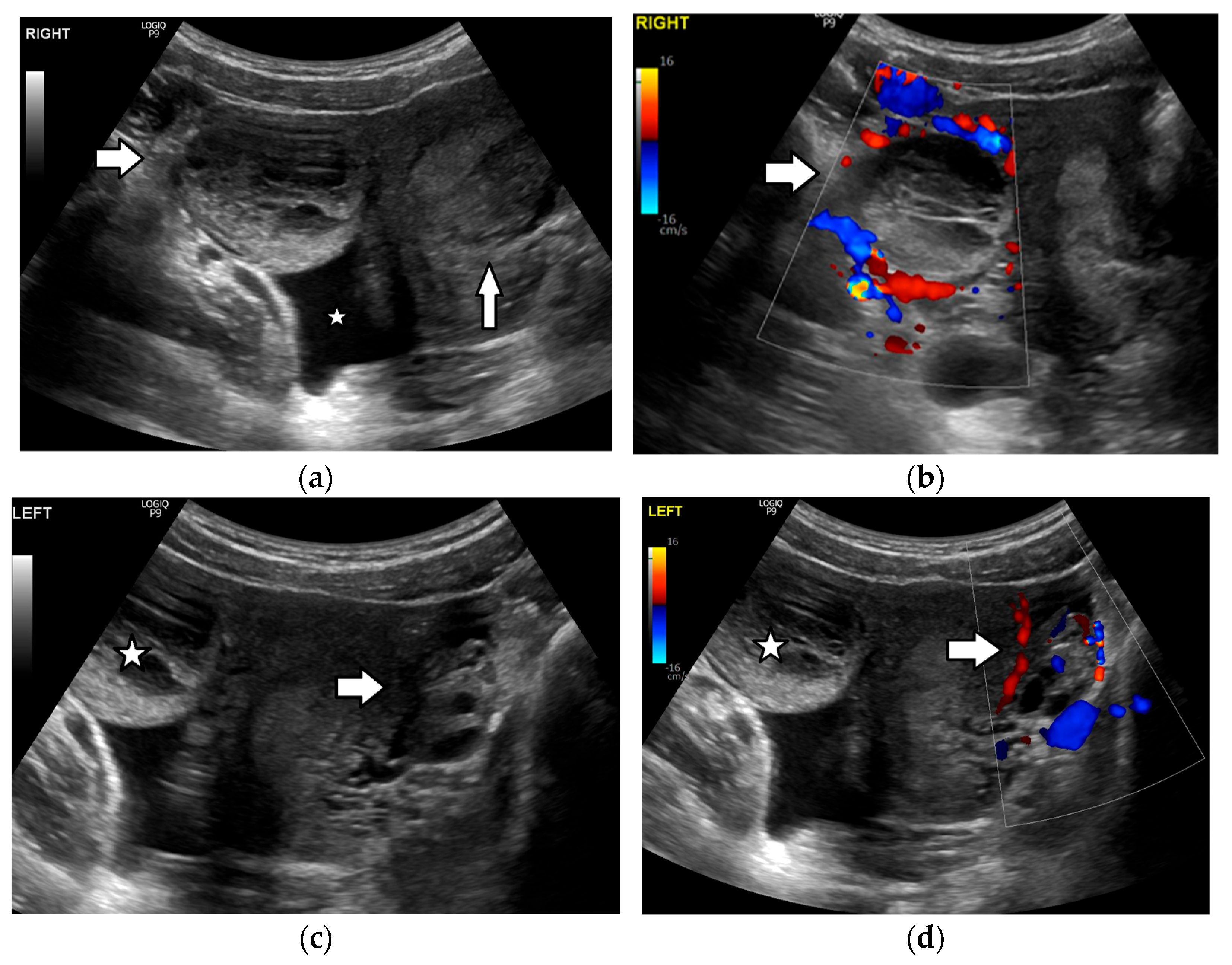
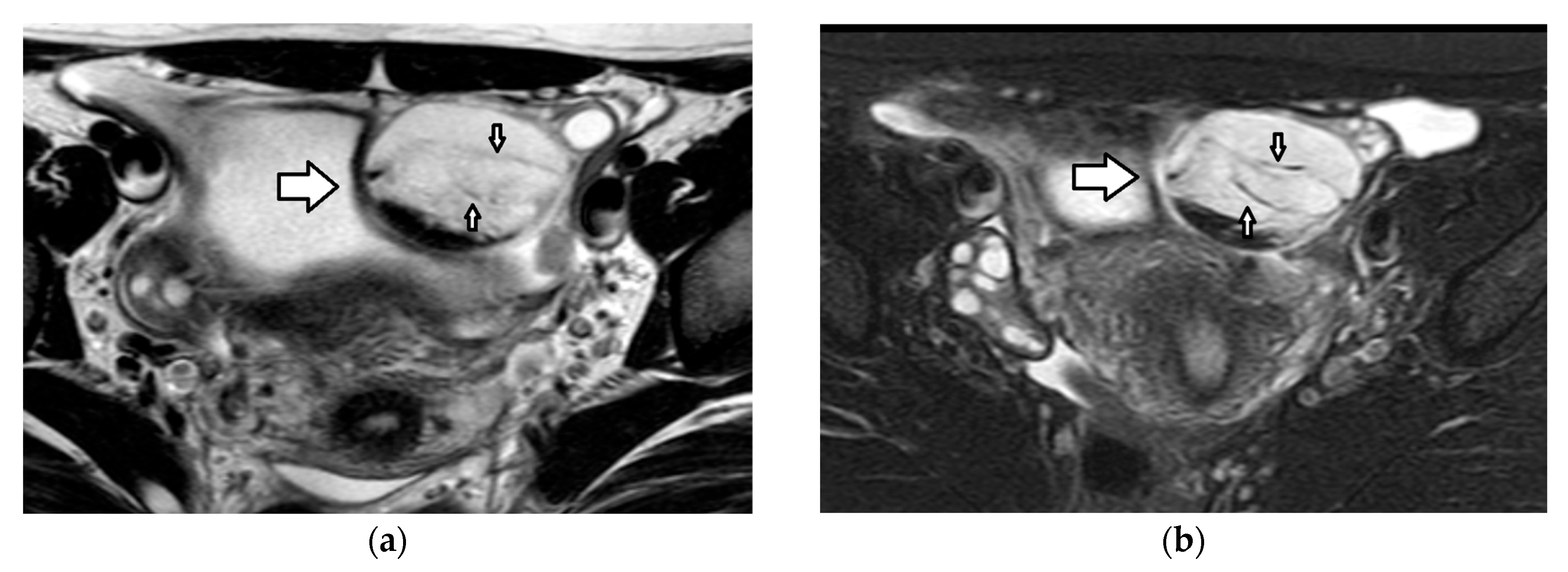
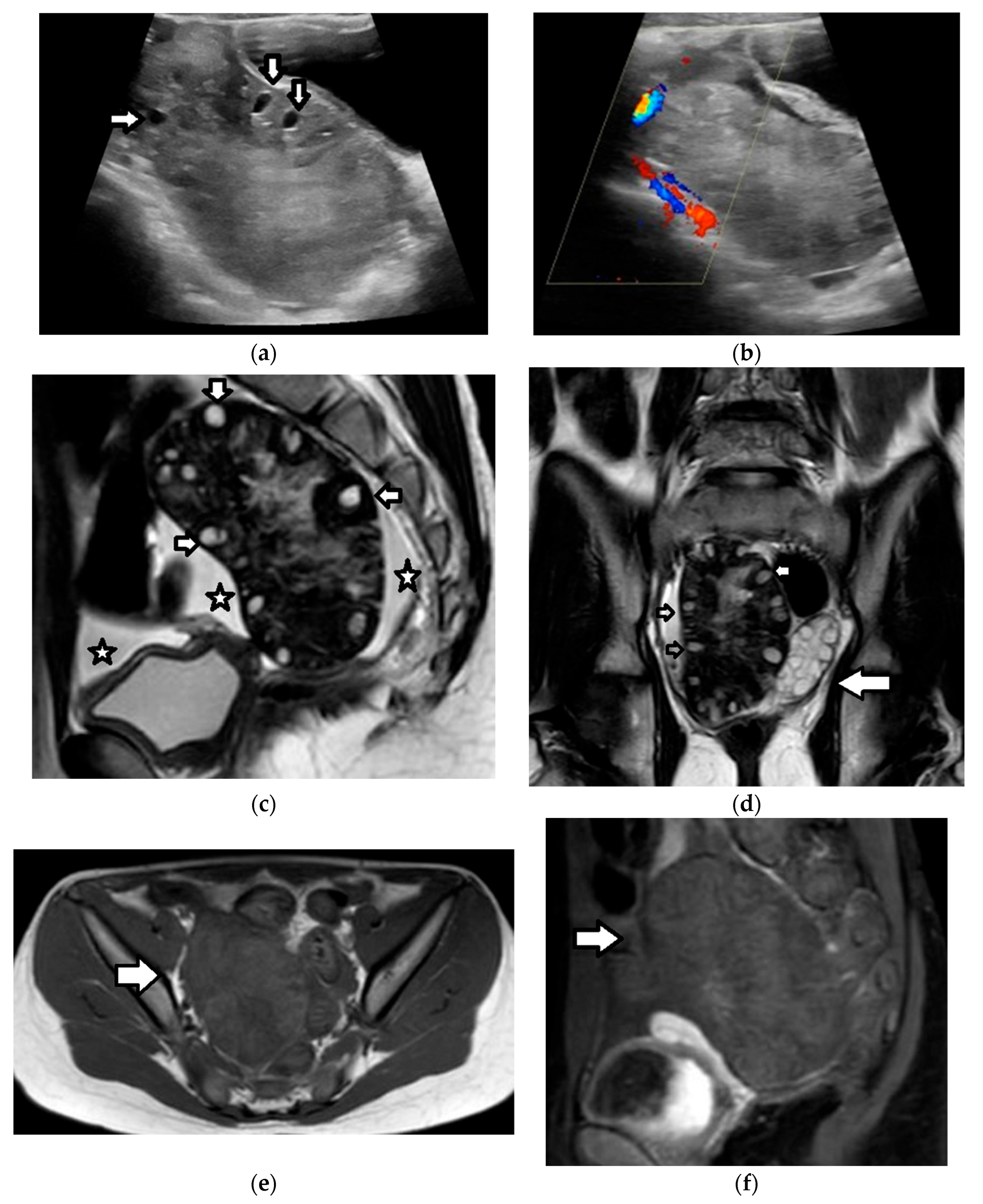
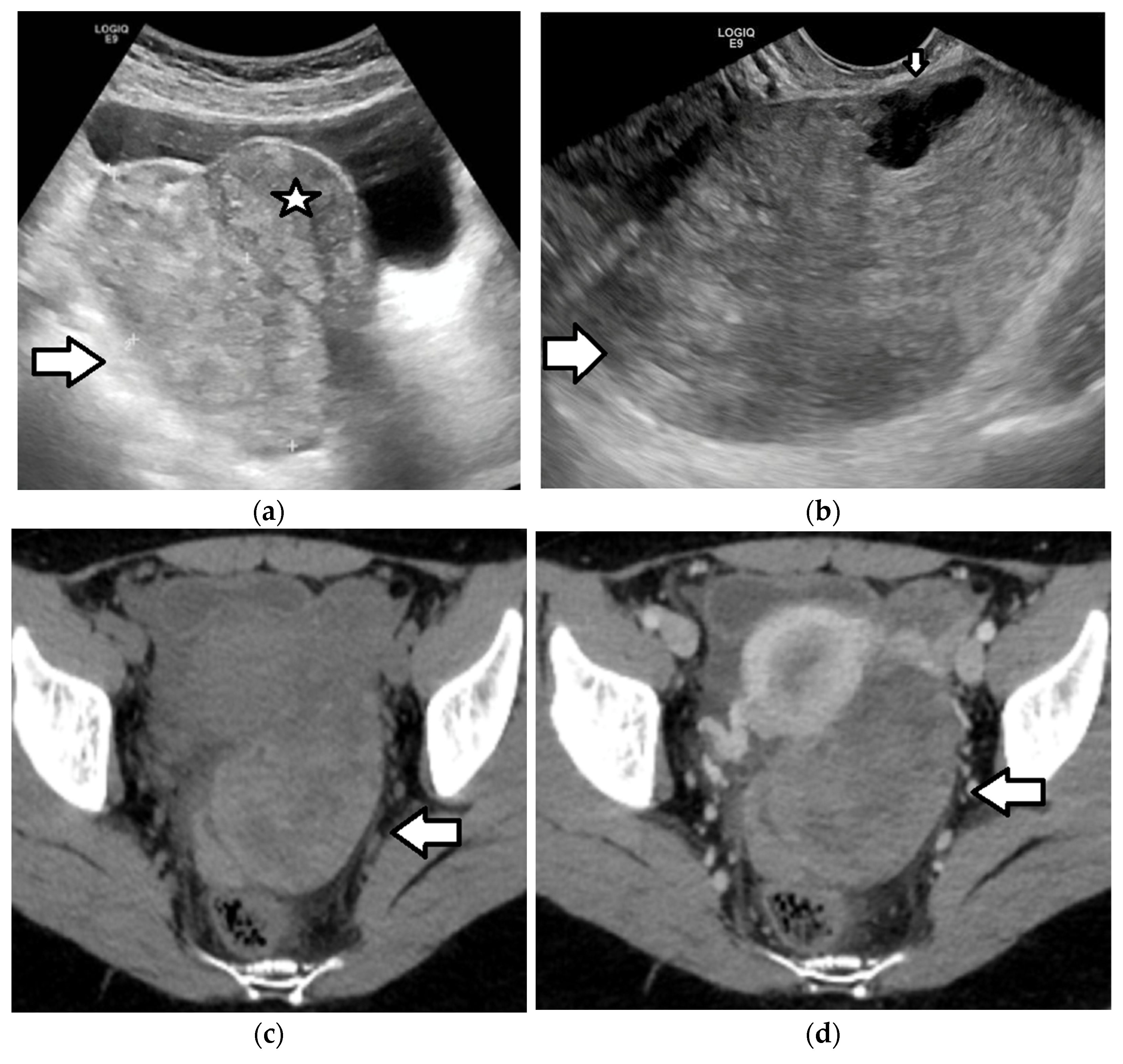
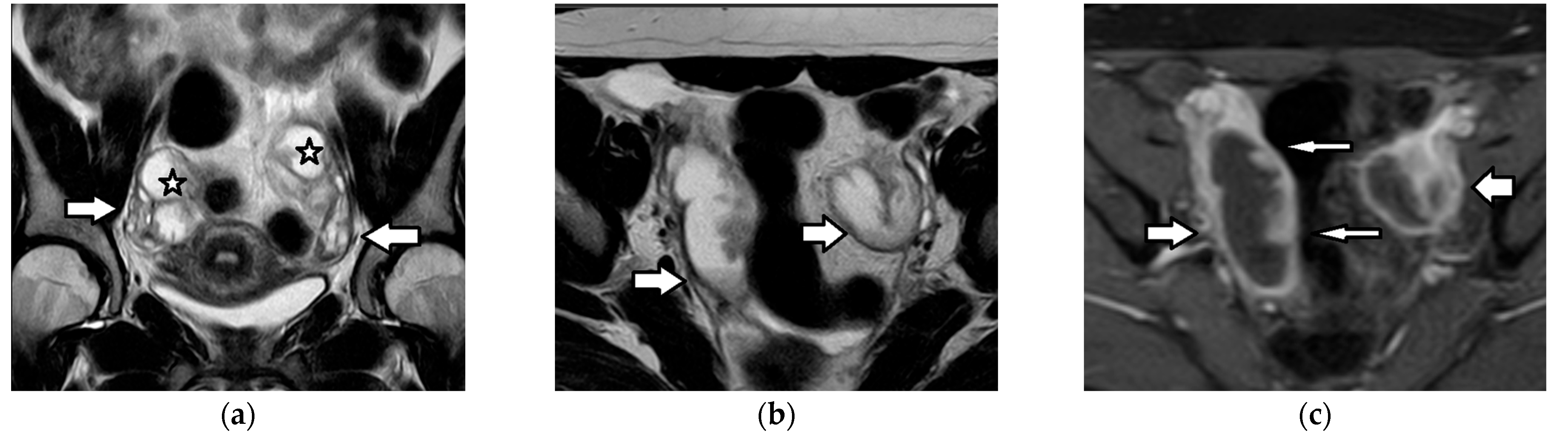

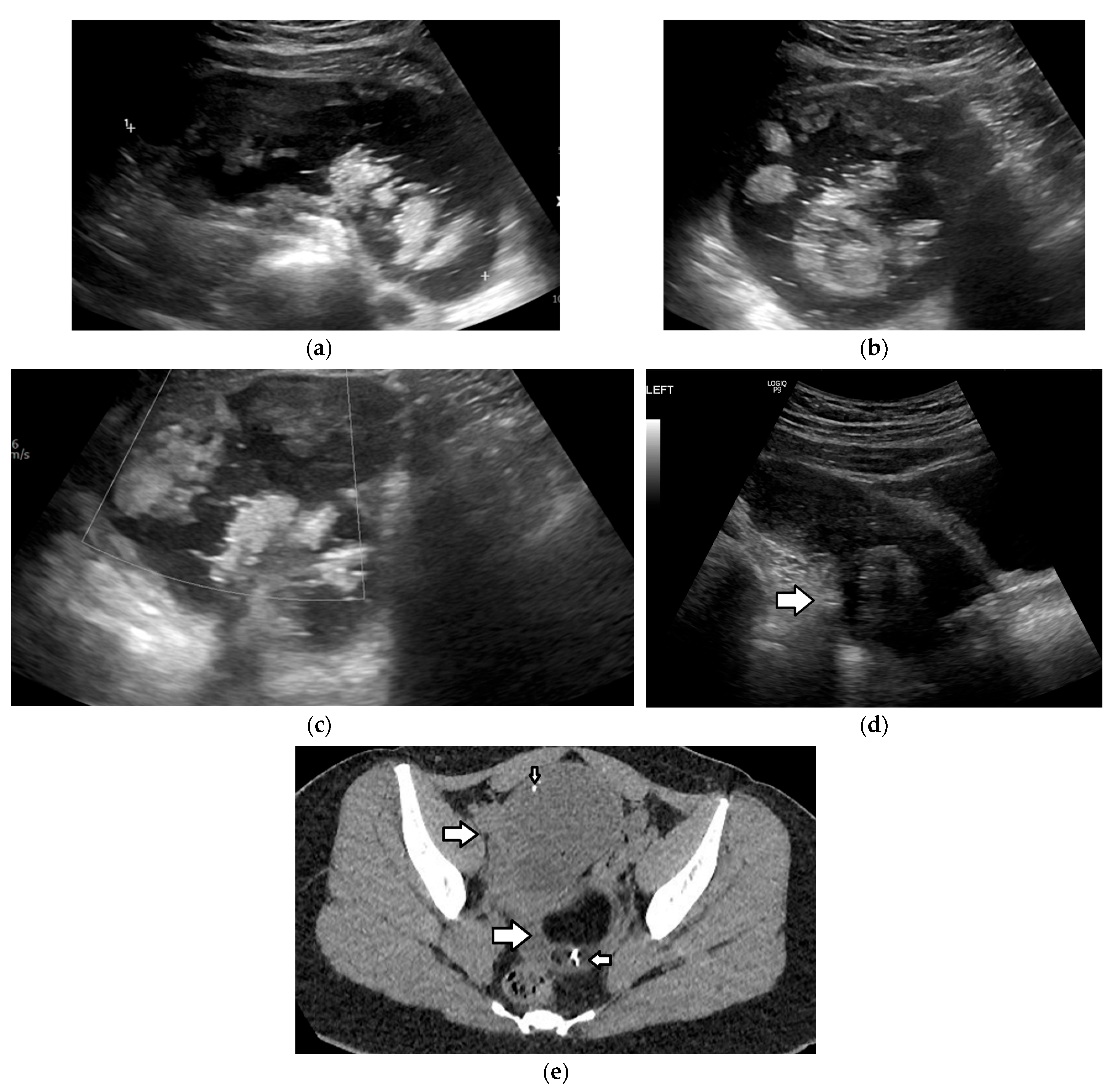


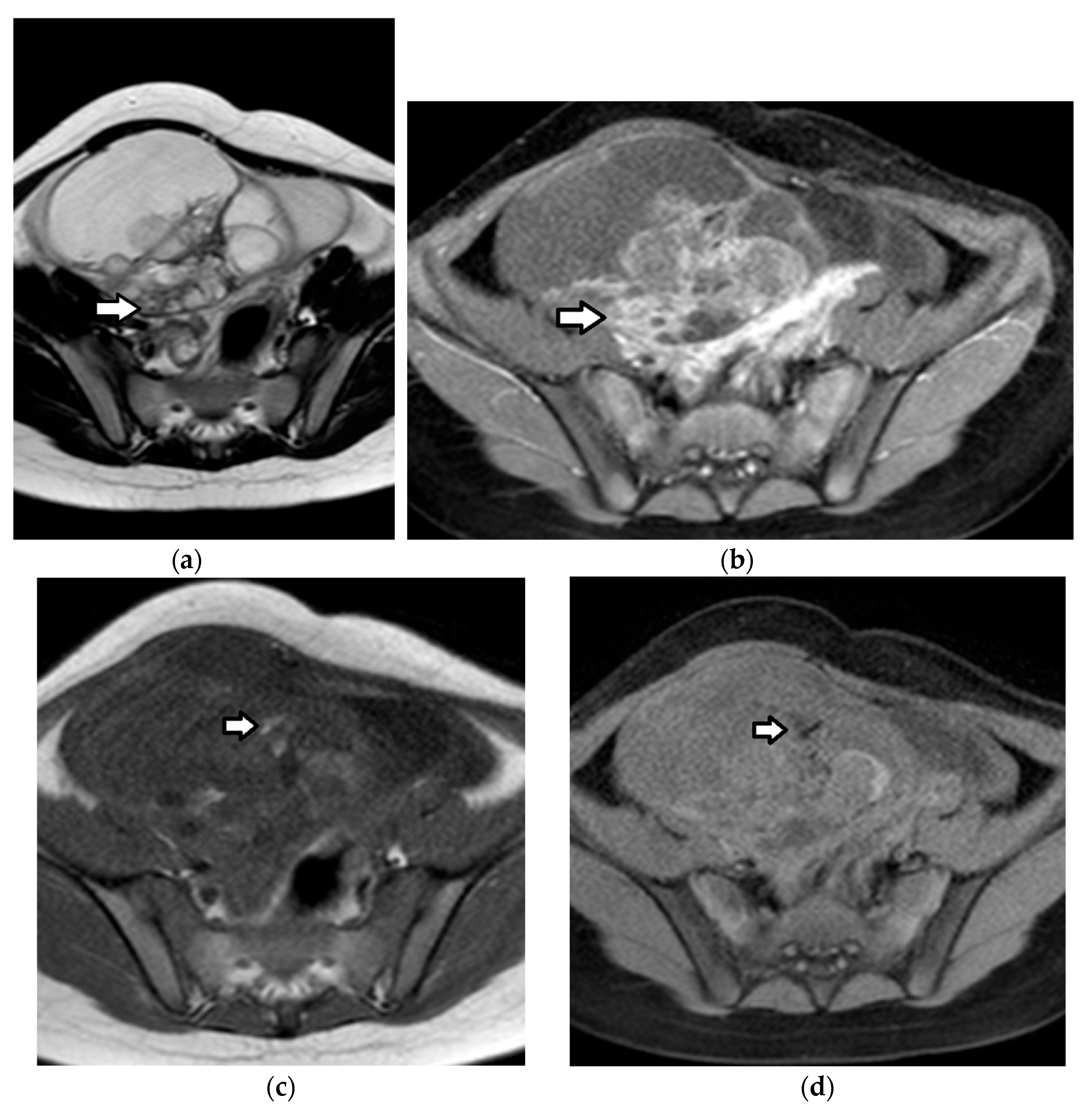
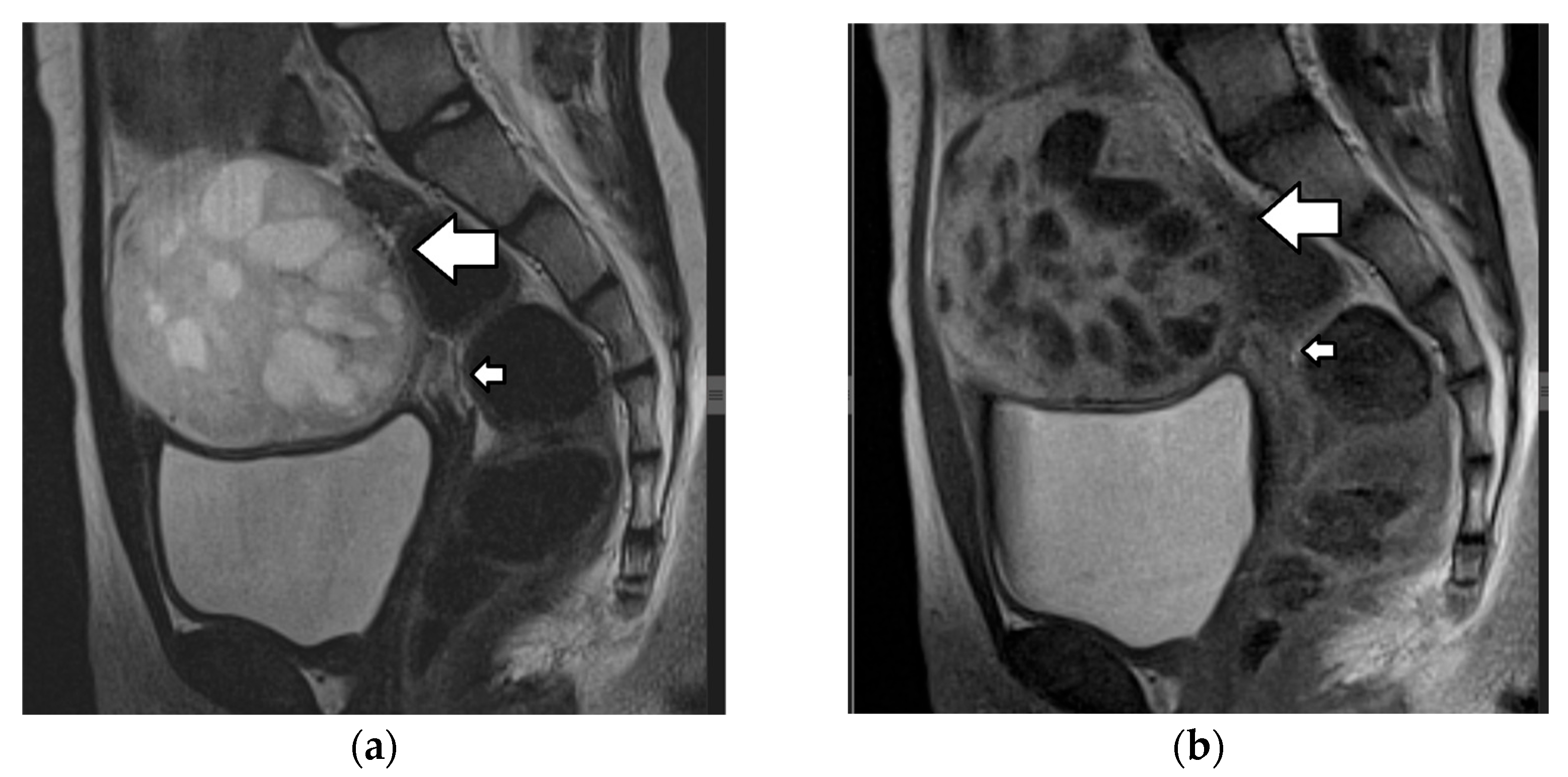

| Histology | Clinical Features | Biologic Behavior | Laboratory Findings | Imaging Characteristics |
|---|---|---|---|---|
| Mature teratoma | most common benign ovarian tumor most common GCT mean age: first, second decades often asymptomatic bilateral: 10–25% 10% of MOGCTs, in contralateral ovary | benign complications: torsion, rupture, infection, malignant transformation (rare) | mean diameter: 6.5 cm US:
| |
| Immature teratoma | 10–20% of ovarian malignancies second most common MOGCT mean age: 10 years ipsilateral MCT: 26% contralateral MCT: 10% | more aggressive compared to MCT often diagnosed at an early stage favorable prognosis | increased AFP (yolk sac tumor elements): 33–65% | large size (mean diameter: 16 cm) extensive solid parts with rich vascularity small cystic areas scattered foci of fat multiple, small, irregular calcifications |
| Dysgerminoma | most common ovarian malignancy 30% of MOGCTs mean age: second, third decades; 10%: first decade bilateral: 10–15% associated with gonadal dysgenesis, gonadoblastoma, chromosomal abnormalities, e.g., Turner syndrome | often diagnosed at an early stage favorable prognosis involvement of pelvic or retroperitoneal lymph nodes recurrence: 13–20% | increased LDH: 95% increased β-hCG (syncytiotrophoblastic giant cells): 5% | large, well-defined, lobulated, mainly solid tumor fibrovascular septa: characteristic
|
| Yolk sac tumor | second most common MOGCT mean age: second, third decades | rapid growth aggressive often diagnosed at an early stage | increased AFP | large, predominantly solid tumor, highly vascular or solid and cystic mass, with intratumoral hemorrhage and necrosis MRI
|
| Non-gestational choriocarcinoma | very rare MOGCT often part of mixed GCT | aggressive poor prognosis | increased β-hCG | few data complex, well-defined, highly vascular tumor |
| Embryonal carcinoma | rare MOGCT often part of mixed GCT mean age: 14 years | highly malignant | increased AFP or β-hCG: 60% | nonspecific large, predominantly solid mass, extensive hemorrhagic and necrotic areas, cystic components with mucoid content |
| Polyembryoma | extremely rare MOGCT often part of mixed GCT | nonspecific | ||
| Mixed GCT | often a combination of dysgerminoma, immature teratoma, and yolk sac tumor | nonspecific large, predominantly solid tumor areas of hemorrhage and necrosis | ||
| Gonadoblastoma | rare association with disorders of sex development and Y chromosome material (Turner syndrome variant) + WT1-related disorders such as Frasier syndrome and Denys–Drash syndrome bilateral: 50% may coexist with MOGCTs, often dysgerminoma | good prognosis | nonspecific small size: difficult to detect or large, solid tumor, with mottled or punctate calcifications |
| Histology | Clinical Features | Biologic Behavior | Laboratory Findings | Imaging Characteristics |
|---|---|---|---|---|
| Juvenile granulosa cell tumor | 75% of SCSTs mean age: 13 years bilateral: 4–5% secretes estrogens: signs of isosexual peripheral precocious puberty or menstrual irregularities secretes androgens: virilization (rarely) possible association with enchondromatosis syndromes: Maffuci syndrome, Ollier disease | malignant often diagnosed at an early stage excellent prognosis recurrence: rare | estrogens androgens (rarely) inhibin, especially inhibin B Müllerian Inhibiting Substance | nonspecific large size (mean diameter, 12.5 cm) multicystic tumor, with solid vascular components MRI
|
| Sertoli–Leydig cell tumor | 15% of SCSTs mean age: 14 years mostly unilateral secretes androgens: virilization secretes estrogens (rarely) association with DICER1 syndrome(moderately and poorly differentiated tumors) possible association with Peutz–Jeghers syndrome | moderately and poorly differentiated tumors: clinically malignant in 10% and 60% of cases, respectively often diagnosed at an early stage favorable prognosis recurrence rate: may be high | increased AFP (occasionally): poorly differentiated tumors with heterologous hepatocyte elements | nonspecific predominantly solid vascular tumor, with numerous peripheral or intratumoral cysts or cystic, with solid components MRI
|
| Histology | Clinical Features | Biologic Behavior | Laboratory Findings | Imaging Characteristics |
|---|---|---|---|---|
| Benign epithelial tumors: serous, mucinous, and mixed cystadenoma | Most common (47–58%) of epithelial tumors After menarche | Benign Recurrence: rarely in contralateral ovary | Serous cystadenoma: unilocular or multilocular, homogeneous, cystic tumor with serous fluid, absence of internal vascularity on Doppler US, and contrast enhancement on CT/MRI, except for thin, smooth walls or septa (≤ 3 mm) Mucinous cystadenoma:multilocular cystic tumor, large size, absence of internal vascularity on Doppler US, and contrast enhancement on CT/MRI, except for thin, smooth wall or septa (≤3 mm), and presence of mucinous fluid, with higher than water CT density (>20 HU) and high T1 signal (proteinaceous content); “stained glass” appearance:different signal intensity in the cystic parts of the tumor due to variable concentrations of mucin and hemorrhage as a characteristic finding | |
| Borderline and malignant epithelial tumors | Borderline: 21–38% of epithelial tumors, and more common in children and young women compared to in adults Malignant: rare (<5%) | Borderline: may recur (7.7%) Malignant: early-stage and low-grade | Large size, bilateral masses, cystic-solid tumor, with solid, vascular components, thick and irregular walls and septa, and/or papillary projections, with blood flow on Doppler US and enhancement on CT/MRI, as well as solid tumor with areas of necrosis Ancillary findings: pelvic organ or pelvic sidewall invasion, ascites, peritoneal metastases Papillary projections: intermediate T1 and T2 signal, variable vascularity, and may have a central hypointense fibrovascular stromal core and hyperintense periphery on T2WI, which are mostly characteristic of borderline tumors |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bourgioti, C.; Konidari, M.; Giantsouli, A.; Kafritsa, A.; Xydis, V.; Moulopoulos, L.-A.; Argyropoulou, M.I.; Tsili, A.C. Imaging Evaluation of Ovarian Masses in a Pediatric Population: A Comprehensive Overview. Cancers 2025, 17, 2316. https://doi.org/10.3390/cancers17142316
Bourgioti C, Konidari M, Giantsouli A, Kafritsa A, Xydis V, Moulopoulos L-A, Argyropoulou MI, Tsili AC. Imaging Evaluation of Ovarian Masses in a Pediatric Population: A Comprehensive Overview. Cancers. 2025; 17(14):2316. https://doi.org/10.3390/cancers17142316
Chicago/Turabian StyleBourgioti, Charis, Marianna Konidari, Anastasia Giantsouli, Afroditi Kafritsa, Vassilis Xydis, Lia-Angela Moulopoulos, Maria I. Argyropoulou, and Athina C. Tsili. 2025. "Imaging Evaluation of Ovarian Masses in a Pediatric Population: A Comprehensive Overview" Cancers 17, no. 14: 2316. https://doi.org/10.3390/cancers17142316
APA StyleBourgioti, C., Konidari, M., Giantsouli, A., Kafritsa, A., Xydis, V., Moulopoulos, L.-A., Argyropoulou, M. I., & Tsili, A. C. (2025). Imaging Evaluation of Ovarian Masses in a Pediatric Population: A Comprehensive Overview. Cancers, 17(14), 2316. https://doi.org/10.3390/cancers17142316






