Evaluating the Effectiveness of Neoadjuvant Therapy in Her2-Positive Invasive Breast Cancer: A Comprehensive Analysis of 167 Cases in Romania
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| pCR | Pathological complete response |
| IBC | Invasive breast cancer |
| Her2 | Human epidermal growth factor receptor 2 |
| CEP17 | Centromeric enumeration probe of chromosome 17 |
| NAT | Neoadjuvant therapy |
| ISH | In situ hybridization |
| IHC | Immunohistochemistry |
| EU | European Union |
| ASCO/CAP | American Society of Clinical Oncology/College of American Pathologists |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Rüschoff, J.; Lebeau, A.; Kreipe, H.; Sinn, P.; Gerharz, C.D.; Koch, W.; Morris, S.; Ammann, J.; Untch, M. Assessing HER2 testing quality in breast cancer: Variables that influence HER2 positivity rate from a large, multicenter, observational study in Germany. Mod. Pathol. 2017, 30, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Carneal, E.E.; Lichtensztajn, D.Y.; Gomez, S.L.; Clarke, C.A.; Jensen, K.C.; Kurian, A.W.; Allison, K.H. Regional Variability in Percentage of Breast Cancers Reported as Positive for HER2 in California: Implications of Patient Demographics on Laboratory Benchmarks. Am. J. Clin. Pathol. 2017, 148, 199–207. [Google Scholar] [CrossRef]
- Kim, M.C.; Cho, E.Y.; Park, S.Y.; Lee, H.J.; Lee, J.S.; Kim, J.Y.; Lee, H.C.; Yoo, J.Y.; Kim, H.S.; Kim, B.; et al. A Nationwide Study on HER2-Low Breast Cancer in South Korea: Its Incidence of 2022 Real World Data and the Importance of Immunohistochemical Staining Protocols. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2024, 56, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; André, F.; Bachelot, T.; Barrios, C.; Bergh, J.; Burstein, H.; Cardoso, M.; Carey, L.; Dawood, S.; Del Mastro, L. Early breast cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2024, 35, 159–182. [Google Scholar] [CrossRef]
- Trastuzumab for early-stage, HER2-positive breast cancer: A meta-analysis of 13 864 women in seven randomised trials. Lancet Oncol. 2021, 22, 1139–1150. [CrossRef]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef]
- Gianni, L.; Pienkowski, T.; Im, Y.H.; Roman, L.; Tseng, L.M.; Liu, M.C.; Lluch, A.; Staroslawska, E.; de la Haba-Rodriguez, J.; Im, S.A.; et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): A randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012, 13, 25–32. [Google Scholar] [CrossRef]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef]
- Elmadani, M.; Mokaya, P.O.; Omer, A.A.; Kiptulon, E.K.; Klara, S.; Orsolya, M. Cancer burden in Europe: A systematic analysis of the GLOBOCAN database (2022). BMC Cancer 2025, 25, 447. [Google Scholar] [CrossRef]
- Pantelimon, I.; Stancu, A.M.; Coniac, S.; Ionescu, A.I.; Atasiei, D.I.; Georgescu, D.E.; Galeș, L.N. Local Control of Advanced Breast Cancer-Debate in Multidisciplinary Tumor Board. J. Clin. Med. 2025, 14, 510. [Google Scholar] [CrossRef] [PubMed]
- Ionescu Miron, A.I.; Anghel, A.V.; Antone-Iordache, I.L.; Atasiei, D.I.; Anghel, C.A.; Barnonschi, A.A.; Bobolocu, A.M.; Verga, C.; Șandru, F.; Lișcu, H.D. Assessing the Impact of Organ Failure and Metastases on Quality of Life in Breast Cancer Patients: A Prospective Study Based on Utilizing EORTC QLQ-C30 and EORTC QLQ-BR45 Questionnaires in Romania. J. Pers. Med. 2024, 14, 214. [Google Scholar] [CrossRef] [PubMed]
- Miron, A.-I.; Anghel, A.-V.; Barnonschi, A.-A.; Mitre, R.; Liscu, H.-D.; Găinariu, E.; Pătru, R.; Coniac, S. Real-world outcomes of CDK4/6 inhibitors treatment in metastatic breast cancer in Romania. Diagnostics 2023, 13, 1938. [Google Scholar] [CrossRef] [PubMed]
- Coca, R.; Moisin, A.; Coca, R.; Diter, A.; Racheriu, M.; Tanasescu, D.; Popa, C.; Cerghedean-Florea, M.E.; Boicean, A.; Tanasescu, C. Exploring Therapeutic Challenges in Patients with HER2-Positive Breast Cancer-A Single-Center Experience. Life 2024, 14, 1025. [Google Scholar] [CrossRef]
- Simionescu, A.A.; Horobeț, A.; Belaşcu, L.; Median, D.M. Real-world data analysis of pregnancy-associated breast cancer at a tertiary-level hospital In Romania. Medicina 2020, 56, 522. [Google Scholar] [CrossRef]
- Lungulescu, C.V.; Camen, G.-C.; Naidin, M.-S.; Berisha, T.-C.; Bita, A.; Dinescu, V.-C.; Buteica, S.A.; Dimulescu, M.-D.; Volovat, S.R.; Turcu-Stiolica, A. Real-World Efficacy and Adherence to Palbociclib in HR-Positive, HER2-Negative Advanced Breast Cancer: Insights from a Romanian Cohort. Cancers 2024, 16, 4161. [Google Scholar] [CrossRef]
- Oprean, C.M.; Badau, L.M.; Petrita, R.; Median, M.D.; Dema, A. Real-World, National Study of Palbociclib in HR+/HER2− Metastatic Breast Cancer: A 2.5-Year Follow-Up PALBO01/2021. Diagnostics 2025, 15, 1173. [Google Scholar] [CrossRef]
- Cătană, A.; Trifa, A.P.; Achimas-Cadariu, P.A.; Bolba-Morar, G.; Lisencu, C.; Kutasi, E.; Chelaru, V.F.; Muntean, M.; Martin, D.L.; Antone, N.Z. Hereditary Breast Cancer in Romania—Molecular Particularities and Genetic Counseling Challenges in an Eastern European Country. Biomedicines 2023, 11, 1386. [Google Scholar] [CrossRef]
- Pop, L.-A.; Cojocneanu-Petric, R.-M.; Pileczki, V.; Morar-Bolba, G.; Irimie, A.; Lazar, V.; Lombardo, C.; Paradiso, A.; Berindan-Neagoe, I. Genetic alterations in sporadic triple negative breast cancer. Breast 2018, 38, 30–38. [Google Scholar] [CrossRef]
- Mustata, L.M.; Peltecu, G.; Mugescu, D.C.; Nedelea, F.M.; Median, M.D. Single Center Experience of Genetic Testing in Patients Undergoing Breast Cancer Treatment. Maedica 2024, 19, 239–246. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2018, 36, 2105–2122. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Somerfield, M.R.; Dowsett, M.; Hammond, M.E.H.; Hayes, D.F.; McShane, L.M.; Saphner, T.J.; Spears, P.A.; Allison, K.H. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer. Arch. Pathol. Lab. Med. 2023, 147, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Lester, S.C. Pathology of breast carcinomas after neoadjuvant chemotherapy: An overview with recommendations on specimen processing and reporting. Arch. Pathol. Lab. Med. 2009, 133, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Luz, P.; Lopes-Brás, R.; de Pinho, I.S.; Patel, V.; Esperança-Martins, M.; Gonçalves, L.; Gonçalves, J.; Freitas, R.; Simão, D.; Galnares, M.R. Predictive factors for pCR and relapse following neoadjuvant dual HER2-blockade in HER2+ breast cancer: An international cohort study. Clin. Transl. Oncol. 2025, 1–10. [Google Scholar] [CrossRef]
- Nierenberg, T.C.; Thomas, S.M.; Halliday, I.; Botty van den Bruele, A.; Chiba, A.; Modell Parrish, K.J.; Woriax, H.E.; DiNome, M.L.; Westbrook, K.E.; Plichta, J.K. Survival outcomes after pathologic complete response with neoadjuvant endocrine therapy vs. neoadjuvant chemotherapy: A retrospective national database study. Breast Cancer Res. Treat. 2025, 212, 1–12. [Google Scholar] [CrossRef]
- Huober, J.; van Mackelenbergh, M.; Schneeweiss, A.; Seither, F.; Blohmer, J.U.; Denkert, C.; Tesch, H.; Hanusch, C.; Salat, C.; Rhiem, K.; et al. Identifying breast cancer patients at risk of relapse despite pathological complete response after neoadjuvant therapy. NPJ Breast Cancer 2023, 9, 23. [Google Scholar] [CrossRef]
- Krystel-Whittemore, M.; Xu, J.; Brogi, E.; Ventura, K.; Patil, S.; Ross, D.S.; Dang, C.; Robson, M.; Norton, L.; Morrow, M.; et al. Pathologic complete response rate according to HER2 detection methods in HER2-positive breast cancer treated with neoadjuvant systemic therapy. Breast Cancer Res. Treat. 2019, 177, 61–66. [Google Scholar] [CrossRef]
- Hurvitz, S.A.; Martin, M.; Symmans, W.F.; Jung, K.H.; Huang, C.S.; Thompson, A.M.; Harbeck, N.; Valero, V.; Stroyakovskiy, D.; Wildiers, H.; et al. Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): A randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2018, 19, 115–126. [Google Scholar] [CrossRef]
- Rodríguez, M.; González, D.M.; El-Sharkawy, F.; Castaño, M.; Madrid, J. Complete pathological response in patients with HER2 positive breast cancer treated with neoadjuvant therapy in Colombia. Biomedica 2023, 43, 396–405. [Google Scholar] [CrossRef]
- Hännikäinen, E.-N.; Mattson, J.; Karihtala, P. Predictors of successful neoadjuvant treatment in HER2-positive breast cancer. Oncol. Lett. 2023, 26, 434. [Google Scholar] [CrossRef]
- Gianni, L.; Pienkowski, T.; Im, Y.H.; Tseng, L.M.; Liu, M.C.; Lluch, A.; Starosławska, E.; de la Haba-Rodriguez, J.; Im, S.A.; Pedrini, J.L.; et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): A multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016, 17, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Jiao, D.; Li, G.; Dai, H.; Wang, J.; Zhang, J.; Hou, Y.; Guo, X.; Zhao, Y.; Gong, X.; Liu, Z. Comparison of the response to neoadjuvant therapy between immunohistochemistry HER2 (3+) and HER2 (2+)/ISH+ early-stage breast cancer: A retrospective multicenter cohort study. Oncologist 2024, 29, e877–e886. [Google Scholar] [CrossRef] [PubMed]
- Antolín, S.; García-Caballero, L.; Reboredo, C.; Molina, A.; Mosquera, J.; Vázquez-Boquete, Á.; Gallego, R.; Santiago, M.P.; Concha, Á.; Pérez, E.; et al. Is there a correlation between HER2 gene amplification level and response to neoadjuvant treatment with trastuzumab and chemotherapy in HER2-positive breast cancer? Virchows Arch. 2021, 479, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Jeon, C.W.; Kim, Y.O.; Jung, S. Pathological complete response to neoadjuvant trastuzumab and pertuzumab therapy is related to human epidermal growth factor receptor 2 (HER2) amplification level in HER2-amplified breast cancer. Medicine 2020, 99, e23053. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Ju, Q.; Gao, C.; Li, J.; Wang, X.; Yan, M.; Zhang, L.; Huang, M.; Long, Q.; Jin, X. Association of HER-2/CEP17 ratio and HER-2 copy number with pCR rate in HER-2-positive breast cancer after dual-target neoadjuvant therapy with trastuzumab and pertuzumab. Front. Oncol. 2022, 12, 819818. [Google Scholar] [CrossRef]
- Venet, D.; Rediti, M.; Maetens, M.; Fumagalli, D.; Brown, D.N.; Majjaj, S.; Salgado, R.; Pusztai, L.; Harbeck, N.; El-Abed, S. Copy number aberration analysis to predict response to neoadjuvant Anti-HER2 therapy: Results from the NeoALTTO Phase III clinical trial. Clin. Cancer Res. 2021, 27, 5607–5618. [Google Scholar] [CrossRef]
- Li, Z.; Metzger Filho, O.; Viale, G.; dell’Orto, P.; Russo, L.; Goyette, M.-A.; Kamat, A.; Yardley, D.A.; Abramson, V.G.; Arteaga, C.L. HER2 heterogeneity and treatment response–associated profiles in HER2-positive breast cancer in the NCT02326974 clinical trial. J. Clin. Investig. 2024, 134, e176454. [Google Scholar] [CrossRef]
- Samiei, S.; Simons, J.M.; Engelen, S.M.E.; Beets-Tan, R.G.H.; Classe, J.M.; Smidt, M.L. Axillary Pathologic Complete Response After Neoadjuvant Systemic Therapy by Breast Cancer Subtype in Patients With Initially Clinically Node-Positive Disease: A Systematic Review and Meta-analysis. JAMA Surg. 2021, 156, e210891. [Google Scholar] [CrossRef]
- Papazisis, K.T.; Liappis, T.; Kontovinis, L.; Pouptsis, A.; Intzes, S.; Natsiopoulos, I. Real-world experience of neoadjuvant chemotherapy for early breast cancer patients: An observational non-interventional study in Thessaloniki, Greece. J. BUON 2020, 25, 634. [Google Scholar]
- Fabbri, A.; Nelli, F.; Botticelli, A.; Giannarelli, D.; Marrucci, E.; Fiore, C.; Virtuoso, A.; Scagnoli, S.; Pisegna, S.; Alesini, D. Pathlogic response and survival after neoadjuvant hemotherapy with or without pertuzumab in patients with HER2-Positive breast cancer: The neopearl nationwide collaborative study. Front. Oncol. 2023, 13, 1177681. [Google Scholar] [CrossRef]
- Boér, K.; Kahán, Z.; Landherr, L.; Csőszi, T.; Máhr, K.; Ruzsa, Á.; Horváth, Z.; Budai, B.; Rubovszky, G. Pathologic complete response rates after neoadjuvant pertuzumab and trastuzumab with chemotherapy in early stage HER2-positive breast cancer- increasing rates of breast conserving surgery: A real-world experience. Pathol. Oncol. Res. 2021, 27, 1609785. [Google Scholar] [CrossRef] [PubMed]
- Gulmez, A.; Harputluoglu, H. Effect of Inflammatory Markers on the Pathologic Complete Response in the Neoadjuvant Treament of HER-2 Positive Local Advanced Breast Cancer. Eurasian J. Med. Investig. 2022, 6, 417–423. [Google Scholar]
- IQVIA; EFPIA. EFPIA Patients W.A.I.T. Indicator 2024 Survey. 2025. Available online: https://efpia.eu/media/oeganukm/efpia-patients-wait-indicator-2024-final-110425.pdf (accessed on 2 July 2025).
- National Institute of Public Health. Cancer Country Profile—Romania 2023. 2023. Available online: https://insp.gov.ro/wp-content/uploads/2024/03/Profil-de-tara-privind-cancerul-2023.pdf (accessed on 2 July 2025).
- Fetica, B.; Blaga, M.L.; Trifa, A.P.; Bocean, C.M.; Balacescu, O.; Fulop, A.; Pop, B. FICTION Technique—A Candidate for the Assessment of HER2 Status in Breast Invasive Carcinomas. Medicina 2025, 61, 1069. [Google Scholar] [CrossRef] [PubMed]
| Clinical Stage | Stage II | Stage III | |||
| 45% (n = 75) | 55% (n = 92) | ||||
| IIA | IIB | IIIA | IIIB | IIIC | |
| 21% (n = 35) | 24% (n = 40) | 29.3% (n = 49) | 23.3% (n = 39) | 2.3% (n = 4) | |
| Lymph node category (N) | N0 | N1 | N2 | ||
| 18% (n = 30) | 49% (n = 82) | 33% (n = 55) | |||
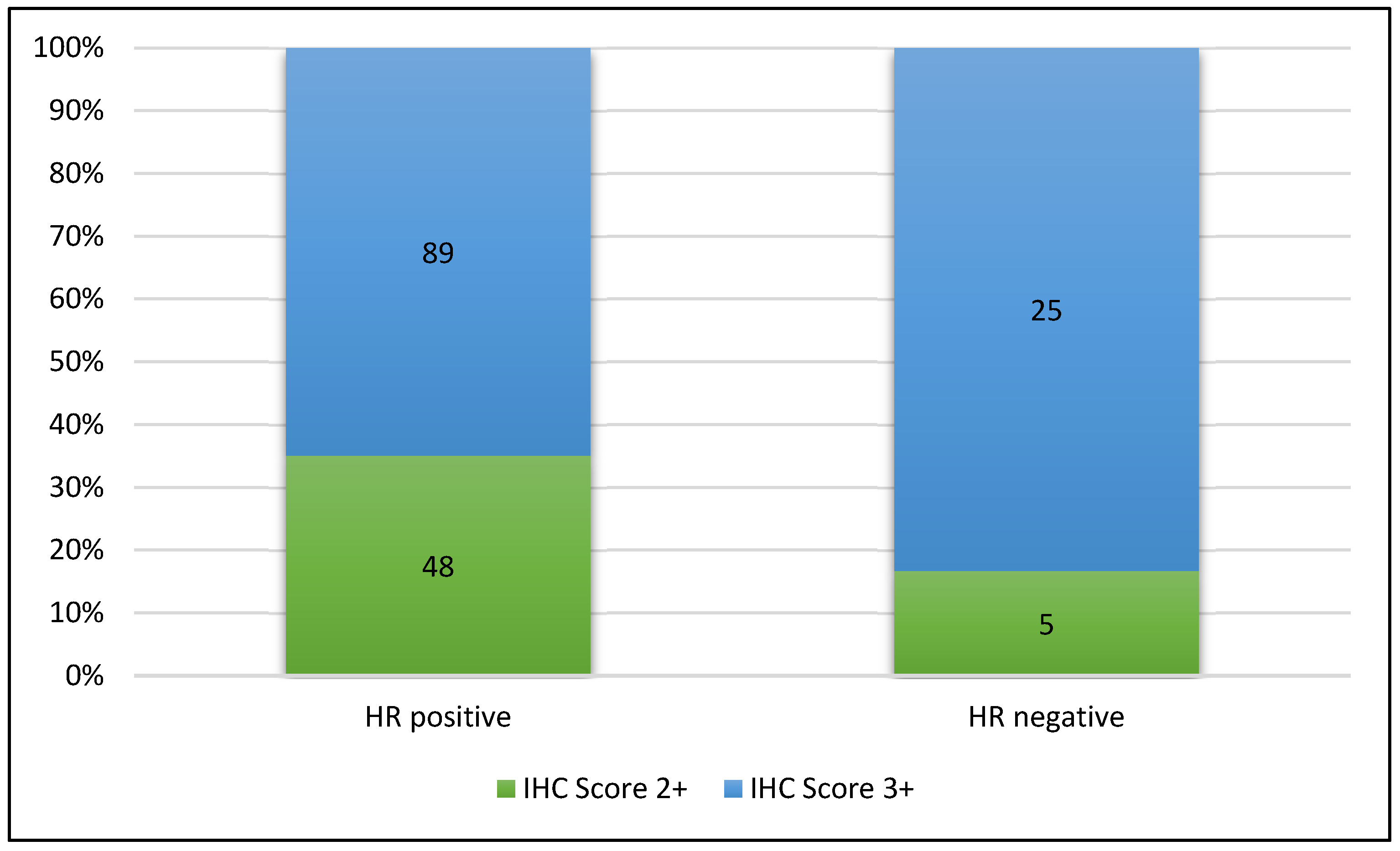
| Hormone receptor expression | HR positive | HR negative | |||
| 82% (n = 137) | 18% (n = 30) | ||||
| Her2 IHC expression | 3+ | 2+ | |||
| 68% (n = 114) | 32% (n = 53) | ||||
| Her2 ISH Group | Group 1 | Group 3 | |||
| 75% (n = 39) | 25% (n = 13) | ||||
| Neoadjuvant therapy | Neoadjuvant chemotherapy + HER2 Dual Blockade (Pertuzumab + Trastuzumab) | Neoadjuvant chemotherapy + Trastuzumab | |||
| 92% (n = 153) | 8% (n = 14) | ||||
| Characteristic | Category | pCR Rates (%) | pCR Cases Count (n) | Statistical Significance (Fisher’s Exact Test p Value) |
|---|---|---|---|---|
| Overall | - | 50.29 | 114 | - |
| Her2 protein expression | 3+ Her2-IHC | 62.28 | 71 | <0.001 95% CI [0.09, 0.43] |
| 2+ Her2-IHC | 24.53 | 13 | ||
| Hormone receptor expression | HR (+) | 45.25 | 62 | 0.008 95% CI [0.10, 0.76] |
| HR (−) | 73.33 | 22 | ||
| Estrogen receptor expression | ER (+) | 10.84 | 9 | 0.001 95% CI [0.10, 0.62] |
| ER (−) | 32.14 | 27 | ||
| Progesterone receptor expression | PR (+) | 33.73 | 28 | 0.059 95% CI [0.27, 1.04] |
| PR (−) | 32.14 | 27 |
| Variable | Estimate | OR | 95% CI | p Value |
|---|---|---|---|---|
| Age category (>50) | 0.057 | 1.06 | [0.52–2.13] | 0.875 |
| Clinical stage (Stage III) | −0.712 | 0.49 | [0.24–0.99] | 0.046 |
| HR_status (HR(+)cases) | −1.101 | 0.33 | [0.13–0.86] | 0.023 |
| Ki-67 (>25%) | 1.122 | 3.07 | [1.41–6.66] | 0.005 |
| IHC Score (3+ cases) | 1.690 | 5.42 | [2.45–11.99] | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pop, B.; Popa, C.; Antone, N.Z.; Achimas-Cadariu, P.-A.; Vlad, I.-C.; Morar-Bolba, G.; Martin, D.L.; Lisencu, C.; Cainap, C.; Pintican, R.; et al. Evaluating the Effectiveness of Neoadjuvant Therapy in Her2-Positive Invasive Breast Cancer: A Comprehensive Analysis of 167 Cases in Romania. Cancers 2025, 17, 2312. https://doi.org/10.3390/cancers17142312
Pop B, Popa C, Antone NZ, Achimas-Cadariu P-A, Vlad I-C, Morar-Bolba G, Martin DL, Lisencu C, Cainap C, Pintican R, et al. Evaluating the Effectiveness of Neoadjuvant Therapy in Her2-Positive Invasive Breast Cancer: A Comprehensive Analysis of 167 Cases in Romania. Cancers. 2025; 17(14):2312. https://doi.org/10.3390/cancers17142312
Chicago/Turabian StylePop, Bogdan, Carmen Popa, Nicoleta Zenovia Antone, Patriciu-Andrei Achimas-Cadariu, Ioan-Cătălin Vlad, Gabriela Morar-Bolba, Daniela Laura Martin, Carmen Lisencu, Călin Cainap, Roxana Pintican, and et al. 2025. "Evaluating the Effectiveness of Neoadjuvant Therapy in Her2-Positive Invasive Breast Cancer: A Comprehensive Analysis of 167 Cases in Romania" Cancers 17, no. 14: 2312. https://doi.org/10.3390/cancers17142312
APA StylePop, B., Popa, C., Antone, N. Z., Achimas-Cadariu, P.-A., Vlad, I.-C., Morar-Bolba, G., Martin, D. L., Lisencu, C., Cainap, C., Pintican, R., Fulop, A., Lisencu, C. I., Nistor-Ciurba, C. C., Muntean, M. V., Cătană, A., & Fetica, B. (2025). Evaluating the Effectiveness of Neoadjuvant Therapy in Her2-Positive Invasive Breast Cancer: A Comprehensive Analysis of 167 Cases in Romania. Cancers, 17(14), 2312. https://doi.org/10.3390/cancers17142312







