Feasibility of Automated Image-Based Red Bone Marrow Dosimetry for [177Lu]Lu-PSMA Radiopharmaceutical Therapy of Metastatic Castration-Resistant Prostate Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
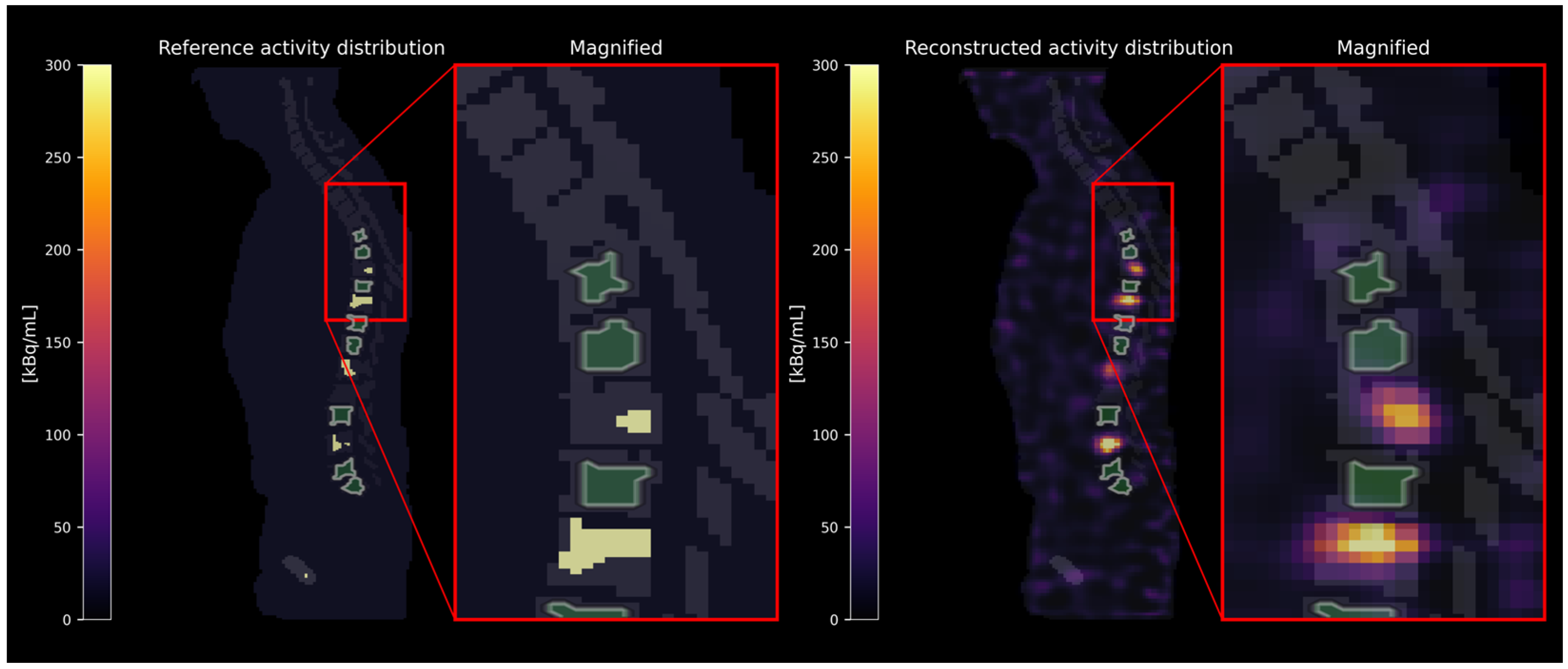
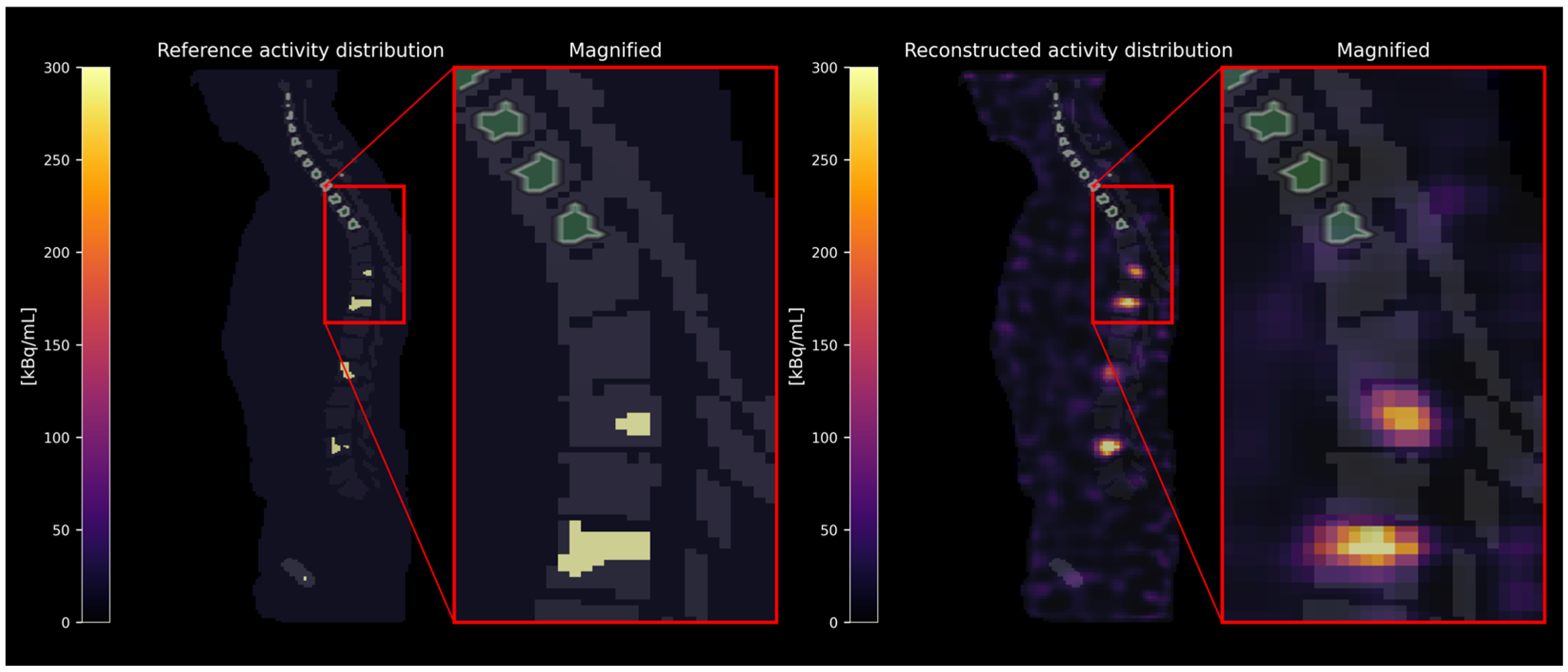
2.1. Virtual Phantom Study
2.1.1. Simulation and Reconstruction
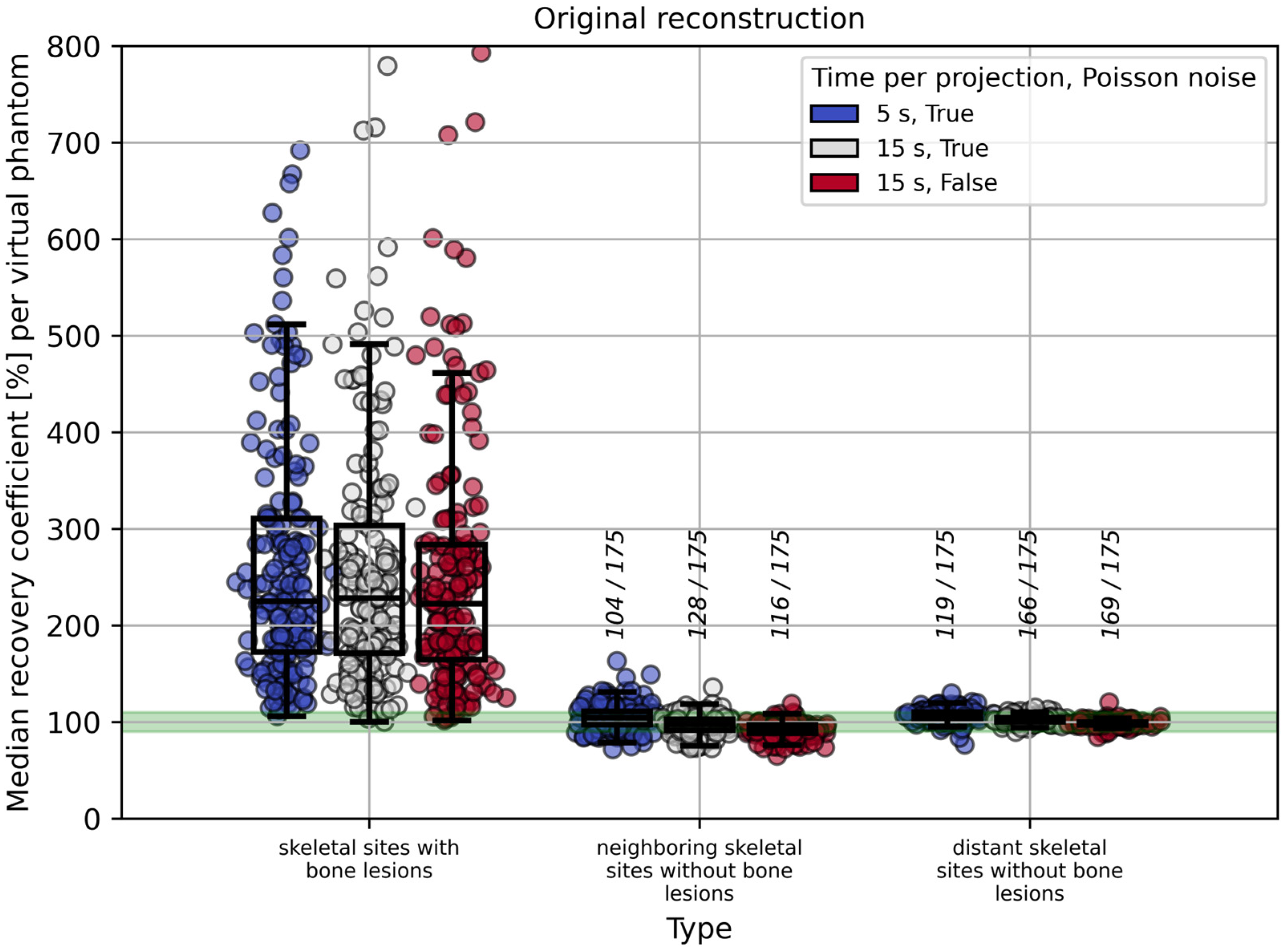
2.1.2. Evaluation
2.1.3. Application of Spill-Over Reduction Techniques
2.1.4. Influence of Time per Projection, Poisson Noise, and Volume Threshold on RC Estimation in the Red Bone Marrow
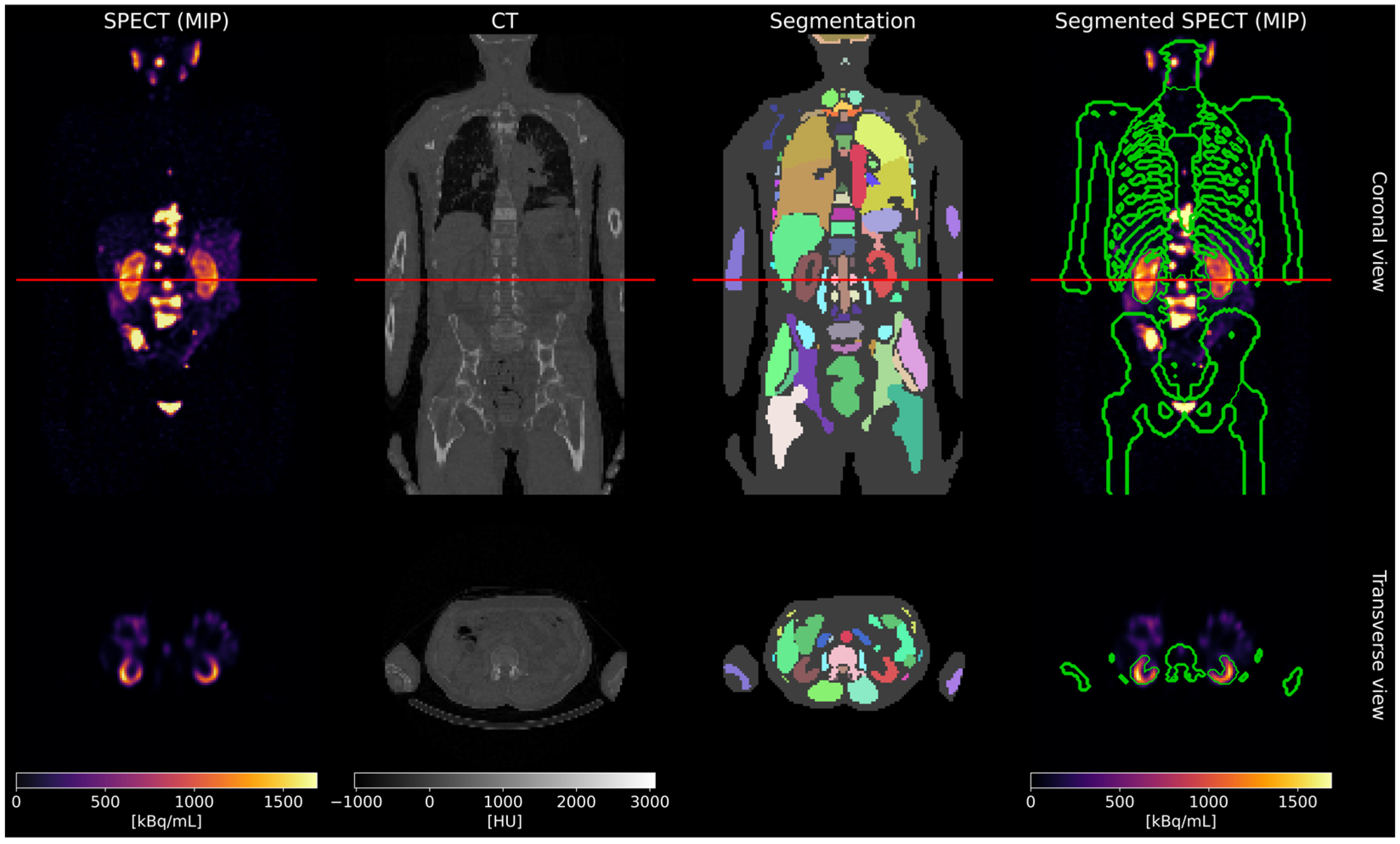
2.2. Patient Study
2.2.1. Self-Absorbed Dose
2.2.2. Cross-Absorbed Dose
3. Results
3.1. Virtual Phantom Study
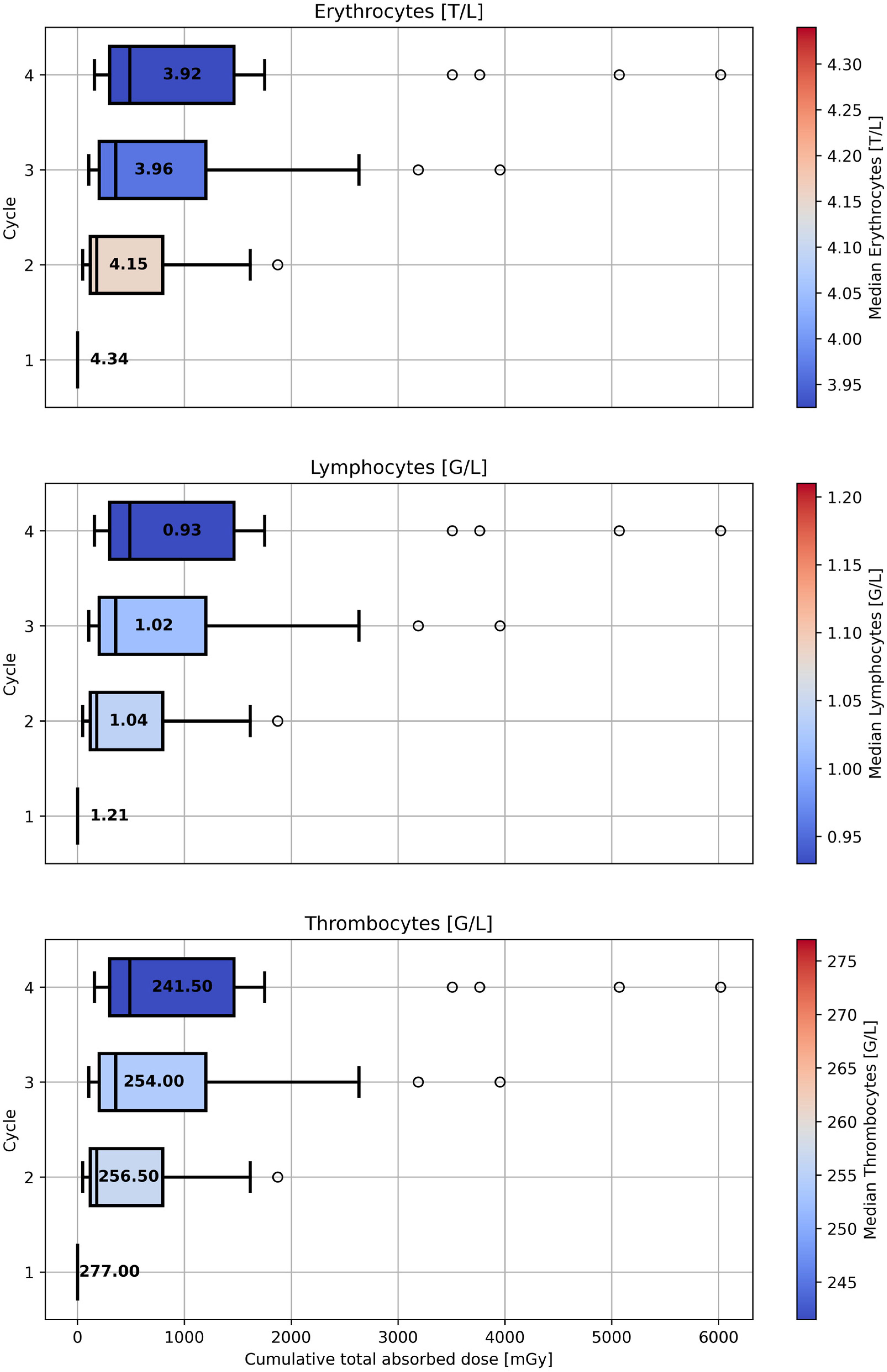
3.2. Patient Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CNR | Contrast-to-Noise Ratio |
| CT | Computed Tomography |
| DEQCT | Dual-Energy Quantitative Computed Tomography |
| EANM | European Association of Nuclear Medicine |
| FWHM | Full Width at Half Maximum |
| HU | Hounsfield Units |
| ICRP | International Commission on Radiological Protection |
| IY | Iterative Yang |
| LR | Lucy–Richardson |
| mCRPC | Metastatic Castration-Resistant Prostate Cancer |
| mHSPC | Metastatic Hormone-Sensitive Prostate Cancer |
| MRI | Magnetic Resonance Imaging |
| NEMA | National Electrical Manufacturers Association |
| NET | Neuroendocrine Tumors |
| OLINDA/EXM | Organ Level Internal Dose Assessment/Exponential Modeling |
| p.i. | Post-Injection |
| PET | Positron Emission Tomography |
| PSF | Point Spread Function |
| PSMA | Prostate-Specific Membrane Antigen |
| PVC | Partial Volume Correction |
| RC | Recovery Coefficient |
| SPECT | Single Photon Emission Computed Tomography |
| SUV | Standardized Uptake Value |
| TAC | Time-Activity Curve |
| TIA | Time-Integrated Activity |
| VOI | Volume of Interest |
| VSV | Voxel-S-Value |
References
- Wang, L.; Lu, B.; He, M.; Wang, Y.; Wang, Z.; Du, L. Prostate Cancer Incidence and Mortality: Global Status and Temporal Trends in 89 Countries from 2000 to 2019. Front. Public Health 2022, 10, 811044. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Giesel, F.L.; Stefanova, M.; Benesova, M.; Bronzel, M.; Afshar-Oromieh, A.; Mier, W.; Eder, M.; Kopka, K.; Haberkorn, U. PSMA-Targeted Radionuclide Therapy of Metastatic Castration-Resistant Prostate Cancer with 177Lu-Labeled PSMA-617. J. Nucl. Med. 2016, 57, 1170–1176. [Google Scholar] [CrossRef] [PubMed]
- Tauber, R.; Knorr, K.; Retz, M.; Rauscher, I.; Grigorascu, S.; Hansen, K.; D’Alessandria, C.; Wester, H.J.; Gschwend, J.; Weber, W.; et al. Safety and Efficacy of [(177)Lu]-PSMA-I&T Radioligand Therapy in Octogenarians with Metastatic Castration-Resistant Prostate Cancer: Report on 80 Patients over the Age of 80 Years. J. Nucl. Med. 2023, 64, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- Groener, D.; Nguyen, C.T.; Baumgarten, J.; Bockisch, B.; Davis, K.; Happel, C.; Mader, N.; Nguyen Ngoc, C.; Wichert, J.; Banek, S.; et al. Hematologic safety of (177)Lu-PSMA-617 radioligand therapy in patients with metastatic castration-resistant prostate cancer. EJNMMI Res. 2021, 11, 61. [Google Scholar] [CrossRef]
- Hagmarker, L.; Svensson, J.; Ryden, T.; van Essen, M.; Sundlov, A.; Gleisner, K.S.; Gjertsson, P.; Bernhardt, P. Bone Marrow Absorbed Doses and Correlations with Hematologic Response During (177)Lu-DOTATATE Treatments Are Influenced by Image-Based Dosimetry Method and Presence of Skeletal Metastases. J. Nucl. Med. 2019, 60, 1406–1413. [Google Scholar] [CrossRef]
- Grob, D.; Prive, B.M.; Muselaers, C.H.J.; Mehra, N.; Nagarajah, J.; Konijnenberg, M.W.; Peters, S.M.B. Bone marrow dosimetry in low volume mHSPC patients receiving Lu-177-PSMA therapy using SPECT/CT. EJNMMI Phys. 2024, 11, 34. [Google Scholar] [CrossRef]
- Hindorf, C.; Glatting, G.; Chiesa, C.; Linden, O.; Flux, G.; Committee, E.D. EANM Dosimetry Committee guidelines for bone marrow and whole-body dosimetry. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1238–1250. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, Z.; Chen, G.; Shi, K.; Mok, G.S.P. Partial volume correction for Lu-177-PSMA SPECT. EJNMMI Phys. 2024, 11, 93. [Google Scholar] [CrossRef]
- Leube, J.; Gustafsson, J.; Lassmann, M.; Salas-Ramirez, M.; Tran-Gia, J. A Deep-Learning-Based Partial-Volume Correction Method for Quantitative (177)Lu SPECT/CT Imaging. J. Nucl. Med. 2024, 65, 980–987. [Google Scholar] [CrossRef]
- Lu, Z.; Liu, Y.; Chen, G.; Jiang, H.; Afshar-Oromieh, A.; Rominger, A.; Shi, K.; Mok, G. Automatic image-based segmentation and partial volume correction for Lu-177-PSMA-617 bone marrow dosimetry. J. Nucl. Med. 2023, 64, P827. [Google Scholar]
- Marquis, H.; Schmidtlein, C.; Carter, L.; Ocampo Ramos, J.; Kesner, A. MIRDpvc: A Software Tool for PET & SPECT Resolution Characterization and Partial Volume Correction. J. Nucl. Med. 2024, 65, 242232. [Google Scholar]
- Marquis, H.; Schmidtlein, C.R.; de Nijs, R.; Mínguez Gabiña, P.; Gustafsson, J.; Kayal, G.; Ocampo Ramos, J.C.; Carter, L.M.; Bailey, D.L.; Kesner, A.L. MIRD Pamphlet No. 32: A MIRD Recovery Coefficient Model for Resolution Characterization and Shape-Specific Partial-Volume Correction. J. Nucl. Med. 2025, 66, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Hu, J.; Chen, G.; Jiang, H.; Shih, C.T.; Afshar-Oromieh, A.; Rominger, A.; Shi, K.; Mok, G.S.P. Automatic bone marrow segmentation for precise [(177)Lu]Lu-PSMA-617 dosimetry. Med. Phys. 2025, 52, 3970–3980. [Google Scholar] [CrossRef] [PubMed]
- Wasserthal, J.; Breit, H.C.; Meyer, M.T.; Pradella, M.; Hinck, D.; Sauter, A.W.; Heye, T.; Boll, D.T.; Cyriac, J.; Yang, S.; et al. TotalSegmentator: Robust Segmentation of 104 Anatomic Structures in CT Images. Radiol. Artif. Intell. 2023, 5, e230024. [Google Scholar] [CrossRef]
- Otsu, N. A Threshold Selection Method from Gray-Level Histograms. IEEE Trans. Syst. Man. Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef]
- Ljungberg, M.; Strand, S.-E. A Monte Carlo program for the simulation of scintillation camera characteristics. Comput. Methods Programs Biomed. 1989, 29, 257–272. [Google Scholar] [CrossRef]
- Gosewisch, A.; Ilhan, H.; Tattenberg, S.; Mairani, A.; Parodi, K.; Brosch, J.; Kaiser, L.; Gildehaus, F.J.; Todica, A.; Ziegler, S.; et al. 3D Monte Carlo bone marrow dosimetry for Lu-177-PSMA therapy with guidance of non-invasive 3D localization of active bone marrow via Tc-99m-anti-granulocyte antibody SPECT/CT. EJNMMI Res. 2019, 9, 76. [Google Scholar] [CrossRef]
- Lucy, L.B. An iterative technique for the rectification of observed distributions. Astron. J. 1974, 79, 745. [Google Scholar] [CrossRef]
- Richardson, W.H. Bayesian-Based Iterative Method of Image Restoration. J. Opt. Soc. Am. 1972, 62, 55–59. [Google Scholar] [CrossRef]
- Yang, J.; Huang, S.C.; Mega, M.; Lin, K.P.; Toga, A.W.; Small, G.W.; Phelps, M.E. Investigation of partial volume correction methods for brain FDG PET studies. IEEE Trans. Nucl. Sci. 1996, 43, 3322–3327. [Google Scholar] [CrossRef]
- Erlandsson, K.; Buvat, I.; Pretorius, P.H.; Thomas, B.A.; Hutton, B.F. A review of partial volume correction techniques for emission tomography and their applications in neurology, cardiology and oncology. Phys. Med. Biol. 2012, 57, R119. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.A.; Cuplov, V.; Bousse, A.; Mendes, A.; Thielemans, K.; Hutton, B.F.; Erlandsson, K. PETPVC: A toolbox for performing partial volume correction techniques in positron emission tomography. Phys. Med. Biol. 2016, 61, 7975–7993. [Google Scholar] [CrossRef] [PubMed]
- Tran-Gia, J.; Lassmann, M. Characterization of Noise and Resolution for Quantitative (177)Lu SPECT/CT with xSPECT Quant. J. Nucl. Med. 2019, 60, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.M.B.; Prive, B.M.; de Bakker, M.; de Lange, F.; Jentzen, W.; Eek, A.; Muselaers, C.H.J.; Mehra, N.; Witjes, J.A.; Gotthardt, M.; et al. Intra-therapeutic dosimetry of [(177)Lu]Lu-PSMA-617 in low-volume hormone-sensitive metastatic prostate cancer patients and correlation with treatment outcome. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 460–469. [Google Scholar] [CrossRef]
- Violet, J.; Jackson, P.; Ferdinandus, J.; Sandhu, S.; Akhurst, T.; Iravani, A.; Kong, G.; Kumar, A.R.; Thang, S.P.; Eu, P.; et al. Dosimetry of (177)Lu-PSMA-617 in Metastatic Castration-Resistant Prostate Cancer: Correlations Between Pretherapeutic Imaging and Whole-Body Tumor Dosimetry with Treatment Outcomes. J. Nucl. Med. 2019, 60, 517–523. [Google Scholar] [CrossRef]
- Okamoto, S.; Thieme, A.; Allmann, J.; D’Alessandria, C.; Maurer, T.; Retz, M.; Tauber, R.; Heck, M.M.; Wester, H.J.; Tamaki, N.; et al. Radiation Dosimetry for (177)Lu-PSMA I&T in Metastatic Castration-Resistant Prostate Cancer: Absorbed Dose in Normal Organs and Tumor Lesions. J. Nucl. Med. 2017, 58, 445–450. [Google Scholar] [CrossRef]
- Delker, A.; Fendler, W.P.; Kratochwil, C.; Brunegraf, A.; Gosewisch, A.; Gildehaus, F.J.; Tritschler, S.; Stief, C.G.; Kopka, K.; Haberkorn, U.; et al. Dosimetry for (177)Lu-DKFZ-PSMA-617: A new radiopharmaceutical for the treatment of metastatic prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 42–51. [Google Scholar] [CrossRef]
- Hough, M.; Johnson, P.; Rajon, D.; Jokisch, D.; Lee, C.; Bolch, W. An image-based skeletal dosimetry model for the ICRP reference adult male--internal electron sources. Phys. Med. Biol. 2011, 56, 2309–2346. [Google Scholar] [CrossRef]
- ICRP. Basic anatomical and physiological data for use in radiological protection: Reference values. Ann. ICRP 2002, 32, 5. [Google Scholar]
- Stabin, M.G.; Sparks, R.B.; Crowe, E. OLINDA/EXM: The Second-Generation Personal Computer Software for Internal Dose Assessment in Nuclear Medicine. J. Nucl. Med. 2005, 46, 1023–1027. [Google Scholar] [CrossRef]
- Beauregard, J.-M.; Bergeron, M.; Bouvet, G.; Beaulieu, A.; Arseneult, F.; April, G.; Buteau, F.-A. Body size and renal function are predictors of healthy tissue dosimetry in PSMA radiopharmaceutical therapy with 177Lu-PSMA-I&T. J. Nucl. Med. 2022, 63, 2630. [Google Scholar]
- Salas-Ramirez, M.; Tran-Gia, J.; Kesenheimer, C.; Weng, A.M.; Kosmala, A.; Heidemeier, A.; Kostler, H.; Lassmann, M. Quantification of fat fraction in lumbar vertebrae: Correlation with age and implications for bone marrow dosimetry in molecular radiotherapy. Phys. Med. Biol. 2018, 63, 025029. [Google Scholar] [CrossRef] [PubMed]
- Salas-Ramirez, M.; Lassmann, M.; Tran-Gia, J. Quantification of the volume fraction of fat, water and bone mineral in spongiosa for red marrow dosimetry in molecular radiotherapy by using a dual-energy (SPECT/)CT. Z. Med. Phys. 2022, 32, 428–437. [Google Scholar] [CrossRef] [PubMed]






| Method | Skeletal Site | Assumed VOI for the Red Bone Marrow |
|---|---|---|
| 1 | with bone lesions | eroded bone compartment VOI excluding dilated bone lesion VOIs |
| 2 | without bone lesions, neighboring | eroded bone compartment VOI |
| 3 | without bone lesions, distant | eroded bone compartment VOI |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rumiantcev, M.; Resch, S.; Liubchenko, G.; Sheikh, G.; Zacherl, M.; Werner, R.A.; Ziegler, S.I.; Böning, G.; Delker, A. Feasibility of Automated Image-Based Red Bone Marrow Dosimetry for [177Lu]Lu-PSMA Radiopharmaceutical Therapy of Metastatic Castration-Resistant Prostate Cancer. Cancers 2025, 17, 2313. https://doi.org/10.3390/cancers17142313
Rumiantcev M, Resch S, Liubchenko G, Sheikh G, Zacherl M, Werner RA, Ziegler SI, Böning G, Delker A. Feasibility of Automated Image-Based Red Bone Marrow Dosimetry for [177Lu]Lu-PSMA Radiopharmaceutical Therapy of Metastatic Castration-Resistant Prostate Cancer. Cancers. 2025; 17(14):2313. https://doi.org/10.3390/cancers17142313
Chicago/Turabian StyleRumiantcev, Mikhail, Sandra Resch, Grigory Liubchenko, Gabriel Sheikh, Mathias Zacherl, Rudolf A. Werner, Sibylle I. Ziegler, Guido Böning, and Astrid Delker. 2025. "Feasibility of Automated Image-Based Red Bone Marrow Dosimetry for [177Lu]Lu-PSMA Radiopharmaceutical Therapy of Metastatic Castration-Resistant Prostate Cancer" Cancers 17, no. 14: 2313. https://doi.org/10.3390/cancers17142313
APA StyleRumiantcev, M., Resch, S., Liubchenko, G., Sheikh, G., Zacherl, M., Werner, R. A., Ziegler, S. I., Böning, G., & Delker, A. (2025). Feasibility of Automated Image-Based Red Bone Marrow Dosimetry for [177Lu]Lu-PSMA Radiopharmaceutical Therapy of Metastatic Castration-Resistant Prostate Cancer. Cancers, 17(14), 2313. https://doi.org/10.3390/cancers17142313






