Colorectal Cancer Risk Following Herpes Zoster Reactivation in COVID-19 Survivors: Global Multicenter Study Using TriNetX
Simple Summary
Abstract
1. Introduction
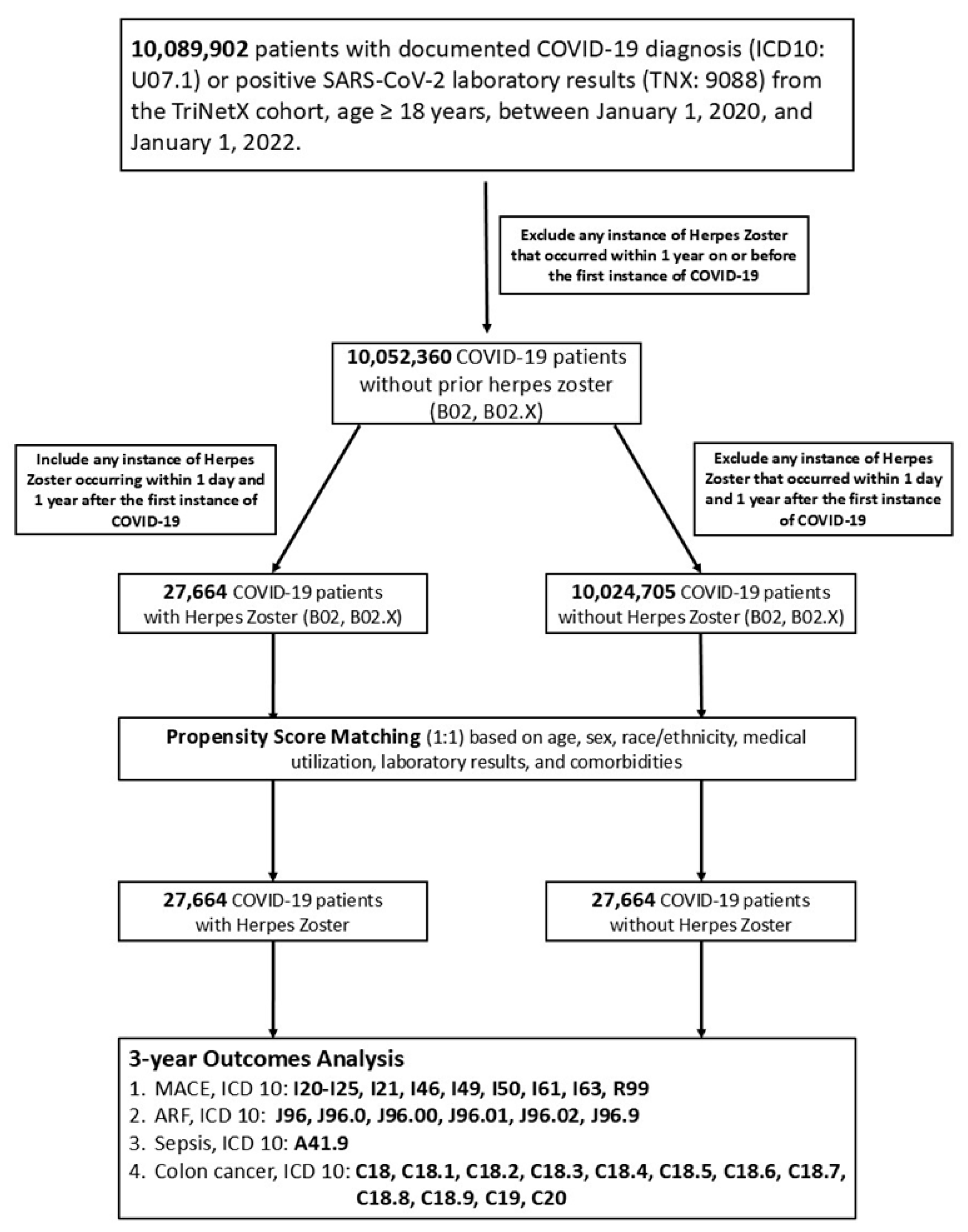
2. Materials and Methods
2.1. Study Design and Data Source
2.2. Study Population
2.3. Index Date and Follow-Up Period
2.4. Outcome Measures
2.5. Propensity Score Matching (PSM) in an Age-Restricted Group
2.6. Statistical Analyses
3. Results
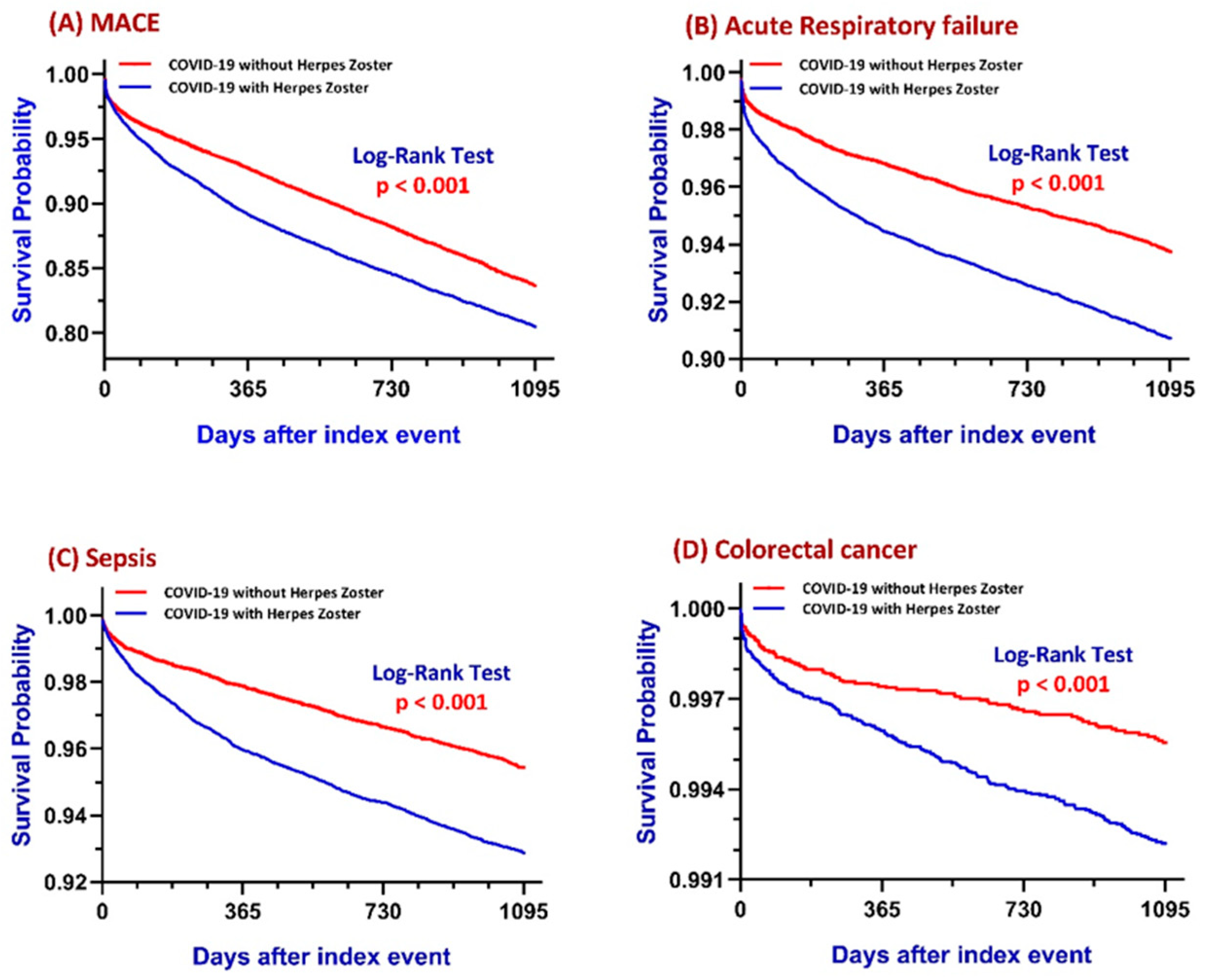
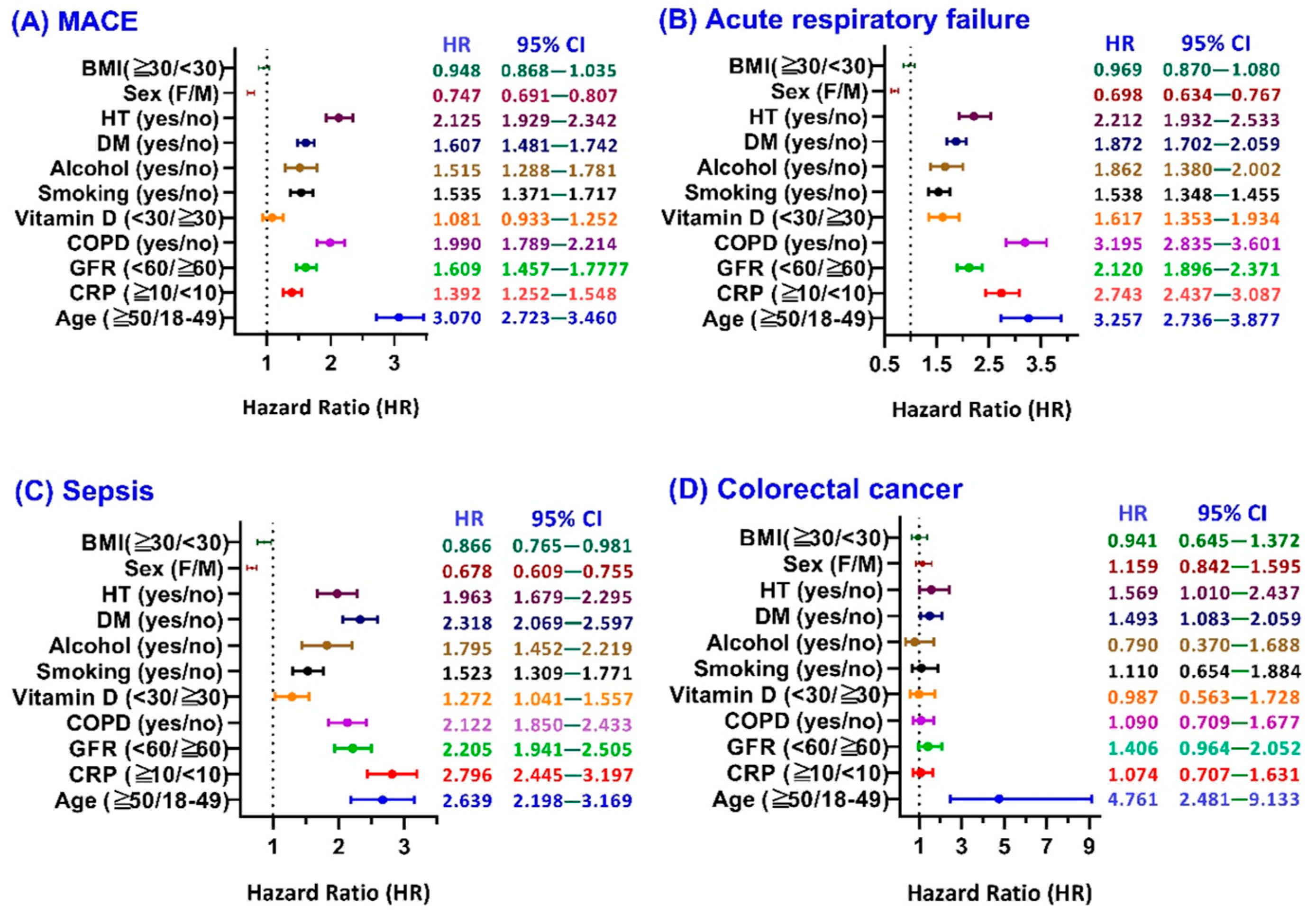
3.1. Major Adverse Cardiovascular Events (MACE)
3.2. Acute Respiratory Failure (ARF)
3.3. Sepsis
3.4. Colorectal Cancer (CRC)
3.5. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiao, T.; Huang, Y.; Sun, H.; Yang, L. Research progress of post-acute sequelae after SARS-CoV-2 infection. Cell Death Dis. 2024, 15, 257. [Google Scholar] [CrossRef] [PubMed]
- Tanriverdi, O.; Alkan, A.; Karaoglu, T.; Kitaplı, S.; Yildiz, A. COVID-19 and Carcinogenesis: Exploring the Hidden Links. Cureus 2024, 16, e68303. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wherry, E.J. T cell responses in patients with COVID-19. Nat. Rev. Immunol. 2020, 20, 529–536. [Google Scholar] [CrossRef]
- Saini, K.S.; Tagliamento, M.; Lambertini, M.; McNally, R.; Romano, M.; Leone, M.; Curigliano, G.; de Azambuja, E. Mortality in patients with cancer and coronavirus disease 2019: A systematic review and pooled analysis of 52 studies. Eur. J. Cancer 2020, 139, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, A.; Shrivastav, S.; Kushwaha, H.R.; Chaturvedi, R.; Singh, R.P. Oncogenic potential of SARS-CoV-2-targeting hallmarks of cancer pathways. Cell Commun. Signal 2024, 22, 447. [Google Scholar] [CrossRef]
- Wang, W.; Xu, Y.; Gao, R.; Lu, R.; Han, K.; Wu, G.; Tan, W. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA 2020, 323, 1843–1844. [Google Scholar] [CrossRef]
- Kim, D.; Quinn, J.; Pinsky, B.; Shah, N.H.; Brown, I. Rates of Co-infection Between SARS-CoV-2 and Other Respiratory Pathogens. JAMA 2020, 323, 2085–2086. [Google Scholar] [CrossRef]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-infections in people with COVID-19: A systematic review and meta-analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef]
- Narasimhan, M.; Ramakrishnan, R.; Durai, P.C.T.; Sneha, B. Association between COVID-19 infection and HZ: A case series. J. Fam. Med. Prim. Care 2023, 12, 2516–2519. [Google Scholar] [CrossRef]
- Wang, F.; Gao, Y.; Wagner, A.L.; Lu, Y. A systematic review and meta-analysis of HZ occurrence/recurrence after COVID-19 infection and vaccination. J. Med. Virol. 2024, 96, e29629. [Google Scholar] [CrossRef]
- Cotton, S.J.; Belcher, J.; Rose, P.K.; Jagadeesan, S.; Neal, R.D. The risk of a subsequent cancer diagnosis after herpes zoster infection. Br. J. Cancer 2013, 108, 721–726. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sim, J.H.; Cho, H.S.; Kim, Y.D.; Mun, J.; Kim, S.B.; Lee, J.H.; Leem, J.G. The Association between Herpes Zoster and Increased Cancer Risk: A Nationwide Population-Based Matched Control Study. Curr. Oncol. 2021, 28, 2720–2730. [Google Scholar] [CrossRef]
- Almutairi, N.; Almutairi, A.N.; Almazyad, M.; Alwazzan, S. HZ in the era of COVID 19: A prospective observational study to probe the association of HZ with COVID 19 infection and vaccination. Dermatol. Ther. 2022, 35, e15521. [Google Scholar] [PubMed]
- Soyuncu, S.; Berk, Y.; Eken, C.; Gulen, B.; Oktay, C. HZ as a useful clinical marker of underlying cell-mediated immune disorders. Ann. Acad. Med. Singap. 2009, 38, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Liapis, I.; Baritaki, S. COVID-19 vs. Cancer Immunosurveillance: A Game of Thrones within an Inflamed Microenviroment. Cancers 2022, 14, 4330. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Abdulle, A.E.; Timens, W.; Hillebrands, J.-L.; Navis, G.J.; Gordijn, S.J.; Bolling, M.C.; Dijkstra, G.; Voors, A.A.; Osterhaus, A.D.; et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J. Pathol. 2020, 251, 228–248. [Google Scholar] [CrossRef]
- Rottoli, M.; Gori, A.; Pellino, G.; Flacco, M.E.; Martellucci, C.; Spinelli, A.; Poggioli, G.; COVID–Colorectal Cancer (CRC) Study Group; Romano, A.; Belvedere, A.; et al. Colorectal Cancer Stage at Diagnosis Before vs During the COVID-19 Pandemic in Italy. JAMA Netw. Open 2022, 5, e2243119. [Google Scholar] [CrossRef]
- Han, Y.D.; Bae, S.U.; Kim, W.R.; Lim, D.R.; Kim, C.W. The COVID-19 pandemic and clinical characteristics of colorectal cancer: A multicenter retrospective study. Sci. Rep. 2025, 15, 8903. [Google Scholar] [CrossRef]
- Watson, N.F.; Ramage, J.M.; Madjd, Z.; Spendlove, I.; Ellis, I.O.; Scholefield, J.H.; Durrant, L.G. Immunosurveillance is active in colorectal cancer as downregulation but not complete loss of MHC class I expression correlates with a poor prognosis. Int. J. Cancer 2006, 118, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Terzić, J.; Grivennikov, S.; Karin, E.; Karin, M. Inflammation and colon cancer. Gastroenterology 2010, 138, 2101–2114.e5. [Google Scholar] [CrossRef] [PubMed]
- Diez-Domingo, J.; Parikh, R.; Bhavsar, A.B.; Cisneros, E.; McCormick, N.; Lecrenier, N. Can COVID-19 Increase the Risk of HZ? A Narrative Review. Dermatol. Ther. 2021, 11, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Gatenbee, C.D.; Baker, A.-M.; Schenck, R.O.; Strobl, M.; West, J.; Neves, M.P.; Hasan, S.Y.; Lakatos, E.; Martinez, P.; Cross, W.C.H.; et al. Immunosuppressive niche engineering at the onset of human colorectal cancer. Nat. Commun. 2022, 13, 1798. [Google Scholar] [CrossRef] [PubMed]
- Phetsouphanh, C.; Darley, D.R.; Wilson, D.B.; Howe, A.; Munier, C.M.L.; Patel, S.K.; Juno, J.A.; Burrell, L.M.; Kent, S.J.; Dore, G.J.; et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat. Immunol. 2022, 23, 210–216. [Google Scholar] [CrossRef] [PubMed]
| Before Matching | After Matching | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | COVID-19 with HZ (n = 3155) | COVID-19 Without HZ (n = 963,799) | COVID-19 with HZ (n = 3154) | COVID-19 Without HZ (n = 3154) | ||||
| +HZ | −HZ | p-Value | Std Diff. | +HZ | −HZ | p-Value | Std Diff. | |
| % of Cohort | % of Cohort | % of Cohort | % of Cohort | |||||
| Demographics (Mean ± SD) | ||||||||
| Age | with HZ | without HZ | with HZ | without HZ | ||||
| 53.1 ± 1.8 | 53.1 ± 1.8 | 53.1 ± 1.8 | 53.1 ± 1.9 | |||||
| +HZ | −HZ | 0.460 | 0.013 | +HZ | −HZ | 0.919 | 0.003 | |
| 100% | 100% | 100% | 100% | |||||
| White | 71.0% | 57.9% | <0.001 | 0.277 | 71.0% | 72.0% | 0.403 | 0.021 |
| Female | 66.5% | 54.4% | <0.001 | 0.250 | 66.5% | 66.6% | 0.894 | 0.003 |
| Black or African American | 11.7% | 15.0% | <0.001 | 0.096 | 11.7% | 11.3% | 0.581 | 0.014 |
| Male | 33.5% | 45.6% | <0.001 | 0.250 | 33.5% | 33.4% | 0.894 | 0.003 |
| Asian | 4.8% | 4.1% | 0.051 | 0.033 | 4.8% | 4.7% | 0.906 | 0.003 |
| Diagnosis | ||||||||
| +HZ | −HZ | p-Value | Std diff. | +HZ | −HZ | p-Value | Std diff. | |
| Diabetes mellitus | 14.3% | 7.5% | <0.001 | 0.218 | 14.2% | 14.0% | 0.800 | 0.006 |
| Hypertensive diseases | 25.4% | 15.0% | <0.001 | 0.261 | 25.4% | 25.7% | 0.795 | 0.007 |
| Cerebrovascular diseases | 2.0% | 1.2% | <0.001 | 0.061 | 2.0% | 1.9% | 0.855 | 0.005 |
| Noninfective enteritis and colitis | 2.7% | 1.1% | <0.001 | 0.117 | 2.7% | 2.5% | 0.692 | 0.010 |
| Ischemic heart diseases | 4.0% | 2.8% | <0.001 | 0.067 | 4.0% | 3.5% | 0.288 | 0.027 |
| Medication | ||||||||
| +HZ | −HZ | p-Value | Std diff. | +HZ | −HZ | p-Value | Std diff. | |
| BETA BLOCKERS/RELATED | 9.3% | 5.7% | <0.001 | 0.136 | 9.3% | 9.2% | 0.862 | 0.004 |
| DIURETICS | 11.0% | 6.3% | <0.001 | 0.166 | 11.0% | 10.3% | 0.414 | 0.021 |
| CALCIUM CHANNEL BLOCKERS | 7.1% | 4.5% | <0.001 | 0.114 | 7.1% | 6.8% | 0.585 | 0.014 |
| ACE INHIBITORS | 6.7% | 4.6% | <0.001 | 0.093 | 6.7% | 6.8% | 0.802 | 0.006 |
| ANGIOTENSIN II INHIBITOR | 5.8% | 3.6% | <0.001 | 0.106 | 5.8% | 5.9% | 0.914 | 0.003 |
| ALPHA BLOCKERS | 2.1% | 1.4% | <0.001 | 0.059 | 2.1% | 1.9% | 0.472 | 0.018 |
| BLOOD GLUCOSE REGULATION AGENTS | 12.8% | 6.8% | <0.001 | 0.203 | 12.8% | 11.8% | 0.206 | 0.032 |
| ANTILIPEMIC AGENTS | 13.2% | 8.3% | <0.001 | 0.159 | 13.2% | 13.1% | 0.941 | 0.002 |
| Laboratory (Mean ± SD) | ||||||||
| with HZ | without HZ | with HZ | without HZ | |||||
| +HZ, % of Cohort | −HZ, % of Cohort | p-Value | Std diff. | +HZ, % of Cohort | −HZ, % of Cohort | p-Value | Std diff. | |
| Ferritin, ng/mL | 368.3 ± 767.2 | 290.1 ± 828.5 | 368.3 ± 767.2 | 240.8 ± 404.6 | ||||
| 4.5% | 2.6% | 0.262 | 0.098 | 4.5% | 4.9% | 0.072 | 0.208 | |
| C reactive protein, mg/L | 16.6 ± 47.1 | 23.9 ± 50.3 | 16.7 ± 47.3 | 19.7 ± 43.2 | ||||
| 6.0% | 3.2% | 0.045 | 0.151 | 6.0% | 6.5% | 0.517 | 0.065 | |
| Sodium, mmol/L | 138.8 ± 3.0 | 139.0 ± 3.0 | 138.8 ± 3.0 | 139.0 ± 3.0 | ||||
| 43.1% | 26.1% | 0.029 | 0.059 | 43.1% | 43.1% | 0.191 | 0.050 | |
| Potassium, mmol/L | 4.1 ± 0.5 | 4.2 ± 0.5 | 4.1 ± 0.5 | 4.2 ± 0.4 | ||||
| 42.3% | 25.5% | 0.230 | 0.033 | 42.3% | 42.3% | 0.203 | 0.049 | |
| Urea nitrogen, mg/dL | 17.1 ± 11.8 | 16.2 ± 9.5 | 17.1 ± 11.8 | 16.2 ± 9.2 | ||||
| 40.8% | 23.0% | 0.001 | 0.083 | 40.7% | 40.4% | 0.032 | 0.085 | |
| Creatinine, mg/dL | 1.1 ± 1.3 | 1.0 ± 1.4 | 1.1 ± 1.3 | 1.0 ± 1.0 | ||||
| 43.1% | 25.7% | 0.450 | 0.022 | 43.1% | 42.9% | 0.148 | 0.056 | |
| Glucose, mg/dL | 119.6 ± 58.8 | 117.4 ± 55.3 | 119.6 ± 58.8 | 120.6 ± 58.2 | ||||
| 42.1% | 25.8% | 0.154 | 0.038 | 42.1% | 2.4% | 0.674 | 0.016 | |
| Calcium, mg/dL | 9.4 ± 0.6 | 9.3 ± 0.6 | 9.4 ± 0.6 | 9.4 ± 0.5 | ||||
| 41.9% | 24.3% | 0.145 | 0.040 | 41.9% | 41.6% | 0.730 | 0.013 | |
| Hemoglobin, g/dL | 13.3 ± 2.0 | 13.5 ± 2.0 | 13.3 ± 2.0 | 13.4 ± 1.9 | ||||
| 39.8% | 23.5% | <0.001 | 0.120 | 39.8% | 39.8% | 0.172 | 0.055 | |
| ALT, U/L | 28.0 ± 23.2 | 31.0 ± 69.4 | 28.0 ± 23.2 | 28.3 ± 21.3 | ||||
| 37.5% | 21.7% | 0.129 | 0.059 | 37.5% | 37.8% | 0.755 | 0.013 | |
| AST, U/L | 27.3 ± 25.0 | 30.1 ± 94.6 | 27.3 ± 25.0 | 27.1 ± 22.0 | ||||
| 37.4% | 21.3% | 0.307 | 0.041 | 37.4% | 37.5% | 0.829 | 0.009 | |
| Bilirubin.total, mg/dL | 0.6 ± 1.6 | 0.6 ± 1.0 | 0.6 ± 1.6 | 0.6 ± 1.1 | ||||
| 35.7% | 20.3% | 0.433 | 0.018 | 35.7% | 35.9% | 0.768 | 0.012 | |
| Albumin, g/dL | 4.1 ± 0.5 | 4.1 ± 0.5 | 4.1 ± 0.5 | 4.1 ± 0.5 | ||||
| 36.3% | 20.7% | 0.913 | 0.003 | 36.3% | 36.3% | 0.363 | 0.038 | |
| Cholesterol, mg/dL | 192.2 ± 45.0 | 190.7 ± 45.1 | 192.2 ± 45.1 | 195.0 ± 43.6 | ||||
| 22.2% | 12.2% | 0.371 | 0.034 | 22.2% | 22.3% | 0.239 | 0.063 | |
| Cholesterol in LDL, mg/dL | 110.6 ± 37.2 | 110.8 ± 37.8 | 110.6 ± 37.2 | 113.6 ± 38.1 | ||||
| 21.7% | 12.3% | 0.924 | 0.004 | 21.7% | 21.7% | 0.149 | 0.078 | |
| Cholesterol in HDL, mg/dL | 50.9 ± 21.0 | 49.5 ± 19.8 | 50.9 ± 21.0 | 49.5 ± 20.6 | ||||
| 22.0% | 12.5% | 0.063 | 0.069 | 22.0% | 22.1% | 0.211 | 0.067 | |
| Triglyceride, mg/dL | 146.2 ± 107.5 | 149.1 ± 136.1 | 146.2 ± 107.5 | 154.9 ± 110.0 | ||||
| 21.7% | 12.5% | 0.580 | 0.024 | 21.7% | 22.3% | 0.138 | 0.080 | |
| Hemoglobin A1c/Hemoglobin.total | 6.7 ± 1.9 | 6.6 ± 1.8 | 6.7 ± 1.9 | 6.7 ± 1.8 | ||||
| 18.3% | 10.1% | 0.107 | 0.066 | 18.3% | 17.5% | 0.875 | 0.009 | |
| Calcidiol, ng/mL | 35.4 ± 18.0 | 33.4 ± 17.1 | 35.4 ± 18.0 | 34.0 ± 17.7 | ||||
| 5.0% | 2.3% | 0.139 | 0.115 | 5.0% | 5.0% | 0.491 | 0.078 | |
| Iron, ug/dL | 73.0 ± 47.4 | 74.5 ± 44.2 | 73.0 ± 47.4 | 77.4 ± 53.7 | ||||
| 4.3% | 2.3% | 0.695 | 0.032 | 4.3% | 4.6% | 0.465 | 0.087 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, T.-J.; Lu, C.-L.; Wang, J.; Tsai, K.-W.; Chen, I.-H.; Lu, K.-C. Colorectal Cancer Risk Following Herpes Zoster Reactivation in COVID-19 Survivors: Global Multicenter Study Using TriNetX. Cancers 2025, 17, 2306. https://doi.org/10.3390/cancers17142306
Lu T-J, Lu C-L, Wang J, Tsai K-W, Chen I-H, Lu K-C. Colorectal Cancer Risk Following Herpes Zoster Reactivation in COVID-19 Survivors: Global Multicenter Study Using TriNetX. Cancers. 2025; 17(14):2306. https://doi.org/10.3390/cancers17142306
Chicago/Turabian StyleLu, Tzung-Ju, Chien-Lin Lu, Joshua Wang, Kuo-Wang Tsai, I-Hung Chen, and Kuo-Cheng Lu. 2025. "Colorectal Cancer Risk Following Herpes Zoster Reactivation in COVID-19 Survivors: Global Multicenter Study Using TriNetX" Cancers 17, no. 14: 2306. https://doi.org/10.3390/cancers17142306
APA StyleLu, T.-J., Lu, C.-L., Wang, J., Tsai, K.-W., Chen, I.-H., & Lu, K.-C. (2025). Colorectal Cancer Risk Following Herpes Zoster Reactivation in COVID-19 Survivors: Global Multicenter Study Using TriNetX. Cancers, 17(14), 2306. https://doi.org/10.3390/cancers17142306






