Immune Checkpoint Molecules in Hodgkin Lymphoma and Other Hematological Malignancies
Simple Summary
Abstract
1. Introduction
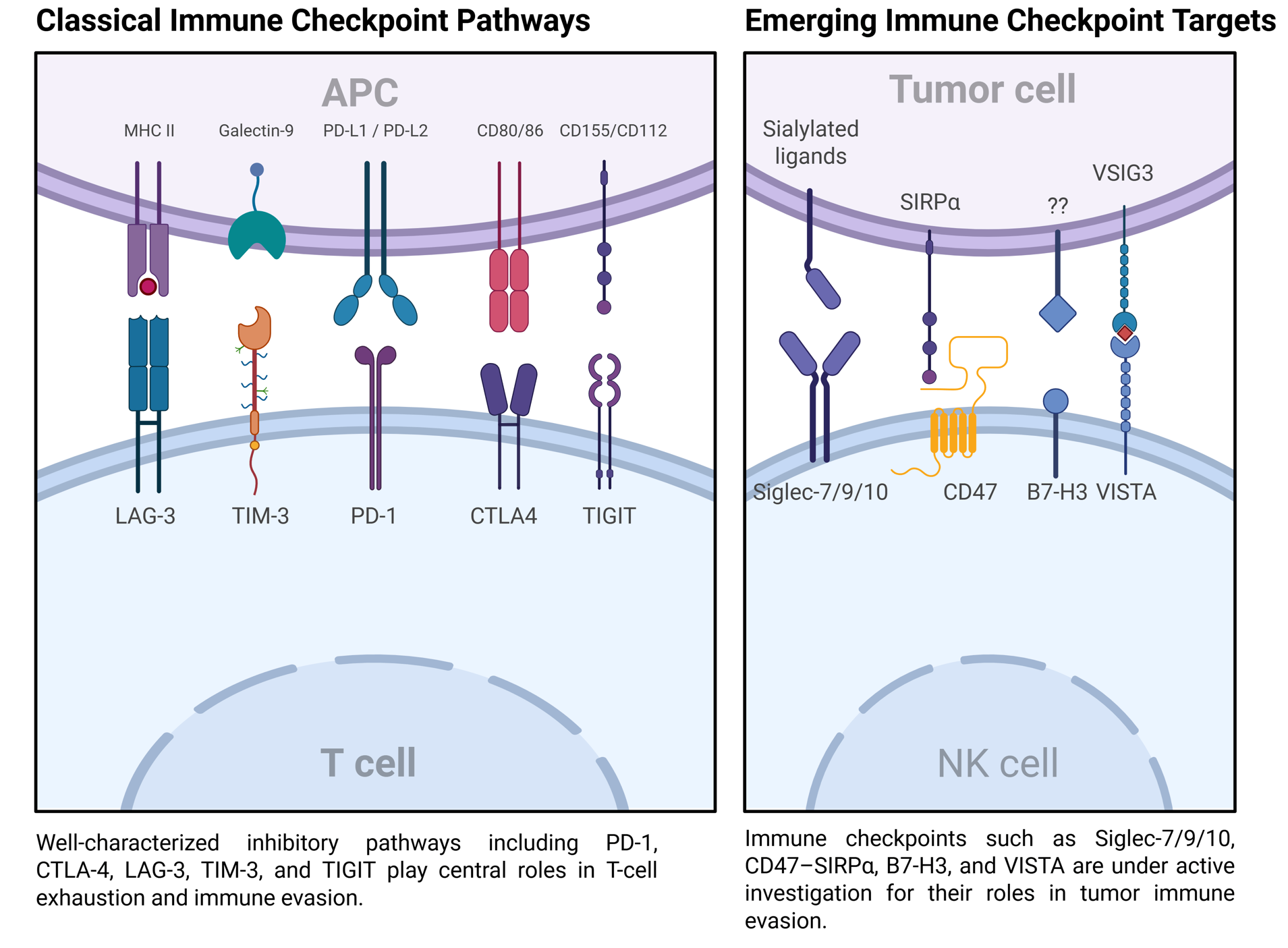
2. Mechanisms of Immune Checkpoint Regulation
2.1. PD-1/PD-L1 Axis
2.2. CTLA-4 Pathway
2.3. LAG-3
2.4. Other Checkpoints
2.5. Immune-Related Adverse Events Associated with Immune Checkpoint Inhibitors
3. Clinical Applications of Immune Checkpoint Inhibitors
3.1. Hodgkin Lymphoma
3.2. Non-Hodgkin Lymphoma
| Regimen | Population | Checkpoint Target | PFS | CR | Side Effects | Reference |
|---|---|---|---|---|---|---|
| Nivolumab 3 mg/kg every 2 weeks | Patients with R/R DLBCL ineligible for or who failed ASCT | PD-1 | Median PFS: 1.9 months (ASCT failed), 1.4 months (ASCT ineligible) | 3% (ASCT-failed cohort only) | Grade 3–4 AEs in 24% of patients: neutropenia (4%), thrombocytopenia (3%), increased lipase (3%) | [131] |
| Nivolumab 3 mg/kg every 2 weeks | Patients with R/R FL (≥2 prior lines, including CD20 antibody and alkylating agent) | PD-1 | Median PFS: 2.2 months (95% CI: 1.9–3.6) | 1% | TRAEs in 54%, with Grade 3–4 TRAEs in 15%. Most common: fatigue (13%), diarrhea (11%), nausea (10%). Grade 3–4 AEs: neoplasm progression (5%), neutropenia (5%), abdominal pain (4%), anemia (4%). Serious immune-related AEs: pneumonitis, rash, colitis, and toxic epidermal necrolysis (each ~1%). 3 treatment-related deaths occurred | [132] |
| Durvalumab + R-CHOP (Arm A) Durvalumab + R2-CHOP (Arm B) | Previously untreated, high/high-intermediate risk DLBCL (IPI ≥ 3/NCCN-IPI ≥ 4) | PD-L1 | 68% (Arm A) and 67% (Arm B) progression-free at 12 months | 54% (Arm A); 67% (Arm B) | Fatigue (61%), neutropenia (52%), neuropathy (50%), nausea (46%), diarrhea (28%). Grade 3–4 AEs in 84% (A) and 100% (B). No treatment-related deaths. | [133] |
| Ipilimumab (3 mg/kg loading, then 1–3 mg/kg monthly × 3–4 months) | R/R B-cell lymphoma (DLBCL and FL) | CTLA-4 | - | 1 CR (DLBCL, >31 months); 1 PR (FL, 19 months) | Generally well tolerated | [128] |
| Rituximab + Ipilimumab | R/R CD20+ B-cell NHL (including FL) | CTLA-4 | Median PFS: 2.6 months overall; 5.6 months in FL subset | Not specified (ORR 58% in FL) | Manageable toxicity. No dose-limiting toxicities. Common AEs not detailed | [129] |
| Nivolumab + Ipilimumab | R/R B-NHL (DLBCL, FL) | PD-1 + CTLA-4 | Median PFS: 1–2 months | 6% (FL: 0%; DLBCL: 9%) | TRAEs occurred in up to 79% of NHL patients, with grade 3–4 events in 15–29%. Common AEs included skin toxicity, fatigue, diarrhea, fever, and infusion reactions. Serious AEs (~3–5%) included pneumonitis, neutropenia, tumor flare, and autoimmune complications. Discontinuation occurred in 8% (nivo/ipi); none with nivo/liri. No treatment-related deaths. | [130] |
| Nivolumab + Lirilumab | R/R B-NHL (DLBCL, FL) | PD-1 + KIR | Median PFS: 1–2 months | 3% (FL: 17%; DLBCL: 0%) | ||
| Nivolumab + Ipilimumab/Lirilumab | R/R T-NHL | PD-1 + CTLA-4 or KIR | Median PFS: ~6 months (liri); ~1–2 mo (ipi) | 0% (both combinations) | tolerability acceptable. | |
| Nivolumab + Ipilimumab/Lirilumab | R/R MM | PD-1 + CTLA-4 or KIR | Median PFS: ~1 month | 0% | No clinical activity in MM; no unexpected toxicity. | |
| Pembrolizumab 200 mg every 3 weeks | R/R PMBCL, post ≥ 2 lines of therapy, including patients ineligible for ASCT | PD-1 | Median PFS: 4.3 months; 4-year PFS rate: 33.0% | 20.8% | 56.6% had treatment-related AEs; most common: neutropenia (18.9%), asthenia (9.4%), hypothyroidism (7.5%), fatigue (5.7%). Grade 3/4 AEs in 22.6% (mostly neutropenia). 1 case of grade 4 pneumonitis. No treatment-related deaths. | [134] |
| Nivolumab + Brentuximab Vedotin | R/R PMBCL; post-ASCT or ≥2 prior lines | PD-1 + CD30 | Median PFS not reached (follow-up: 11.1 mo) | 37% (investigator), 43% (indep. review) | 83% had treatment-related AEs. Grade 3–4 in 53%: neutropenia (30%), thrombocytopenia (10%), peripheral neuropathy (10%). No treatment-related deaths. | [135] |
| Magrolimab + Rituximab | R/R indolent NHL (FL or MZL); median 3 prior lines; 65% rituximab-refractory | CD47 + CD20 | Median PFS: 7.4 months; 2-year PFS rate: 27.4% | 30.4% | 100% had any-grade TEAEs; 95.7% treatment-related. Common: infusion reactions (60.9%), headache (52.2%), fatigue (45.7%). Grade ≥ 3: anemia (21.7%), thrombocytopenia (17.4%), neutropenia (10.9%). No treatment-related deaths. | [136] |
| TTI-622 (0.8–18 mg/kg weekly) | R/R lymphomas: DLBCL, CTCL-MF, PTCL, HL, FL; 27 evaluable patients (median 3 prior therapies; incl. CAR-T/HSCT) | CD47 | Not reported (early-phase) | 7.4% (2/27) | 47% had treatment-related AEs. Most common: thrombocytopenia (21%), neutropenia (12%), anemia & fatigue (9% each). Grade ≥ 3 AEs in 5–9% range. No clear dose-related toxicity trend. | [137] |
3.3. Myeloid Malignancies
3.4. Multiple Myeloma
4. Mechanisms of Immune Resistance to Checkpoint Inhibitors
4.1. Tumor-Intrinsic Mechanisms
4.2. Tumor-Extrinsic Mechanisms
5. Strategies to Overcome Resistance
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Krummel, M.F.; Allison, J.P. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J. Exp. Med. 1996, 183, 2533–2540. [Google Scholar]
- Latchman, Y.; Wood, C.R.; Chernova, T.; Chaudhary, D.; Borde, M.; Chernova, I.; Iwai, Y.; Long, A.J.; Brown, J.A.; Nunes, R. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2001, 2, 261–268. [Google Scholar] [PubMed]
- Walunas, T.L.; Bakker, C.Y.; Bluestone, J.A. CTLA-4 ligation blocks CD28-dependent T cell activation. J. Exp. Med. 1996, 183, 2541–2550. [Google Scholar] [PubMed]
- Sun, W.; Hu, S.; Wang, X. Advances and clinical applications of immune checkpoint inhibitors in hematological malignancies. Cancer Commun. 2024, 44, 1071–1097. [Google Scholar]
- Dong, H.; Strome, S.E.; Salomao, D.R.; Tamura, H.; Hirano, F.; Flies, D.B.; Roche, P.C.; Lu, J.; Zhu, G.; Tamada, K. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat. Med. 2002, 8, 793–800. [Google Scholar]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar]
- Ansell, S.M.; Lesokhin, A.M.; Borrello, I.; Halwani, A.; Scott, E.C.; Gutierrez, M.; Schuster, S.J.; Millenson, M.M.; Cattry, D.; Freeman, G.J. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N. Engl. J. Med. 2015, 372, 311–319. [Google Scholar]
- Anderson, A.C.; Joller, N.; Kuchroo, V.K. Lag-3, Tim-3, and TIGIT: Co-inhibitory receptors with specialized functions in immune regulation. Immunity 2016, 44, 989–1004. [Google Scholar]
- Postow, M.A.; Callahan, M.K.; Wolchok, J.D. Immune checkpoint blockade in cancer therapy. J. Clin. Oncol. 2015, 33, 1974–1982. [Google Scholar]
- Campo, E.; Swerdlow, S.H.; Harris, N.L.; Pileri, S.; Stein, H.; Jaffe, E.S. The 2008 WHO classification of lymphoid neoplasms and beyond: Evolving concepts and practical applications. Blood J. Am. Soc. Hematol. 2011, 117, 5019–5032. [Google Scholar]
- Timmerman, J.M.; Armand, P.; Fanale, M.; Ratanatharathorn, V.; Kuruvilla, J.; Cohen, J.B.; Collins, G.; Savage, K.J.; Kato, K.; Farsaci, B. Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: A multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016, 17, 1283–1294. [Google Scholar]
- Tsumura, A.; Levis, D.; Tuscano, J.M. Checkpoint inhibition in hematologic malignancies. Front. Oncol. 2023, 13, 1288172. [Google Scholar]
- Zinzani, P.L.; Santoro, A.; Gritti, G.; Brice, P.; Barr, P.M.; Kuruvilla, J.; Cunningham, D.; Kline, J.; Johnson, N.A.; Mehta-Shah, N. Nivolumab combined with brentuximab vedotin for R/R primary mediastinal large B-cell lymphoma: A 3-year follow-up. Blood Adv. 2023, 7, 5272–5280. [Google Scholar]
- Daver, N.; Garcia-Manero, G.; Basu, S.; Boddu, P.C.; Alfayez, M.; Cortes, J.E.; Konopleva, M.; Ravandi-Kashani, F.; Jabbour, E.; Kadia, T. Efficacy, safety, and biomarkers of response to azacitidine and nivolumab in relapsed/refractory acute myeloid leukemia: A nonrandomized, open-label, phase II study. Cancer Discov. 2019, 9, 370–383. [Google Scholar]
- Mateos, M.-V.; Blacklock, H.; Schjesvold, F.; Oriol, A.; Simpson, D.; George, A.; Goldschmidt, H.; Larocca, A.; Chanan-Khan, A.; Sherbenou, D. Pembrolizumab plus pomalidomide and dexamethasone for patients with relapsed or refractory multiple myeloma (KEYNOTE-183): A randomised, open-label, phase 3 trial. Lancet Haematol. 2019, 6, e459–e469. [Google Scholar]
- Raab, M.S.; Podar, K.; Breitkreutz, I.; Richardson, P.G.; Anderson, K.C. Multiple myeloma. Lancet 2009, 374, 324–339. [Google Scholar]
- Lenschow, D.J.; Walunas, T.L.; Bluestone, J.A. CD28/B7 system of T cell costimulation. Annu. Rev. Immunol. 1996, 14, 233–258. [Google Scholar]
- Liechtenstein, T.; Dufait, I.; Lanna, A.; Breckpot, K.; Escors, D. Modulating co-stimulation during antigen presentation to enhance cancer immunotherapy. Immunol. Endocr. Metab. Agents Med. Chem. 2012, 12, 224–235. [Google Scholar]
- Chen, L.; Flies, D.B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013, 13, 227–242. [Google Scholar]
- Rudd, C.E.; Taylor, A.; Schneider, H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol. Rev. 2009, 229, 12–26. [Google Scholar]
- Lin, X.; Kang, K.; Chen, P.; Zeng, Z.; Li, G.; Xiong, W.; Yi, M.; Xiang, B. Regulatory mechanisms of PD-1/PD-L1 in cancers. Mol. Cancer 2024, 23, 108. [Google Scholar] [PubMed]
- Shibru, B.; Fey, K.; Fricke, S.; Blaudszun, A.-R.; Fürst, F.; Weise, M.; Seiffert, S.; Weyh, M.K.; Köhl, U.; Sack, U. Detection of immune checkpoint receptors–a current challenge in clinical flow cytometry. Front. Immunol. 2021, 12, 694055. [Google Scholar]
- Morad, G.; Helmink, B.A.; Sharma, P.; Wargo, J.A. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell 2021, 184, 5309–5337. [Google Scholar] [PubMed]
- Bally, A.P.; Tang, Y.; Lee, J.T.; Barwick, B.G.; Martinez, R.; Evavold, B.D.; Boss, J.M. Conserved region C functions to regulate PD-1 expression and subsequent CD8 T cell memory. J. Immunol. 2017, 198, 205–217. [Google Scholar]
- Bally, A.P.; Lu, P.; Tang, Y.; Austin, J.W.; Scharer, C.D.; Ahmed, R.; Boss, J.M. NF-κB regulates PD-1 expression in macrophages. J. Immunol. 2015, 194, 4545–4554. [Google Scholar]
- Oestreich, K.J.; Yoon, H.; Ahmed, R.; Boss, J.M. NFATc1 regulates PD-1 expression upon T cell activation. J. Immunol. 2008, 181, 4832–4839. [Google Scholar]
- Austin, J.W.; Lu, P.; Majumder, P.; Ahmed, R.; Boss, J.M. STAT3, STAT4, NFATc1, and CTCF regulate PD-1 through multiple novel regulatory regions in murine T cells. J. Immunol. 2014, 192, 4876–4886. [Google Scholar]
- Agata, Y.; Kawasaki, A.; Nishimura, H.; Ishida, Y.; Tsubat, T.; Yagita, H.; Honjo, T. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int. Immunol. 1996, 8, 765–772. [Google Scholar]
- Brown, K.E.; Freeman, G.J.; Wherry, E.J.; Sharpe, A.H. Role of PD-1 in regulating acute infections. Curr. Opin. Immunol. 2010, 22, 397–401. [Google Scholar]
- Youngblood, B.; Oestreich, K.J.; Ha, S.-J.; Duraiswamy, J.; Akondy, R.S.; West, E.E.; Wei, Z.; Lu, P.; Austin, J.W.; Riley, J.L. Chronic virus infection enforces demethylation of the locus that encodes PD-1 in antigen-specific CD8+ T cells. Immunity 2011, 35, 400–412. [Google Scholar] [PubMed]
- Ahn, E.; Youngblood, B.; Lee, J.; Lee, J.; Sarkar, S.; Ahmed, R. Demethylation of the PD-1 promoter is imprinted during the effector phase of CD8 T cell exhaustion. J. Virol. 2016, 90, 8934–8946. [Google Scholar] [PubMed]
- Nakayamada, S.; Poholek, A.C.; Lu, K.T.; Takahashi, H.; Kato, M.; Iwata, S.; Hirahara, K.; Cannons, J.L.; Schwartzberg, P.L.; Vahedi, G. Type I IFN induces binding of STAT1 to Bcl6: Divergent roles of STAT family transcription factors in the T follicular helper cell genetic program. J. Immunol. 2014, 192, 2156–2166. [Google Scholar]
- Bao, S.; Jiang, X.; Jin, S.; Tu, P.; Lu, J. TGF-β1 induces immune escape by enhancing PD-1 and CTLA-4 expression on T lymphocytes in hepatocellular carcinoma. Front. Oncol. 2021, 11, 694145. [Google Scholar]
- Barber, D.L.; Wherry, E.J.; Masopust, D.; Zhu, B.; Allison, J.P.; Sharpe, A.H.; Freeman, G.J.; Ahmed, R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006, 439, 682–687. [Google Scholar]
- Cloughesy, T.F.; Mochizuki, A.Y.; Orpilla, J.R.; Hugo, W.; Lee, A.H.; Davidson, T.B.; Wang, A.C.; Ellingson, B.M.; Rytlewski, J.A.; Sanders, C.M. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat. Med. 2019, 25, 477–486. [Google Scholar]
- Pan, J.; Li, X.; Shao, B.; Xu, F.; Huang, X.; Guo, X.; Zhou, S. Self-blockade of PD-L1 with bacteria-derived outer-membrane vesicle for enhanced cancer immunotherapy. Adv. Mater. 2022, 34, 2106307. [Google Scholar]
- Sanmamed, M.F.; Chen, L. Inducible expression of B7-H1 (PD-L1) and its selective role in tumor site immune modulation. Cancer J. 2014, 20, 256–261. [Google Scholar]
- Sharpe, A.H.; Wherry, E.J.; Ahmed, R.; Freeman, G.J. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat. Immunol. 2007, 8, 239–245. [Google Scholar]
- Rozali, E.N.; Hato, S.V.; Robinson, B.W.; Lake, R.A.; Lesterhuis, W.J. Programmed death ligand 2 in cancer-induced immune suppression. J. Immunol. Res. 2012, 2012, 656340. [Google Scholar]
- Yang, H.; Zhang, Q.; Xu, M.; Wang, L.; Chen, X.; Feng, Y.; Li, Y.; Zhang, X.; Cui, W.; Jia, X. CCL2-CCR2 axis recruits tumor associated macrophages to induce immune evasion through PD-1 signaling in esophageal carcinogenesis. Mol. Cancer 2020, 19, 1–14. [Google Scholar]
- Ohaegbulam, K.C.; Assal, A.; Lazar-Molnar, E.; Yao, Y.; Zang, X. Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends Mol. Med. 2015, 21, 24–33. [Google Scholar]
- Ji, M.; Liu, Y.; Li, Q.; Li, X.-D.; Zhao, W.-Q.; Zhang, H.; Zhang, X.; Jiang, J.-T.; Wu, C.-P. PD-1/PD-L1 pathway in non-small-cell lung cancer and its relation with EGFR mutation. J. Transl. Med. 2015, 13, 1–6. [Google Scholar]
- Blank, C.; Brown, I.; Marks, R.; Nishimura, H.; Honjo, T.; Gajewski, T.F. Absence of programmed death receptor 1 alters thymic development and enhances generation of CD4/CD8 double-negative TCR-transgenic T cells. J. Immunol. 2003, 171, 4574–4581. [Google Scholar] [PubMed]
- Dorfman, D.M.; Brown, J.A.; Shahsafaei, A.; Freeman, G.J. Programmed death-1 (PD-1) is a marker of germinal center-associated T cells and angioimmunoblastic T-cell lymphoma. Am. J. Surg. Pathol. 2006, 30, 802–810. [Google Scholar]
- Ghebeh, H.; Mohammed, S.; Al-Omair, A.; Qattant, A.; Lehe, C.; Al-Qudaihi, G.; Elkum, N.; Alshabanah, M.; Amer, S.B.; Tulbah, A. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: Correlation with important high-risk prognostic factors. Neoplasia 2006, 8, 190–198. [Google Scholar]
- Shimauchi, T.; Kabashima, K.; Nakashima, D.; Sugita, K.; Yamada, Y.; Hino, R.; Tokura, Y. Augmented expression of programmed death-1 in both neoplastic and non-neoplastic CD4+ T-cells in adult T-cell leukemia/lymphoma. Int. J. Cancer 2007, 121, 2585–2590. [Google Scholar]
- Yamamoto, R.; Nishikori, M.; Kitawaki, T.; Sakai, T.; Hishizawa, M.; Tashima, M.; Kondo, T.; Ohmori, K.; Kurata, M.; Hayashi, T. PD-1–PD-1 ligand interaction contributes to immunosuppressive microenvironment of Hodgkin lymphoma. Blood J. Am. Soc. Hematol. 2008, 111, 3220–3224. [Google Scholar]
- Zhou, Q.; Munger, M.E.; Veenstra, R.G.; Weigel, B.J.; Hirashima, M.; Munn, D.H.; Murphy, W.J.; Azuma, M.; Anderson, A.C.; Kuchroo, V.K. Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood J. Am. Soc. Hematol. 2011, 117, 4501–4510. [Google Scholar]
- Christiansson, L.; Söderlund, S.; Svensson, E.; Mustjoki, S.; Bengtsson, M.; Simonsson, B.; Olsson-Strömberg, U.; Loskog, A.S. Increased level of myeloid-derived suppressor cells, programmed death receptor ligand 1/programmed death receptor 1, and soluble CD25 in Sokal high risk chronic myeloid leukemia. PLoS ONE 2013, 8, e55818. [Google Scholar]
- Schwartz, J.-C.D.; Zhang, X.; Fedorov, A.A.; Nathenson, S.G.; Almo, S.C. Structural basis for co-stimulation by the human CTLA-4/B7-2 complex. Nature 2001, 410, 604–608. [Google Scholar]
- Stamper, C.C.; Zhang, Y.; Tobin, J.F.; Erbe, D.V.; Ikemizu, S.; Davis, S.J.; Stahl, M.L.; Seehra, J.; Somers, W.S.; Mosyak, L. Crystal structure of the B7-1/CTLA-4 complex that inhibits human immune responses. Nature 2001, 410, 608–611. [Google Scholar] [PubMed]
- Collins, A.V.; Brodie, D.W.; Gilbert, R.J.; Iaboni, A.; Manso-Sancho, R.; Walse, B.; Stuart, D.I.; van der Merwe, P.A.; Davis, S.J. The interaction properties of costimulatory molecules revisited. Immunity 2002, 17, 201–210. [Google Scholar] [PubMed]
- Walker, L.S.; Sansom, D.M. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat. Rev. Immunol. 2011, 11, 852–863. [Google Scholar]
- Pentcheva-Hoang, T.; Egen, J.G.; Wojnoonski, K.; Allison, J.P. B7-1 and B7-2 selectively recruit CTLA-4 and CD28 to the immunological synapse. Immunity 2004, 21, 401–413. [Google Scholar]
- Yokosuka, T.; Kobayashi, W.; Takamatsu, M.; Sakata-Sogawa, K.; Zeng, H.; Hashimoto-Tane, A.; Yagita, H.; Tokunaga, M.; Saito, T. Spatiotemporal basis of CTLA-4 costimulatory molecule-mediated negative regulation of T cell activation. Immunity 2010, 33, 326–339. [Google Scholar]
- Qureshi, O.S.; Zheng, Y.; Nakamura, K.; Attridge, K.; Manzotti, C.; Schmidt, E.M.; Baker, J.; Jeffery, L.E.; Kaur, S.; Briggs, Z. Trans-endocytosis of CD80 and CD86: A molecular basis for the cell-extrinsic function of CTLA-4. Science 2011, 332, 600–603. [Google Scholar] [PubMed]
- Lingel, H.; Brunner-Weinzierl, M.C. CTLA-4 (CD152): A versatile receptor for immune-based therapy. Semin. Immunol. 2019, 42, 101298. [Google Scholar]
- Shiratori, T.; Miyatake, S.; Ohno, H.; Nakaseko, C.; Isono, K.; Bonifacino, J.S.; Saito, T. Tyrosine phosphorylation controls internalization of CTLA-4 by regulating its interaction with clathrin-associated adaptor complex AP-2. Immunity 1997, 6, 583–589. [Google Scholar]
- Chuang, E.; Alegre, M.-L.; Duckett, C.S.; Noel, P.J.; Vander Heiden, M.; Thompson, C.B. Interaction of CTLA-4 with the clathrin-associated protein AP50 results in ligand-independent endocytosis that limits cell surface expression. J. Immunol. 1997, 159, 144–151. [Google Scholar]
- Lo, B.; Zhang, K.; Lu, W.; Zheng, L.; Zhang, Q.; Kanellopoulou, C.; Zhang, Y.; Liu, Z.; Fritz, J.M.; Marsh, R. Patients with LRBA deficiency show CTLA4 loss and immune dysregulation responsive to abatacept therapy. Science 2015, 349, 436–440. [Google Scholar]
- Banton, M.C.; Inder, K.L.; Valk, E.; Rudd, C.E.; Schneider, H. Rab8 binding to immune cell-specific adaptor LAX facilitates formation of trans-Golgi network-proximal CTLA-4 vesicles for surface expression. Mol. Cell. Biol. 2014, 34, 1486–1499. [Google Scholar]
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 pathways: Similarities, differences, and implications of their inhibition. Am. J. Clin. Oncol. 2016, 39, 98–106. [Google Scholar] [PubMed]
- Rohr, J.; Guo, S.; Huo, J.; Bouska, A.; Lachel, C.; Li, Y.; Simone, P.D.; Zhang, W.; Gong, Q.; Wang, C. Recurrent activating mutations of CD28 in peripheral T-cell lymphomas. Leukemia 2016, 30, 1062–1070. [Google Scholar]
- Kataoka, K.; Nagata, Y.; Kitanaka, A.; Shiraishi, Y.; Shimamura, T.; Yasunaga, J.-i.; Totoki, Y.; Chiba, K.; Sato-Otsubo, A.; Nagae, G. Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat. Genet. 2015, 47, 1304–1315. [Google Scholar] [PubMed]
- Leach, D.R.; Krummel, M.F.; Allison, J.P. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996, 271, 1734–1736. [Google Scholar] [PubMed]
- Van Elsas, A.; Hurwitz, A.A.; Allison, J.P. Combination immunotherapy of B16 melanoma using anti–cytotoxic T lymphocyte–associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J. Exp. Med. 1999, 190, 355–366. [Google Scholar]
- van Elsas, A.; Sutmuller, R.P.; Hurwitz, A.A.; Ziskin, J.; Villasenor, J.; Medema, J.-P.; Overwijk, W.W.; Restifo, N.P.; Melief, C.J.; Offringa, R. Elucidating the autoimmune and antitumor effector mechanisms of a treatment based on cytotoxic T lymphocyte antigen-4 blockade in combination with a B16 melanoma vaccine: Comparison of prophylaxis and therapy. J. Exp. Med. 2001, 194, 481–490. [Google Scholar]
- Ramagopal, U.A.; Liu, W.; Garrett-Thomson, S.C.; Bonanno, J.B.; Yan, Q.; Srinivasan, M.; Wong, S.C.; Bell, A.; Mankikar, S.; Rangan, V.S. Structural basis for cancer immunotherapy by the first-in-class checkpoint inhibitor ipilimumab. Proc. Natl. Acad. Sci. USA 2017, 114, E4223–E4232. [Google Scholar]
- Tarhini, A.A.; Kirkwood, J.M. Tremelimumab, a fully human monoclonal IgG2 antibody against CTLA4 for the potential treatment of cancer. Curr. Opin. Mol. Ther. 2007, 9, 505–514. [Google Scholar]
- Boutros, C.; Tarhini, A.; Routier, E.; Lambotte, O.; Ladurie, F.L.; Carbonnel, F.; Izzeddine, H.; Marabelle, A.; Champiat, S.; Berdelou, A. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat. Rev. Clin. Oncol. 2016, 13, 473–486. [Google Scholar] [PubMed]
- Rowshanravan, B.; Halliday, N.; Sansom, D.M. CTLA-4: A moving target in immunotherapy. Blood J. Am. Soc. Hematol. 2018, 131, 58–67. [Google Scholar]
- Cha, E.; Klinger, M.; Hou, Y.; Cummings, C.; Ribas, A.; Faham, M.; Fong, L. Improved survival with T cell clonotype stability after anti-CTLA-4 treatment in cancer patients. Sci. Transl. Med. 2014, 6, 238ra270. [Google Scholar]
- Oh, D.Y.; Cham, J.; Zhang, L.; Fong, G.; Kwek, S.S.; Klinger, M.; Faham, M.; Fong, L. Immune toxicities elicted by CTLA-4 blockade in cancer patients are associated with early diversification of the T-cell repertoire. Cancer Res. 2017, 77, 1322–1330. [Google Scholar]
- Subudhi, S.K.; Aparicio, A.; Gao, J.; Zurita, A.J.; Araujo, J.C.; Logothetis, C.J.; Tahir, S.A.; Korivi, B.R.; Slack, R.S.; Vence, L. Clonal expansion of CD8 T cells in the systemic circulation precedes development of ipilimumab-induced toxicities. Proc. Natl. Acad. Sci. USA 2016, 113, 11919–11924. [Google Scholar]
- Zhang, A.; Ren, Z.; Tseng, K.-F.; Liu, X.; Li, H.; Lu, C.; Cai, Y.; Minna, J.D.; Fu, Y.-X. Dual targeting of CTLA-4 and CD47 on Treg cells promotes immunity against solid tumors. Sci. Transl. Med. 2021, 13, eabg8693. [Google Scholar]
- Hosseini, A.; Gharibi, T.; Marofi, F.; Babaloo, Z.; Baradaran, B. CTLA-4: From mechanism to autoimmune therapy. Int. Immunopharmacol. 2020, 80, 106221. [Google Scholar]
- Schubert, D.; Bode, C.; Kenefeck, R.; Hou, T.Z.; Wing, J.B.; Kennedy, A.; Bulashevska, A.; Petersen, B.-S.; Schäffer, A.A.; Grüning, B.A. Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations. Nat. Med. 2014, 20, 1410–1416. [Google Scholar]
- Kuehn, H.S.; Ouyang, W.; Lo, B.; Deenick, E.K.; Niemela, J.E.; Avery, D.T.; Schickel, J.-N.; Tran, D.Q.; Stoddard, J.; Zhang, Y. Immune dysregulation in human subjects with heterozygous germline mutations in CTLA4. Science 2014, 345, 1623–1627. [Google Scholar]
- Lopez-Herrera, G.; Tampella, G.; Pan-Hammarström, Q.; Herholz, P.; Trujillo-Vargas, C.M.; Phadwal, K.; Simon, A.K.; Moutschen, M.; Etzioni, A.; Mory, A. Deleterious mutations in LRBA are associated with a syndrome of immune deficiency and autoimmunity. Am. J. Hum. Genet. 2012, 90, 986–1001. [Google Scholar]
- Maruhashi, T.; Sugiura, D.; Okazaki, I.-m.; Shimizu, K.; Maeda, T.K.; Ikubo, J.; Yoshikawa, H.; Maenaka, K.; Ishimaru, N.; Kosako, H. Binding of LAG-3 to stable peptide-MHC class II limits T cell function and suppresses autoimmunity and anti-cancer immunity. Immunity 2022, 55, 912–924.e8. [Google Scholar]
- Triebel, F.; Jitsukawa, S.; Baixeras, E.; Roman-Roman, S.; Genevee, C.; Viegas-Pequignot, E.; Hercend, T. LAG-3, a novel lymphocyte activation gene closely related to CD4. J. Exp. Med. 1990, 171, 1393–1405. [Google Scholar]
- Huard, B.; Prigent, P.; Tournier, M.; Bruniquel, D.; Triebel, F. CD4/major histocompatibility complex class II interaction analyzed with CD4-and lymphocyte activation gene-3 (LAG-3)-Ig fusion proteins. Eur. J. Immunol. 1995, 25, 2718–2721. [Google Scholar]
- Chocarro, L.; Blanco, E.; Zuazo, M.; Arasanz, H.; Bocanegra, A.; Fernández-Rubio, L.; Morente, P.; Fernández-Hinojal, G.; Echaide, M.; Garnica, M. Understanding LAG-3 signaling. Int. J. Mol. Sci. 2021, 22, 5282. [Google Scholar]
- Huard, B.; Prigent, P.; Pagès, F.; Bruniquel, D.; Triebel, F. T cell major histocompatibility complex class II molecules down-regulate CD4+ T cell clone responses following LAG-3 binding. Eur. J. Immunol. 1996, 26, 1180–1186. [Google Scholar] [PubMed]
- Paik, J. Nivolumab plus relatlimab: First approval. Drugs 2022, 82, 925–931. [Google Scholar] [PubMed]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Castillo Gutiérrez, E.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar]
- Okagawa, T.; Konnai, S.; Nishimori, A.; Maekawa, N.; Goto, S.; Ikebuchi, R.; Kohara, J.; Suzuki, Y.; Yamada, S.; Kato, Y. Cooperation of PD-1 and LAG-3 in the exhaustion of CD4+ and CD8+ T cells during bovine leukemia virus infection. Vet. Res. 2018, 49, 1–12. [Google Scholar]
- Kouo, T.; Huang, L.; Pucsek, A.B.; Cao, M.; Solt, S.; Armstrong, T.; Jaffee, E. Galectin-3 shapes antitumor immune responses by suppressing CD8+ T cells via LAG-3 and inhibiting expansion of plasmacytoid dendritic cells. Cancer Immunol. Res. 2015, 3, 412–423. [Google Scholar]
- Wang, J.; Sanmamed, M.F.; Datar, I.; Su, T.T.; Ji, L.; Sun, J.; Chen, L.; Chen, Y.; Zhu, G.; Yin, W. Fibrinogen-like protein 1 is a major immune inhibitory ligand of LAG-3. Cell 2019, 176, 334–347.e12. [Google Scholar]
- Huang, Y.-H.; Zhu, C.; Kondo, Y.; Anderson, A.C.; Gandhi, A.; Russell, A.; Dougan, S.K.; Petersen, B.-S.; Melum, E.; Pertel, T. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature 2015, 517, 386–390. [Google Scholar] [PubMed]
- Zeidan, A.M.; Komrokji, R.S.; Brunner, A.M. TIM-3 pathway dysregulation and targeting in cancer. Expert Rev. Anticancer Ther. 2021, 21, 523–534. [Google Scholar]
- Chiba, S.; Baghdadi, M.; Akiba, H.; Yoshiyama, H.; Kinoshita, I.; Dosaka-Akita, H.; Fujioka, Y.; Ohba, Y.; Gorman, J.V.; Colgan, J.D. Tumor-infiltrating DCs suppress nucleic acid–mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nat. Immunol. 2012, 13, 832–842. [Google Scholar]
- Sakuishi, K.; Apetoh, L.; Sullivan, J.M.; Blazar, B.R.; Kuchroo, V.K.; Anderson, A.C. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 2010, 207, 2187–2194. [Google Scholar]
- Koyama, S.; Akbay, E.A.; Li, Y.Y.; Herter-Sprie, G.S.; Buczkowski, K.A.; Richards, W.G.; Gandhi, L.; Redig, A.J.; Rodig, S.J.; Asahina, H. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat. Commun. 2016, 7, 10501. [Google Scholar] [PubMed]
- Cai, L.; Li, Y.; Tan, J.; Xu, L.; Li, Y. Targeting LAG-3, TIM-3, and TIGIT for cancer immunotherapy. J. Hematol. Oncol. 2023, 16, 101. [Google Scholar] [PubMed]
- Yu, X.; Harden, K.C.; Gonzalez, L.; Francesco, M.; Chiang, E.; Irving, B.; Tom, I.; Ivelja, S.; Refino, C.J.; Clark, H. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat. Immunol. 2009, 10, 48–57. [Google Scholar]
- Levin, S.D.; Taft, D.W.; Brandt, C.S.; Bucher, C.; Howard, E.D.; Chadwick, E.M.; Johnston, J.; Hammond, A.; Bontadelli, K.; Ardourel, D. Vstm3 is a member of the CD28 family and an important modulator of T-cell function. Eur. J. Immunol. 2011, 41, 902–915. [Google Scholar]
- Gur, C.; Ibrahim, Y.; Isaacson, B.; Yamin, R.; Abed, J.; Gamliel, M.; Enk, J.; Bar-On, Y.; Stanietsky-Kaynan, N.; Coppenhagen-Glazer, S. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity 2015, 42, 344–355. [Google Scholar]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar]
- Zhu, Y.; Paniccia, A.; Schulick, A.C.; Chen, W.; Koenig, M.R.; Byers, J.T.; Yao, S.; Bevers, S.; Edil, B.H. Identification of CD112R as a novel checkpoint for human T cells. J. Exp. Med. 2016, 213, 167–176. [Google Scholar] [PubMed]
- Wang, M.; Bu, J.; Zhou, M.; Sido, J.; Lin, Y.; Liu, G.; Lin, Q.; Xu, X.; Leavenworth, J.W.; Shen, E. CD8+ T cells expressing both PD-1 and TIGIT but not CD226 are dysfunctional in acute myeloid leukemia (AML) patients. Clin. Immunol. 2018, 190, 64–73. [Google Scholar] [PubMed]
- Guillerey, C.; Harjunpää, H.; Carrié, N.; Kassem, S.; Teo, T.; Miles, K.; Krumeich, S.; Weulersse, M.; Cuisinier, M.; Stannard, K. TIGIT immune checkpoint blockade restores CD8+ T-cell immunity against multiple myeloma. Blood J. Am. Soc. Hematol. 2018, 132, 1689–1694. [Google Scholar]
- Jin, S.; Zhang, Y.; Zhou, F.; Chen, X.; Sheng, J.; Zhang, J. TIGIT: A promising target to overcome the barrier of immunotherapy in hematological malignancies. Front. Oncol. 2022, 12, 1091782. [Google Scholar]
- Qiu, D.; Liu, X.; Wang, W.; Jiang, X.; Wu, X.; Zheng, J.; Zhou, K.; Kong, X.; Wu, X.; Jin, Z. TIGIT axis: Novel immune checkpoints in anti-leukemia immunity. Clin. Exp. Med. 2023, 23, 165–174. [Google Scholar]
- Houssaini, M.S.; Damou, M.; Ismaili, N. Advances in the management of non-small cell lung cancer (NSCLC): A new practice changing data from asco 2020 annual meeting. Cancer Treat. Res. Commun. 2020, 25, 100239. [Google Scholar]
- Fletcher, K.; Johnson, D.B. Chronic immune-related adverse events arising from immune checkpoint inhibitors: An update. J. Immunother. Cancer 2024, 12, e008591. [Google Scholar]
- Patrinely, J.R.; Johnson, R.; Lawless, A.R.; Bhave, P.; Sawyers, A.; Dimitrova, M.; Yeoh, H.L.; Palmeri, M.; Ye, F.; Fan, R. Chronic immune-related adverse events following adjuvant anti–PD-1 therapy for high-risk resected melanoma. JAMA Oncol. 2021, 7, 744–748. [Google Scholar]
- Barron, C.C.; Stefanova, I.; Cha, Y.; Elsolh, K.; Zereshkian, A.; Gaafour, N.; McWhirter, E. Chronic immune-related adverse events in patients with cancer receiving immune checkpoint inhibitors: A systematic review. J. Immunother. Cancer 2023, 11, e006500. [Google Scholar]
- Johnson, D.B.; Taylor, K.B.; Cohen, J.V.; Ayoubi, N.; Haugh, A.M.; Wang, D.Y.; Schlick, B.D.; Voorhees, A.L.; Gage, K.L.; Fintelmann, F.J. Anti–PD-1–induced Pneumonitis is associated with persistent imaging abnormalities in melanoma patients. Cancer Immunol. Res. 2019, 7, 1755–1759. [Google Scholar]
- Roemer, M.G.; Advani, R.H.; Ligon, A.H.; Natkunam, Y.; Redd, R.A.; Homer, H.; Connelly, C.F.; Sun, H.H.; Daadi, S.E.; Freeman, G.J. PD-L1 and PD-L2 genetic alterations define classical Hodgkin lymphoma and predict outcome. J. Clin. Oncol. 2016, 34, 2690–2697. [Google Scholar]
- Lynch, R.C.; Binkley, M.S. Rising to the Top: How Immune-Checkpoint Inhibitors are Changing the Landscape of Treatment for Classic Hodgkin Lymphoma. Semin. Radiat. Oncol. 2025, 35, 40–46. [Google Scholar] [PubMed]
- Chen, R.; Zinzani, P.L.; Fanale, M.A.; Armand, P.; Johnson, N.A.; Brice, P.; Radford, J.; Ribrag, V.; Molin, D.; Vassilakopoulos, T.P. Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. J. Clin. Oncol. 2017, 35, 2125–2132. [Google Scholar] [PubMed]
- Armand, P.; Zinzani, P.L.; Lee, H.J.; Johnson, N.A.; Brice, P.; Radford, J.; Ribrag, V.; Molin, D.; Vassilakopoulos, T.P.; Tomita, A. Five-year follow-up of KEYNOTE-087: Pembrolizumab monotherapy for relapsed/refractory classical Hodgkin lymphoma. Blood 2023, 142, 878–886. [Google Scholar]
- Ansell, S.M.; Bröckelmann, P.J.; von Keudell, G.; Lee, H.J.; Santoro, A.; Zinzani, P.L.; Collins, G.P.; Cohen, J.B.; de Boer, J.P.; Kuruvilla, J. Nivolumab for relapsed/refractory classical Hodgkin lymphoma: 5-year survival from the pivotal phase 2 CheckMate 205 study. Blood Adv. 2023, 7, 6266–6274. [Google Scholar]
- Kuruvilla, J.; Ramchandren, R.; Santoro, A.; Paszkiewicz-Kozik, E.; Gasiorowski, R.; Johnson, N.A.; Fogliatto, L.M.; Goncalves, I.; de Oliveira, J.S.; Buccheri, V. Pembrolizumab versus brentuximab vedotin in relapsed or refractory classical Hodgkin lymphoma (KEYNOTE-204): An interim analysis of a multicentre, randomised, open-label, phase 3 study. Lancet Oncol. 2021, 22, 512–524. [Google Scholar]
- Broccoli, A.; Zinzani, P.L. The role of transplantation in Hodgkin lymphoma. Br. J. Haematol. 2019, 184, 93–104. [Google Scholar]
- Advani, R.H.; Moskowitz, A.J.; Bartlett, N.L.; Vose, J.M.; Ramchandren, R.; Feldman, T.A.; LaCasce, A.S.; Christian, B.A.; Ansell, S.M.; Moskowitz, C.H. Brentuximab vedotin in combination with nivolumab in relapsed or refractory Hodgkin lymphoma: 3-year study results. Blood J. Am. Soc. Hematol. 2021, 138, 427–438. [Google Scholar]
- Harker-Murray, P.; Mauz-Körholz, C.; Leblanc, T.; Mascarin, M.; Michel, G.; Cooper, S.; Beishuizen, A.; Leger, K.J.; Amoroso, L.; Buffardi, S. Nivolumab and brentuximab vedotin with or without bendamustine for R/R Hodgkin lymphoma in children, adolescents, and young adults. Blood J. Am. Soc. Hematol. 2023, 141, 2075–2084. [Google Scholar]
- Mei, M.G.; Lee, H.J.; Palmer, J.M.; Chen, R.; Tsai, N.-C.; Chen, L.; McBride, K.; Smith, D.L.; Melgar, I.; Song, J.Y. Response-adapted anti-PD-1–based salvage therapy for Hodgkin lymphoma with nivolumab alone or in combination with ICE. Blood J. Am. Soc. Hematol. 2022, 139, 3605–3616. [Google Scholar]
- Bryan, L.J.; Casulo, C.; Allen, P.B.; Smith, S.E.; Savas, H.; Dillehay, G.L.; Karmali, R.; Pro, B.; Kane, K.L.; Bazzi, L.A. Pembrolizumab added to ifosfamide, carboplatin, and etoposide chemotherapy for relapsed or refractory classic Hodgkin lymphoma: A multi-institutional phase 2 investigator-initiated nonrandomized clinical trial. JAMA Oncol. 2023, 9, 683–691. [Google Scholar] [PubMed]
- Moskowitz, A.J.; Shah, G.; Schöder, H.; Ganesan, N.; Hancock, H.; Davey, T.; Perez, L.; Sohail, S.; Santarosa, A.; Capadona, C. T099: High efficacy and durability of second-line therapy with pembrolizumab, gemcitabine, vinorelbine, and liposomal doxorubicin in the phase II study for relapsed and refractory Hodgkin lymphoma. HemaSphere 2022, 6, 45–46. [Google Scholar]
- Bröckelmann, P.J.; Goergen, H.; Keller, U.; Meissner, J.; Ordemann, R.; Halbsguth, T.V.; Sasse, S.; Sökler, M.; Kerkhoff, A.; Mathas, S. Efficacy of nivolumab and AVD in early-stage unfavorable classic Hodgkin lymphoma: The randomized phase 2 German Hodgkin Study Group NIVAHL trial. JAMA Oncol. 2020, 6, 872–880. [Google Scholar]
- Advani, R.H.; Avigdor, A.; Sureda, A.; Lavie, D.; Hohaus, S.; Zaucha, J.M.; Hua, V.M.; Zilioli, V.R.; Gazitua, R.; Özcan, M. Pembrolizumab and chemotherapy in newly-diagnosed, early unfavorable or advanced stage classic Hodgkin lymphoma: The phase 2 Keynote-C11 study. Blood 2022, 140, 1759–1760. [Google Scholar]
- Herrera, A.F.; LeBlanc, M.L.; Castellino, S.M.; Li, H.; Rutherford, S.C.; Evens, A.M.; Davison, K.; Punnett, A.; Hodgson, D.C.; Parsons, S.K. SWOG S1826, a randomized study of nivolumab (N)-AVD versus brentuximab vedotin (BV)-AVD in advanced stage (AS) classic Hodgkin lymphoma (HL). In Proceedings of the 2023 ASCO Annual Meeting, Chicago, IL, USA, 2–6 June 2023. [Google Scholar]
- Song, Y.; Gao, Q.; Zhang, H.; Fan, L.; Zhou, J.; Zou, D.; Li, W.; Yang, H.; Liu, T.; Wang, Q. Tislelizumab for relapsed/refractory classical Hodgkin lymphoma: 3-year follow-up and correlative biomarker analysis. Clin. Cancer Res. 2022, 28, 1147–1156. [Google Scholar]
- Yang, Z.-Z.; Novak, A.J.; Stenson, M.J.; Witzig, T.E.; Ansell, S.M. Intratumoral CD4+ CD25+ regulatory T-cell-mediated suppression of infiltrating CD4+ T cells in B-cell non-Hodgkin lymphoma. Blood 2006, 107, 3639–3646. [Google Scholar]
- Ansell, S.M.; Hurvitz, S.A.; Koenig, P.A.; LaPlant, B.R.; Kabat, B.F.; Fernando, D.; Habermann, T.M.; Inwards, D.J.; Verma, M.; Yamada, R. Phase I study of ipilimumab, an anti–CTLA-4 monoclonal antibody, in patients with relapsed and refractory B-cell non–Hodgkin lymphoma. Clin. Cancer Res. 2009, 15, 6446–6453. [Google Scholar]
- Tuscano, J.M.; Maverakis, E.; Groshen, S.; Tsao-Wei, D.; Luxardi, G.; Merleev, A.A.; Beaven, A.; DiPersio, J.F.; Popplewell, L.; Chen, R. A phase I study of the combination of rituximab and ipilimumab in patients with relapsed/refractory B-cell lymphoma. Clin. Cancer Res. 2019, 25, 7004–7013. [Google Scholar]
- Armand, P.; Lesokhin, A.; Borrello, I.; Timmerman, J.; Gutierrez, M.; Zhu, L.; Popa McKiver, M.; Ansell, S.M. A phase 1b study of dual PD-1 and CTLA-4 or KIR blockade in patients with relapsed/refractory lymphoid malignancies. Leukemia 2021, 35, 777–786. [Google Scholar]
- Ansell, S.M.; Minnema, M.C.; Johnson, P.; Timmerman, J.M.; Armand, P.; Shipp, M.A.; Rodig, S.J.; Ligon, A.H.; Roemer, M.G.; Reddy, N. Nivolumab for relapsed/refractory diffuse large B-cell lymphoma in patients ineligible for or having failed autologous transplantation: A single-arm, phase II study. J. Clin. Oncol. 2019, 37, 481–489. [Google Scholar]
- Armand, P.; Janssens, A.; Gritti, G.; Radford, J.; Timmerman, J.; Pinto, A.; Mercadal Vilchez, S.; Johnson, P.; Cunningham, D.; Leonard, J.P. Efficacy and safety results from CheckMate 140, a phase 2 study of nivolumab for relapsed/refractory follicular lymphoma. Blood J. Am. Soc. Hematol. 2021, 137, 637–645. [Google Scholar]
- Nowakowski, G.S.; Willenbacher, W.; Greil, R.; Larsen, T.S.; Patel, K.; Jäger, U.; Manges, R.F.; Trumper, L.H.; Haioun, C.; Everaus, H. Safety and efficacy of PD-L1 inhibitor durvalumab with R-CHOP or R2-CHOP in subjects with previously untreated, high-risk DLBCL. In Proceedings of the 2019 ASCO Annual Meeting, Chicago, IL, USA, 31 May–4 June 2019. [Google Scholar]
- Zinzani, P.L.; Thieblemont, C.; Melnichenko, V.; Bouabdallah, K.; Walewski, J.; Majlis, A.; Fogliatto, L.; Garcia-Sancho, A.M.; Christian, B.; Gulbas, Z. Pembrolizumab in relapsed or refractory primary mediastinal large B-cell lymphoma: Final analysis of KEYNOTE-170. Blood J. Am. Soc. Hematol. 2023, 142, 141–145. [Google Scholar]
- Zinzani, P.L.; Santoro, A.; Gritti, G.; Brice, P.; Barr, P.M.; Kuruvilla, J.; Cunningham, D.; Kline, J.; Johnson, N.A.; Mehta-Shah, N. Nivolumab combined with brentuximab vedotin for relapsed/refractory primary mediastinal large B-cell lymphoma: Efficacy and safety from the phase II CheckMate 436 study. J. Clin. Oncol. 2019, 37, 3081–3089. [Google Scholar]
- Mehta, A.; Popplewell, L.; Collins, G.P.; Smith, S.M.; Flinn, I.W.; Bartlett, N.L.; Ghosh, N.; Hacohen-Kleiman, G.; Huo, Y.; Su-Feher, L. Magrolimab plus rituximab in relapsed/refractory indolent non-Hodgkin lymphoma: 3-year follow-up of a phase 1/2 trial. Blood Adv. 2024, 8, 5855–5863. [Google Scholar] [PubMed]
- Patel, K.; Zonder, J.A.; Sano, D.; Maris, M.; Lesokhin, A.; von Keudell, G.; Lai, C.; Ramchandren, R.; Catalano, T.; Lin, G.H. CD47-blocker TTI-622 shows single-agent activity in patients with advanced relapsed or refractory lymphoma: Update from the ongoing first-in-human dose escalation study. Blood 2021, 138, 3560. [Google Scholar]
- Kiyasu, J.; Miyoshi, H.; Hirata, A.; Arakawa, F.; Ichikawa, A.; Niino, D.; Sugita, Y.; Yufu, Y.; Choi, I.; Abe, Y. Expression of programmed cell death ligand 1 is associated with poor overall survival in patients with diffuse large B-cell lymphoma. Blood J. Am. Soc. Hematol. 2015, 126, 2193–2201. [Google Scholar]
- Lesokhin, A.M.; Ansell, S.M.; Armand, P.; Scott, E.C.; Halwani, A.; Gutierrez, M.; Millenson, M.M.; Cohen, A.D.; Schuster, S.J.; Lebovic, D. Nivolumab in patients with relapsed or refractory hematologic malignancy: Preliminary results of a phase Ib study. J. Clin. Oncol. 2016, 34, 2698–2704. [Google Scholar] [PubMed]
- Nayak, L.; Iwamoto, F.M.; LaCasce, A.; Mukundan, S.; Roemer, M.G.; Chapuy, B.; Armand, P.; Rodig, S.J.; Shipp, M.A. PD-1 blockade with nivolumab in relapsed/refractory primary central nervous system and testicular lymphoma. Blood J. Am. Soc. Hematol. 2017, 129, 3071–3073. [Google Scholar]
- Kim, S.J.; Lim, J.Q.; Laurensia, Y.; Cho, J.; Yoon, S.E.; Lee, J.Y.; Ryu, K.J.; Ko, Y.H.; Koh, Y.; Cho, D. Avelumab for the treatment of relapsed or refractory extranodal NK/T-cell lymphoma: An open-label phase 2 study. Blood J. Am. Soc. Hematol. 2020, 136, 2754–2763. [Google Scholar]
- Tao, R.; Fan, L.; Song, Y.; Hu, Y.; Zhang, W.; Wang, Y.; Xu, W.; Li, J. Sintilimab for relapsed/refractory extranodal NK/T cell lymphoma: A multicenter, single-arm, phase 2 trial (ORIENT-4). Signal Transduct. Target. Ther. 2021, 6, 365. [Google Scholar]
- Morschhauser, F.; Ghosh, N.; Lossos, I.S.; Palomba, M.L.; Mehta, A.; Casasnovas, O.; Stevens, D.; Katakam, S.; Knapp, A.; Nielsen, T. Obinutuzumab-atezolizumab-lenalidomide for the treatment of patients with relapsed/refractory follicular lymphoma: Final analysis of a Phase Ib/II trial. Blood Cancer J. 2021, 11, 147. [Google Scholar]
- Hirayama, A.V.; Gauthier, J.; Hay, K.A.; Sheih, A.; Cherian, S.; Chen, X.; Pender, B.S.; Hawkins, R.M.; Vakil, A.; Steinmetz, R.N. Efficacy and toxicity of JCAR014 in combination with durvalumab for the treatment of patients with relapsed/refractory aggressive B-cell non-Hodgkin lymphoma. Blood 2018, 132, 1680. [Google Scholar]
- Jäger, U.; Worel, N.; McGuirk, J.; Riedell, P.A.; Fleury, I.; Borchmann, P.; Du, Y.; Abdelhady, A.M.; Han, X.; Martinez-Prieto, M. Safety and efficacy of tisagenlecleucel (tisa-cel) plus pembrolizumab (pembro) in patients (pts) with relapsed/refractory diffuse large B-cell lymphoma (r/r DLBCL): Updated analysis of the phase 1b PORTIA study. In Proceedings of the 2021 ASH Annual Meeting, Atlanta, GA, USA, 11–14 December 2021. [Google Scholar]
- Jacobson, C.A.; Westin, J.R.; Miklos, D.B.; Herrera, A.F.; Lee, J.; Seng, J.; Rossi, J.M.; Sun, J.; Dong, J.; Roberts, Z.J. Abstract CT055: Phase 1/2 primary analysis of ZUMA-6: Axicabtagene ciloleucel (Axi-Cel) in combination with atezolizumab (Atezo) for the treatment of patients (Pts) with refractory diffuse large B cell lymphoma (DLBCL). Cancer Res. 2020, 80, CT055. [Google Scholar]
- Chong, E.A.; Svoboda, J.; Nasta, S.D.; Landsburg, D.J.; Winchell, N.; Napier, E.; Mato, A.R.; Melenhorst, J.J.; Ruella, M.; Lacey, S.F. Sequential anti-CD19 directed chimeric antigen receptor modified T-cell therapy (CART19) and PD-1 blockade with pembrolizumab in patients with relapsed or refractory B-cell non-Hodgkin lymphomas. Blood 2018, 132, 4198. [Google Scholar]
- Osborne, W.; Marzolini, M.; Tholouli, E.; Ramakrishnan, A.; Bachier, C.R.; McSweeney, P.A.; Irvine, D.; Zhang, M.; Al-Hajj, M.A.; Pule, M. Phase I Alexander study of AUTO3, the first CD19/22 dual targeting CAR T cell therapy, with pembrolizumab in patients with relapsed/refractory (r/r) DLBCL. In Proceedings of the 2020 ASCO Annual Meeting, Virtual Meeting, 29–31 May 2020. [Google Scholar]
- Ansell, S.; Gutierrez, M.E.; Shipp, M.A.; Gladstone, D.; Moskowitz, A.; Borello, I.; Popa-Mckiver, M.; Farsaci, B.; Zhu, L.; Lesokhin, A.M. A phase 1 study of nivolumab in combination with ipilimumab for relapsed or refractory hematologic malignancies (CheckMate 039). Blood 2016, 128, 183. [Google Scholar]
- Witzig, T.E.; Maddocks, K.J.; De Vos, S.; Lyons, R.M.; Edenfield, W.J.; Sharman, J.P.; Vose, J.; Yimer, H.A.; Wei, H.; Chan, E.M. Phase 1/2 trial of acalabrutinib plus pembrolizumab (Pem) in relapsed/refractory (r/r) diffuse large B-cell lymphoma (DLBCL). In Proceedings of the 2019 ASH Annual Meeting, Orlando, FL, USA, 7–10 December 2019. [Google Scholar]
- Casulo, C.; Santoro, A.; Cartron, G.; Ando, K.; Munoz, J.; Le Gouill, S.; Izutsu, K.; Rule, S.; Lugtenburg, P.; Ruan, J. Durvalumab as monotherapy and in combination therapy in patients with lymphoma or chronic lymphocytic leukemia: The FUSION NHL 001 trial. Cancer Rep. 2023, 6, e1662. [Google Scholar]
- Gregory, G.P.; Kumar, S.; Wang, D.; Mahadevan, D.; Walker, P.; Wagner-Johnston, N.; Escobar, C.; Bannerji, R.; Bhutani, D.; Chang, J. Pembrolizumab plus dinaciclib in patients with hematologic malignancies: The phase 1b KEYNOTE-155 study. Blood Adv. 2022, 6, 1232–1242. [Google Scholar] [PubMed]
- Herrera, A.F.; Goy, A.; Mehta, A.; Ramchandren, R.; Pagel, J.M.; Svoboda, J.; Guan, S.; Hill, J.S.; Kwei, K.; Liu, E.A. Safety and activity of ibrutinib in combination with durvalumab in patients with relapsed or refractory follicular lymphoma or diffuse large B-cell lymphoma. Am. J. Hematol. 2020, 95, 18–27. [Google Scholar] [PubMed]
- Younes, A.; Brody, J.; Carpio, C.; Lopez-Guillermo, A.; Ben-Yehuda, D.; Ferhanoglu, B.; Nagler, A.; Ozcan, M.; Avivi, I.; Bosch, F. Safety and activity of ibrutinib in combination with nivolumab in patients with relapsed non-Hodgkin lymphoma or chronic lymphocytic leukaemia: A phase 1/2a study. Lancet Haematol. 2019, 6, e67–e78. [Google Scholar]
- Reinhardt, C.; Ochsenbein, A.F. Immune checkpoints regulate acute myeloid leukemia stem cells. Leukemia 2025, 39, 1277–1293. [Google Scholar]
- Radpour, R.; Riether, C.; Simillion, C.; Höpner, S.; Bruggmann, R.; Ochsenbein, A.F. CD8+ T cells expand stem and progenitor cells in favorable but not adverse risk acute myeloid leukemia. Leukemia 2019, 33, 2379–2392. [Google Scholar]
- Radpour, R.; Stucki, M.; Riether, C.; Ochsenbein, A.F. Epigenetic silencing of immune-checkpoint receptors in bone marrow-infiltrating T cells in acute myeloid leukemia. Front. Oncol. 2021, 11, 663406. [Google Scholar]
- Soltani, M.; Vosoughi, M.; Ganjalikhani-Hakemi, M.; Shapoorian, H.; Beshkar, P.; Eskandari, N.; Ghezelbash, B. PD-1/PD-L1 Interaction Regulates BCL2, KI67, BAX, and CASP3, Altering Proliferation, Survival, and Apoptosis in Acute Myeloid Leukemia. Iran. J. Allergy Asthma Immunol. 2023, 22. [Google Scholar]
- Soltani, M.; Ghanadian, M.; Ghezelbash, B.; Shokouhi, A.; Zamyatnin Jr, A.A.; Bazhin, A.V.; Ganjalikhani-Hakemi, M. PD-L1 stimulation can promote proliferation and survival of leukemic cells by influencing glucose and fatty acid metabolism in acute myeloid leukemia. BMC Cancer 2023, 23, 447. [Google Scholar]
- Ma, P.; Xing, M.; Han, L.; Gan, S.; Ma, J.; Wu, F.; Huang, Y.; Chen, Y.; Tian, W.; An, C. High PD-L1 expression drives glycolysis via an Akt/mTOR/HIF-1α axis in acute myeloid leukemia. Oncol. Rep. 2020, 43, 999–1009. [Google Scholar] [PubMed]
- Kikushige, Y.; Miyamoto, T.; Yuda, J.; Jabbarzadeh-Tabrizi, S.; Shima, T.; Takayanagi, S.-i.; Niiro, H.; Yurino, A.; Miyawaki, K.; Takenaka, K. A TIM-3/Gal-9 autocrine stimulatory loop drives self-renewal of human myeloid leukemia stem cells and leukemic progression. Cell Stem Cell 2015, 17, 341–352. [Google Scholar]
- Wang, Z.; Chen, J.; Wang, M.; Zhang, L.; Yu, L. One stone, two birds: The roles of Tim-3 in acute myeloid leukemia. Front. Immunol. 2021, 12, 618710. [Google Scholar]
- Pophali, P.; Varela, J.C.; Rosenblatt, J. Immune checkpoint blockade in hematological malignancies: Current state and future potential. Front. Oncol. 2024, 14, 1323914. [Google Scholar]
- Berger, R.; Rotem-Yehudar, R.; Slama, G.; Landes, S.; Kneller, A.; Leiba, M.; Koren-Michowitz, M.; Shimoni, A.; Nagler, A. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin. Cancer Res. 2008, 14, 3044–3051. [Google Scholar]
- Garcia-Manero, G.; Tallman, M.S.; Martinelli, G.; Ribrag, V.; Yang, H.; Balakumaran, A.; Chlosta, S.; Zhang, Y.; Smith, B.D. Pembrolizumab, a PD-1 inhibitor, in patients with myelodysplastic syndrome (MDS) after failure of hypomethylating agent treatment. Blood 2016, 128, 345. [Google Scholar]
- Zeidan, A.M.; Knaus, H.A.; Robinson, T.M.; Towlerton, A.M.; Warren, E.H.; Zeidner, J.F.; Blackford, A.L.; Duffield, A.S.; Rizzieri, D.; Frattini, M.G. A multi-center phase I trial of ipilimumab in patients with myelodysplastic syndromes following hypomethylating agent failure. Clin. Cancer Res. 2018, 24, 3519–3527. [Google Scholar]
- Davids, M.S.; Kim, H.T.; Bachireddy, P.; Costello, C.; Liguori, R.; Savell, A.; Lukez, A.P.; Avigan, D.; Chen, Y.-B.; McSweeney, P. Ipilimumab for patients with relapse after allogeneic transplantation. N. Engl. J. Med. 2016, 375, 143–153. [Google Scholar]
- Garcia, J.S.; Flamand, Y.; Penter, L.; Keng, M.; Tomlinson, B.K.; Mendez, L.M.; Koller, P.; Cullen, N.; Arihara, Y.; Pfaff, K. Ipilimumab plus decitabine for patients with MDS or AML in posttransplant or transplant-naïve settings. Blood 2023, 141, 1884–1888. [Google Scholar]
- Zeidner, J.F.; Vincent, B.G.; Ivanova, A.; Moore, D.; McKinnon, K.P.; Wilkinson, A.D.; Mukhopadhyay, R.; Mazziotta, F.; Knaus, H.A.; Foster, M.C. Phase II trial of pembrolizumab after high-dose cytarabine in relapsed/refractory acute myeloid leukemia. Blood Cancer Discov. 2021, 2, 616–629. [Google Scholar]
- Chien, K.S.; Borthakur, G.; Naqvi, K.; Daver, N.; Bravo, G.M.; Cortes, J.E.; DiNardo, C.D.; Jabbour, E.; Alvarado, Y.; Andreeff, M. Updated results from a phase II study combining azacitidine and pembrolizumab in previously-untreated patients with higher-risk myelodysplastic syndrome. Blood 2020, 136, 38–39. [Google Scholar]
- Daver, N.G.; Basu, S.; Garcia-Manero, G.; Cortes, J.E.; Ravandi, F.; Jabbour, E.; Hendrickson, S.; Brandt, M.; Pierce, S.; Gordon, T. Phase IB/II study of nivolumab with azacytidine (AZA) in patients (pts) with relapsed AML. In Proceedings of the 2017 ASH Annual Meeting, Atlanta, GA, USA, 9–12 December 2017. [Google Scholar]
- Brunner, A.M.; Esteve, J.; Porkka, K.; Knapper, S.; Traer, E.; Scholl, S.; Garcia-Manero, G.; Vey, N.; Wermke, M.; Janssen, J. Efficacy and safety of sabatolimab (MBG453) in combination with hypomethylating agents (HMAs) in patients (Pts) with very high/high-risk myelodysplastic syndrome (vHR/HR-MDS) and acute myeloid leukemia (AML): Final analysis from a phase Ib study. Blood 2021, 138, 244. [Google Scholar]
- Sallman, D.A.; Al Malki, M.M.; Asch, A.S.; Wang, E.S.; Jurcic, J.G.; Bradley, T.J.; Flinn, I.W.; Pollyea, D.A.; Kambhampati, S.; Tanaka, T.N. Magrolimab in combination with azacitidine in patients with higher-risk myelodysplastic syndromes: Final results of a phase Ib study. J. Clin. Oncol. 2023, 41, 2815–2826. [Google Scholar] [PubMed]
- Gojo, I.; Stuart, R.K.; Webster, J.; Blackford, A.; Varela, J.C.; Morrow, J.; DeZern, A.E.; Foster, M.C.; Levis, M.J.; Coombs, C.C. Multi-center phase 2 study of pembroluzimab (Pembro) and azacitidine (AZA) in patients with relapsed/refractory acute myeloid leukemia (AML) and in newly diagnosed (≥ 65 years) AML patients. Blood 2019, 134, 832. [Google Scholar]
- Liu, Z.; Xu, X.; Liu, H.; Zhao, X.; Yang, C.; Fu, R. Immune checkpoint inhibitors for multiple myeloma immunotherapy. Exp. Hematol. Oncol. 2023, 12, 99. [Google Scholar]
- Liu, Z.; Mi, F.; Han, M.; Tian, M.; Deng, L.; Meng, N.; Luo, J.; Fu, R. Bone marrow-derived mesenchymal stem cells inhibit CD8+ T cell immune responses via PD-1/PD-L1 pathway in multiple myeloma. Clin. Exp. Immunol. 2021, 205, 53–62. [Google Scholar]
- Huang, S.; Zhao, Y.; Liao, P.; Wang, J.; Li, Z.; Tan, J.; Zha, X.; Chen, S.; Li, Y.; Zhong, L. Different expression patterns of VISTA concurrent with PD-1, Tim-3, and TIGIT on T cell subsets in peripheral blood and bone marrow from patients with multiple myeloma. Front. Oncol. 2022, 12, 1014904. [Google Scholar]
- Shayan, G.; Srivastava, R.; Li, J.; Schmitt, N.; Kane, L.P.; Ferris, R.L. Adaptive resistance to anti-PD1 therapy by Tim-3 upregulation is mediated by the PI3K-Akt pathway in head and neck cancer. Oncoimmunology 2017, 6, e1261779. [Google Scholar] [PubMed]
- Karabon, L.; Pawlak-Adamska, E.; Tomkiewicz, A.; Jedynak, A.; Kielbinski, M.; Woszczyk, D.; Potoczek, S.; Jonkisz, A.; Kuliczkowski, K.; Frydecka, I. Variations in suppressor molecule ctla-4 gene are related to susceptibility to multiple myeloma in a polish population. Pathol. Oncol. Res. 2012, 18, 219–226. [Google Scholar] [PubMed]
- Zheng, C.; Huang, D.; Liu, L.; Björkholm, M.; Holm, G.; Yi, Q.; Sundblad, A. Cytotoxic T-lymphocyte antigen-4 microsatellite polymorphism is associated with multiple myeloma. Br. J. Haematol. 2001, 112, 216–218. [Google Scholar] [PubMed]
- Liu, J.; Hamrouni, A.; Wolowiec, D.; Coiteux, V.; Kuliczkowski, K.; Hetuin, D.; Saudemont, A.; Quesnel, B. Plasma cells from multiple myeloma patients express B7-H1 (PD-L1) and increase expression after stimulation with IFN-γ and TLR ligands via a MyD88-, TRAF6-, and MEK-dependent pathway. Blood J. Am. Soc. Hematol. 2007, 110, 296–304. [Google Scholar]
- Braga, W.M.T.; Vettore, A.L.; Carvalho, A.C.; Atanackovic, D.; Colleoni, G.W. Overexpression of CTLA-4 in the bone marrow of patients with multiple myeloma as a sign of local accumulation of immunosuppressive tregs–perspectives for novel treatment strategies. Blood 2011, 118, 1829. [Google Scholar]
- Dhodapkar, M.V.; Sexton, R.; Das, R.; Dhodapkar, K.M.; Zhang, L.; Sundaram, R.; Soni, S.; Crowley, J.J.; Orlowski, R.Z.; Barlogie, B. Prospective analysis of antigen-specific immunity, stem-cell antigens, and immune checkpoints in monoclonal gammopathy. Blood J. Am. Soc. Hematol. 2015, 126, 2475–2478. [Google Scholar]
- Usmani, S.Z.; Schjesvold, F.; Oriol, A.; Karlin, L.; Cavo, M.; Rifkin, R.M.; Yimer, H.A.; LeBlanc, R.; Takezako, N.; McCroskey, R.D. Pembrolizumab plus lenalidomide and dexamethasone for patients with treatment-naive multiple myeloma (KEYNOTE-185): A randomised, open-label, phase 3 trial. Lancet Haematol. 2019, 6, e448–e458. [Google Scholar]
- Ribrag, V.; Avigan, D.E.; Green, D.J.; Wise-Draper, T.; Posada, J.G.; Vij, R.; Zhu, Y.; Farooqui, M.Z.; Marinello, P.; Siegel, D.S. Phase 1b trial of pembrolizumab monotherapy for relapsed/refractory multiple myeloma: KEYNOTE-013. Br. J. Haematol. 2019, 186, e41–e44. [Google Scholar]
- Cho, H.J.; Richard, S.; Lesokhin, A.; Biran, N.; Paul, B.; Vij, R.; Pianko, M.; Doxie, D.; Martillo, M.; Wozniak, K. Abstract CT262: Durable responses following anti-TIGIT (BMS-986207) and anti-LAG3 (BMS-980616) in combination with pomalidomide in relapsed myeloma: MMRF MyCheckpoint trial. Cancer Res. 2023, 83, CT262. [Google Scholar]
- Nakamura, K.; Smyth, M.J.; Martinet, L. Cancer immunoediting and immune dysregulation in multiple myeloma. Blood J. Am. Soc. Hematol. 2020, 136, 2731–2740. [Google Scholar]
- Zhao, Y.; Shao, Q.; Peng, G. Exhaustion and senescence: Two crucial dysfunctional states of T cells in the tumor microenvironment. Cell. Mol. Immunol. 2020, 17, 27–35. [Google Scholar] [PubMed]
- Bailur, J.K.; McCachren, S.S.; Doxie, D.B.; Shrestha, M.; Pendleton, K.; Nooka, A.K.; Neparidze, N.; Parker, T.L.; Bar, N.; Kaufman, J.L. Early alterations in stem-like/marrow-resident T cells and innate and myeloid cells in preneoplastic gammopathy. JCI Insight 2019, 4, e127807. [Google Scholar]
- Chung, D.J.; Pronschinske, K.B.; Shyer, J.A.; Sharma, S.; Leung, S.; Curran, S.A.; Lesokhin, A.M.; Devlin, S.M.; Giralt, S.A.; Young, J.W. T-cell exhaustion in multiple myeloma relapse after autotransplant: Optimal timing of immunotherapy. Cancer Immunol. Res. 2016, 4, 61–71. [Google Scholar]
- Nakamura, K.; Kassem, S.; Cleynen, A.; Chretien, M.-L.; Guillerey, C.; Putz, E.M.; Bald, T.; Foerster, I.; Vuckovic, S.; Hill, G.R. Dysregulated IL-18 is a key driver of immunosuppression and a possible therapeutic target in the multiple myeloma microenvironment. Cancer Cell 2018, 33, 634–648.e5. [Google Scholar] [PubMed]
- Bae, J.; Accardi, F.; Hideshima, T.; Tai, Y.-T.; Prabhala, R.; Shambley, A.; Wen, K.; Rowell, S.; Richardson, P.G.; Munshi, N.C. Targeting LAG3/GAL-3 to overcome immunosuppression and enhance anti-tumor immune responses in multiple myeloma. Leukemia 2022, 36, 138–154. [Google Scholar]
- Cristescu, R.; Mogg, R.; Ayers, M.; Albright, A.; Murphy, E.; Yearley, J.; Sher, X.; Liu, X.Q.; Lu, H.; Nebozhyn, M. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade–based immunotherapy. Science 2018, 362, eaar3593. [Google Scholar]
- Galanina, N.; Bejar, R.; Choi, M.; Goodman, A.; Wieduwilt, M.; Mulroney, C.; Kim, L.; Yeerna, H.; Tamayo, P.; Vergilio, J.-A. Comprehensive genomic profiling reveals diverse but actionable molecular portfolios across hematologic malignancies: Implications for next generation clinical trials. Cancers 2018, 11, 11. [Google Scholar] [CrossRef]
- Anagnostou, V.; Smith, K.N.; Forde, P.M.; Niknafs, N.; Bhattacharya, R.; White, J.; Zhang, T.; Adleff, V.; Phallen, J.; Wali, N. Evolution of neoantigen landscape during immune checkpoint blockade in non–small cell lung cancer. Cancer Discov. 2017, 7, 264–276. [Google Scholar]
- Pereira, C.; Gimenez-Xavier, P.; Pros, E.; Pajares, M.J.; Moro, M.; Gomez, A.; Navarro, A.; Condom, E.; Moran, S.; Gomez-Lopez, G. Genomic profiling of patient-derived xenografts for lung cancer identifies B2M inactivation impairing immunorecognition. Clin. Cancer Res. 2017, 23, 3203–3213. [Google Scholar]
- Zaretsky, J.M.; Garcia-Diaz, A.; Shin, D.S.; Escuin-Ordinas, H.; Hugo, W.; Hu-Lieskovan, S.; Torrejon, D.Y.; Abril-Rodriguez, G.; Sandoval, S.; Barthly, L. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N. Engl. J. Med. 2016, 375, 819–829. [Google Scholar] [PubMed]
- Haddad, A.F.; Young, J.S.; Gill, S.; Aghi, M.K. Resistance to immune checkpoint blockade: Mechanisms, counter-acting approaches, and future directions. Semin. Cancer Biol. 2022, 86, 532–541. [Google Scholar]
- Benci, J.L.; Xu, B.; Qiu, Y.; Wu, T.J.; Dada, H.; Twyman-Saint Victor, C.; Cucolo, L.; Lee, D.S.; Pauken, K.E.; Huang, A.C. Tumor interferon signaling regulates a multigenic resistance program to immune checkpoint blockade. Cell 2016, 167, 1540–1554.e12. [Google Scholar]
- Yu, M.; Peng, Z.; Qin, M.; Liu, Y.; Wang, J.; Zhang, C.; Lin, J.; Dong, T.; Wang, L.; Li, S. Interferon-γ induces tumor resistance to anti-PD-1 immunotherapy by promoting YAP phase separation. Mol. Cell 2021, 81, 1216–1230.e9. [Google Scholar] [PubMed]
- Shin, D.S.; Zaretsky, J.M.; Escuin-Ordinas, H.; Garcia-Diaz, A.; Hu-Lieskovan, S.; Kalbasi, A.; Grasso, C.S.; Hugo, W.; Sandoval, S.; Torrejon, D.Y. Primary resistance to PD-1 blockade mediated by JAK1/2 mutations. Cancer Discov. 2017, 7, 188–201. [Google Scholar] [PubMed]
- Restifo, N.P.; Marincola, F.M.; Kawakami, Y.; Taubenberger, J.; Yannelli, J.R.; Rosenberg, S.A. Loss of functional beta2-microglobulin in metastatic melanomas from five patients receiving immunotherapy. JNCI J. Natl. Cancer Inst. 1996, 88, 100–108. [Google Scholar]
- Peng, D.; Kryczek, I.; Nagarsheth, N.; Zhao, L.; Wei, S.; Wang, W.; Sun, Y.; Zhao, E.; Vatan, L.; Szeliga, W. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature 2015, 527, 249–253. [Google Scholar]
- Burr, M.L.; Sparbier, C.E.; Chan, K.L.; Chan, Y.-C.; Kersbergen, A.; Lam, E.Y.; Azidis-Yates, E.; Vassiliadis, D.; Bell, C.C.; Gilan, O. An evolutionarily conserved function of polycomb silences the MHC class I antigen presentation pathway and enables immune evasion in cancer. Cancer Cell 2019, 36, 385–401.e8. [Google Scholar]
- Yoshihama, S.; Roszik, J.; Downs, I.; Meissner, T.B.; Vijayan, S.; Chapuy, B.; Sidiq, T.; Shipp, M.A.; Lizee, G.A.; Kobayashi, K.S. NLRC5/MHC class I transactivator is a target for immune evasion in cancer. Proc. Natl. Acad. Sci. USA 2016, 113, 5999–6004. [Google Scholar]
- Gao, Y.; Nihira, N.T.; Bu, X.; Chu, C.; Zhang, J.; Kolodziejczyk, A.; Fan, Y.; Chan, N.T.; Ma, L.; Liu, J. Acetylation-dependent regulation of PD-L1 nuclear translocation dictates the efficacy of anti-PD-1 immunotherapy. Nat. Cell Biol. 2020, 22, 1064–1075. [Google Scholar]
- Alsaafeen, B.H.; Ali, B.R.; Elkord, E. Resistance mechanisms to immune checkpoint inhibitors: Updated insights. Mol. Cancer 2025, 24, 20. [Google Scholar] [PubMed]
- Peranzoni, E.; Lemoine, J.; Vimeux, L.; Feuillet, V.; Barrin, S.; Kantari-Mimoun, C.; Bercovici, N.; Guérin, M.; Biton, J.; Ouakrim, H. Macrophages impede CD8 T cells from reaching tumor cells and limit the efficacy of anti–PD-1 treatment. Proc. Natl. Acad. Sci. USA 2018, 115, E4041–E4050. [Google Scholar]
- Arlauckas, S.P.; Garris, C.S.; Kohler, R.H.; Kitaoka, M.; Cuccarese, M.F.; Yang, K.S.; Miller, M.A.; Carlson, J.C.; Freeman, G.J.; Anthony, R.M. In vivo imaging reveals a tumor-associated macrophage–mediated resistance pathway in anti–PD-1 therapy. Sci. Transl. Med. 2017, 9, eaal3604. [Google Scholar]
- Huang, R.-Y.; Francois, A.; McGray, A.R.; Miliotto, A.; Odunsi, K. Compensatory upregulation of PD-1, LAG-3, and CTLA-4 limits the efficacy of single-agent checkpoint blockade in metastatic ovarian cancer. Oncoimmunology 2017, 6, e1249561. [Google Scholar]
- Fourcade, J.; Sun, Z.; Chauvin, J.-M.; Ka, M.; Davar, D.; Pagliano, O.; Wang, H.; Saada, S.; Menna, C.; Amin, R. CD226 opposes TIGIT to disrupt Tregs in melanoma. JCI Insight 2018, 3, e121157. [Google Scholar]
- Chauvin, J.-M.; Pagliano, O.; Fourcade, J.; Sun, Z.; Wang, H.; Sander, C.; Kirkwood, J.M.; Chen, T.-h.T.; Maurer, M.; Korman, A.J. TIGIT and PD-1 impair tumor antigen–specific CD8+ T cells in melanoma patients. J. Clin. Investig. 2015, 125, 2046–2058. [Google Scholar]
- Almawash, S. Revolutionary Cancer Therapy for Personalization and Improved Efficacy: Strategies to Overcome Resistance to Immune Checkpoint Inhibitor Therapy. Cancers 2025, 17, 880. [Google Scholar] [CrossRef] [PubMed]
- Alsaafeen, B.H.; Ali, B.R.; Elkord, E. Combinational therapeutic strategies to overcome resistance to immune checkpoint inhibitors. Front. Immunol. 2025, 16, 1546717. [Google Scholar]
- Xiao, Y.; Li, Z.-Z.; Zhong, N.-N.; Cao, L.-M.; Liu, B.; Bu, L.-L. Charting new frontiers: Co-inhibitory immune checkpoint proteins in therapeutics, biomarkers, and drug delivery systems in cancer care. Transl. Oncol. 2023, 38, 101794. [Google Scholar]
- Wojtukiewicz, M.Z.; Rek, M.M.; Karpowicz, K.; Górska, M.; Polityńska, B.; Wojtukiewicz, A.M.; Moniuszko, M.; Radziwon, P.; Tucker, S.C.; Honn, K.V. Inhibitors of immune checkpoints—PD-1, PD-L1, CTLA-4—New opportunities for cancer patients and a new challenge for internists and general practitioners. Cancer Metastasis Rev. 2021, 40, 949–982. [Google Scholar]
- Dhasmana, A.; Dhasmana, S.; Haque, S.; Cobos, E.; Yallapu, M.M.; Chauhan, S.C. Next-generation immune checkpoint inhibitors as promising functional molecules in cancer therapeutics. Cancer Metastasis Rev. 2023, 42, 597–600. [Google Scholar]
- Lee, J.B.; Kim, H.R.; Ha, S.-J. Immune checkpoint inhibitors in 10 years: Contribution of basic research and clinical application in cancer immunotherapy. Immune Netw. 2022, 22, e2. [Google Scholar] [PubMed]
- Luo, N.; Nixon, M.J.; Gonzalez-Ericsson, P.I.; Sanchez, V.; Opalenik, S.R.; Li, H.; Zahnow, C.A.; Nickels, M.L.; Liu, F.; Tantawy, M.N. DNA methyltransferase inhibition upregulates MHC-I to potentiate cytotoxic T lymphocyte responses in breast cancer. Nat. Commun. 2018, 9, 248. [Google Scholar] [PubMed]
- Magner, W.J.; Kazim, A.L.; Stewart, C.; Romano, M.A.; Catalano, G.; Grande, C.; Keiser, N.; Santaniello, F.; Tomasi, T.B. Activation of MHC class I, II, and CD40 gene expression by histone deacetylase inhibitors. J. Immunol. 2000, 165, 7017–7024. [Google Scholar] [PubMed]
- Rossi, L.E.; Avila, D.E.; Spallanzani, R.G.; Ziblat, A.; Fuertes, M.B.; Lapyckyj, L.; Croci, D.O.; Rabinovich, G.A.; Domaica, C.I.; Zwirner, N.W. Histone deacetylase inhibitors impair NK cell viability and effector functions through inhibition of activation and receptor expression. J. Leukoc. Biol. 2012, 91, 321–331. [Google Scholar]
- Shi, X.; Li, M.; Cui, M.; Niu, C.; Xu, J.; Zhou, L.; Li, W.; Gao, Y.; Kong, W.; Cui, J. Epigenetic suppression of the antitumor cytotoxicity of NK cells by histone deacetylase inhibitor valproic acid. Am. J. Cancer Res. 2016, 6, 600. [Google Scholar]



| Regimen | PFS, % | CR % | Reference |
|---|---|---|---|
| BV-nivolumab | 77 (3-years) | 67 | [118] |
| Nivolumab–BV ± BV–Bendamustine | 91 (1-year) | 59 (after induction) 94 (pre-consolidation) | [119] |
| nivolumab\nivolumab ± ICE | 72 (2-years) | 91 | [120] |
| Pembrolizumab-ICE | 87 (2-years) | 87 | [121] |
| Pembrolizumab-GVD | 96 (30-months) | 95 | [122] |
| Regimen | Population | Checkpoint Target | PFS | CR | Side Effects | Reference |
|---|---|---|---|---|---|---|
| CT-011 (0.2–6 mg/kg) | Advanced myeloid malignancies (subset) | PD-1 | - | 1 CR in total | Safe and well tolerated; no maximum tolerated dose reached; no major immune toxicities noted | [164] |
| Pembrolizumab (10 mg/kg q2w) | R/R MDS post-HMA failure (IPSS int-1 to high risk) | PD-1 | Not directly reported; 24-week OS: 49% | 0% | 36% had treatment-related AEs. Most frequent: hypothyroidism (14%), fatigue (11%). Grade 3/4 AEs in 7% (e.g., tumor lysis syndrome, gastroenteritis). No treatment-related deaths reported. | [165] |
| High-dose cytarabine → Pembrolizumab 200 mg IV on Day 14 (±maintenance) | R/R AML (n = 37) | PD-1 | Median OS: 11.1 months 13.2 months in R/R | CRc: 38% (composite CR) ORR: 46% | Grade ≥ 3 irAEs: 14% (rare and self-limited); generally tolerable and feasible in post-chemotherapy setting | [169] |
| Azacitidine (75 mg/m2 × 7 days/cycle) + Pembrolizumab (200 mg IV q21d) | Previously untreated, higher-risk MDS (n = 17) | PD-1 | OS not reached (median FU: 13.8 mo) | 3/17 (18%) CR Overall Response Rate: 80% | Most common AEs: arthralgia (40%), pneumonia (33%), nausea (27%), rash (27%); 1 early death unrelated to treatment | [170] |
| Azacitidine (75 mg/m2 Days 1–7) + Nivolumab (3 mg/kg Days 1 & 14) | Patients with relapsed AML (n = 53), median age 68; 43% secondary AML, 43% poor-risk cytogenetics | PD-1 | Median OS: 5.7 mo overall; 9.3 mo in salvage-1 subgroup | CR/CRi: 21% Overall Response Rate: 35% | Grade 3/4 immune AEs in 14%, Grade 2 AEs in 12%; most resolved with steroids; 12/13 rechallenged successfully | [171] |
| Sabatolimab + HMA | vHR/HR-MDS (n = 53) and ND-AML (n = 48) patients | TIM-3 | MDS: 12-mo PFS: 51.9% AML: 12-mo PFS: 27.9% | - | Gr ≥ 3 AEs similar to HMA alone: thrombocytopenia (~44%), neutropenia (~48%), anemia (~30%), febrile neutropenia (~33%); few immune AEs; no Gr ≥ 3 imAEs in MDS patients | [172] |
| Magrolimab + Azacitidine | Untreated high-risk MDS | CD47 | Median PFS: 11.6 months; Median OS: not reached (17.1 mo follow-up) | 33% | Most common: constipation (68%), thrombocytopenia (55%), anemia (52%). Manageable anemia (median Hb drop: −0.7 g/dL). No major immune-related AEs reported. | [173] |
| Ipilimumab + Decitabine | R/R or secondary MDS/AML (n = 48: 25 post-HSCT, 23 transplant-naïve) | CTLA-4 | No significant OS/PFS difference between arms; 1-year OS in transplant-naïve with irAE: 72.7% | 52% in transplant-naïve (5 CR, 2 CRi, 5 mCR ± HI); 20% in post-HSCT (4 CR, 1 mCR) | Grade ≥ 3 neutropenia (32–48%), thrombocytopenia (28–48%), febrile neutropenia (36–61%). irAEs in ~45%; GVHD in post-HSCT; dermatitis/colitis in transplant-naïve. | [168] |
| Regimen | Population | Checkpoint Target | PFS | CR | Side Effects | Reference |
|---|---|---|---|---|---|---|
| Nivolumab 1 or 3 mg/kg every 2 weeks | R/R MM (n = 27), median 3 prior therapies | PD-1 | - | 0% | 63% had treatment-related AEs (mostly grade 1–2); stable disease in 63%. No objective responses. Only 1/14 had PD-L1+ tumors. | [139] |
| Nivolumab (3 mg/kg) + Ipilimumab (1 mg/kg) Q3W × 4, then Nivolumab Q2W | R/R MM (n = 7), median 5 prior therapies | PD-1 + CTLA-4 | - | 0% | Common AEs: fatigue (26%), pyrexia (23%), diarrhea (18%). Grade ≥ 3 AEs in 29% of patients overall. 4 MM patients died (all from disease progression). No treatment-related deaths. | [149] |
| Pembrolizumab monotherapy (10 mg/kg Q2W or 200 mg Q3W) | 30 patients with R/R MM (median 4 prior therapies; 53% post-SCT; 67% lenalidomide-refractory) | PD-1 | Median PFS: 2.7 months | 0% (No CRs or PRs) | 40% had TRAEs; most common: asthenia (17%), arthralgia (7%). One grade 3 TRAE (myalgia). No grade 4/5 TRAEs. 1 patient discontinued due to AE. | [185] |
| Pembrolizumab + Pomalidomide + Dexamethasone | 125 patients with R/R MM (≥2 prior therapies, refractory to last line) | PD-1 | Median PFS: 5.6 months vs. 8.4 months in control arm | - | Serious AEs in 63% (vs. 46% control); 4 treatment-related deaths: myocarditis, Stevens-Johnson syndrome, neutropenic sepsis, unknown cause. Study halted due to safety | [16] |
| Anti-LAG-3 (BMS-980616) or Anti-TIGIT (BMS-986207) + pomalidomide | R/R MM after ≥3 lines including anti-CD38 mAb | LAG-3/TIGIT | - | 0% | Common AEs: anemia, dyspnea. Grade 3–4 AEs: dyspnea, neutropenia, thrombocytopenia. No autoimmune AEs. 1 pneumonia-related death (unrelated). | [186] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alibrahim, M.N.; Carbone, A.; Alsaleh, N.; Gloghini, A. Immune Checkpoint Molecules in Hodgkin Lymphoma and Other Hematological Malignancies. Cancers 2025, 17, 2292. https://doi.org/10.3390/cancers17142292
Alibrahim MN, Carbone A, Alsaleh N, Gloghini A. Immune Checkpoint Molecules in Hodgkin Lymphoma and Other Hematological Malignancies. Cancers. 2025; 17(14):2292. https://doi.org/10.3390/cancers17142292
Chicago/Turabian StyleAlibrahim, Mohamed Nazem, Antonino Carbone, Noor Alsaleh, and Annunziata Gloghini. 2025. "Immune Checkpoint Molecules in Hodgkin Lymphoma and Other Hematological Malignancies" Cancers 17, no. 14: 2292. https://doi.org/10.3390/cancers17142292
APA StyleAlibrahim, M. N., Carbone, A., Alsaleh, N., & Gloghini, A. (2025). Immune Checkpoint Molecules in Hodgkin Lymphoma and Other Hematological Malignancies. Cancers, 17(14), 2292. https://doi.org/10.3390/cancers17142292








