The Incidence, Mortality, and Survival Trends of Pancreatic Cancer in Kazakhstan: Data from the National Electronic Registry of Oncological Patients (2014–2023)
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design and Population
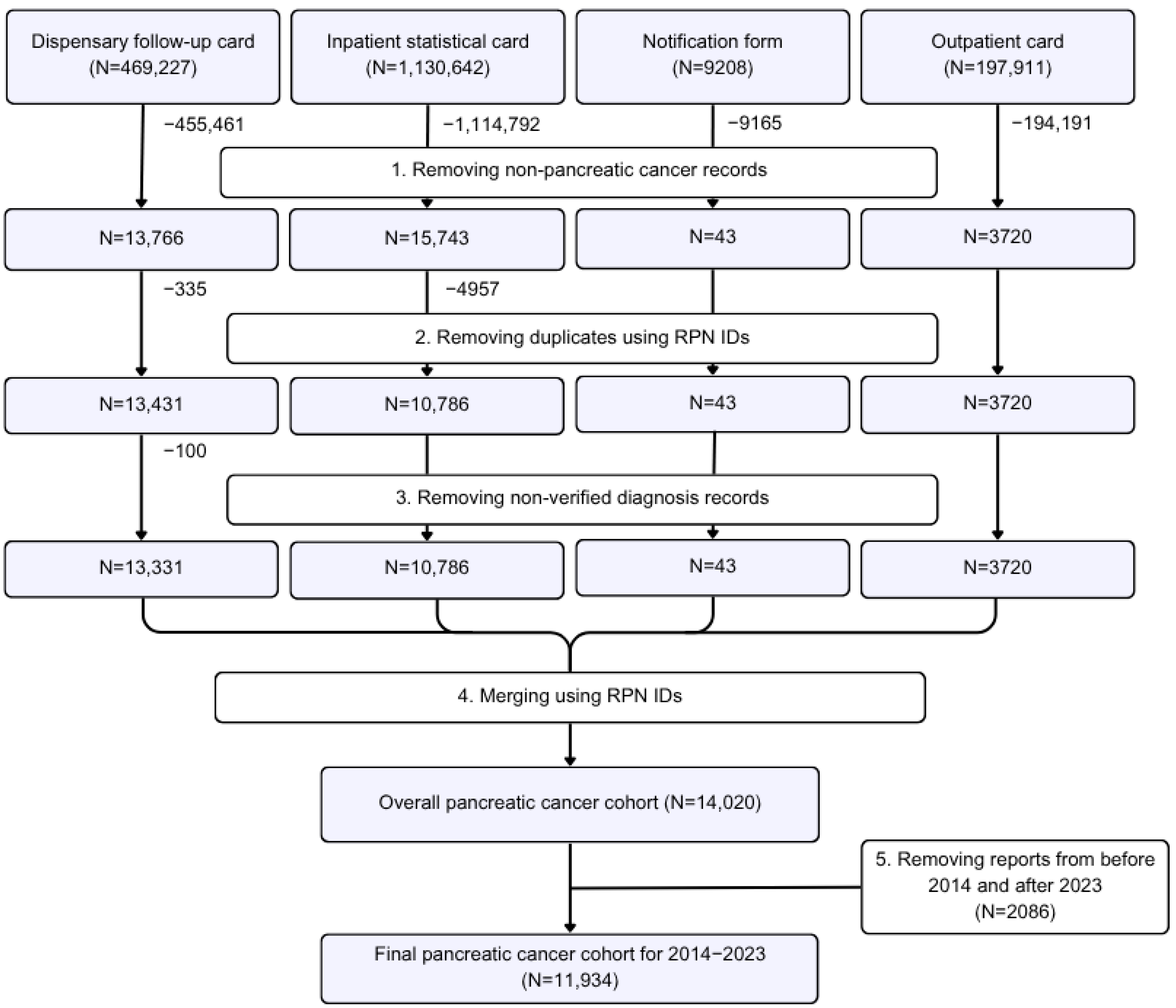
2.2. Study Sample
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
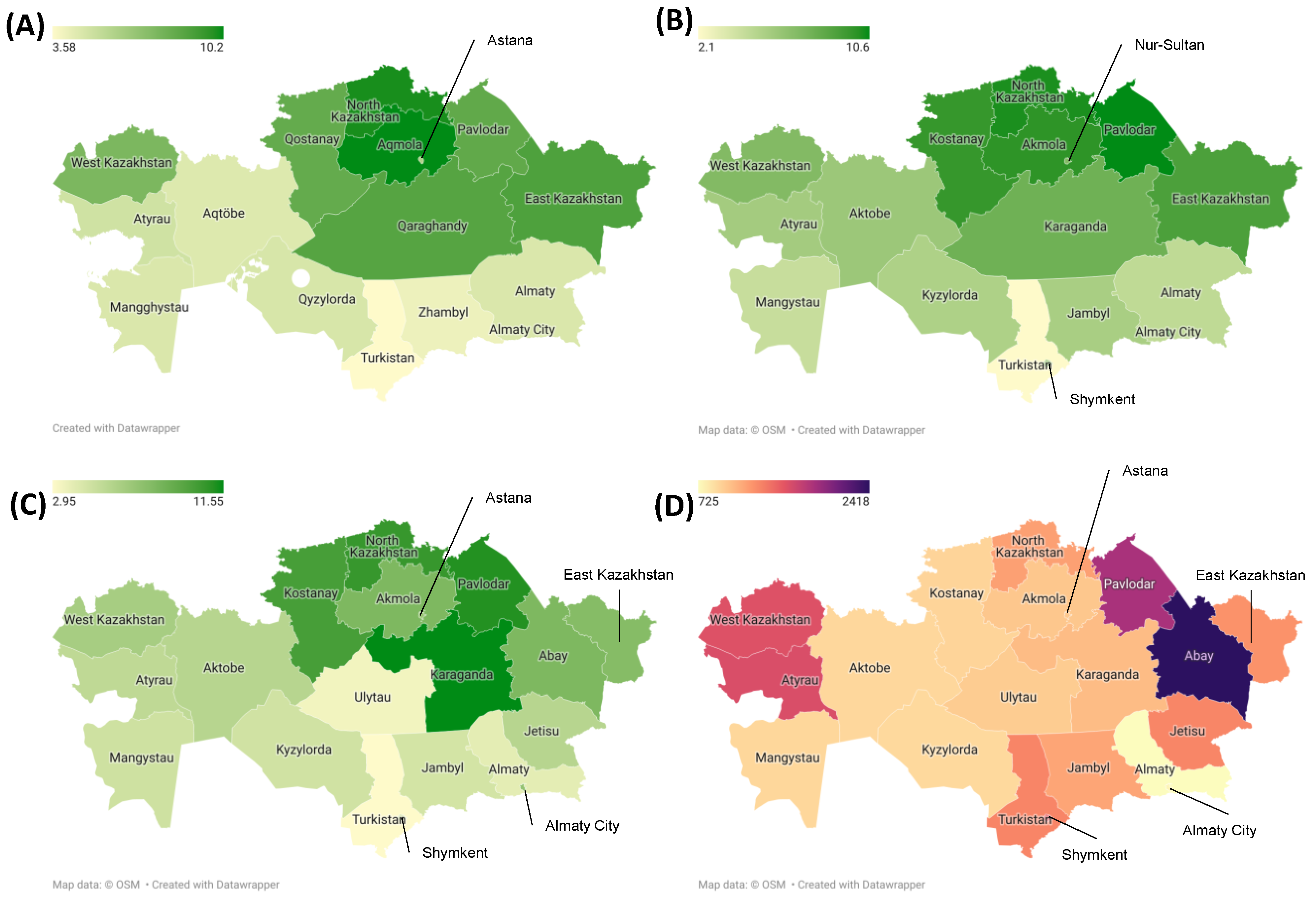
3.2. Incidence, Prevalence and Mortality Trends over Time
3.3. Survival Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, W.; Chawla, A.; O’Reilly, E.M. PC: A Review. JAMA 2021, 326, 851–862, Erratum in JAMA 2021, 326, 2081. https://doi.org/10.1001/jama.2021.19984. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stoffel, E.M.; Brand, R.E.; Goggins, M. PC: Changing Epidemiology and New Approaches to Risk Assessment, Early Detection, and Prevention. Gastroenterology 2023, 164, 752–765. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- European Cancer Patient Coalition 15 Key Facts on PC. Available online: https://ecpc.org/15-key-facts-on-pancreatic-cancer/ (accessed on 3 October 2024).
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory. In Cancer Today; International Agency for Research on Cancer: Lyon, France, 2024; Available online: https://gco.iarc.who.int/today (accessed on 6 September 2024).
- Semenova, Y.; Lim, L.; Salpynov, Z.; Gaipov, A.; Jakovljevic, M. Historical evolution of healthcare systems of post-soviet Russia, Belarus, Kazakhstan, Kyrgyzstan, Tajikistan, Turkmenistan, Uzbekistan, Armenia, and Azerbaijan: A scoping review. Heliyon 2024, 10, e29550. [Google Scholar] [CrossRef]
- Government of the Republic of Kazakhstan. On the Approval of the List of Guaranteed Volume of Free Medical Care and Invalidation of Some Decisions of the Government of the Republic of Kazakhstan; Government of the Republic of Kazakhstan: Astana, Kazakhstan, 2020; No. 672.
- World Health Organization. Mortality Database, Kazakhstan, 2021. Available online: https://platform.who.int/mortality/countries/country-details/MDB/kazakhstan?countryProfileId=3ac951f5-81f9-44f4-bb1f-8b9bcf7339d0 (accessed on 3 October 2024).
- Xiang, X.; Chen, X.; He, Y.; Wang, Y.; Xia, W.; Ye, S.; Wang, S.; Xiao, Y.; Li, Q.; Wang, X.; et al. Pancreatic cancer challenge in 52 Asian countries: Age-centric insights and the role of modifiable risk factors (1990–2019). Front. Oncol. 2023, 13, 1271370. [Google Scholar] [CrossRef]
- Kazakh Institute of Oncology and Radiology. Indicators of the Oncology Service of the Republic of Kazakhstan, 2023 (Statistical and Analytical Materials). Available online: https://onco.kz/pokazateli-onkologicheskoj-sluzhby/ (accessed on 3 October 2024).
- Aitbayev, C.; Fazyl, F.; Batyrbekov, K.; Spataev Zh Sarbassov, D. Main Epidemiological Aspects of PC in Kazakhstan. Eur. J. Appl. Biotechnol. 2022, 3, 75–81. [Google Scholar]
- Pourhoseingholi, M.A.; Ashtari, S.; Hajizadeh, N.; Fazeli, Z.; Zali, M.R. Systematic Review of PC Epidemiology in Asia-Pacific Region: Major Patterns in GLOBACON 2012. Gastroenterol. Hepatol. Bed Bench. 2017, 10, 245–257. [Google Scholar] [PubMed] [PubMed Central]
- Ranganath, R.; Chu, Q. Global Trends in Pancreas Cancer Among Asia-Pacific Population. Gastrointest. Oncol. 2021, 12 (Suppl. S2), S374–S386. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goodarzi, E.; Dehkordi, A.H.; Beiranvand, R.; Naemi, H.; Khazaei, Z. Epidemiology of the Incidence and Mortality of Pancreas Cancer and Its Relationship with the Human Development Index (HDI) in the World: An Ecological Study in 2018. Curr. Pharm. Des. 2020, 26, 5163–5173. [Google Scholar] [CrossRef] [PubMed]
- Akhmedullin, R.; Aimyshev, T.; Zhakhina, G.; Yerdessov, S.; Beyembetova, A.; Ablayeva, A.; Biniyazova, A.; Seyil, T.; Abdukhakimova, D.; Segizbayeva, A.; et al. In-depth analysis and trends of cancer mortality in Kazakhstan: A joinpoint analysis of nationwide healthcare data 2014–2022. BMC Cancer 2024, 24, 1340. [Google Scholar] [CrossRef]
- Beyembetova, A.; Ablayeva, A.; Akhmedullin, R.; Abdukhakimova, D.; Biniyazova, A.; Gaipov, A. National Electronic Oncology Registry in Kazakhstan: Patient’s Journey. Epidemiol. Health Data Insights 2025, 1, ehdi004. [Google Scholar] [CrossRef]
- Gusmanov, A.; Zhakhina, G.; Yerdessov, S.; Sakko, Y.; Mussina, K.; Alimbayev, A.; Syssoyev, D.; Sarria-Santamera, A.; Gaipov, A. Review of the research databases on population-based Registries of Unified electronic Healthcare system of Kazakhstan (UNEHS): Possibilities and limitations for epidemiological research and Real-World Evidence. Int. J. Med. Inform. 2022, 170, 104950. [Google Scholar] [PubMed]
- Demographic Statistics Bureau of National Statistics Agency for Strategic Planning and Reforms of the Republic of Kazakhstan; 2023. Available online: https://taldau.stat.gov.kz/ru/NewIndex/GetIndex/703831 (accessed on 25 February 2025).
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The strengthening the reporting of Observational studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2023. CA A Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Partyka, O.; Pajewska, M.; Kwaśniewska, D.; Czerw, A.; Deptała, A.; Budzik, M.; Cipora, E.; Gąska, I.; Gazdowicz, L.; Mielnik, A.; et al. Overview of Pancreatic Cancer Epidemiology in Europe and Recommendations for Screening in High-Risk Populations. Cancers 2023, 15, 3634. [Google Scholar] [CrossRef]
- Gugenheim, J.; Crovetto, A.; Petrucciani, N. Neoadjuvant therapy for PC. Updates Surg. 2021, 74, 35–42. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ilic, M.; Ilic, I. Epidemiology of PC. World J. Gastroenterol. 2016, 22, 9694–9705. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Hu, J.-X.; Zhao, C.-F.; Chen, W.-B.; Liu, Q.-C.; Li, Q.-W.; Lin, Y.-Y.; Gao, F. Pancreatic cancer: A review of epidemiology, trend, and risk factors. World J. Gastroenterol. 2021, 27, 4298–4321. [Google Scholar] [CrossRef]
- GBD Cancer Compare, 2021. Available online: https://vizhub.healthdata.org/gbd-compare/cancer (accessed on 3 October 2024).
- Decker, K.M.; Feely, A.; Bucher, O.; Czaykowski, P.; Hebbard, P.; Kim, J.O.; Pitz, M.; Singh, H.; Thiessen, M.; Lambert, P. New Cancer Diagnoses Before and During the COVID-19 Pandemic. JAMA Netw. Open 2023, 6, e2332363. [Google Scholar]
- Graus, M.U.; de Hingh, I.H.; Besselink, M.G.; Bruno, M.J.; Wilmink, J.W.; de Meijer, V.E.; van Velthuysen, M.-L.F.; Iersel, L.B.V.-V.; van der Geest, L.G.; de Vos-Geelen, J.; et al. Population-based impact of COVID-19 on incidence, treatment, and survival of patients with pancreatic cancer. HPB 2023, 25, 1195–1202. [Google Scholar] [CrossRef]
- Lemanska, A.; Andrews, C.; Fisher, L.; Bacon, S.; E Frampton, A.; Mehrkar, A.; Inglesby, P.; Davy, S.; Roberts, K.; Patalay, P.; et al. Healthcare in England was affected by the COVID-19 pandemic across the pancreatic cancer pathway: A cohort study using OpenSAFELY-TPP. eLife 2023, 12, e85332. [Google Scholar] [CrossRef] [PubMed]
- Yoo, B.S.; Patel, A.; Houston, K.V.; Vargas, A.; Sangay, A.R.V.; D’souza, S.M.; Johnson, D.A. The impact of COVID-19 pandemic on diagnosis and management of gastrointestinal cancers. Explor. Med. 2023, 4, 356–362. [Google Scholar] [CrossRef]
- Hidalgo, M. Pancreatic Cancer. N. Engl. J. Med. 2010, 362, 1605–1617. [Google Scholar] [CrossRef]
- Didier, A.J.; Nandwani, S.; Fahoury, A.M.; Craig, D.J.; Watkins, D.; Campbell, A.; Spencer, C.T.; Batten, M.; Vijendra, D.; Sutton, J.M. Trends in pancreatic cancer mortality in the United States 1999–2020: A CDC database population-based study. Cancer Causes Control 2024, 35, 1509–1516. [Google Scholar] [CrossRef] [PubMed]
- Kleeff, J.; Korc, M.; Apte, M.; La Vecchia, C.; Johnson, C.D.; Biankin, A.V.; Neale, R.E.; Tempero, M.; Tuveson, D.A.; Hruban, R.H.; et al. Pancreatic cancer. Nat. Rev. Dis. Primers 2016, 2, 16022. [Google Scholar] [CrossRef] [PubMed]
- Wiest, N.E.; Moktan, V.P.; Oman, S.P.; Chirilă, R.M. Screening for pancreatic cancer: A review for general clinicians. Rom. J. Intern. Med. 2020, 58, 119–128. [Google Scholar] [CrossRef]
- Aslanian, H.R.; Lee, J.H.; Canto, M.I. AGA Clinical Practice Update on Pancreas Cancer Screening in High-Risk Individuals: Expert Review. Gastroenterology 2020, 159, 358–362. [Google Scholar] [CrossRef]
- Saad, A.M.; Turk, T.; Al-Husseini, M.J.; Abdel-Rahman, O. Trends in pancreatic adenocarcinoma incidence and mortality in the United States in the last four decades; a SEER-based study. BMC Cancer 2018, 18, 688. [Google Scholar] [CrossRef]

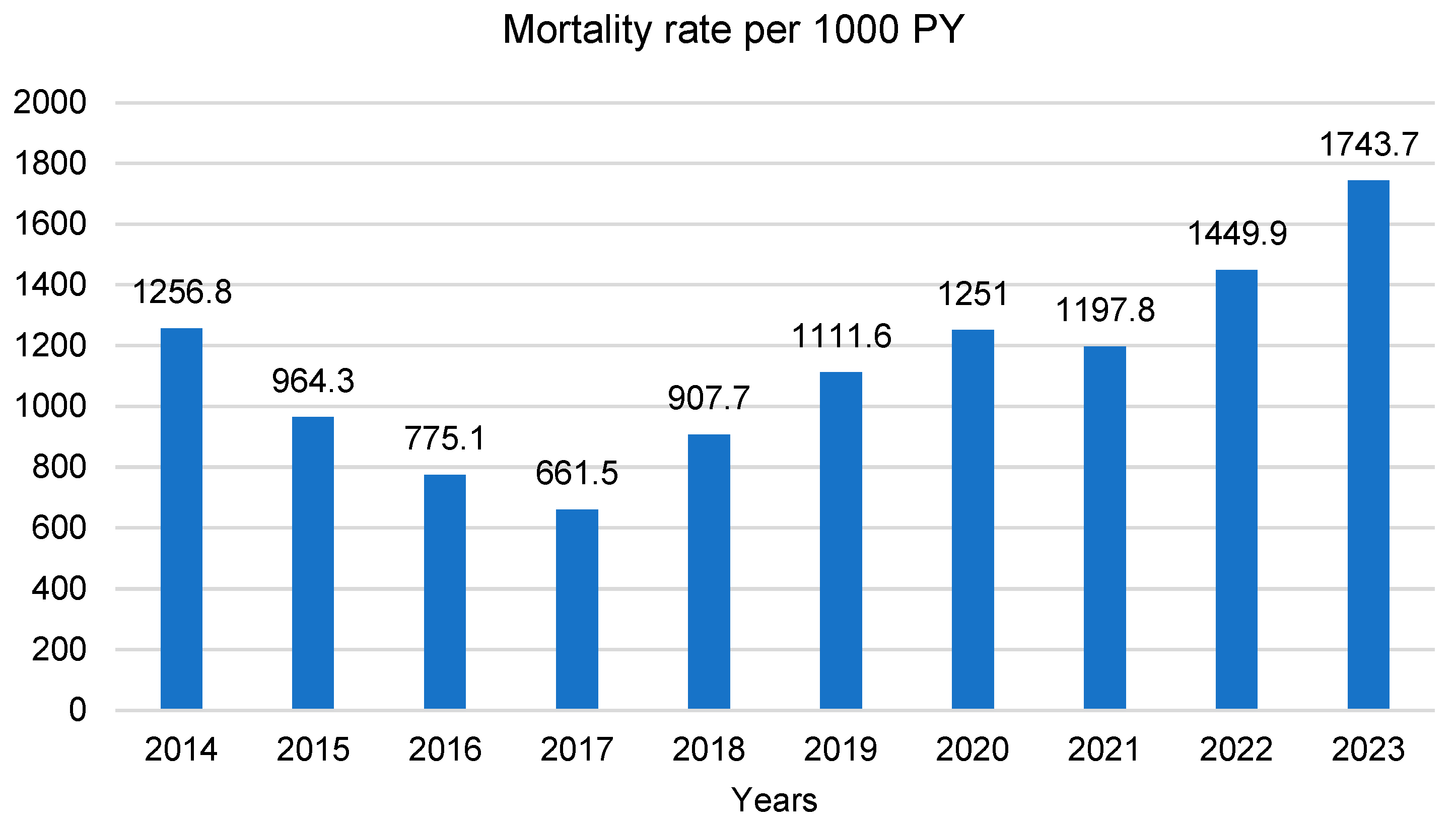

| Characteristics | Total n = 11,934 | Alive n = 1807 (15.1%) | Died n = 10,127 (84.9%) | p-Value | Mortality per 1000 PY (95% CI) |
|---|---|---|---|---|---|
| Age, mean ± SD | 64.5 ± 11.3 | 60.2 ± 13.2 | 65.1 ± 10.8 | <0.001 | |
| Age group, n (%) | <0.001 | ||||
| 0–29 | 62 (0.5) | 47 (75.8) | 15 (24.2) | 78 (47–130) | |
| 30–44 | 491 (4.2) | 139 (28.3) | 352 (71.7) | 526 (474–584) | |
| 45–59 | 3032 (25.9) | 475 (15.7) | 2557 (84.3) | 927 (892–964) | |
| 60–74 | 5872 (50.1) | 754 (12.8) | 5118 (87.2) | 1334 (1298–1371) | |
| ≥75 | 2267 (19.3) | 184 (8.1) | 2083 (91.9) | 1984 (1901–2071) | |
| Sex, n (%) | <0.001 | ||||
| Male | 5669 (49.9) | 522 (9.2) | 5147 (90.8) | 1590 (1277–1350) | |
| Female | 5636 (50.1) | 673 (11.9) | 4963 (88.1) | 1313 (1548–1635) | |
| Ethnicity, n (%) | 0.011 | ||||
| Other | 1720 (15.2) | 192 (11.2) | 1528 (88.8) | 1308 (1244–1375) | |
| Kazakh | 5952 (52.7) | 665 (11.2) | 5287 (88.8) | 1414 (1377–1453) | |
| Russian | 3633 (32.1) | 338 (9.3) | 3295 (90.7) | 1560 (1508–1615) | |
| Clinical stage, n (%) | <0.001 | ||||
| Stage I | 429 (3.7) | 252 (58.7) | 177 (41.3) | 159 (137–184) | |
| Stage II | 2214 (19.2) | 492 (22.2) | 1722 (77.8) | 693 (661–726) | |
| Stage III | 4369 (37.8) | 458 (10.5) | 3911 (89.5) | 1363 (1321–1406) | |
| Stage IV | 4224 (36.5) | 206 (4.9) | 4018 (95.1) | 2641 (2561–2724) | |
| Unknown | 196 (1.7) | 8 (4.1) | 188 (95.9) | 4781 (4144–5515) | |
| Not applicable | 128 (1.1) | 24 (18.8) | 104 (81.3) | 985 (813–1193) | |
| Tumor site in the pancreas, n (%) | <0.001 | ||||
| Diffuse lesion (“C25”) | 1007 (8.4) | 118 (11.7) | 889 (88.3) | 864 (809–923) | |
| Head of pancreas | 7118 (59.6) | 981 (13.8) | 6137 (86.2) | 1109 (1081–1137) | |
| Body of pancreas | 1600 (13.4) | 247 (15.4) | 1353 (84.6) | 1140 (1081–1202) | |
| Tail of pancreas | 1070 (9.0) | 188 (17.6) | 882 (82.4) | 1005 (941–1074) | |
| Pancreatic duct | 30 (0.3) | 6 (20) | 24 (80) | 535 (359–799) | |
| Endocrine part | 3 (0.03) | 0 (0) | 3 (100) | 2706 (873–8389) | |
| Other parts | 66 (0.5) | 12 (18.2) | 54 (81.8) | 1293 (990–1688) | |
| Overlapping sites | 631 (5.3) | 61 (9.7) | 570 (90.3) | 1651 (1521–1792) | |
| Unspecified part | 244 (2.1) | 34 (13.9) | 210 (86.1) | 1095 (957–1254) | |
| Benign neoplasms | 165 (1.4) | 160 (97) | 5 (3) | 14 (6–33) |
| Variables | Crude HR (95% CI) | p-Value | Adjusted HR (95% CI) | p-Value |
|---|---|---|---|---|
| Age | 1.21 [1.19–1.23] | <0.001 | 1.21 [1.19–1.24] | <0.001 |
| Sex | ||||
| Female | 1.00 (reference) | 1.00 (reference) | ||
| Male | 1.13 [1.09–1.19] | <0.001 | 1.17 [1.06–1.20] | <0.001 |
| Ethnicity | ||||
| Other | 1.00 (reference) | 1.00 (reference) | ||
| Kazakh | 0.99 [0.94–1.05] | 0.725 | 1.05 [0.99–1.11] | 0.130 |
| Russian | 1.16 [1.10–1.24] | <0.001 | 1.13 [1.06–1.20] | <0.001 |
| Clinical stage | ||||
| Stage I | 1.00 (reference) | 1.00 (reference) | ||
| Stage II | 2.85 [2.44–3.33] | <0.001 | 2.15 [1.83–2.51] | <0.001 |
| Stage III | 4.58 [3.93–5.31] | <0.001 | 3.29 [2.82–3.83] | <0.001 |
| Stage IV | 6.66 [5.72–7.75] | <0.001 | 4.80 [4.13–5.59] | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biniyazova, A.; Akhmedullin, R.; Ablayeva, A.; Beyembetova, A.; Abdukhakimova, D.; Zhumabekov, A.; Aimyshev, T.; Zhakhina, G.; Seyil, T.; Semenova, Y.; et al. The Incidence, Mortality, and Survival Trends of Pancreatic Cancer in Kazakhstan: Data from the National Electronic Registry of Oncological Patients (2014–2023). Cancers 2025, 17, 2277. https://doi.org/10.3390/cancers17142277
Biniyazova A, Akhmedullin R, Ablayeva A, Beyembetova A, Abdukhakimova D, Zhumabekov A, Aimyshev T, Zhakhina G, Seyil T, Semenova Y, et al. The Incidence, Mortality, and Survival Trends of Pancreatic Cancer in Kazakhstan: Data from the National Electronic Registry of Oncological Patients (2014–2023). Cancers. 2025; 17(14):2277. https://doi.org/10.3390/cancers17142277
Chicago/Turabian StyleBiniyazova, Aigerim, Ruslan Akhmedullin, Ayana Ablayeva, Altynay Beyembetova, Diyora Abdukhakimova, Abzal Zhumabekov, Temirgali Aimyshev, Gulnur Zhakhina, Temirlan Seyil, Yuliya Semenova, and et al. 2025. "The Incidence, Mortality, and Survival Trends of Pancreatic Cancer in Kazakhstan: Data from the National Electronic Registry of Oncological Patients (2014–2023)" Cancers 17, no. 14: 2277. https://doi.org/10.3390/cancers17142277
APA StyleBiniyazova, A., Akhmedullin, R., Ablayeva, A., Beyembetova, A., Abdukhakimova, D., Zhumabekov, A., Aimyshev, T., Zhakhina, G., Seyil, T., Semenova, Y., & Gaipov, A. (2025). The Incidence, Mortality, and Survival Trends of Pancreatic Cancer in Kazakhstan: Data from the National Electronic Registry of Oncological Patients (2014–2023). Cancers, 17(14), 2277. https://doi.org/10.3390/cancers17142277







