Real-World Outcomes of Adjuvant Paclitaxel and Trastuzumab Therapy in Lymph Node-Negative, HER2-Positive Early-Stage Breast Cancer: A Multicenter Retrospective Data Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Control Groups
- Histopathological diagnosis of invasive breast cancer
- HER2 positivity confirmed by IHC 3 + or FISH amplification
- Clinical stage I or II disease
- Completion of adjuvant therapy after surgery and at least 6 months of follow-up
- Stage IV (metastatic) disease at diagnosis
- HER2-negative tumor profile
- Incomplete treatment or insufficient follow-up
2.2. Study Endpoints
2.3. Statistical Analysis
3. Results
3.1. Patient and Treatment Characteristics
| Variables | n | % |
|---|---|---|
| Age | ||
| Mean (SD) | 54.42 (13.01) | |
| Median (min-max) | 54.00 (29–83) | |
| ≤65 | 103 | 79.8 |
| >65 | 26 | 20.2 |
| Menopause | ||
| Pre | 52 | 40.3 |
| Post | 77 | 59.7 |
| Breast Laterality | ||
| Right | 58 | 45.0 |
| Left | 71 | 55.0 |
| Tcat | ||
| T1a | 11 | 8.5 |
| T1b | 37 | 28.7 |
| T1c | 62 | 48.1 |
| T2 | 19 | 14.7 |
| Hgrad | ||
| Grade1 | 10 | 7.8 |
| Grade2 | 55 | 42.6 |
| Grade3 | 64 | 49.6 |
| ER | ||
| Negative | 41 | 31.8 |
| Positive | 88 | 68.2 |
| PR | ||
| Negative | 61 | 47.3 |
| Positive | 68 | 52.7 |
| HER2 | ||
| Positive | 129 | 100.0 |
| Ki67 | ||
| <20 | 49 | 38.0 |
| >20 | 80 | 62.0 |
| LI | ||
| Absent | 56 | 43.4 |
| Present | 73 | 56.6 |
| VI | ||
| Absent | 99 | 76.7 |
| Present | 30 | 23.3 |
| PI | ||
| Absent | 109 | 84.5 |
| Present | 20 | 15.5 |
| ADJ-RT | ||
| Absent | 20 | 15.5 |
| Present | 109 | 84.5 |
| ADJ-ET | ||
| Absent | 46 | 35.7 |
| Present | 83 | 64.3 |
| Neuropathy | ||
| Absent | 60 | 46.5 |
| Present | 69 | 53.5 |
| Neuropathy grade | ||
| Grade0 | 29 | 42.0 |
| Grade1 | 33 | 47.8 |
| Grade2 | 7 | 10.1 |
| Recurrence | ||
| Absent | 125 | 96.9 |
| Present | 4 | 3.1 |
| Mortality | ||
| Right | 123 | 95.3 |
| Ex | 6 | 4.7 |
| Follow-up Duration (months) | ||
| Mean (SD) | 69.05 (34.01) | |
| Median(min-max) | 70.90 (5.57–187.47) | |
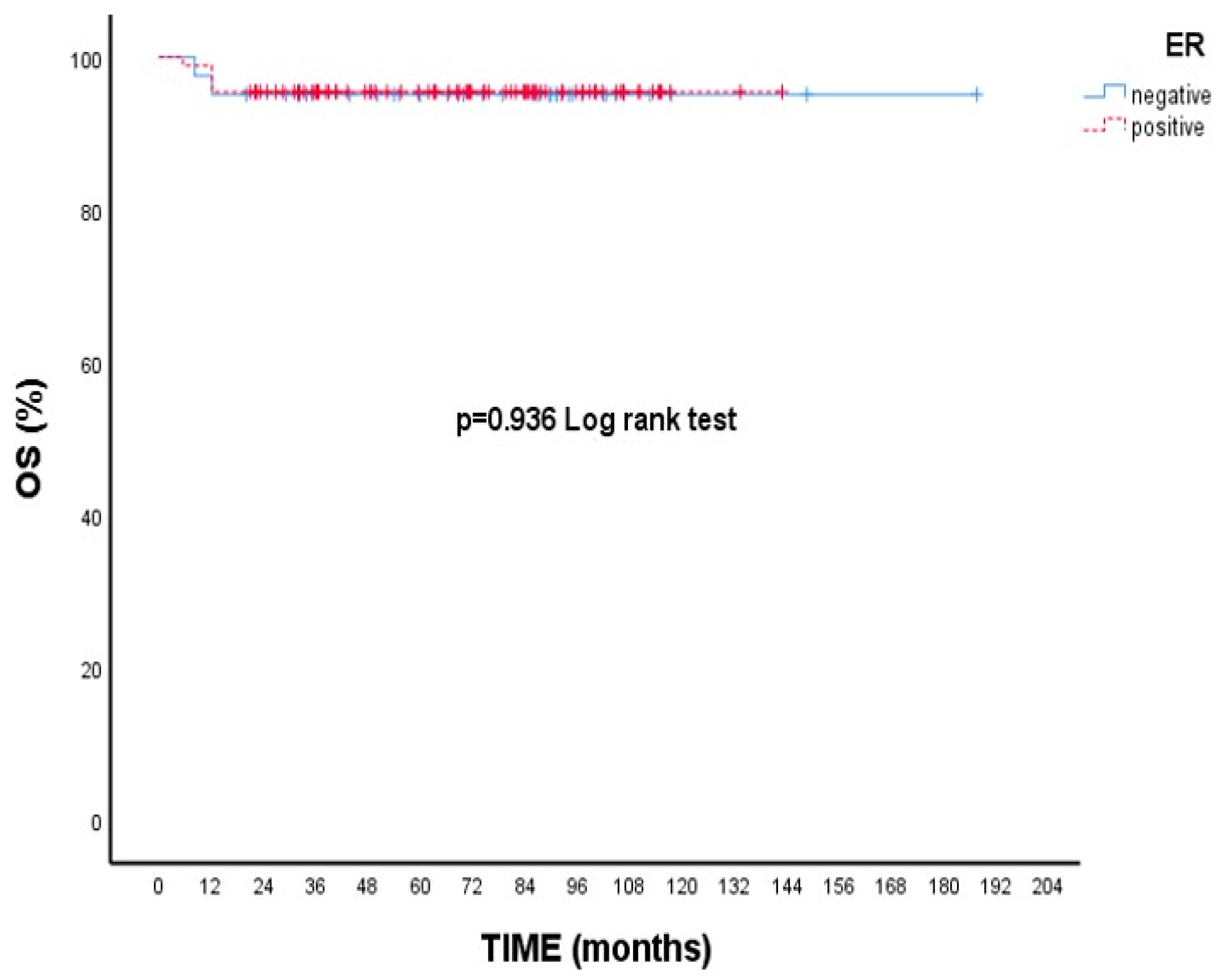
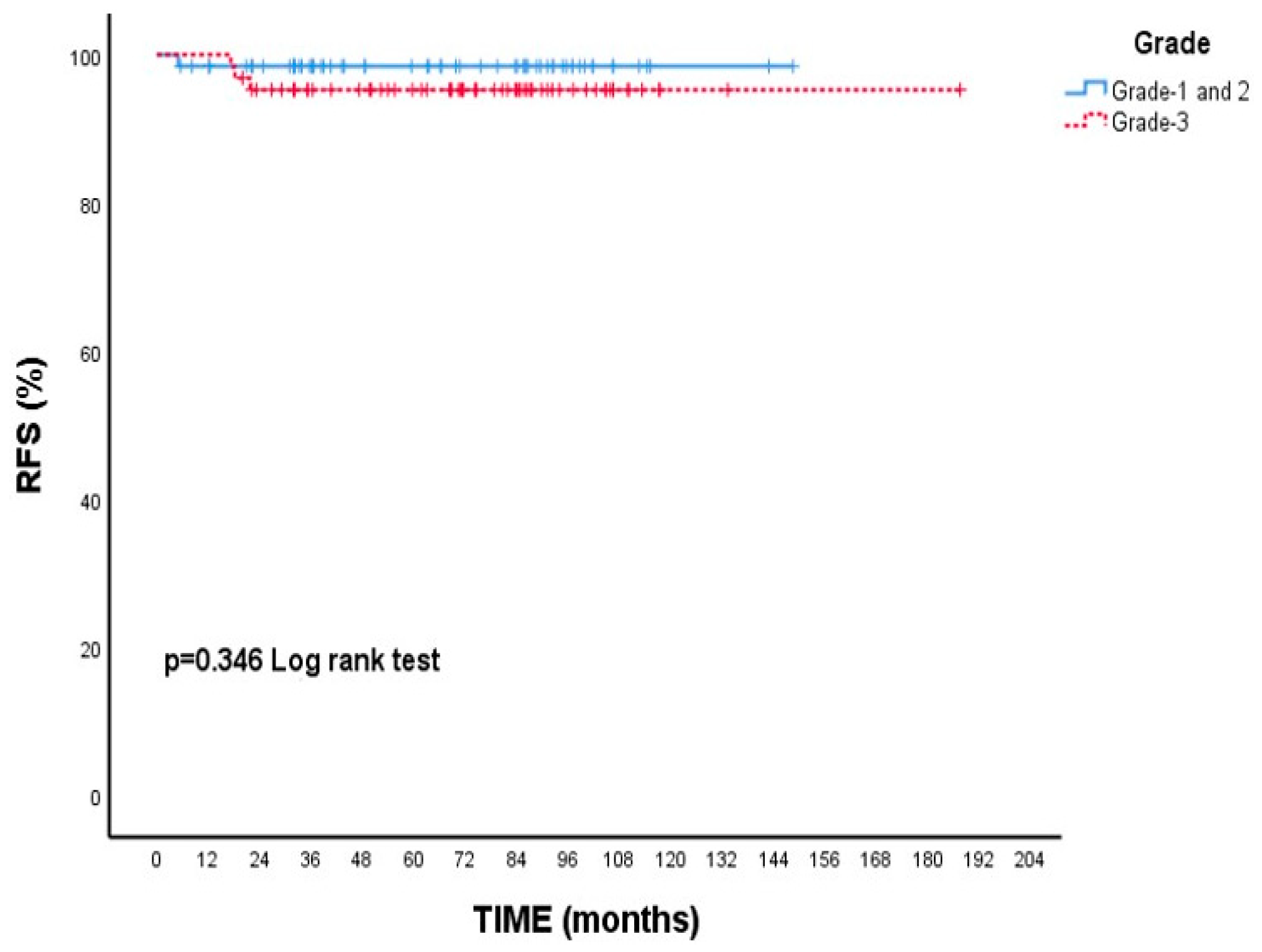
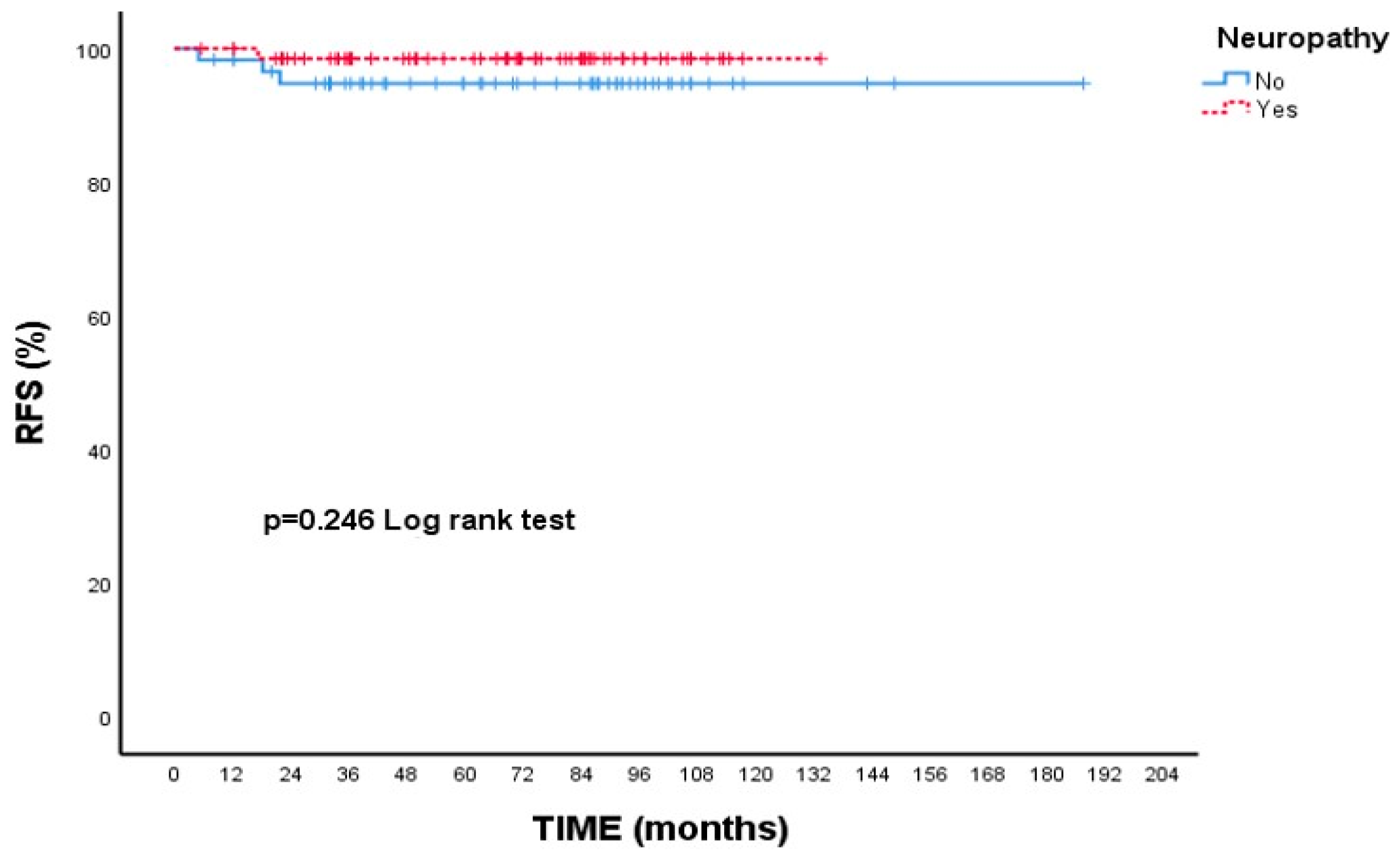
3.2. Survival Outcomes
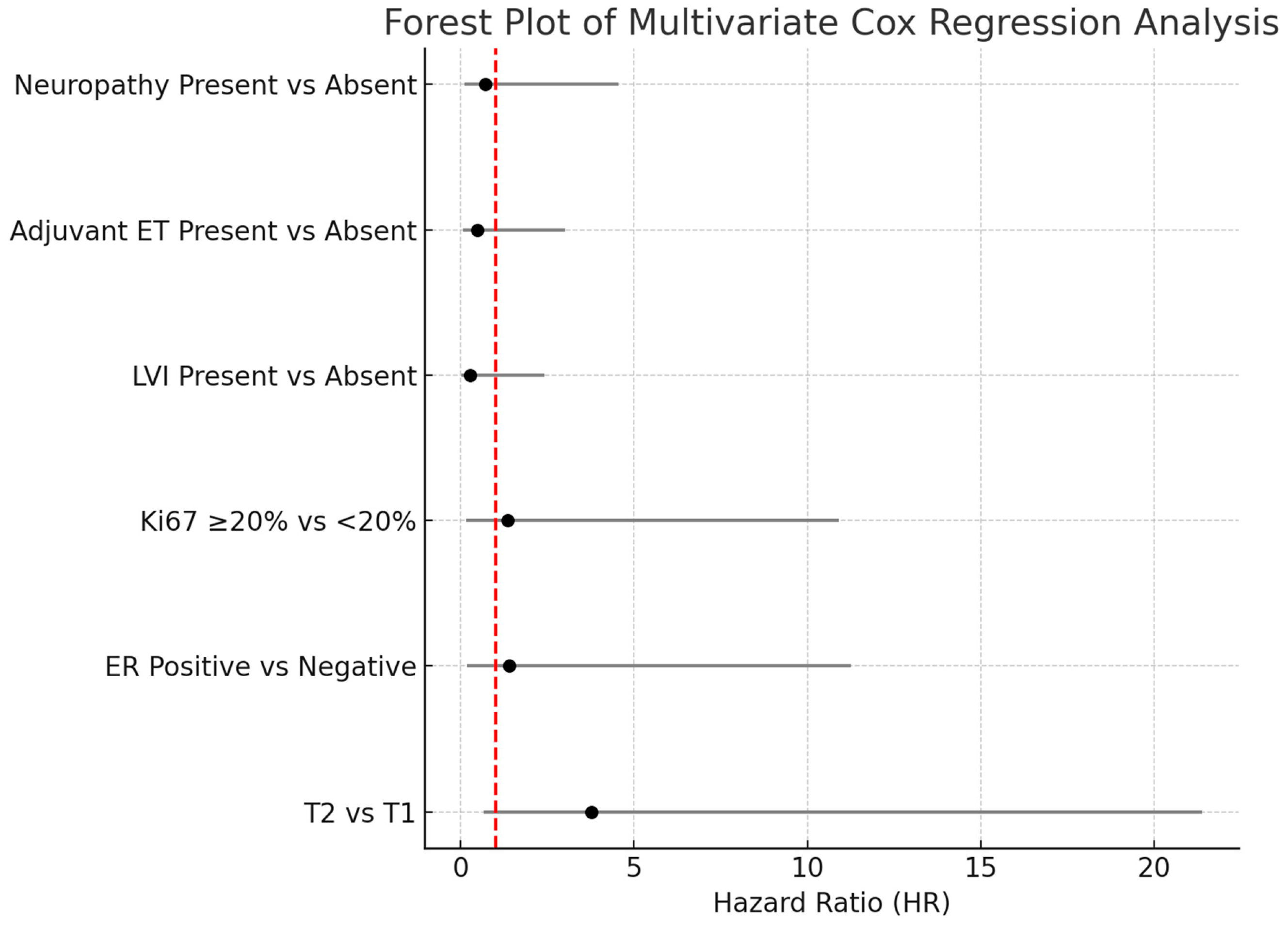
3.3. Multivariate Cox Regression Analysis
3.4. Safety
| Adverse Event * | N (%) |
|---|---|
| Neuropathy (any grade) | 69 (53.5) |
| Neuropathy Grade 0 | 29 (42.0) |
| Neuropathy Grade 1 | 33 (47.8) |
| Neuropathy Grade 2 | 7 (10.1) |
| Cardiotoxicity | 0 (0.0) |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Slamon, D.J.; Clark, G.M.; Wong, S.G.; Levin, W.J.; Ullrich, A.; McGuire, W.L. Human Breast Cancer: Correlation of Relapse and Survival with Amplification of the HER-2/neu Oncogene. Science 1987, 235, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: ASCO/CAP Clinical Practice Guideline Update. Arch. Pathol. Lab. Med. 2018, 142, 1364–1382. [Google Scholar] [CrossRef] [PubMed]
- Tolaney, S.M.; Barry, W.T.; Dang, C.T.; Yardley, D.A.; Moy, B.; Marcom, P.K.; Albain, K.S.; Rugo, H.S.; Ellis, M.; Shapira, I.; et al. Adjuvant Paclitaxel and Trastuzumab for Node-Negative, HER2-Positive Breast Cancer. N. Engl. J. Med. 2015, 372, 134–141. [Google Scholar] [CrossRef]
- Goldhirsch, A.; Winer, E.P.; Coates, A.S.; Gelber, R.D.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.-J. Personalizing the Treatment of Women with Early Breast Cancer: St. Gallen Consensus 2013. Ann. Oncol. 2013, 24, 2206–2223. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. Version 4.2024. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed on 1 July 2025).
- Tolaney, S.M.; Tayob, N.; Dang, C.; Yardley, D.A.; Isakoff, S.J.; Valero, V.; Faggen, M.; Mulvey, T.; Bose, R.; Hu, J.; et al. Adjuvant Trastuzumab Emtansine Versus Paclitaxel in Combination With Trastuzumab for Stage I HER2-Positive Breast Cancer (ATEMPT): A Randomized Clinical Trial. J. Clin. Oncol. 2021, 39, 2375–2385. [Google Scholar] [CrossRef]
- Piccart-Gebhart, M.; Holmes, E.; Baselga, J.; de Azambuja, E.; Dueck, A.C.; Viale, G.; Zujewski, J.A.; Goldhirsch, A.; Armour, A.; Pritchard, K.I.; et al. Adjuvant Lapatinib and Trastuzumab for Early HER2-Positive Breast Cancer (ALTTO Trial). J. Clin. Oncol. 2016, 34, 1034–1042. [Google Scholar] [CrossRef]
- Kumar, A.; Sohal, D.P.S.; Walters, I.; Clancy, J. Real-World Evidence: An Essential Component of Healthcare Decision Making. J. Comp. Eff. Res. 2020, 9, 989–992. [Google Scholar] [CrossRef]
- Makady, A.; de Boer, A.; Hillege, H.; Klungel, O.; Goettsch, W. What is Real-World Data? A Review of Definitions Based on Literature and Stakeholder Interviews. Value Health 2017, 20, 858–865. [Google Scholar] [CrossRef]
- Romond, E.H.; Perez, E.A.; Bryant, J.; Suman, V.J.; Geyer, C.E., Jr.; Davidson, N.E.; Tan-Chiu, E.; Martino, S.; Paik, S.; Kaufman, P.A.; et al. Trastuzumab plus Chemotherapy for Operable HER2-Positive Breast Cancer: NSABP B-31/N9831. N. Engl. J. Med. 2005, 353, 1673–1684. [Google Scholar] [CrossRef]
- Swain, S.M.; Baselga, J.; Kim, S.-B.; Ro, J.; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.-M.; Schneeweiss, A.; Heeson, S.; et al. Pertuzumab, Trastuzumab, and Docetaxel in HER2-Positive Metastatic Breast Cancer. N. Engl. J. Med. 2015, 372, 724–734. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) v5.0; National Cancer Institute: Bethesda, MD, USA, 2017. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm (accessed on 1 July 2025).
- Burstein, H.J.; Curigliano, G.; Loibl, S.; Dubsky, P.; Gnant, M.; Poortmans, P.; Colleoni, M.; Denkert, C.; Piccart, M.; Winer, E.P.; et al. Estimating the benefits of therapy for early-stage HER2-positive breast cancer: The St. Gallen International Consensus Guidelines. Ann. Oncol. 2019, 30, 1541–1557. [Google Scholar] [CrossRef] [PubMed]
- von Minckwitz, G.; Procter, M.; de Azambuja, E.; Zardavas, D.; Benyunes, M.C.; Viale, G.; Suter, T.M.; Arahmani, A.; Rouchet, N.; Clark, E.; et al. APHINITY: Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N. Engl. J. Med. 2017, 377, 122–131. [Google Scholar] [CrossRef]
- Swain, S.M.; Ewer, M.S.; Viale, G.; Amadori, D.; Ciruelos, E.; Láng, I.; Delaloge, S.; Schneeweiss, A.; Brain, E.; Diéras, V.; et al. Incidence and Outcomes of Cardiac Events with Adjuvant Trastuzumab: NSABP B-31. J. Clin. Oncol. 2011, 29, 3366–3373. [Google Scholar] [CrossRef]
- Narui, K.; Miura, D.; Hasegawa, Y.; Akazawa, K.; Kohno, N.; Ishikawa, T.A. Randomized Controlled Phase 2 Study of Neoadjuvant Eribulin versus Paclitaxel in Women with Operable Breast Cancer: The JONIE-3 Study. Clin. Breast Cancer 2022, 22, e881–e891. [Google Scholar] [CrossRef] [PubMed]
- Schneeweiss, A.; Chia, S.; Hickish, T.; Harvey, V.; Eniu, A.; Hegg, R.; Tausch, C.; Seo, J.H.; Tsai, Y.F.; Ratnayake, J.; et al. Pertuzumab plus Trastuzumab in Combination with Standard Neoadjuvant Anthracycline-Containing and Anthracycline-Free Chemotherapy Regimens in Patients with HER2-Positive Early Breast Cancer: A Randomized Phase II Cardiac Safety Study (TRYPHAENA). Ann. Oncol. 2013, 24, 2278–2284. [Google Scholar] [CrossRef] [PubMed]
- von Minckwitz, G.; Huang, C.S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef]
- Gianni, L.; Pienkowski, T.; Im, Y.-H.; Tseng, L.-M.; Liu, M.-C.; Lluch, A.; Starosławska, E.; De La Haba-Rodríguez, J.R.; Im, S.-A.; Pedrini, J.L.; et al. 5-Year Analysis of Neoadjuvant Pertuzumab and Trastuzumab in Patients with Locally Advanced, Inflammatory, or Early-Stage HER2-Positive Breast Cancer (NeoSphere): A Multicentre, Open-Label, Phase 2 Randomised Trial. Lancet Oncol. 2016, 17, 791–800. [Google Scholar] [CrossRef]
- Cameron, D.; Piccart-Gebhart, M.J.; Gelber, R.D.; Procter, M.; Goldhirsch, A.; de Azambuja, E.; Castro, G.; Untch, M.; Smith, I.; Gianni, L.; et al. 11 Years’ Follow-Up of Trastuzumab after Adjuvant Chemotherapy in HER2-Positive Early Breast Cancer: Final Analysis of the HERceptin Adjuvant (HERA) Trial. Lancet 2017, 389, 1195–1205. [Google Scholar] [CrossRef]
- Baselga, J.; Cortés, J.; Kim, S.B.; Im, S.A.; Hegg, R.; Im, Y.H.; Roman, L.; Pedrini, J.L.; Pienkowski, T.; Knott, A.; et al. Pertuzumab plus Trastuzumab plus Docetaxel for Metastatic Breast Cancer. N. Engl. J. Med. 2012, 366, 109–119. [Google Scholar] [CrossRef]
- Pivot, X.; Gligorov, J.; Müller, V.; Curigliano, G.; Knoop, A.; Verma, S.; Jenkins, V.; Scotto, N.; Osborne, S.; Fallowfield, L. Patients’ Preferences for Subcutaneous Trastuzumab versus Conventional Intravenous Infusion for the Adjuvant Treatment of HER2-Positive Early Breast Cancer: Final Analysis of 488 Patients in the International, Randomized, Two-Cohort PrefHer Study. Ann. Oncol. 2014, 25, 1979–1987. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Iwase, H.; Saito, T.; Takahashi, M.; Yamaguchi, K.; Takashima, T.; Niikura, N.; Hozumi, Y.; Tokunaga, E.; Masuda, N.; et al. Real-World Safety of Trastuzumab in Patients with HER2-Positive Breast Cancer in Japan: A Post-Marketing Surveillance Study. Breast Cancer 2020, 27, 487–495. [Google Scholar] [CrossRef]
- Pivot, X.; Romieu, G.; Debled, M.; Pierga, J.-Y.; Kerbrat, P.; Bachelot, T.; Ferrero, J.-M.; Lortholary, A.; Patsouris, A.; Dalenc, F.; et al. Six Months versus Twelve Months of Adjuvant Trastuzumab for Patients with HER2-Positive Early Breast Cancer (PHARE): A Randomised Phase 3 Trial. Lancet Oncol. 2013, 14, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Van Ramshorst, M.S.; van der Heiden-van der Loo, M.; Dackus, G.M.H.E.; Linn, S.C.; Sonke, G.S. The Effect of Trastuzumab-Based Chemotherapy in Small Node-Negative HER2-Positive Breast Cancer: A Population-Based Cohort Study. Breast Cancer Res. Treat. 2016, 158, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Eiermann, W.; Robert, N.J.; Pienkowski, T.; Martin, M.; Press, M.; Mackey, J.; Glaspy, J.; Chan, A.; Pawlicki, M.; et al. Adjuvant Trastuzumab in HER2-Positive Early Breast Cancer: Final Analysis of Overall Survival in the BCIRG 006 Trial. N. Engl. J. Med. 2011, 365, 1273–1283. [Google Scholar] [CrossRef]
- Perez, E.A.; Romond, E.H.; Suman, V.J.; Jeong, J.-H.; Davidson, N.E.; Geyer, C.E., Jr.; Martino, S.; Mamounas, E.P.; Rastogi, P.; Swain, S.M.; et al. Trastuzumab Plus Chemotherapy for HER2-Positive Early Breast Cancer: 10-Year Follow-Up of the N9831 Trial. J. Clin. Oncol. 2014, 32, 3744–3752. [Google Scholar] [CrossRef]
- Ponde, N.F.; Lambertini, M.; Agbor-Tarh, D.; Desmet, M.; de Azambuja, E.; Bradbury, I.; Ellis, P.; Di Cosimo, S.; Gelber, R.D.; Piccart, M.; et al. Prognostic Value of Nodal Status in HER2-Positive Early Breast Cancer: A Pooled Analysis of Randomised Trials. Ann. Oncol. 2019, 30, 761–767. [Google Scholar] [CrossRef]
- Dalyca, S.L.; Mandelker, E.; Mayer, I.A.; McArthur, H.L.; Tolaney, S.M.; Barcenas, C.H.; Gomez, H.L.; Im, S.A.; Iwata, H.; Pivot, X.; et al. Real-World 5-Year Outcomes of Patients Treated with the Adjuvant APT Regimen in Stage I HER2-Positive Early Breast Cancer: An International Retrospective Study. Ann. Oncol. 2025, 36, 556–566. [Google Scholar] [CrossRef]
- Tsai, Y.F.; Hsu, C.Y.; Chiu, Y.N.; Huang, C.C.; Chou, S.H.; Lin, Y.S.; Chao, T.C.; Liu, C.Y.; Chiu, J.H.; Tseng, L.M. Genetic Alterations in HER2-Positive and Equivocal Breast Cancer by Immunohistochemistry. Breast Cancer Targets Ther. 2025, 17, 253–263. [Google Scholar] [CrossRef]

| Variables | 2 Years % | 5 Years % | Median (95% CI) | p |
|---|---|---|---|---|
| Overall | 95.3 | 95.3 | - (-) | |
| Age | ||||
| ≤65 | 96.1 | 96.1 | - (-) | 0.394 |
| >65 | 92.3 | 92.3 | - (-) | |
| Menopause | ||||
| Pre | 94.2 | 94.2 | - (-) | 0.634 |
| Post | 96.1 | 96.1 | - (-) | |
| Breast Laterality | ||||
| Right | 96.6 | 96.6 | - (-) | 0.549 |
| Left | 94.4 | 94.4 | - (-) | |
| Tcat | ||||
| T1 | 96.4 | 96.4 | - (-) | 0.173 |
| T2 | 89.5 | 89.5 | - (-) | |
| ER | ||||
| Negative | 95.1 | 95.1 | - (-) | 0.936 |
| Positive | 95.5 | 95.5 | - (-) | |
| PR | ||||
| Negative | 95.1 | 95.1 | - (-) | 0.895 |
| Positive | 95.6 | 95.6 | - (-) | |
| Ki67 | ||||
| <20 | 93.9 | 93.9 | - (-) | 0.524 |
| >20 | 96.3 | 96.3 | - (-) | |
| LI | ||||
| Absent | 92.9 | 92.9 | - (-) | 0.248 |
| Present | 97.3 | 97.3 | - (-) | |
| VI | ||||
| Absent | 97.0 | 97.0 | - (-) | 0.110 |
| Present | 90.0 | 90.0 | - (-) | |
| PI | ||||
| Absent | 95.4 | 95.4 | - (-) | 0.952 |
| Present | 95.0 | 95.0 | - (-) | |
| ADJ-RT | ||||
| Absent | 90.0 | 90.0 | - (-) | 0.192 |
| Present | 96.3 | 96.3 | - (-) | |
| ADJ-ET | ||||
| Absent | 93.5 | 93.5 | - (-) | 0.443 |
| Present | 96.4 | 96.4 | - (-) | |
| Neuropathy | ||||
| Absent | 95.0 | 95.0 | - (-) | 0.864 |
| Present | 95.7 | 95.7 | - (-) |
| Variables | 2 Years % | 5 Years % | Median (95% CI) | p |
|---|---|---|---|---|
| Overall | 96.8 | 96.8 | - (-) | |
| Age | ||||
| ≤65 | 97.0 | 97.0 | - (-) | 0.776 |
| >65 | 95.8 | 95.8 | - (-) | |
| Breast Laterality | ||||
| Right | 96.4 | 96.4 | - (-) | 0.857 |
| Left | 97.1 | 97.1 | - (-) | |
| Tcat | ||||
| T1 | 97.2 | 97.2 | - (-) | 0.519 |
| T2 | 94.1 | 94.1 | - (-) | |
| Hgrade | ||||
| Grade-1 and 2 | 98.5 | 98.5 | - (-) | 0.346 |
| Grade-3 | 95.3 | 95.3 | - (-) | |
| ER | ||||
| Negative | 94.9 | 94.9 | - (-) | 0.423 |
| Positive | 97.7 | 97.7 | - (-) | |
| PR | ||||
| Negative | 96.6 | 96.6 | - (-) | 0.909 |
| Positive | 97.0 | 97.0 | - (-) | |
| Ki67 | ||||
| <20 | 97.8 | 97.8 | - (-) | 0.604 |
| >20 | 96.1 | 96.1 | - (-) | |
| LI | ||||
| Absent | 98.1 | 98.1 | - (-) | 0.476 |
| Present | 95.8 | 95.8 | - (-) | |
| ADJ-ET | ||||
| Absent | 97.7 | 97.7 | - (-) | 0.672 |
| Present | 96.3 | 96.3 | - (-) | |
| Neuropathy | ||||
| Absent | 94.8 | 94.8 | - (-) | 0.246 |
| Present | 98.5 | 98.5 | - (-) |
| Variables | HR (%95 CI) | |
|---|---|---|
| Tcat | ||
| T1 | ref | 0.132 |
| T2 | 3.78 (0.66–21.37) | |
| ER | ||
| Negative | ref | 0.747 |
| Positive | 1.40 (0.17–11.24) | |
| Ki67 | ||
| <20 | ref | 0.773 |
| >20 | 1.35 (0.16–10.90) | |
| LI | ||
| Absent | ref | 0.243 |
| Present | 0.27 (0.03–2.42) | |
| ADJ-ET | ||
| Absent | ref | 0.438 |
| Present | 0.48 (0.07–3.01) | |
| Nöropati | ||
| Absent | ref | 0.721 |
| Present | 0.72 (0.11–4.56) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Şahin Çelik, B.; Peker, P.; Özçelik, E.E.; Kuzu, Ö.F.; Gökmen, E.; Başaran, G.; Evrensel, T. Real-World Outcomes of Adjuvant Paclitaxel and Trastuzumab Therapy in Lymph Node-Negative, HER2-Positive Early-Stage Breast Cancer: A Multicenter Retrospective Data Analysis. Cancers 2025, 17, 2271. https://doi.org/10.3390/cancers17142271
Şahin Çelik B, Peker P, Özçelik EE, Kuzu ÖF, Gökmen E, Başaran G, Evrensel T. Real-World Outcomes of Adjuvant Paclitaxel and Trastuzumab Therapy in Lymph Node-Negative, HER2-Positive Early-Stage Breast Cancer: A Multicenter Retrospective Data Analysis. Cancers. 2025; 17(14):2271. https://doi.org/10.3390/cancers17142271
Chicago/Turabian StyleŞahin Çelik, Buket, Pınar Peker, Ender Eren Özçelik, Ömer Faruk Kuzu, Erhan Gökmen, Gül Başaran, and Türkkan Evrensel. 2025. "Real-World Outcomes of Adjuvant Paclitaxel and Trastuzumab Therapy in Lymph Node-Negative, HER2-Positive Early-Stage Breast Cancer: A Multicenter Retrospective Data Analysis" Cancers 17, no. 14: 2271. https://doi.org/10.3390/cancers17142271
APA StyleŞahin Çelik, B., Peker, P., Özçelik, E. E., Kuzu, Ö. F., Gökmen, E., Başaran, G., & Evrensel, T. (2025). Real-World Outcomes of Adjuvant Paclitaxel and Trastuzumab Therapy in Lymph Node-Negative, HER2-Positive Early-Stage Breast Cancer: A Multicenter Retrospective Data Analysis. Cancers, 17(14), 2271. https://doi.org/10.3390/cancers17142271






