Recent Advances in Immunotherapy for Melanoma: Perspectives on the Development of Novel Treatments: A Mini Review
Simple Summary
Abstract
1. Introduction
2. Aim
3. Methods
Search Strategy for the Literature Search
4. Previous Immunotherapy for Melanoma
4.1. Type I Interferon Therapy
4.2. High Dose IL-2 Therapy
5. Immune Checkpoint Inhibitors (ICIs) for Advanced Melanoma
5.1. Anti-CTLA-4 Ab Monotherapy
| Treatment | * ORR | PFS | OS | Phase | Reference | ||
|---|---|---|---|---|---|---|---|
| First line | Monotherapy | ipilimumab | 11% | the 3-year OS: 21–25% | phase III | [22,23] | |
| nivolumab | 42% | the 5-year PFS: 28% | the 5-year OS: 39% | phase III | [24] | ||
| pembrolizumab | 51.1% | the 7-year PFS: 23.8% | the 7-year OS: 37.8% | phase III | [25] | ||
| Combination therapy | nivolumab + ipilimumab | 58% | the 6.5-year PFS: 34% | the 6.5-year OS: 49% | phase III | [26] | |
| nivolumab + relatlimab | 43.7% | the 36-month PFS: 31.8% | the 36-month OS: 54.6% | phase III | [27] | ||
| Second line | Combination therapy | nivolumab + ipilimumab | 28% | the 6-month PFS: 34% | phase II | [28] | |
| nivolumab + relatlimab | 9.2–12% | the 6-month PFS: 27.7–29.1% | phase I/IIa | [29] | |||
| nivolumab + TM5614 | 25.7% | phase Ⅱ | [30] |
5.2. Anti-PD-1Ab Monotherapy
6. Combination Therapy
6.1. Combination with Anti-PD-1 and Anti-CTLA-4 Antibody
6.2. Combination with Anti-PD-1 and Anti-LAG-3 Antibodies
6.3. Combination with Anti-PD-1 Antibody and Pegylated Interleukin-2 (IL-2) Cytokine Prodrug
7. Future Perspectives
7.1. KIT Inhibitor Therapy Plus Pembrolizumab
7.2. TM5614 Plus Nivolumab
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
| TLA | Three letter acronym |
| LD | Linear dichroism |
| PD-1 | Programmed cell death protein 1 |
| CTLA4 | Cytotoxic T-lymphocyte antigen-4 |
| BRAF | B-Raf proto-oncogene, serine/threonine kinase |
| IL-2 | Interleukin-2 |
| IFN | Interferon |
| NK cells | Natural killer cells |
| MEK | Mitogen-activated protein kinase |
References
- Young, A.M.; Marsden, J.; Goodman, A.; Burton, A.; Dunn, J.A. Prospective randomized comparison of dacarbazine (DTIC) versus DTIC plus interferon-alpha (IFN-alpha) in metastatic melanoma. Clin. Oncol. 2001, 13, 458–465. [Google Scholar] [CrossRef]
- Namikawa, K.; Tsutsumida, A.; Mizutani, T.; Shibata, T.; Takenouchi, T.; Yoshikawa, S.; Kiyohara, Y.; Uchi, H.; Furue, M.; Ogata, D.; et al. Randomized phase III trial of adjuvant therapy with locoregional interferon beta versus surgery alone in stage II/III cutaneous melanoma: Japan Clinical Oncology Group Study (JCOG1309, J-FERON). Jpn. J. Clin. Oncol. 2017, 47, 664–667. [Google Scholar] [CrossRef][Green Version]
- Keilholz, U.; Conradt, C.; Legha, S.S.; Khayat, D.; Scheibenbogen, C.; Thatcher, N.; Goey, S.H.; Gore, M.; Dorval, T.; Hancock, B.; et al. Results of interleukin-2-based treatment in advanced melanoma: A case record-based analysis of 631 patients. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1998, 16, 2921–2929. [Google Scholar] [CrossRef]
- Hauschild, A.; Garbe, C.; Stolz, W.; Ellwanger, U.; Seiter, S.; Dummer, R.; Ugurel, S.; Sebastian, G.; Nashan, D.; Linse, R.; et al. Dacarbazine and interferon alpha with or without interleukin 2 in metastatic melanoma: A randomized phase III multicentre trial of the Dermatologic Cooperative Oncology Group (DeCOG). Br. J. Cancer 2001, 84, 1036–1042. [Google Scholar] [CrossRef]
- Legha, S.S. The role of interferon alfa in the treatment of metastatic melanoma. Semin. Oncol. 1997, 24, S24–S31. [Google Scholar]
- Joshi, U.M.; Hundal, J.; Mata, J.R.; Schollenberger, M.D.; Warrier, G.; Luke, J.J.; Lipson, E.J.; Funchain, P. Beyond Checkpoint Inhibition: Keeping Therapeutic Options Open. Am. Soc. Clin. Oncol. Educ. Book Am. Soc. Clin. Oncol. Annu. Meet. 2025, 45, e473856. [Google Scholar] [CrossRef]
- Fujimura, T.; Muto, Y.; Asano, Y. Immunotherapy for Melanoma: The Significance of Immune Checkpoint Inhibitors for the Treatment of Advanced Melanoma. Int. J. Mol. Sci. 2022, 23, 15720. [Google Scholar] [CrossRef]
- Namikawa, K.; Yamazaki, N. Targeted Therapy and Immunotherapy for Melanoma in Japan. Curr. Treat. Options Oncol. 2019, 20, 7. [Google Scholar] [CrossRef]
- Swetter, S.M.; Johnson, D.; Albertini, M.R.; Barker, C.A.; Bateni, S.; Baumgartner, J.; Bhatia, S.; Bichakjian, C.; Boland, G.; Chandra, S.; et al. NCCN Guidelines® Insights: Melanoma: Cutaneous, Version 2.2024. J. Natl. Compr. Cancer Netw. 2024, 22, 290–298. [Google Scholar] [CrossRef]
- Di Trolio, R.; Simeone, E.; Di Lorenzo, G.; Buonerba, C.; Ascierto, P.A. The use of interferon in melanoma patients: A systematic review. Cytokine Growth Factor Rev. 2015, 26, 203–212. [Google Scholar] [CrossRef]
- Egberts, F.; Gutzmer, R.; Ugurel, S.; Becker, J.C.; Trefzer, U.; Degen, A.; Schenck, F.; Frey, L.; Wilhelm, T.; Hassel, J.C.; et al. Sorafenib and pegylated interferon-α2b in advanced metastatic melanoma: A multicenter phase II DeCOG trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2011, 22, 1667–1674. [Google Scholar] [CrossRef]
- Ishihara, K. [Clinical trials of human fibroblast interferon (Hu IFN-beta) in the treatment of malignant tumors of the skin]. Nihon Gan Chiryo Gakkai Shi 1983, 18, 41–53. [Google Scholar]
- Rosenberg, S.A.; Lotze, M.T.; Muul, L.M.; Leitman, S.; Chang, A.E.; Ettinghausen, S.E.; Matory, Y.L.; Skibber, J.M.; Shiloni, E.; Vetto, J.T.; et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N. Engl. J. Med. 1985, 313, 1485–1492. [Google Scholar] [CrossRef]
- Atkins, M.B.; Lotze, M.T.; Dutcher, J.P.; Fisher, R.I.; Weiss, G.; Margolin, K.; Abrams, J.; Sznol, M.; Parkinson, D.; Hawkins, M.; et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: Analysis of 270 patients treated between 1985 and 1993. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1999, 17, 2105–2116. [Google Scholar] [CrossRef]
- Raeber, M.E.; Sahin, D.; Karakus, U.; Boyman, O. A systematic review of interleukin-2-based immunotherapies in clinical trials for cancer and autoimmune diseases. eBioMedicine 2023, 90, 104539. [Google Scholar] [CrossRef]
- Diab, A.; Gogas, H.; Sandhu, S.; Long, G.V.; Ascierto, P.A.; Larkin, J.; Sznol, M.; Franke, F.; Ciuleanu, T.E.; Pereira, C.; et al. Bempegaldesleukin Plus Nivolumab in Untreated Advanced Melanoma: The Open-Label, Phase III PIVOT IO 001 Trial Results. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2023, 41, 4756–4767. [Google Scholar] [CrossRef]
- Sabel, M.S.; Sondak, V.K. Is there a role for adjuvant high-dose interferon-alpha-2b in the management of melanoma? Drugs 2003, 63, 1053–1058. [Google Scholar] [CrossRef]
- Davar, D.; Ding, F.; Saul, M.; Sander, C.; Tarhini, A.A.; Kirkwood, J.M.; Tawbi, H.A. High-dose interleukin-2 (HD IL-2) for advanced melanoma: A single center experience from the University of Pittsburgh Cancer Institute. J. Immunother. Cancer 2017, 5, 74. [Google Scholar] [CrossRef]
- Leach, D.R.; Krummel, M.F.; Allison, J.P. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996, 271, 1734–1736. [Google Scholar] [CrossRef]
- Rowshanravan, B.; Halliday, N.; Sansom, D.M. CTLA-4: A moving target in immunotherapy. Blood 2018, 131, 58–67. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- McDermott, D.; Haanen, J.; Chen, T.T.; Lorigan, P.; O’Day, S. Efficacy and safety of ipilimumab in metastatic melanoma patients surviving more than 2 years following treatment in a phase III trial (MDX010-20). Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2013, 24, 2694–2698. [Google Scholar] [CrossRef]
- Schadendorf, D.; Hodi, F.S.; Robert, C.; Weber, J.S.; Margolin, K.; Hamid, O.; Patt, D.; Chen, T.T.; Berman, D.M.; Wolchok, J.D. Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 1889–1894. [Google Scholar] [CrossRef]
- Robert, C.; Long, G.V.; Larkin, J.; Wolchok, J.D.; Hassel, J.C.; Schadendorf, D.; Hodi, F.S.; Lebbé, C.; Grob, J.J.; Hyngstrom, J.R.; et al. Long-term outcomes among patients who respond within the first year to nivolumab plus ipilimumab or nivolumab monotherapy: A pooled analysis in 935 patients. Eur. J. Cancer 2025, 214, 115119. [Google Scholar] [CrossRef]
- Robert, C.; Carlino, M.S.; McNeil, C.; Ribas, A.; Grob, J.J.; Schachter, J.; Nyakas, M.; Kee, D.; Petrella, T.M.; Blaustein, A.; et al. Seven-Year Follow-Up of the Phase III KEYNOTE-006 Study: Pembrolizumab Versus Ipilimumab in Advanced Melanoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2023, 41, 3998–4003. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Long-Term Outcomes with Nivolumab Plus Ipilimumab or Nivolumab Alone Versus Ipilimumab in Patients with Advanced Melanoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 127–137. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Hodi, F.S.; Lipson, E.J.; Schadendorf, D.; Ascierto, P.A.; Matamala, L.; Castillo Gutiérrez, E.; Rutkowski, P.; Gogas, H.; Lao, C.D.; et al. Three-Year Overall Survival with Nivolumab Plus Relatlimab in Advanced Melanoma From RELATIVITY-047. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2025, 43, 1546–1552. [Google Scholar] [CrossRef]
- VanderWalde, A.; Bellasea, S.L.; Kendra, K.L.; Khushalani, N.I.; Campbell, K.M.; Scumpia, P.O.; Kuklinski, L.F.; Collichio, F.; Sosman, J.A.; Ikeguchi, A.; et al. Ipilimumab with or without nivolumab in PD-1 or PD-L1 blockade refractory metastatic melanoma: A randomized phase 2 trial. Nat. Med. 2023, 29, 2278–2285. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Lipson, E.J.; Dummer, R.; Larkin, J.; Long, G.V.; Sanborn, R.E.; Chiarion-Sileni, V.; Dréno, B.; Dalle, S.; Schadendorf, D.; et al. Nivolumab and Relatlimab in Patients with Advanced Melanoma that Had Progressed on Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy: Results from the Phase I/IIa RELATIVITY-020 Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2023, 41, 2724–2735. [Google Scholar] [CrossRef]
- Fujimura, T.; Yoshino, K.; Kato, H.; Fukushima, S.; Ishizuki, S.; Otsuka, A.; Matsushita, S.; Amagai, R.; Muto, Y.; Yamazaki, E.; et al. A phase II multicentre study of plasminogen activator inhibitor-1 inhibitor (TM5614) plus nivolumab for treating anti-programmed cell death 1 antibody-refractory malignant melanoma: TM5614-MM trial. Br. J. Dermatol. 2024, 191, 691–697. [Google Scholar] [CrossRef]
- Attia, P.; Phan, G.Q.; Maker, A.V.; Robinson, M.R.; Quezado, M.M.; Yang, J.C.; Sherry, R.M.; Topalian, S.L.; Kammula, U.S.; Royal, R.E.; et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 6043–6053. [Google Scholar] [CrossRef]
- Tang, Q.; Chen, Y.; Li, X.; Long, S.; Shi, Y.; Yu, Y.; Wu, W.; Han, L.; Wang, S. The role of PD-1/PD-L1 and application of immune-checkpoint inhibitors in human cancers. Front. Immunol. 2022, 13, 964442. [Google Scholar] [CrossRef]
- Ghosh, C.; Luong, G.; Sun, Y. A snapshot of the PD-1/PD-L1 pathway. J. Cancer 2021, 12, 2735–2746. [Google Scholar] [CrossRef]
- Ai, L.; Xu, A.; Xu, J. Roles of PD-1/PD-L1 Pathway: Signaling, Cancer, and Beyond. Adv. Exp. Med. Biol. 2020, 1248, 33–59. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Drake, C.G.; Wollner, I.; Powderly, J.D.; Picus, J.; Sharfman, W.H.; Stankevich, E.; Pons, A.; Salay, T.M.; McMiller, T.L.; et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 3167–3175. [Google Scholar] [CrossRef]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Di Giacomo, A.M.; Mortier, L.; Rutkowski, P.; Hassel, J.C.; McNeil, C.M.; Kalinka, E.A.; et al. Five-Year Outcomes with Nivolumab in Patients with Wild-Type BRAF Advanced Melanoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 3937–3946. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Chiarion-Sileni, V.; Rutkowski, P.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Queirolo, P.; Dummer, R.; Butler, M.O.; Hill, A.G.; et al. Final, 10-Year Outcomes with Nivolumab plus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2025, 392, 11–22. [Google Scholar] [CrossRef]
- Cybulska-Stopa, B.; Piejko, K.; Ostaszewski, K.; Dziura, R.; Galus, Ł.; Ziółkowska, B.; Kempa-Kamińska, N.; Ziętek, M.; Bal, W.; Kamycka, A.; et al. Long-term clinical evidence of comparable efficacy and toxicity of nivolumab and pembrolizumab in advanced melanoma treatment. Melanoma Res. 2023, 33, 208–217. [Google Scholar] [CrossRef]
- Bai, X.; Shoushtari, A.N.; Betof Warner, A.; Si, L.; Tang, B.; Cui, C.; Yang, X.; Wei, X.; Quach, H.T.; Cann, C.G.; et al. Benefit and toxicity of programmed death-1 blockade vary by ethnicity in patients with advanced melanoma: An international multicentre observational study. Br. J. Dermatol. 2022, 187, 401–410. [Google Scholar] [CrossRef]
- Nakamura, Y.; Namikawa, K.; Yoshino, K.; Yoshikawa, S.; Uchi, H.; Goto, K.; Nakamura, Y.; Fukushima, S.; Kiniwa, Y.; Takenouchi, T.; et al. Anti-PD1 checkpoint inhibitor therapy in acral melanoma: A multicenter study of 193 Japanese patients. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020, 31, 1198–1206. [Google Scholar] [CrossRef]
- Weber, J.; Mandala, M.; Del Vecchio, M.; Gogas, H.J.; Arance, A.M.; Cowey, C.L.; Dalle, S.; Schenker, M.; Chiarion-Sileni, V.; Marquez-Rodas, I.; et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N. Engl. J. Med. 2017, 377, 1824–1835. [Google Scholar] [CrossRef]
- Larkin, J.; Del Vecchio, M.; Mandalá, M.; Gogas, H.; Arance Fernandez, A.M.; Dalle, S.; Cowey, C.L.; Schenker, M.; Grob, J.J.; Chiarion-Sileni, V.; et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III/IV Melanoma: 5-Year Efficacy and Biomarker Results from CheckMate 238. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2023, 29, 3352–3361. [Google Scholar] [CrossRef]
- Eggermont, A.M.; Kicinski, M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.; Dalle, S.; Haydon, A.; Meshcheryakov, A.; Khattak, A.; et al. Seven-year analysis of adjuvant pembrolizumab versus placebo in stage III melanoma in the EORTC1325/KEYNOTE-054 trial. Eur. J. Cancer 2024, 211, 114327. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Blank, C.U.; Mandalà, M.; Long, G.V.; Atkinson, V.G.; Dalle, S.; Haydon, A.M.; Meshcheryakov, A.; Khattak, A.; Carlino, M.S.; et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma (EORTC 1325-MG/KEYNOTE-054): Distant metastasis-free survival results from a double-blind, randomised, controlled, phase 3 trial. Lancet. Oncol. 2021, 22, 643–654. [Google Scholar] [CrossRef]
- Winge-Main, A.; Robsahm, T.E.; Nyakas, M.; Festervoll, G.; Torkilseng, E.; Thybo, S.; Pati, S.; Carroll, R. Long-term outcomes of stage IIB-IV melanoma patients: Nationwide data from Norway. Future Oncol. 2023, 19, 205–215. [Google Scholar] [CrossRef]
- Luke, J.J.; Rutkowski, P.; Queirolo, P.; Del Vecchio, M.; Mackiewicz, J.; Chiarion-Sileni, V.; de la Cruz Merino, L.; Khattak, M.A.; Schadendorf, D.; Long, G.V.; et al. Pembrolizumab versus placebo as adjuvant therapy in completely resected stage IIB or IIC melanoma (KEYNOTE-716): A randomised, double-blind, phase 3 trial. Lancet 2022, 399, 1718–1729. [Google Scholar] [CrossRef]
- Luke, J.J.; Ascierto, P.A.; Khattak, M.A.; de la Cruz Merino, L.; Del Vecchio, M.; Rutkowski, P.; Spagnolo, F.; Mackiewicz, J.; Chiarion-Sileni, V.; Kirkwood, J.M.; et al. Pembrolizumab Versus Placebo as Adjuvant Therapy in Resected Stage IIB or IIC Melanoma: Final Analysis of Distant Metastasis-Free Survival in the Phase III KEYNOTE-716 Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2024, 42, 1619–1624. [Google Scholar] [CrossRef]
- Hilke, F.J.; Sinnberg, T.; Gschwind, A.; Niessner, H.; Demidov, G.; Amaral, T.; Ossowski, S.; Bonzheim, I.; Röcken, M.; Riess, O.; et al. Distinct Mutation Patterns Reveal Melanoma Subtypes and Influence Immunotherapy Response in Advanced Melanoma Patients. Cancers 2020, 12, 2359. [Google Scholar] [CrossRef]
- Yu, J.; Yan, J.; Guo, Q.; Chi, Z.; Tang, B.; Zheng, B.; Yu, J.; Yin, T.; Cheng, Z.; Wu, X.; et al. Genetic Aberrations in the CDK4 Pathway Are Associated with Innate Resistance to PD-1 Blockade in Chinese Patients with Non-Cutaneous Melanoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 6511–6523. [Google Scholar] [CrossRef]
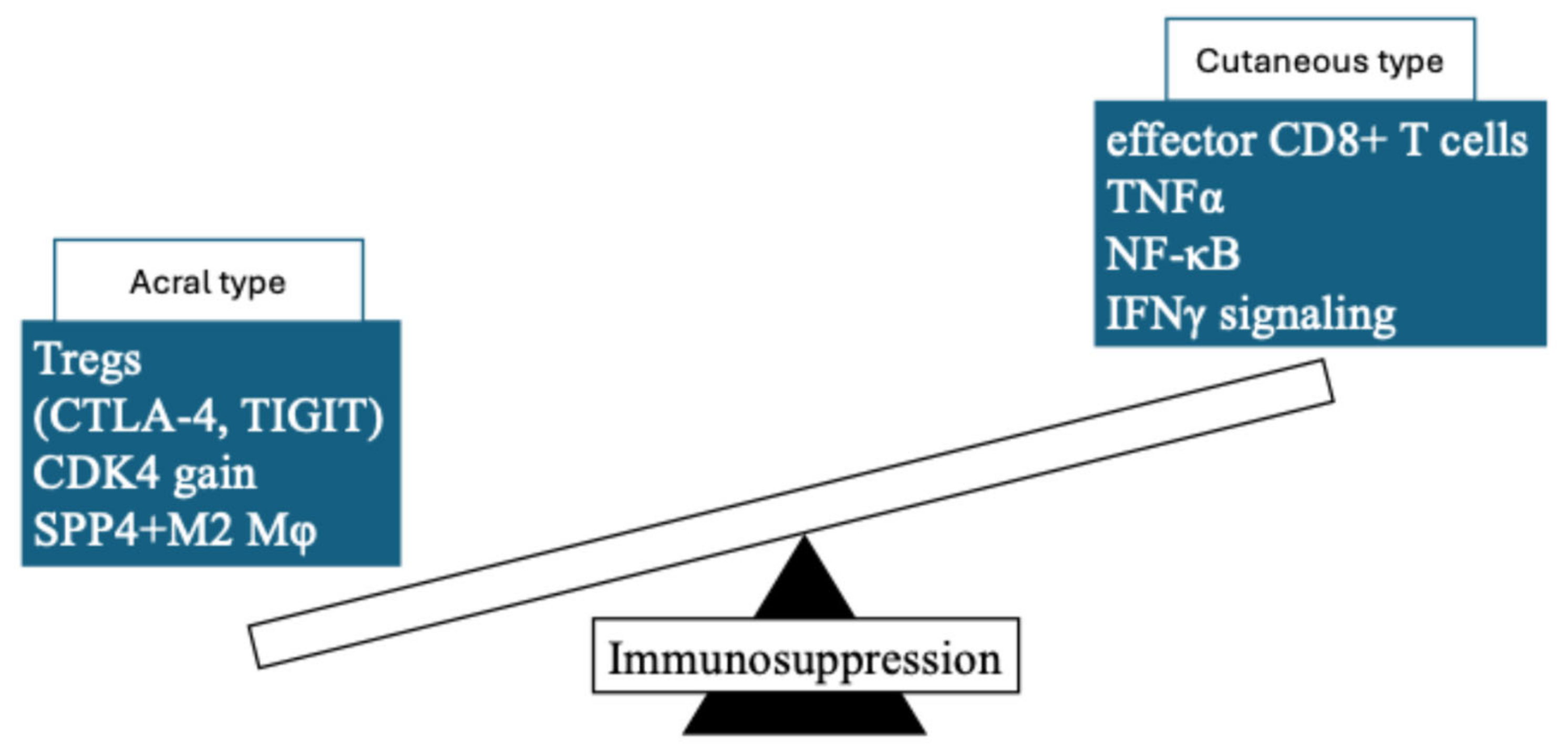
- Minowa, T.; Murata, K.; Mizue, Y.; Murai, A.; Nakatsugawa, M.; Sasaki, K.; Tokita, S.; Kubo, T.; Kanaseki, T.; Tsukahara, T.; et al. Single-cell profiling of acral melanoma infiltrating lymphocytes reveals a suppressive tumor microenvironment. Sci. Transl. Med. 2024, 16, eadk8832. [Google Scholar] [CrossRef]
- Kirkwood, J.M.; Del Vecchio, M.; Weber, J.; Hoeller, C.; Grob, J.J.; Mohr, P.; Loquai, C.; Dutriaux, C.; Chiarion-Sileni, V.; Mackiewicz, J.; et al. Adjuvant nivolumab in resected stage IIB/C melanoma: Primary results from the randomized, phase 3 CheckMate 76K trial. Nat. Med. 2023, 29, 2835–2843. [Google Scholar] [CrossRef]
- Muto, Y.; Kambayashi, Y.; Kato, H.; Fukushima, S.; Ito, T.; Maekawa, T.; Fujisawa, Y.; Yoshino, K.; Uchi, H.; Matsushita, S.; et al. Adjuvant Anti-PD-1 Antibody Therapy for Advanced Melanoma: A Multicentre Study of 78 Japanese Cases. Acta Derm.-Venereol. 2022, 102, adv00756. [Google Scholar] [CrossRef]
- Muto, Y.; Kambayashi, Y.; Kato, H.; Fukushima, S.; Ito, T.; Maekawa, T.; Shoichiro, I.; Uchi, H.; Matsushita, S.; Yamamoto, Y.; et al. Postoperative adjuvant therapy for 120 patients with melanoma, including acral and mucosal subtypes: A multicentre, observational study of 2-year follow-up results. Br. J. Dermatol. 2023, 189, 476–478. [Google Scholar] [CrossRef]
- Muto, Y.; Kambayashi, Y.; Kato, H.; Mizuhashi, S.; Ito, T.; Maekawa, T.; Ishizuki, S.; Uchi, H.; Matsushita, S.; Yamamoto, Y.; et al. Three-Year Analysis of Adjuvant Therapy in Postoperative Melanoma including Acral and Mucosal Subtypes. Cancers 2024, 16, 2755. [Google Scholar] [CrossRef]
- Huang, A.C.; Orlowski, R.J.; Xu, X.; Mick, R.; George, S.M.; Yan, P.K.; Manne, S.; Kraya, A.A.; Wubbenhorst, B.; Dorfman, L.; et al. A single dose of neoadjuvant PD-1 blockade predicts clinical outcomes in resectable melanoma. Nat. Med. 2019, 25, 454–461. [Google Scholar] [CrossRef]
- Sharon, C.E.; Tortorello, G.N.; Ma, K.L.; Huang, A.C.; Xu, X.; Giles, L.R.; McGettigan, S.; Kreider, K.; Schuchter, L.M.; Mathew, A.J.; et al. Long-term outcomes to neoadjuvant pembrolizumab based on pathological response for patients with resectable stage III/IV cutaneous melanoma. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2023, 34, 806–812. [Google Scholar] [CrossRef]
- Patel, S.P.; Othus, M.; Chen, Y.; Wright, G.P., Jr.; Yost, K.J.; Hyngstrom, J.R.; Hu-Lieskovan, S.; Lao, C.D.; Fecher, L.A.; Truong, T.G.; et al. Neoadjuvant-Adjuvant or Adjuvant-Only Pembrolizumab in Advanced Melanoma. N. Engl. J. Med. 2023, 388, 813–823. [Google Scholar] [CrossRef]
- Nakamura, Y.; Namikawa, K.; Kiniwa, Y.; Kato, H.; Yamasaki, O.; Yoshikawa, S.; Maekawa, T.; Matsushita, S.; Takenouchi, T.; Inozume, T.; et al. Efficacy comparison between anti-PD-1 antibody monotherapy and anti-PD-1 plus anti-CTLA-4 combination therapy as first-line immunotherapy for advanced acral melanoma: A retrospective, multicenter study of 254 Japanese patients. Eur. J. Cancer 2022, 176, 78–87. [Google Scholar] [CrossRef]
- Nakamura, Y.; Namikawa, K.; Yoshikawa, S.; Kiniwa, Y.; Maekawa, T.; Yamasaki, O.; Isei, T.; Matsushita, S.; Nomura, M.; Nakai, Y.; et al. Anti-PD-1 antibody monotherapy versus anti-PD-1 plus anti-CTLA-4 combination therapy as first-line immunotherapy in unresectable or metastatic mucosal melanoma: A retrospective, multicenter study of 329 Japanese cases (JMAC study). ESMO Open 2021, 6, 100325. [Google Scholar] [CrossRef]
- Draghi, A.; Chamberlain, C.A.; Furness, A.; Donia, M. Acquired resistance to cancer immunotherapy. Semin. Immunopathol. 2019, 41, 31–40. [Google Scholar] [CrossRef]
- Mori, T.; Namikawa, K.; Yamazaki, N.; Kiniwa, Y.; Yamasaki, O.; Yoshikawa, S.; Inozume, T.; Kato, H.; Nakai, Y.; Fukushima, S.; et al. Efficacy of salvage therapies for advanced acral melanoma after anti-PD-1 monotherapy failure: A multicenter retrospective study of 108 Japanese patients. Front. Med. 2023, 10, 1229937. [Google Scholar] [CrossRef]
- Blank, C.U.; Rozeman, E.A.; Fanchi, L.F.; Sikorska, K.; van de Wiel, B.; Kvistborg, P.; Krijgsman, O.; van den Braber, M.; Philips, D.; Broeks, A.; et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat. Med. 2018, 24, 1655–1661. [Google Scholar] [CrossRef]
- Rozeman, E.A.; Menzies, A.M.; van Akkooi, A.C.J.; Adhikari, C.; Bierman, C.; van de Wiel, B.A.; Scolyer, R.A.; Krijgsman, O.; Sikorska, K.; Eriksson, H.; et al. Identification of the optimal combination dosing schedule of neoadjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma (OpACIN-neo): A multicentre, phase 2, randomised, controlled trial. Lancet Oncol. 2019, 20, 948–960. [Google Scholar] [CrossRef]
- Blank, C.U.; Lucas, M.W.; Scolyer, R.A.; van de Wiel, B.A.; Menzies, A.M.; Lopez-Yurda, M.; Hoeijmakers, L.L.; Saw, R.P.M.; Lijnsvelt, J.M.; Maher, N.G.; et al. Neoadjuvant Nivolumab and Ipilimumab in Resectable Stage III Melanoma. N. Engl. J. Med. 2024, 391, 1696–1708. [Google Scholar] [CrossRef]
- Aggarwal, V.; Workman, C.J.; Vignali, D.A.A. LAG-3 as the third checkpoint inhibitor. Nat. Immunol. 2023, 24, 1415–1422. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Castillo Gutiérrez, E.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; De Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef]
- Long, G.V.; Lipson, E.J.; Hodi, F.S.; Ascierto, P.A.; Larkin, J.; Lao, C.; Grob, J.J.; Ejzykowicz, F.; Moshyk, A.; Garcia-Horton, V.; et al. First-Line Nivolumab Plus Relatlimab Versus Nivolumab Plus Ipilimumab in Advanced Melanoma: An Indirect Treatment Comparison Using RELATIVITY-047 and CheckMate 067 Trial Data. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2024, 42, 3926–3934. [Google Scholar] [CrossRef]
- Amaria, R.N.; Postow, M.; Burton, E.M.; Tetzlaff, M.T.; Ross, M.I.; Torres-Cabala, C.; Glitza, I.C.; Duan, F.; Milton, D.R.; Busam, K.; et al. Neoadjuvant relatlimab and nivolumab in resectable melanoma. Nature 2022, 611, 155–160. [Google Scholar] [CrossRef]
- Bentebibel, S.E.; Hurwitz, M.E.; Bernatchez, C.; Haymaker, C.; Hudgens, C.W.; Kluger, H.M.; Tetzlaff, M.T.; Tagliaferri, M.A.; Zalevsky, J.; Hoch, U.; et al. A First-in-Human Study and Biomarker Analysis of NKTR-214, a Novel IL2Rβγ-Biased Cytokine, in Patients with Advanced or Metastatic Solid Tumors. Cancer Discov. 2019, 9, 711–721. [Google Scholar] [CrossRef]
- Charych, D.; Khalili, S.; Dixit, V.; Kirk, P.; Chang, T.; Langowski, J.; Rubas, W.; Doberstein, S.K.; Eldon, M.; Hoch, U.; et al. Modeling the receptor pharmacology, pharmacokinetics, and pharmacodynamics of NKTR-214, a kinetically-controlled interleukin-2 (IL2) receptor agonist for cancer immunotherapy. PLoS ONE 2017, 12, e0179431. [Google Scholar] [CrossRef]
- Charych, D.H.; Hoch, U.; Langowski, J.L.; Lee, S.R.; Addepalli, M.K.; Kirk, P.B.; Sheng, D.; Liu, X.; Sims, P.W.; VanderVeen, L.A.; et al. NKTR-214, an Engineered Cytokine with Biased IL2 Receptor Binding, Increased Tumor Exposure, and Marked Efficacy in Mouse Tumor Models. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 680–690. [Google Scholar] [CrossRef]
- Pham, D.D.M.; Guhan, S.; Tsao, H. KIT and Melanoma: Biological Insights and Clinical Implications. Yonsei Med. J. 2020, 61, 562–571. [Google Scholar] [CrossRef]
- Larkin, J.; Marais, R.; Porta, N.; Gonzalez de Castro, D.; Parsons, L.; Messiou, C.; Stamp, G.; Thompson, L.; Edmonds, K.; Sarker, S.; et al. Nilotinib in KIT-driven advanced melanoma: Results from the phase II single-arm NICAM trial. Cell Rep. Med. 2024, 5, 101435. [Google Scholar] [CrossRef]
- Janku, F.; Bauer, S.; Shoumariyeh, K.; Jones, R.L.; Spreafico, A.; Jennings, J.; Psoinos, C.; Meade, J.; Ruiz-Soto, R.; Chi, P. Efficacy and safety of ripretinib in patients with KIT-altered metastatic melanoma. ESMO Open 2022, 7, 100520. [Google Scholar] [CrossRef]
- Hirai, I.; Tanese, K.; Fukuda, K.; Fusumae, T.; Nakamura, Y.; Sato, Y.; Amagai, M.; Funakoshi, T. Imatinib mesylate in combination with pembrolizumab in patients with advanced KIT-mutant melanoma following progression on standard therapy: A phase I/II trial and study protocol. Medicine 2021, 100, e27832. [Google Scholar] [CrossRef]
- Ibrahim, A.A.; Fujimura, T.; Uno, T.; Terada, T.; Hirano, K.I.; Hosokawa, H.; Ohta, A.; Miyata, T.; Ando, K.; Yahata, T. Plasminogen activator inhibitor-1 promotes immune evasion in tumors by facilitating the expression of programmed cell death-ligand 1. Front. Immunol. 2024, 15, 1365894. [Google Scholar] [CrossRef]
- Sadik, A.; Somarribas Patterson, L.F.; Öztürk, S.; Mohapatra, S.R.; Panitz, V.; Secker, P.F.; Pfänder, P.; Loth, S.; Salem, H.; Prentzell, M.T.; et al. IL4I1 Is a Metabolic Immune Checkpoint that Activates the AHR and Promotes Tumor Progression. Cell 2020, 182, 1252–1270.e1234. [Google Scholar] [CrossRef]
| # | Retrieval Style | No. of References |
|---|---|---|
| #01 | “Melanoma/immunotherapy” [TIAB] | 22,100 |
| #02 | “Melanoma/immune checkpoint inhibitors” [TIAB] and/or “ICIs” [TIAB]) | 8775 |
| #03 | “Melanoma/anti-PD-1 antibody” [TIAB] and/or “anti-PD-1 Ab” [TIAB]) | 2745 |
| #04 | “Melanoma/nivolumab” [TIAB] and/or “pembrolizumab” [TIAB] | 14,168 |
| #05 | “Melanoma/nivolumab” [TIAB] and/or “ipilimumab” [TIAB] | 7910 |
| #06 | “Melanoma/nivolumab” [TIAB] and/or “relatlimab” [TIAB] | 3182 |
| #07 | “Melanoma/anti-PD-1 antibody resistant” [TIAB] | 262 |
| #08 | “Melanoma/clinical trial” [TIAB] and “nivolumab” [TIAB] and/or “pembrolizumab” [TIAB] | 12,495 |
| #09 | “Melanoma/clinical trial” [TIAB] and “nivolumab” [TIAB] | 495 |
| #10 | “Melanoma” [TIAB] and “pembrolizumab” [TIAB] | 453 |
| #11 | “Melanoma” [TIAB] and “anti-PD-1 antibody” [TIAB] | 254 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muto, Y.; Fujimura, T.; Asano, Y. Recent Advances in Immunotherapy for Melanoma: Perspectives on the Development of Novel Treatments: A Mini Review. Cancers 2025, 17, 2265. https://doi.org/10.3390/cancers17132265
Muto Y, Fujimura T, Asano Y. Recent Advances in Immunotherapy for Melanoma: Perspectives on the Development of Novel Treatments: A Mini Review. Cancers. 2025; 17(13):2265. https://doi.org/10.3390/cancers17132265
Chicago/Turabian StyleMuto, Yusuke, Taku Fujimura, and Yoshihide Asano. 2025. "Recent Advances in Immunotherapy for Melanoma: Perspectives on the Development of Novel Treatments: A Mini Review" Cancers 17, no. 13: 2265. https://doi.org/10.3390/cancers17132265
APA StyleMuto, Y., Fujimura, T., & Asano, Y. (2025). Recent Advances in Immunotherapy for Melanoma: Perspectives on the Development of Novel Treatments: A Mini Review. Cancers, 17(13), 2265. https://doi.org/10.3390/cancers17132265







