Endocervical Curettage and Extended HPV Genotyping as Predictors of Residual Disease After Hysterectomy in Postmenopausal Women Previously Treated with LEEP for CIN3: A Multivariate Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. HPV Test and Genotyping
2.2. Surgical Procedures
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Centers for Disease Control and Prevention. How Many Cancers Are Linked with HPV Each Year? 2014. Available online: https://www.cdc.gov/cancer/hpv/cases.html (accessed on 12 January 2025).
- Aupérin, A. Epidemiology of head and neck cancers: An update. Curr. Opin. Oncol. 2020, 32, 178–186. [Google Scholar] [CrossRef]
- Bruno, M.T.; Boemi, S.; Caruso, G.; Sgalambro, F.; Ferlito, S.; Cavallaro, A.; Sudano, M.C.; Palumbo, M. Oral HPV Infection in Women with HPV-Positive Cervix Is Closely Related to Oral Sex. Diagnostics 2023, 13, 2096. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of worldwide incidence and mortality for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, S.N.; Brotherton, J.M.; Kaldor, J.M.; Skinner, S.R.; Cummins, E.; Liu, B.; Bateson, D.; McNamee, K.; Garefalakis, M.; Garland, S.M. Fall in human papillomavirus prevalence following a national vaccination program. J. Infect. Dis. 2012, 206, 1645–1651. [Google Scholar] [CrossRef] [PubMed]
- Soutter, W.P.; Sasieni, P.; Panoskaltsis, T. Long-term risk of invasive cervical cancer after treatment of squamous cervical intraepithelial neoplasia. Int. J. Cancer 2006, 118, 2048–2055. [Google Scholar] [CrossRef]
- Hermansson, M.; Olovsson, E.H.; Lindström, A.K.; Ruth, S. HPV prevalence and HPV-related dysplasia in elderly women. PLoS ONE 2018, 1, e0189300. [Google Scholar] [CrossRef]
- Wright, T.C., Jr.; Cox, J.T.; Massad, L.S.; Carlson, J.; Twiggs, L.B.; Wilkinson, E.J.; American Society for Colposcopy and Cervical Pathology. 2001 consensus guidelines for the management of women with cervical intraepithelial neoplasia. Am. J. Obstet. Gynecol. 2003, 189, 295–304. [Google Scholar] [CrossRef]
- Kesic, V.; Dokic, M.; Atanackovic, J.; Milenkovic, S.; Kalezic, I.; Vukovic, S. Hysterectomy for Treatment of CIN. J. Low. Genit. Tract. Dis. 2003, 7, 32–35. [Google Scholar] [CrossRef]
- Bruno, M.T.; Scalia, G.; Cassaro, N.; Costanzo, M.; Boemi, S. Conservative management of CIN2 p16 positive lesions in women with multiple HPV infection. BMC Infect. Dis. 2020, 20, 801. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bornstein, J.; Bentley, J.; Bösze, P.; Girardi, F.; Haefner, H.; Menton, M.; Perrotta, M.; Prendiville, W.; Russell, P.; Sideri, M.; et al. 2011 colposcopic terminology of the International Federation for Cervical Pathology and Colposcopy. Obstet. Gynecol. 2012, 120, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Perkins, R.B.; Guido, R.S.; Castle, P.E.; Chelmow, D.; Einstein, M.H.; Garcia, F.; Huh, W.K.; Kim, J.J.; Moscicki, A.B.; Nayar, R.; et al. 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors. J. Low. Genit. Tract. Dis. 2020, 24, 102–131. [Google Scholar] [CrossRef]
- Bruno, M.T.; Bonanno, G.; Sgalambro, F.; Cavallaro, A.; Boemi, S. Overexpression of E6/E7 mRNA HPV Is a Prognostic Biomarker for Residual Disease Progression in Women Undergoing LEEP for Cervical Intraepithelial Neoplasia 3. Cancers 2023, 15, 4203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ni, Z.; Wei, T.; Liu, Q. Persistent HPV infection after conization of cervical intraepithelial neoplasia a systematic review and meta-analysis. BMC Women’s Health 2023, 23, 216. [Google Scholar] [CrossRef] [PubMed]
- Bilibio, J.P.; Monego, H.I.; Binda, M.L.A.; Dos Reis, R. Menopausal status is associated with a high risk for residual disease after cervical conization with positive margins. PLoS ONE 2019, 14, e0217562. [Google Scholar] [CrossRef]
- Bruno, M.T.; Valenti, G.; Ruggeri, Z.; Incognito, G.G.; Coretti, P.; Montana, G.D.; Panella, M.M.; Mereu, L. Correlation of the HPV 16 Genotype Persistence in Women Undergoing LEEP for CIN3 with the Risk of CIN2+ Relapses in the First 18 Months of Follow-Up: A Multicenter Retrospective Study. Diagnostics 2024, 14, 509. [Google Scholar] [CrossRef]
- Vieira-Baptista, P.; Preti, M.; Bornstein, J. Human Papillomavirus Infection and Cancer Risk in Peri- and Postmenopausal Women. In Postmenopausal Diseases and Disorders; Pérez-López, F., Ed.; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Ciavattini, A.; Di Giuseppe, J.; Marconi, C.; Giannella, L.; Delli Carpini, G.; Paolucci, M.; Fichera, M.; De Vincenzo, R.P.; Scambia, G.; Evangelista, M.T.; et al. Hysterectomy for cervical intraepithelial neoplasia: A retrospective observational multi-institutional study. Int. J. Gynaecol. Obstet. 2022, 159, 679–688. [Google Scholar] [CrossRef]
- Ruengkhachorn, I.; Phithakwatchara, N.; Viriyapak, B.; Sangkarat, S.; Hanamornroongruang, S.; Petsuksiri, J. Comparison of oncologic outcomes of unanticipated cervical carcinoma in women undergoing inadvertent simple hysterectomy and those undergoing surgical treatment after preoperative diagnosis. Gynecol. Oncol. 2019, 153, 248–254. [Google Scholar] [CrossRef]
- Gupta, R.; Sodhani, P.; Mehrotra, R.; Gupta, S. Cervical high-grade squamous intraepithelial lesion on conventional cytology: Cytological patterns, pitfalls, and diagnostic clues. Diagn. Cytopathol. 2019, 47, 1267–1276. [Google Scholar] [CrossRef]
- Yamamoto, R.; Sekiyama, K.; Higuchi, T.; Ikeda, M.; Mikami, M.; Kobayashi, Y.; Nagase, S.; Yokoyama, M.; Enomoto, T.; Katabuchi, H. Value and limitation of conization as a diagnostic procedure for cervical neoplasm. J. Obstet. Gynaecol. Res. 2019, 45, 2419–2424. [Google Scholar] [CrossRef]
- Srisomboon, J.; Pantusart, A.; Phongnarisorn, C.; Suprasert, P. Reasons for improper simple hysterectomy in patients with invasive cervical cancer in the northern region of Thailand. J. Obstet. Gynaecol. Res. 2000, 26, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Behtash, N.; Mousavi, A.; Mohit, M.; Modares, M.; Khanafshar, N.; Hanjani, P. Simple hysterectomy in the presence of invasive cervical cancer in Iran. Int. J. Gynecol. Cancer 2003, 13, 177–181. [Google Scholar] [CrossRef]
- Leath, C.A., 3rd; Straughn, J.M.; Bhoola, S.M.; Partridge, E.E.; Kilgore, L.C.; Alvarez, R.D. The role of radical parametrectomy in the treatment of occult cervical carcinoma after extrafascial hysterectomy. Gynecol. Oncol. 2004, 92, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Kim, D.Y.; Kim, J.H.; Kim, Y.M.; Kim, Y.T.; Nam, J.H. Management of occult invasive cervical cancer found after simple hysterectomy. Ann. Oncol. 2010, 21, 994–1000. [Google Scholar] [CrossRef]
- Suh, D.H.; Chung, H.H.; Kim, J.W.; Park, N.H.; Song, Y.S.; Kang, S.B. An occult invasive cervical cancer found after a simple hysterectomy: A 10-year experience in a single institution. Int. J. Gynecol. Cancer 2011, 21, 1646–1653. [Google Scholar] [CrossRef]
- Kang, W.D.; Ju, U.C.; Kim, S.M. A human papillomavirus (HPV)-16 or HPV-18 genotype is a reliable predictor of residual disease in a subsequent hysterectomy following a loop electrosurgical excision procedure for cervical intraepithelial neoplasia 3. J. Gynecol. Oncol. 2015, 27, e2. [Google Scholar] [CrossRef]
- Bruno, M.T.; Panella, M.M.; Valenti, G.; Ruggeri, Z.; Sgalambro, F.; Reina, S.; Mereu, L. Cervical Intraepithelial Neoplasia Grade 3 (CIN3) in Women Younger than 30 Years Was Significantly Associated with HPV16/18 Genotypes. Cancers 2024, 16, 2043. [Google Scholar] [CrossRef]
- Giannella, L.; Giorgi Rossi, P.; Delli Carpini, G.; Di Giuseppe, J.; Bogani, G.; Gardella, B.; Monti, E.; Liverani, C.A.; Ghelardi, A.; Insinga, S.; et al. Age-related distribution of uncommon HPV genotypes in cervical intraepithelial neoplasia grade 3. Gynecol. Oncol. 2021, 161, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Guardado-Estrada, M.; Juárez-Torres, E.; Román-Bassaure, E.; Medina-Martinez, I.; Alfaro, A.; Benuto, R.E.; Dean, M.; Villegas-Sepulveda, N.; Berumen, J. The distribution of high-risk human papillomaviruses is different in young and old patients with cervical cancer. PLoS ONE 2014, 9, e109406. [Google Scholar] [CrossRef]
- Aro, K.; Nieminen, P.; Louvanto, K.; Jakobsson, M.; Virtanen, S.; Lehtinen, M.; Dillner, J.; Kalliala, I. Age-specific HPV type distribution in high-grade cervical disease in screened and unvaccinated women. Gynecol. Oncol. 2019, 154, 354–359. [Google Scholar] [CrossRef]
- Bruno, M.T.; Valenti, G.; Cavallaro, A.G.; Palermo, I.; Aiello, T.; Farina, J.; Panella, M.M.; Mereu, L. Extended Genotyping to Stratify the Risk of CIN2+ in Women with Persistent HPV Infection, Negative Cytology and Type 3 Transformation Zone. Cancers 2024, 16, 1816. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Cho, T.; Mogami, T.; Yokota, N.R.; Matsunaga, T.; Asai-Sato, M.; Hirahara, F.; Nojima, M.; Mori, M.; Miyagi, E. Evaluation of endocervical curettage with conization in diagnosis of endocervical lesions. J. Obstet. Gynaecol. Res. 2017, 43, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Lea, J.S.; Shin, C.H.; Sheets, E.E.; Coleman, R.L.; Gehrig, P.A.; Duska, L.R.; Miller, D.S.; Schorge, J.O. Endocervical curettage at conization to predict residual cervical adenocarcinoma in situ. Gynecol. Oncol. 2002, 87, 129–132. [Google Scholar] [CrossRef]
- Akgor, U.; Ozgul, N.; Gunes, A.C.; Turkyılmaz, M.; Gultekin, M. Evaluation of Endocervical Curettage in Colposcopy in the Turkish Cervical Cancer Screening Program. J. Clin. Med. 2024, 13, 4417. [Google Scholar] [CrossRef] [PubMed]
- Driggers, R.W.; Zahn, C.M. To ECC or not to ECC: The question remains. Obstet. Gynecol. Clin. N. Am. 2008, 35, 583–597. [Google Scholar] [CrossRef]
- Fu, Y.; Chen, C.; Feng, S.; Cheng, X.; Wang, X.; Xie, X.; Lü, W. Residual disease and risk factors in patients with high-grade cervical intraepithelial neoplasia and positive margins after initial conization. Ther. Clin. Risk Manag. 2015, 11, 851–856. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, D.Y.; Kim, J.H.; Kim, Y.M.; Kim, Y.T.; Nam, J.H. Human Papillomavirus Test After Conization in Predicting Residual Disease in Subsequent Hysterectomy Specimens Joo-Hyun. Obstet. Gynecol. 2009, 114, 87–92. [Google Scholar] [CrossRef]
- Kalliala, I.; Athanasiou, A.; Veroniki, A.A.; Salanti, G.; Efthimiou, O.; Raftis, N.; Bowden, S.; Paraskevaidi, M.; Aro, K.; Arbyn, M.; et al. Incidence and mortality from cervical cancer and other malignancies after treatment of cervical intraepithelial neoplasia: A systematic review and meta-analysis of the literature. Ann. Oncol. 2020, 31, 213–227. [Google Scholar] [CrossRef]
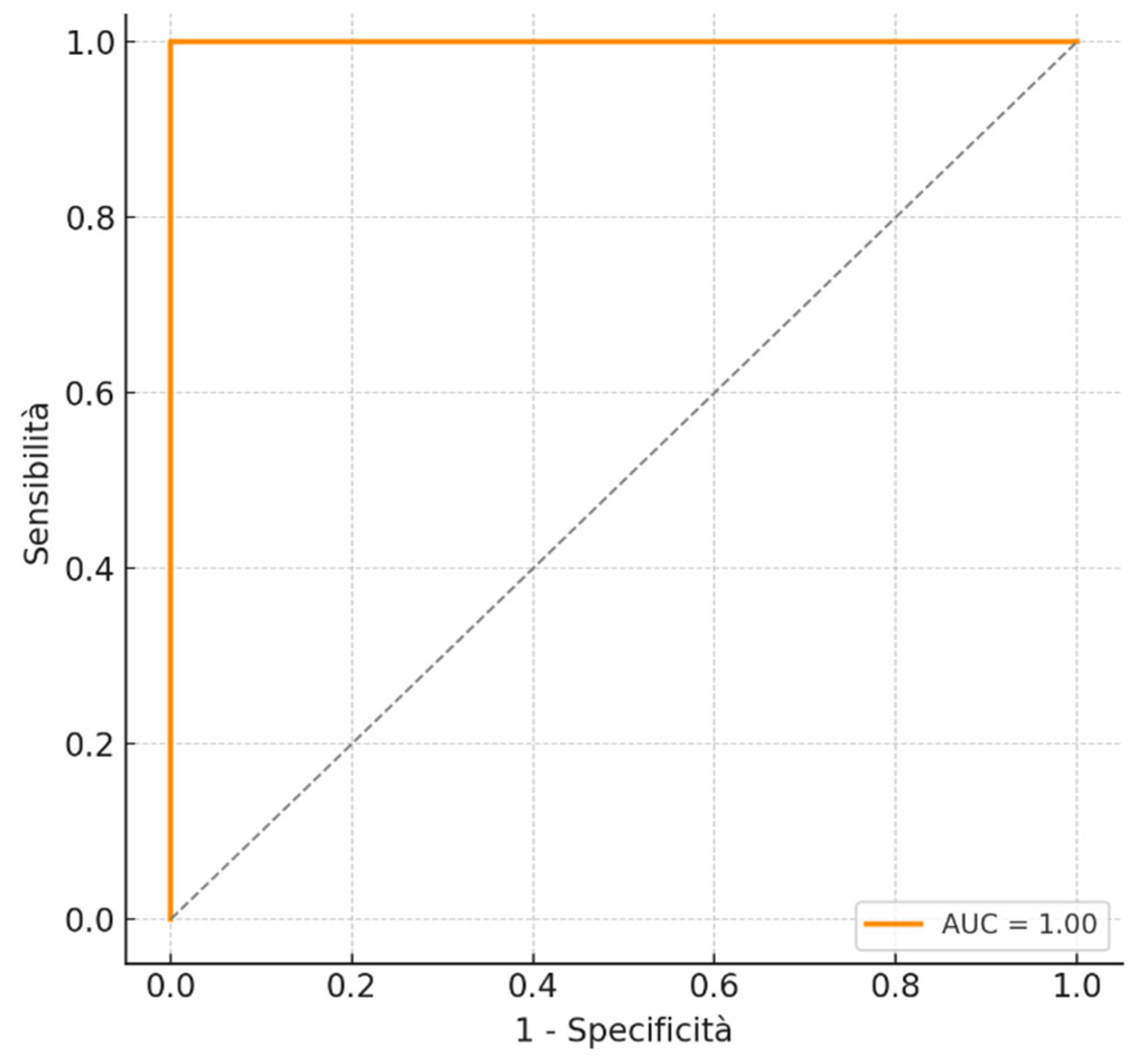
| Variable | Total N (%) | Positive Residual N (%) | Negative Residual N (%) | p-Value | Cramer’s V |
|---|---|---|---|---|---|
| Negative Margins | 93 (60.4) | 11 (28.2) | 82 (71.3) | <0.001 | 0.383 |
| Positive Margins | 61 (39.6) | 28 (71.8) | 33 (28.7) | ||
| Negative HPV post-LEEP | 90 (58.4) | 2 (5.1) | 88 (76.5) | <0.001 | 0.630 |
| HPV no 16/18 | 38 (24.7) | 22 (56.4) | 16 (13.9) | ||
| HPV 16/18 | 26 (16.9) | 15 (38.5) | 11 (9.6) | ||
| Negative ECC | 67 (54.5) | 9 (25.0) | 58 (66.7) | <0.001 | 0.381 |
| Positive ECC | 56 (45.5) | 27 (75.0) | 29 (33.3) | ||
| TZ type 3 | 115 (74.7) | 31 (79.5) | 84 (73.0) | 0.273 | 0.130 |
| Variable | Crude OR (IC 95%) | p-Value | Adjusted OR (IC 95%) | p-Value |
|---|---|---|---|---|
| Positive Margins | 6.325 (2.825–14) | <0.001 | 1.757 (0.519–5.952) | 0.365 |
| Non-HPV 16/18 post-LEEP | 60 (12–298) | <0.001 | 68 (7.8–610) | <0.001 |
| HPV 16/18 post-LEEP | 60 (13–283) | <0.001 | 74 (8.0–694) | <0.001 |
| Positive ECC | 6.000 (2.498–14) | <0.001 | 3.642 (1.154–11) | 0.028 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruno, M.T.; Cavallaro, A.G.; Fiore, M.; Ruggeri, Z.; Somma, M.; Pagana, A.; Mascellino, G.; Laganà, A.S. Endocervical Curettage and Extended HPV Genotyping as Predictors of Residual Disease After Hysterectomy in Postmenopausal Women Previously Treated with LEEP for CIN3: A Multivariate Analysis. Cancers 2025, 17, 2264. https://doi.org/10.3390/cancers17132264
Bruno MT, Cavallaro AG, Fiore M, Ruggeri Z, Somma M, Pagana A, Mascellino G, Laganà AS. Endocervical Curettage and Extended HPV Genotyping as Predictors of Residual Disease After Hysterectomy in Postmenopausal Women Previously Treated with LEEP for CIN3: A Multivariate Analysis. Cancers. 2025; 17(13):2264. https://doi.org/10.3390/cancers17132264
Chicago/Turabian StyleBruno, Maria Teresa, Antonino Giovanni Cavallaro, Maria Fiore, Zaira Ruggeri, Martina Somma, Alessia Pagana, Giuseppe Mascellino, and Antonio Simone Laganà. 2025. "Endocervical Curettage and Extended HPV Genotyping as Predictors of Residual Disease After Hysterectomy in Postmenopausal Women Previously Treated with LEEP for CIN3: A Multivariate Analysis" Cancers 17, no. 13: 2264. https://doi.org/10.3390/cancers17132264
APA StyleBruno, M. T., Cavallaro, A. G., Fiore, M., Ruggeri, Z., Somma, M., Pagana, A., Mascellino, G., & Laganà, A. S. (2025). Endocervical Curettage and Extended HPV Genotyping as Predictors of Residual Disease After Hysterectomy in Postmenopausal Women Previously Treated with LEEP for CIN3: A Multivariate Analysis. Cancers, 17(13), 2264. https://doi.org/10.3390/cancers17132264








