Comparative Analysis of Gut Microbiota Responses to New SN-38 Derivatives, Irinotecan, and FOLFOX in Mice Bearing Colorectal Cancer Patient-Derived Xenografts
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tumor Engraftment
2.2. Administration of Chemotherapy
2.3. Statistical Methods
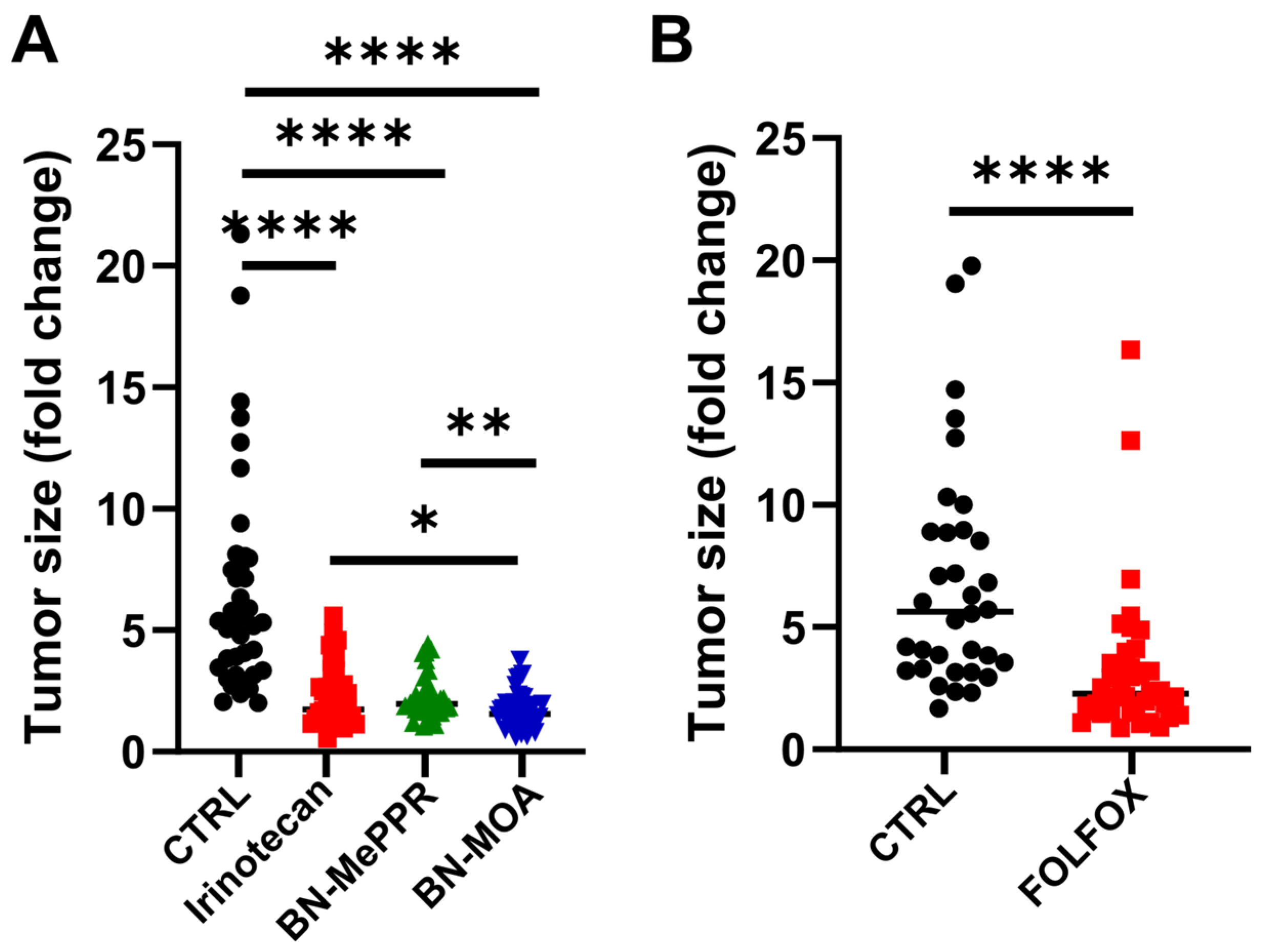
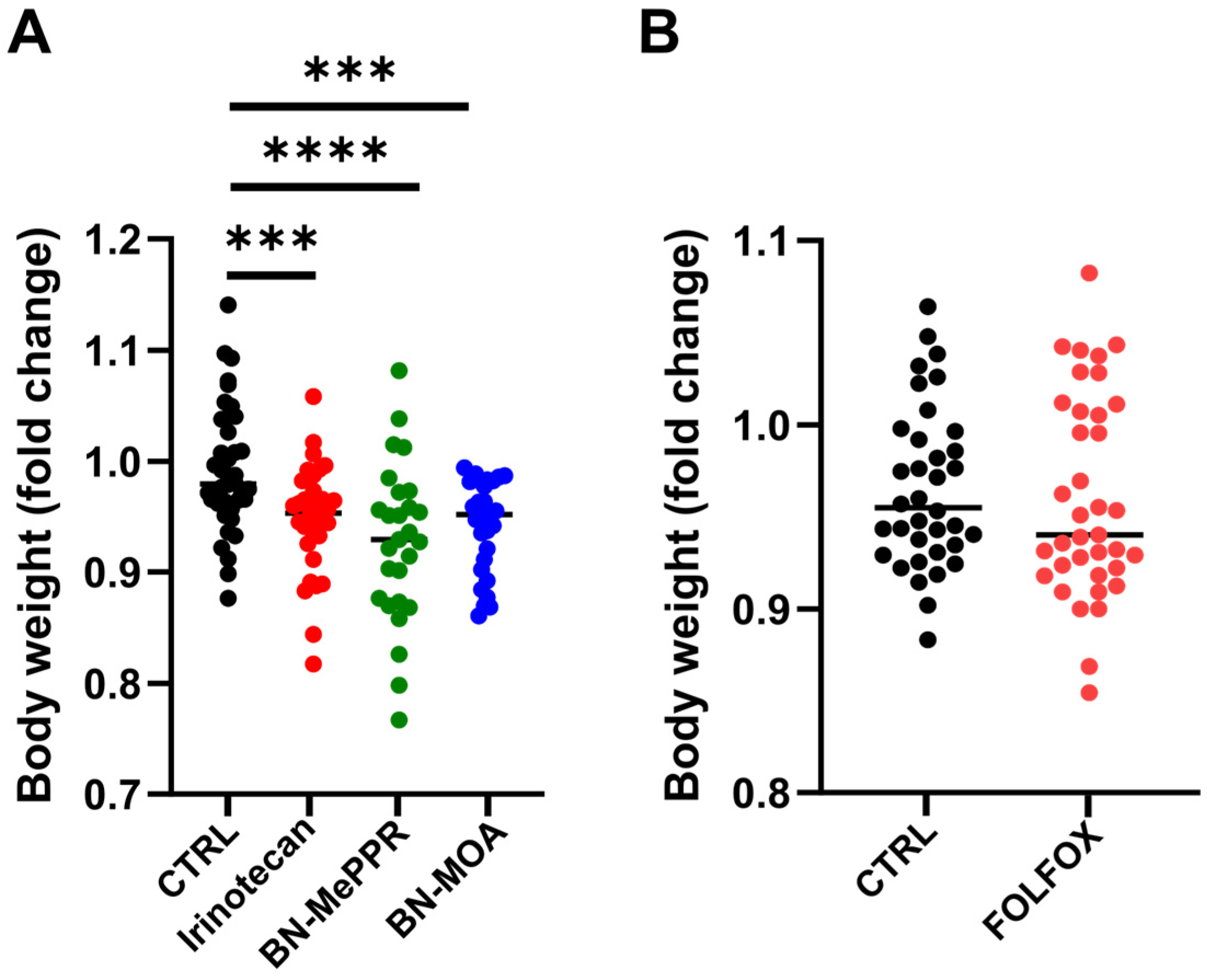
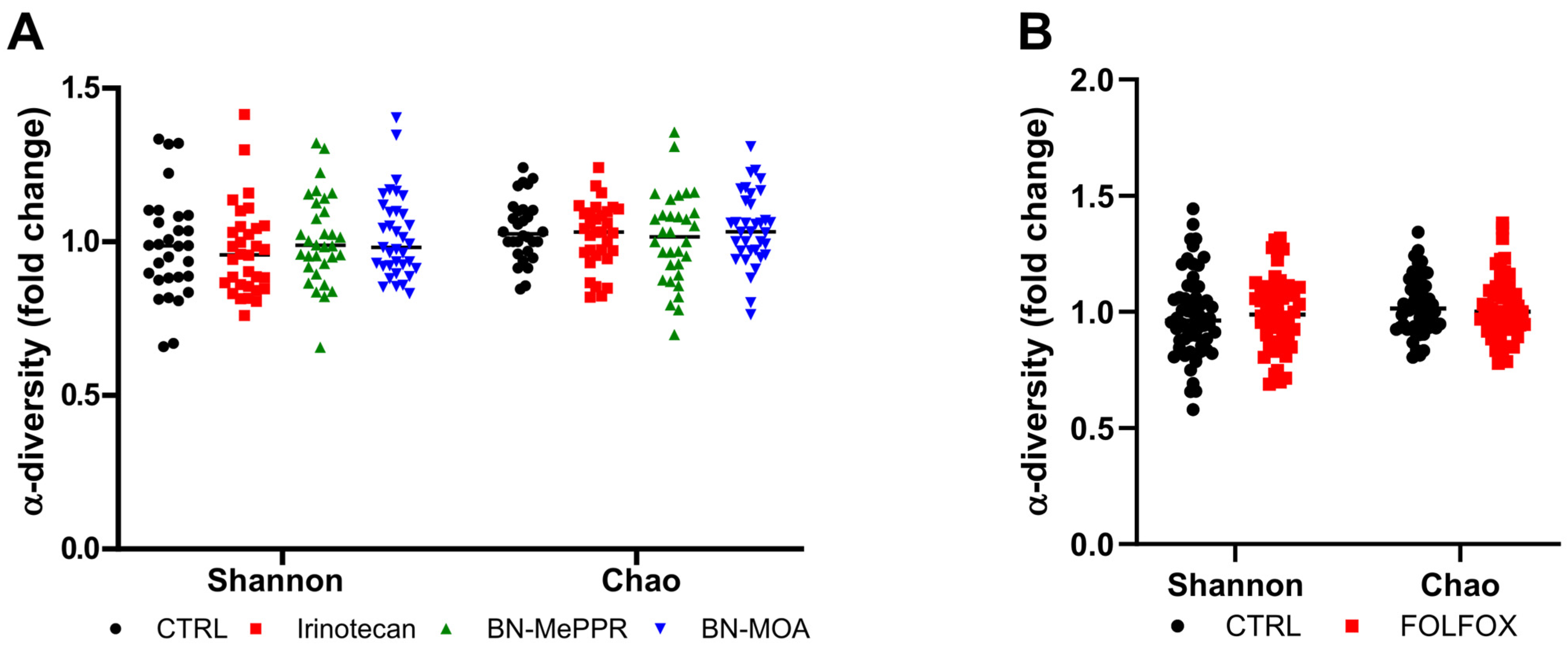
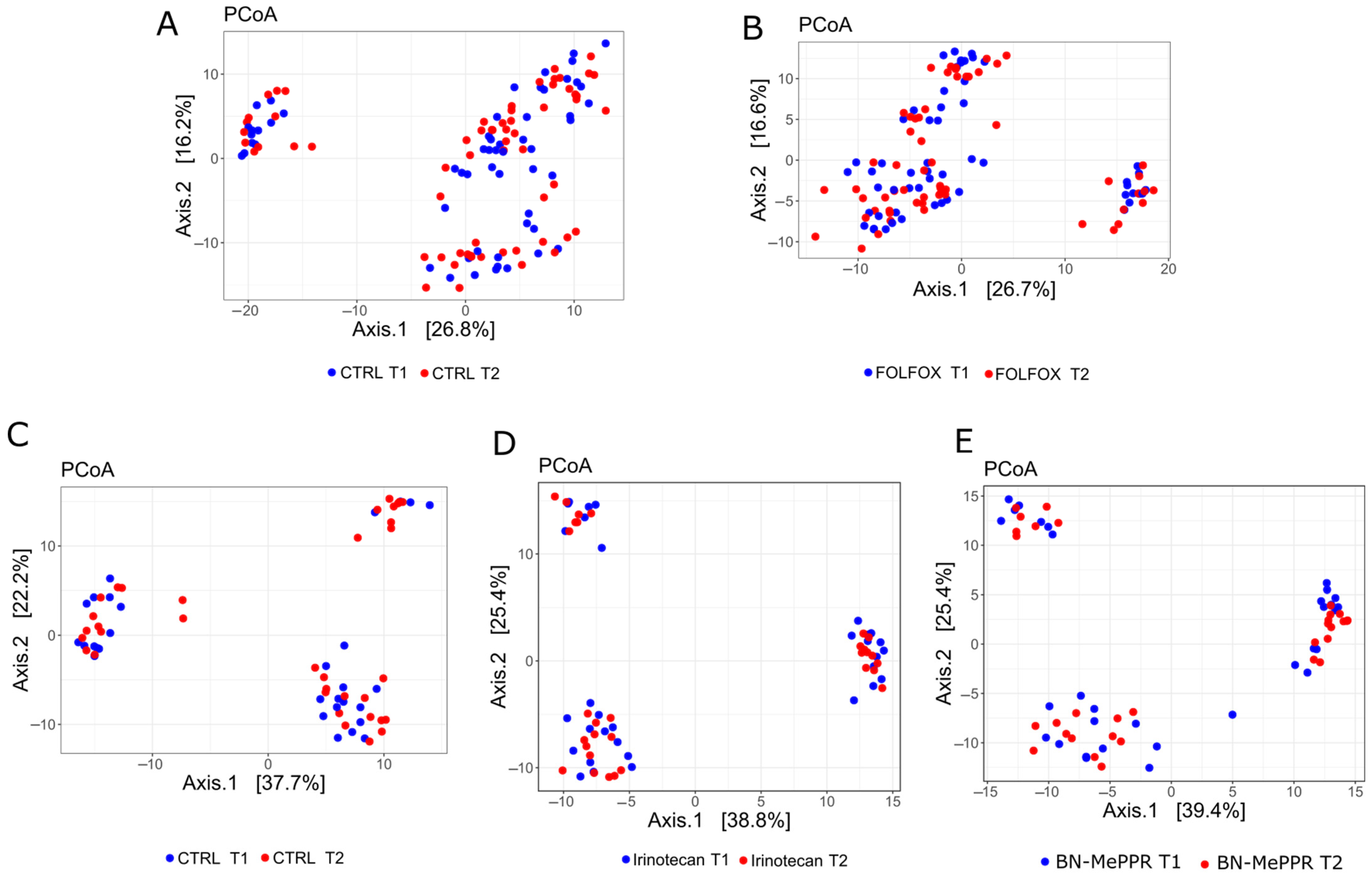
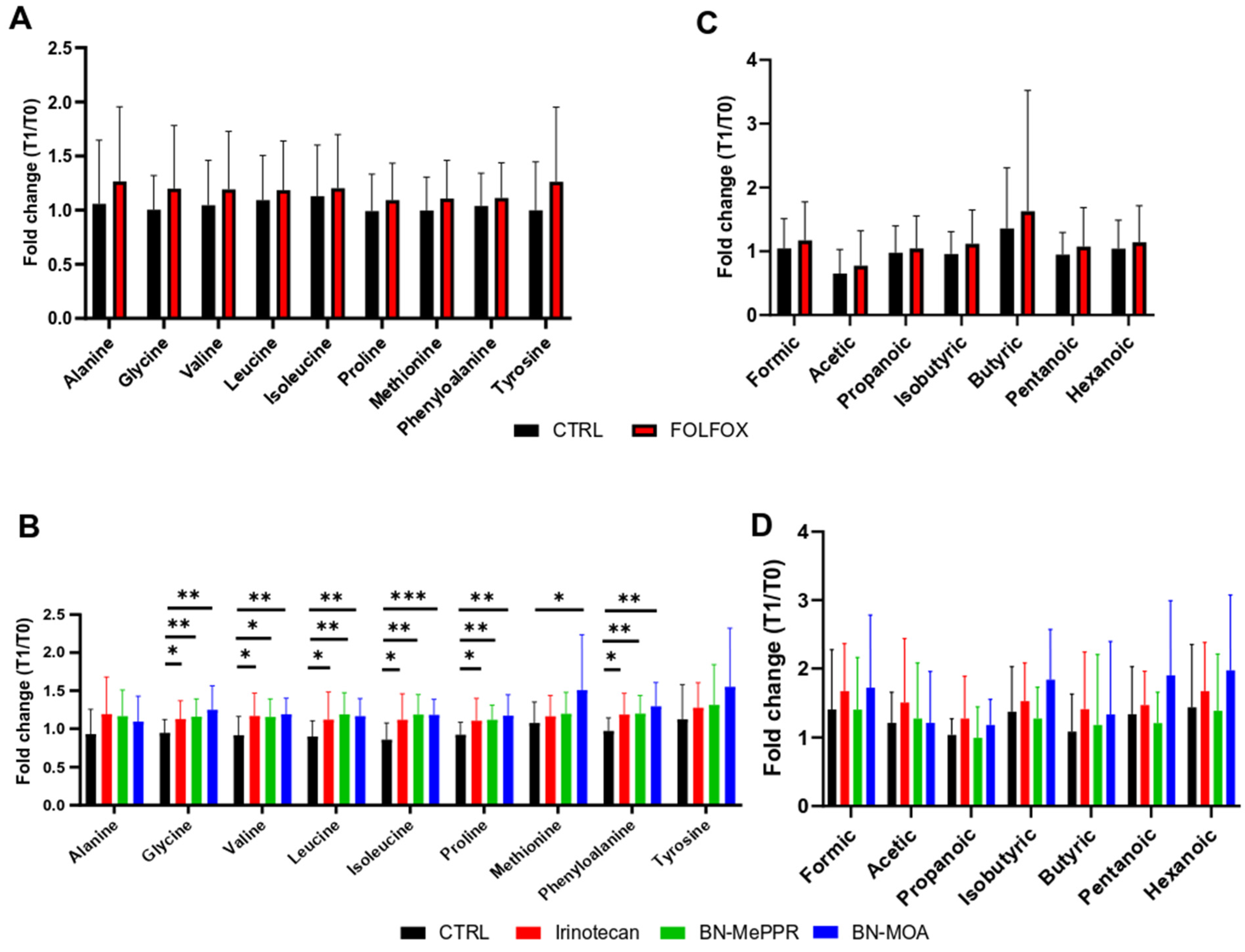
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-FU | 5-fluorouracil |
| AAs | amino acids |
| BN-MePPR | 7-ethyl-9-(N-(N-methylpiperazinyl)methyl-10-hydroxycamptothecin |
| BN-MOA | 7-ethyl-9-(N-morpholinyl)metyl-10-hydroxycamptothecin |
| CES | carboxylesterase |
| CPT | plant alkaloid camptothecin |
| CRC | colon adenocarcinoma |
| NOD | non-obese diabetic mouse |
| NSG | NOD scid gamma mouse |
| PDX | patient-derived xenografts |
| SCFAs | short-chain fatty acids |
| SN-38 | 7-ethyl-10-hydroxycamptothecin |
| TOP1 | topoisomerase I |
| UGT | uridine diphosphate glucuronosyltransferases |
References
- Rothenberg, M.L. Irinotecan (CPT-11): Recent Developments and Future Directions–Colorectal Cancer and Beyond. Oncologist 2001, 6, 66–80. [Google Scholar] [CrossRef]
- Gallotti, B.; Galvao, I.; Leles, G.; Quintanilha, M.F.; Souza, R.O.; Miranda, V.C.; Rocha, V.M.; Trindade, L.M.; Jesus, L.C.L.; Mendes, V.; et al. Effects of Dietary Fibre Intake in Chemotherapy-Induced Mucositis in Murine Model. Br. J. Nutr. 2021, 126, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Merali, Z.; Ross, S.; Paré, G. The Pharmacogenetics of Carboxylesterases: CES1 and CES2 Genetic Variants and Their Clinical Effect. Drug Metab. Drug Interact. 2014, 29, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Takakura, A.; Kurita, A.; Asahara, T.; Yokoba, M.; Yamamoto, M.; Ryuge, S.; Igawa, S.; Yasuzawa, Y.; Sasaki, J.; Kobayashi, H.; et al. Rapid Deconjugation of SN-38 Glucuronide and Adsorption of Released Free SN-38 by Intestinal Microorganisms in Rat. Oncol. Lett. 2012, 3, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Chen, J.; Jia, Y.; Chen, Y.; Li, Y.; Yan, R. Fecal Microbial SN-38G Metabotypes in Colorectal Cancer Patients Are Associated with Irinotecan-Induced Delayed Diarrhea. J. Pharmacol. Exp. Ther. 2023, 385, 53–54. [Google Scholar] [CrossRef]
- Mathijssen, R.H.J.; Loos, W.J.; Verweij, J.; Sparreboom, A. Pharmacology of Topoisomerase I Inhibitors Irinotecan (CPT-11) and Topotecan. Curr. Cancer Drug Targets 2002, 2, 103–123. [Google Scholar] [CrossRef]
- Liu, L.F.; Desai, S.D.; Li, T.K.; Mao, Y.; Sun, M.; Sim, S.P. Mechanism of Action of Camptothecin. Ann. N. Y. Acad. Sci. 2000, 922, 1–10. [Google Scholar] [CrossRef]
- Bao, X.; Wu, J.; Kim, S.; LoRusso, P.; Li, J. Pharmacometabolomics Reveals Irinotecan Mechanism of Action in Cancer Patients. J. Clin. Pharmacol. 2019, 59, 20–34. [Google Scholar] [CrossRef]
- Casadó, A.; Sagristá, M.L.; Mora Giménez, M. A Novel Microfluidic Liposomal Formulation for the Delivery of the SN-38 Camptothecin: Characterization and in Vitro Assessment of Its Cytotoxic Effect on Two Tumor Cell Lines. Int. J. Nanomed. 2018, 13, 5301–5320. [Google Scholar] [CrossRef]
- Yue, B.; Gao, R.; Wang, Z.; Dou, W. Microbiota-Host-Irinotecan Axis: A New Insight Toward Irinotecan Chemotherapy. Front. Cell. Infect. Microbiol. 2021, 11, 710945. [Google Scholar] [CrossRef]
- Unrug-Bielawska, K.; Earnshaw, D.; Cybulska-Lubak, M.; Kaniuga, E.; Sandowska-Markiewicz, Z.; Statkiewicz, M.; Rumienczyk, I.; Dąbrowska, M.; Kocik-Krol, J.; Klimkiewicz, K.; et al. Therapeutic Responses to Two New SN-38 Derivatives in Colorectal Cancer Patient-Derived Xenografts and Respective 3D In Vitro Cultures. Anticancer Res. 2024, 44, 4219–4224. [Google Scholar] [CrossRef] [PubMed]
- Lensu, S.; Pekkala, S. Gut Microbiota, Microbial Metabolites and Human Physical Performance. Metabolites 2021, 11, 716. [Google Scholar] [CrossRef] [PubMed]
- Perillo, F.; Amoroso, C.; Strati, F.; Giuffrè, M.R.; Díaz-Basabe, A.; Lattanzi, G.; Facciotti, F. Gut Microbiota Manipulation as a Tool for Colorectal Cancer Management: Recent Advances in Its Use for Therapeutic Purposes. Int. J. Mol. Sci. 2020, 21, 5389. [Google Scholar] [CrossRef]
- Alexander, J.L.; Wilson, I.D.; Teare, J.; Marchesi, J.R.; Nicholson, J.K.; Kinross, J.M. Gut Microbiota Modulation of Chemotherapy Efficacy and Toxicity. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Wen, X.-S.; Xian, C.J. Chemotherapy-Induced Intestinal Microbiota Dysbiosis Impairs Mucosal Homeostasis by Modulating Toll-like Receptor Signaling Pathways. Int. J. Mol. Sci. 2021, 22, 9474. [Google Scholar] [CrossRef]
- Li, S.; Zhu, S.; Yu, J. The Role of Gut Microbiota and Metabolites in Cancer Chemotherapy. J. Adv. Res. 2024, 64, 223–235. [Google Scholar] [CrossRef]
- Cybulska, M.; Olesinski, T.; Goryca, K.; Paczkowska, K.; Statkiewicz, M.; Kopczynski, M.; Grochowska, A.; Unrug-Bielawska, K.; Tyl-Bielicka, A.; Gajewska, M.; et al. Challenges in Stratifying the Molecular Variability of Patient-Derived Colon Tumor Xenografts. BioMed Res. Int. 2018, 2018, 2954208. [Google Scholar] [CrossRef]
- Zeber-Lubecka, N.; Kulecka, M.; Jagiełło-Gruszfeld, A.; Dąbrowska, M.; Kluska, A.; Piątkowska, M.; Bagińska, K.; Głowienka, M.; Surynt, P.; Tenderenda, M.; et al. Breast Cancer but Not the Menopausal Status Is Associated with Small Changes of the Gut Microbiota. Front. Oncol. 2024, 14, 1279132. [Google Scholar] [CrossRef]
- Kulecka, M.; Czarnowski, P.; Bałabas, A.; Turkot, M.; Kruczkowska-Tarantowicz, K.; Żeber-Lubecka, N.; Dąbrowska, M.; Paszkiewicz-Kozik, E.; Walewski, J.; Ługowska, I.; et al. Microbial and Metabolic Gut Profiling across Seven Malignancies Identifies Fecal Faecalibacillus Intestinalis and Formic Acid as Commonly Altered in Cancer Patients. Int. J. Mol. Sci. 2024, 25, 8026. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing Mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- lmerTest. lmerTest-Package: lmerTest: Tests in Linear Mixed Effects Models. Available online: https://rdrr.io/cran/lmerTest/man/lmerTest-package.html (accessed on 7 May 2025).
- Zhou, H.; He, K.; Chen, J.; Zhang, X. LinDA: Linear Models for Differential Abundance Analysis of Microbiome Compositional Data. Genome Biol. 2022, 23, 95. [Google Scholar] [CrossRef] [PubMed]
- Forsgård, R.A.; Marrachelli, V.G.; Korpela, K.; Frias, R.; Collado, M.C.; Korpela, R.; Monleon, D.; Spillmann, T.; Österlund, P. Chemotherapy-Induced Gastrointestinal Toxicity Is Associated with Changes in Serum and Urine Metabolome and Fecal Microbiota in Male Sprague-Dawley Rats. Cancer Chemother. Pharmacol. 2017, 80, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Crossland, N.A.; Beck, S.; Tan, W.Y.; Lo, M.; Mason, J.B.; Zhang, C.; Guo, W.; Crott, J.W. Fecal Microbiota Transplanted from Old Mice Promotes More Colonic Inflammation, Proliferation, and Tumor Formation in Azoxymethane-Treated A/J Mice than Microbiota Originating from Young Mice. Gut Microbes 2023, 15, 2288187. [Google Scholar] [CrossRef] [PubMed]
- André, T.; Boni, C.; Mounedji-Boudiaf, L.; Navarro, M.; Tabernero, J.; Hickish, T.; Topham, C.; Zaninelli, M.; Clingan, P.; Bridgewater, J.; et al. Oxaliplatin, Fluorouracil, and Leucovorin as Adjuvant Treatment for Colon Cancer. N. Engl. J. Med. 2004, 350, 2343–2351. [Google Scholar] [CrossRef]
- Song, Y.; Wang, X.; Lu, X.; Wang, T. Exposure to Microcystin-LR Promotes Colorectal Cancer Progression by Altering Gut Microbiota and Associated Metabolites in APCmin/+ Mice. Toxins 2024, 16, 212. [Google Scholar] [CrossRef]
- Newsome, R.C.; Gharaibeh, R.Z.; Pierce, C.M.; Da Silva, W.V.; Paul, S.; Hogue, S.R.; Yu, Q.; Antonia, S.; Conejo-Garcia, J.R.; Robinson, L.A.; et al. Interaction of Bacterial Genera Associated with Therapeutic Response to Immune Checkpoint PD-1 Blockade in a United States Cohort. Genome Med. 2022, 14, 35. [Google Scholar] [CrossRef]
- Chen, K.J.; Chen, Y.L.; Ueng, S.H.; Hwang, T.L.; Kuo, L.M.; Hsieh, P.W. Neutrophil Elastase Inhibitor (MPH-966) Improves Intestinal Mucosal Damage and Gut Microbiota in a Mouse Model of 5-Fluorouracil–Induced Intestinal Mucositis. Biomed. Pharmacother. 2021, 134, 111152. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, B.; Hua, Y.; Sun, R.; Tan, X.; Chang, X.; Tang, D.; Gu, J. Astragalus Mongholicus Bunge and Curcuma Aromatica Salisb. Modulate Gut Microbiome and Bile Acid Metabolism to Inhibit Colon Cancer Progression. Front. Microbiol. 2024, 15, 1395634. [Google Scholar] [CrossRef]
- Karami, P.; Goli, H.R.; Abediankenari, S.; Chandani, S.R.; Jafari, N.; Ghasemi, M.; Ahanjan, M. Anti-Tumor Effects of Bacteroides Fragilis and Bifidobacterium Bifidum Culture Supernatants on Mouse Breast Cancer. Gene Rep. 2023, 33, 101815. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, L.; Chen, W.; Chang, Z.; Tu, J.; Qin, Y.; Yao, Y.; Dong, M.; Ding, J.; Li, S.; et al. Microbiota Enterotoxigenic Bacteroides Fragilis -Secreted BFT-1 Promotes Breast Cancer Cell Stemness and Chemoresistance through Its Functional Receptor NOD1. Protein Cell 2024, 15, 419–440. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Mehrabian, P.; Boyajian, S.; Wu, W.-L.; Selicha, J.; Vonderfecht, S.; Mazmanian, S.K. The Protective Role of Bacteroides Fragilis in a Murine Model of Colitis-Associated Colorectal Cancer. mSphere 2018, 3, e00587-18. [Google Scholar] [CrossRef] [PubMed]
- Hills, R.; Pontefract, B.; Mishcon, H.; Black, C.; Sutton, S.; Theberge, C. Gut Microbiome: Profound Implications for Diet and Disease. Nutrients 2019, 11, 1613. [Google Scholar] [CrossRef]
- Niu, H.; Zhou, M.; Zogona, D.; Xing, Z.; Wu, T.; Chen, R.; Cui, D.; Liang, F.; Xu, X. Akkermansia Muciniphila: A Potential Candidate for Ameliorating Metabolic Diseases. Front. Immunol. 2024, 15, 1370658. [Google Scholar] [CrossRef]
- Sheng, Q.-S.; He, K.-X.; Li, J.-J.; Zhong, Z.-F.; Wang, F.-X.; Pan, L.-L.; Lin, J.-J. Comparison of Gut Microbiome in Human Colorectal Cancer in Paired Tumor and Adjacent Normal Tissues. OncoTargets Ther. 2020, 13, 635–646. [Google Scholar] [CrossRef]
- Liu, X.; Cui, S.; Zhang, L.; Wu, S.; Feng, C.; Liu, B.; Yang, H. Gut Microbiota Affects the Activation of STING Pathway and Thus Participates in the Progression of Colorectal Cancer. World J. Surg. Oncol. 2024, 22, 192. [Google Scholar] [CrossRef]
- Lin, X.B.; Dieleman, L.A.; Ketabi, A.; Bibova, I.; Sawyer, M.B.; Xue, H.; Field, C.J.; Baracos, V.E.; Gänzle, M.G. Irinotecan (CPT-11) Chemotherapy Alters Intestinal Microbiota in Tumour Bearing Rats. PLoS ONE 2012, 7, e39764. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, L.; Chen, S.; Guo, S.; Yue, T.; Hou, Q.; Feng, M.; Xu, H.; Liu, Y.; Wang, P.; et al. The Administration of Escherichia Coli Nissle 1917 Ameliorates Irinotecan–Induced Intestinal Barrier Dysfunction and Gut Microbial Dysbiosis in Mice. Life Sci. 2019, 231, 116529. [Google Scholar] [CrossRef]
- Stringer, A.M.; Gibson, R.J.; Logan, R.M.; Bowen, J.M.; Yeoh, A.S.J.; Keefe, D.M.K. Faecal Microflora and Beta-Glucuronidase Expression Are Altered in an Irinotecan-Induced Diarrhea Model in Rats. Cancer Biol. Ther. 2008, 7, 1919–1925. [Google Scholar] [CrossRef] [PubMed]
- Stringer, A.M.; Gibson, R.J.; Bowen, J.M.; Logan, R.M.; Ashton, K.; Yeoh, A.S.J.; Al-Dasooqi, N.; Keefe, D.M.K. Irinotecan-induced Mucositis Manifesting as Diarrhoea Corresponds with an Amended Intestinal Flora and Mucin Profile. Int. J. Exp. Pathol. 2009, 90, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Stringer, A.M.; Gibson, R.J.; Logan, R.M.; Bowen, J.M.; Yeoh, A.S.-J.; Burns, J.; Keefe, D.M.K. Chemotherapy-Induced Diarrhea Is Associated with Changes in the Luminal Environment in the DA Rat. Exp. Biol. Med. 2007, 232, 96–106. [Google Scholar]
- Wang, L.; Wang, R.; Wei, G.; Wang, S.; Du, G. Dihydrotanshinone Attenuates Chemotherapy-Induced Intestinal Mucositis and Alters Fecal Microbiota in Mice. Biomed. Pharmacother. 2020, 128, 110262. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, S.A.; Merenstein, D.; Fraser, C.M.; Marco, M.L. Molecular Mechanisms of Probiotic Prevention of Antibiotic-Associated Diarrhea. Curr. Opin. Biotechnol. 2020, 61, 226–234. [Google Scholar] [CrossRef]
- Fusco, W.; Lorenzo, M.B.; Cintoni, M.; Porcari, S.; Rinninella, E.; Kaitsas, F.; Lener, E.; Mele, M.C.; Gasbarrini, A.; Collado, M.C.; et al. Short-Chain Fatty-Acid-Producing Bacteria: Key Components of the Human Gut Microbiota. Nutrients 2023, 15, 2211. [Google Scholar] [CrossRef]
- Frampton, J.; Murphy, K.G.; Frost, G.; Chambers, E.S. Short-Chain Fatty Acids as Potential Regulators of Skeletal Muscle Metabolism and Function. Nat. Metab. 2020, 2, 840–848. [Google Scholar] [CrossRef]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient Metabolism by the Human Gut Microbiome: Major Fermentation by-Products and Their Impact on Host Health. Microbiome 2019, 7, 91. [Google Scholar] [CrossRef]
- Macfarlane, S.; Quigley, M.E.; Hopkins, M.J.; Newton, D.F.; Macfarlane, G.T. Polysaccharide Degradation by Human Intestinal Bacteria during Growth under Multi-Substrate Limiting Conditions in a Three-Stage Continuous Culture System. FEMS Microbiol. Ecol. 1998, 26, 231–243. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Chain, F.; Martín, R.; Bermúdez-Humarán, L.G.; Courau, S.; Langella, P. Beneficial Effects on Host Energy Metabolism of Short-Chain Fatty Acids and Vitamins Produced by Commensal and Probiotic Bacteria. Microb. Cell Fact. 2017, 16, 79. [Google Scholar] [CrossRef]
- Markowiak-Kopeć, P.; Śliżewska, K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef]
- Lin, X.B.; Farhangfar, A.; Valcheva, R.; Sawyer, M.B.; Dieleman, L.; Schieber, A.; Gänzle, M.G.; Baracos, V. The Role of Intestinal Microbiota in Development of Irinotecan Toxicity and in Toxicity Reduction through Dietary Fibres in Rats. PLoS ONE 2014, 9, e83644. [Google Scholar] [CrossRef] [PubMed]
- Hueso, T.; Ekpe, K.; Mayeur, C.; Gatse, A.; Joncquel-Chevallier Curt, M.; Gricourt, G.; Rodriguez, C.; Burdet, C.; Ulmann, G.; Neut, C.; et al. Impact and Consequences of Intensive Chemotherapy on Intestinal Barrier and Microbiota in Acute Myeloid Leukemia: The Role of Mucosal Strengthening. Gut Microbes 2020, 12, 1800897. [Google Scholar] [CrossRef] [PubMed]
- Ziemons, J.; Aarnoutse, R.; Heuft, A.; Hillege, L.; Waelen, J.; De Vos-Geelen, J.; Valkenburg-van Iersel, L.; Van Hellemond, I.E.G.; Creemers, G.-J.M.; Baars, A.; et al. Fecal Levels of SCFA and BCFA during Capecitabine in Patients with Metastatic or Unresectable Colorectal Cancer. Clin. Exp. Med. 2023, 23, 3919–3933. [Google Scholar] [CrossRef]
- Bai, Z.; Liu, Y.; Zhao, Y.; Yan, R.; Yang, L.; Ma, H.; Wang, J.; Wang, T.; Li, Y.; Zhang, G.; et al. Aspirin Ameliorates Atherosclerotic Immuno-Inflammation through Regulating the Treg/Th17 Axis and CD39–CD73 Adenosine Signaling via Remodeling the Gut Microbiota in ApoE−/− Mice. Int. Immunopharmacol. 2023, 120, 110296. [Google Scholar] [CrossRef]
- Encarnação, J.C.; Pires, A.S.; Amaral, R.A.; Gonçalves, T.J.; Laranjo, M.; Casalta-Lopes, J.E.; Gonçalves, A.C.; Sarmento-Ribeiro, A.B.; Abrantes, A.M.; Botelho, M.F. Butyrate, a Dietary Fiber Derivative That Improves Irinotecan Effect in Colon Cancer Cells. J. Nutr. Biochem. 2018, 56, 183–192. [Google Scholar] [CrossRef]
- Son, M.-Y.; Cho, H.-S. Anticancer Effects of Gut Microbiota-Derived Short-Chain Fatty Acids in Cancers. J. Microbiol. Biotechnol. 2023, 33, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Maria, R.M.; Altei, W.F.; Selistre-de-Araujo, H.S.; Colnago, L.A. Effects of Doxorubicin, Cisplatin, and Tamoxifen on the Metabolic Profile of Human Breast Cancer MCF-7 Cells As Determined by 1H High-Resolution Magic Angle Spinning Nuclear Magnetic Resonance. Biochemistry 2017, 56, 2219–2224. [Google Scholar] [CrossRef]
- Wang, W.; Zou, W. Amino Acids and Their Transporters in T Cell Immunity and Cancer Therapy. Mol. Cell 2020, 80, 384–395. [Google Scholar] [CrossRef]
- Wang, W.; Kryczek, I.; Dostál, L.; Lin, H.; Tan, L.; Zhao, L.; Lu, F.; Wei, S.; Maj, T.; Peng, D.; et al. Effector T Cells Abrogate Stroma-Mediated Chemoresistance in Ovarian Cancer. Cell 2016, 165, 1092–1105. [Google Scholar] [CrossRef]
- Tintelnot, J.; Xu, Y.; Lesker, T.R.; Schönlein, M.; Konczalla, L.; Giannou, A.D.; Pelczar, P.; Kylies, D.; Puelles, V.G.; Bielecka, A.A.; et al. Microbiota-Derived 3-IAA Influences Chemotherapy Efficacy in Pancreatic Cancer. Nature 2023, 615, 168–174. [Google Scholar] [CrossRef]
- Zidi, O.; Souai, N.; Raies, H.; Ben Ayed, F.; Mezlini, A.; Mezrioui, S.; Tranchida, F.; Sabatier, J.-M.; Mosbah, A.; Cherif, A.; et al. Fecal Metabolic Profiling of Breast Cancer Patients during Neoadjuvant Chemotherapy Reveals Potential Biomarkers. Molecules 2021, 26, 2266. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Hou, X.; Fang, Q.; Wang, H.; Li, X.; Hu, Z.; Liu, Z.; Fan, L.; Liu, Y.; Fu, Z.; et al. High-Salt Diet Decreases FOLFOX Efficacy via Gut Bacterial Tryptophan Metabolism in Colorectal Cancer. Mol. Med. 2025, 31, 66. [Google Scholar] [CrossRef]
- Ribeiro, R.A.; Wanderley, C.W.S.; Wong, D.V.T.; Mota, J.M.S.C.; Leite, C.A.V.G.; Souza, M.H.L.P.; Cunha, F.Q.; Lima-Júnior, R.C.P. Irinotecan- and 5-Fluorouracil-Induced Intestinal Mucositis: Insights into Pathogenesis and Therapeutic Perspectives. Cancer Chemother. Pharmacol. 2016, 78, 881–893. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-W.; Lee, H.-C.; Li, L.-H.; Chiang Chiau, J.-S.; Wang, T.-E.; Chuang, W.-H.; Chen, M.-J.; Wang, H.-Y.; Shih, S.-C.; Liu, C.-Y.; et al. Fecal Microbiota Transplantation Prevents Intestinal Injury, Upregulation of Toll-Like Receptors, and 5-Fluorouracil/Oxaliplatin-Induced Toxicity in Colorectal Cancer. Int. J. Mol. Sci. 2020, 21, 386. [Google Scholar] [CrossRef] [PubMed]
- Soufizadeh, P.; Mansouri, V.; Ahmadbeigi, N. A Review of Animal Models Utilized in Preclinical Studies of Approved Gene Therapy Products: Trends and Insights. Lab. Anim. Res. 2024, 40, 17. [Google Scholar] [CrossRef]
- Anonymous. What Differentiates Clinical Trial Statistics from Preclinical Methods and Why Robust Approaches Matter. Nat. Commun. 2024, 15, 7035. [Google Scholar] [CrossRef]
| Phylum | Class | Order | Family | Genus | Fold Change | Adjusted p-Value |
|---|---|---|---|---|---|---|
| Firmicutes | Clostridia | Lachnospirales | Lachnospiraceae | Marvinbryantia | 0.35 | 4.22 × 10−5 |
| Firmicutes | Bacilli | Lactobacillales | Lactobacillaceae | Lactobacillus | 0.40 | 2.98 × 10−4 |
| Firmicutes | Clostridia | Oscillospirales | Ruminococcaceae | Ruminococcus | 0.48 | 2.05 × 10−3 |
| Firmicutes | Clostridia | Peptostreptococcales-Tissierellales | Anaerovoracaceae | [Eubacterium] nodatum group | 0.48 | 1.09 × 10−2 |
| Phylum | Class | Order | Family | Genus | Fold Change | Adjusted p-Value |
|---|---|---|---|---|---|---|
| Firmicutes | Clostridia | Lachnospirales | Lachnospiraceae | Marvinbryantia | 0.39 | 0.0016 |
| Bacteroidota | Bacteroidia | Bacteroidales | Bacteroidaceae | Bacteroides | 0.67 | 0.0056 |
| Verrucomicrobiota | Verrucomicrobiae | Verrucomicrobiales | Akkermansiaceae | Akkermansia | 1.99 | 0.068 |
| Firmicutes | Clostridia | Clostridiales | Clostridiaceae | Candidatus Arthromitus | 0.59 | 0.068 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Unrug-Bielawska, K.; Sandowska-Markiewicz, Z.; Piątkowska, M.; Czarnowski, P.; Goryca, K.; Zeber-Lubecka, N.; Dąbrowska, M.; Kaniuga, E.; Cybulska-Lubak, M.; Bałabas, A.; et al. Comparative Analysis of Gut Microbiota Responses to New SN-38 Derivatives, Irinotecan, and FOLFOX in Mice Bearing Colorectal Cancer Patient-Derived Xenografts. Cancers 2025, 17, 2263. https://doi.org/10.3390/cancers17132263
Unrug-Bielawska K, Sandowska-Markiewicz Z, Piątkowska M, Czarnowski P, Goryca K, Zeber-Lubecka N, Dąbrowska M, Kaniuga E, Cybulska-Lubak M, Bałabas A, et al. Comparative Analysis of Gut Microbiota Responses to New SN-38 Derivatives, Irinotecan, and FOLFOX in Mice Bearing Colorectal Cancer Patient-Derived Xenografts. Cancers. 2025; 17(13):2263. https://doi.org/10.3390/cancers17132263
Chicago/Turabian StyleUnrug-Bielawska, Katarzyna, Zuzanna Sandowska-Markiewicz, Magdalena Piątkowska, Paweł Czarnowski, Krzysztof Goryca, Natalia Zeber-Lubecka, Michalina Dąbrowska, Ewelina Kaniuga, Magdalena Cybulska-Lubak, Aneta Bałabas, and et al. 2025. "Comparative Analysis of Gut Microbiota Responses to New SN-38 Derivatives, Irinotecan, and FOLFOX in Mice Bearing Colorectal Cancer Patient-Derived Xenografts" Cancers 17, no. 13: 2263. https://doi.org/10.3390/cancers17132263
APA StyleUnrug-Bielawska, K., Sandowska-Markiewicz, Z., Piątkowska, M., Czarnowski, P., Goryca, K., Zeber-Lubecka, N., Dąbrowska, M., Kaniuga, E., Cybulska-Lubak, M., Bałabas, A., Statkiewicz, M., Rumieńczyk, I., Pyśniak, K., Mikula, M., & Ostrowski, J. (2025). Comparative Analysis of Gut Microbiota Responses to New SN-38 Derivatives, Irinotecan, and FOLFOX in Mice Bearing Colorectal Cancer Patient-Derived Xenografts. Cancers, 17(13), 2263. https://doi.org/10.3390/cancers17132263






