Discordance Between Radiological and Pathological Responses to Pembrolizumab in Mismatch Repair-Deficient Metastatic Colorectal Cancer: Implications for Precision Oncology
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. MMR Status Assessment
2.3. Radiological Response and Pathological Response
2.4. Statistical Analysis
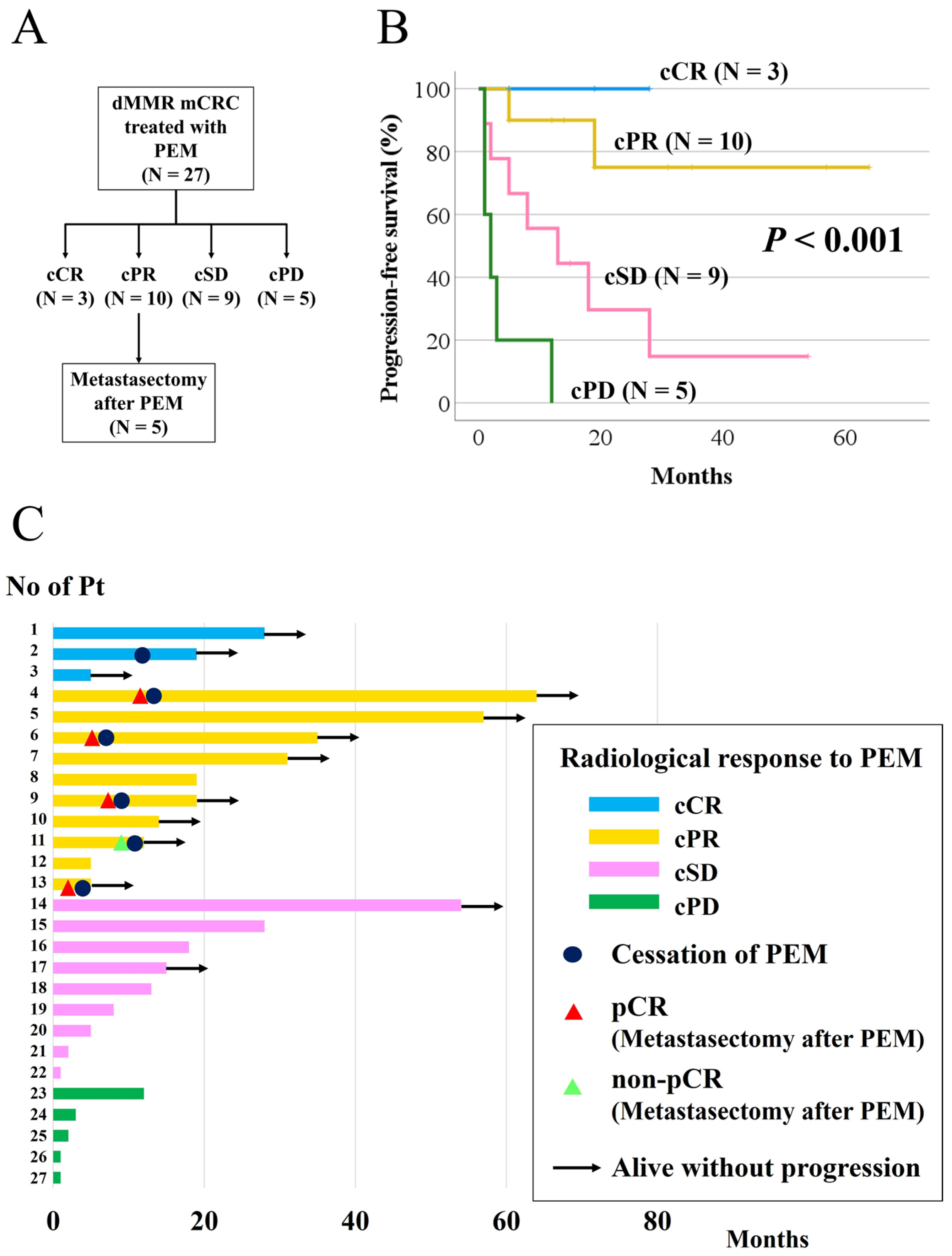
3. Results
3.1. Clinicopathologic Description
3.2. Objective Response and Overall Response
3.3. Progression-Free Survival According to Radiological Response and pCR in Patients with cPR
| Characteristic | n (%) | |
|---|---|---|
| Radiologic response | cPR | 5 (100) |
| Pathological response | pCR | 4 (80) |
| non-PCR | 1 (20) |
| No. of Pt | Age | Sex | Primary Tumor Location | KRAS Status | BRAF Status | Site of Mets | Radiological Response | Histopathological Response | Conversion Therapy | Survival Status |
|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 70’s | M | Transverse colon | Wild | V600E | LN | cPR | pCR | Yes | Alive with NED |
| 6 | 40’s | F | Rectum | Wild | Wild | Peri | cPR | pCR | Yes | Alive with NED |
| 9 | 50’s | M | Sigmoid colon | Wild | Wild | Peri | cPR | pCR | Yes | Alive with NED |
| 11 | 60’s | M | Ascending colon | G13D | Wild | Peri | cPR | non-pCR | Yes | Alive with NED |
| 13 | 70’s | F | Cecum | Wild | Wild | Liver | cPR | pCR | No | Alive with NED |
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| dMMR | Mismatch repair deficient |
| mCRC | Metastatic colorectal cancer |
| ICI | Immune check point inhibitor |
| MSI | Microsatellite instability |
| MSS | Microsatellite stable |
| MSI-H | Microsatellite instability-high |
| pCR | Pathological complete response |
| PCR | Polymerase chain reaction |
| CT | Computed tomography |
| PFS | Progression-free survival |
| cCR | Clinical complete response |
| cPR | Clinical partial response |
| ctDNA | Circulating tumor DNA |
References
- Baretti, M.; Le, D.T. DNA mismatch repair in cancer. Pharmacol. Ther. 2018, 189, 45–62. [Google Scholar] [CrossRef]
- Leone, R.D.; Powell, J.D. Metabolism of immune cells in cancer. Nat. Rev. Cancer 2020, 20, 516–531. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Sha, D.; Jin, Z.; Budczies, J.; Kluck, K.; Stenzinger, A.; Sinicrope, F.A. Tumor mutational burden as a predictive biomarker in solid tumors. Cancer Discov. 2020, 10, 1808–1825. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef]
- Prasad, V.; Kaestner, V.; Mailankody, S. Cancer drugs approved based on biomarkers and not tumor type-FDA approval of pembrolizumab for mismatch repair-deficient solid cancers. JAMA Oncol. 2018, 4, 157–158. [Google Scholar] [CrossRef]
- Thein, K.Z.; Lemery, S.J.; Kummar, S. Tissue-agnostic drug development: A new path to drug approval. Cancer Discov. 2021, 11, 2139–2144. [Google Scholar] [CrossRef]
- André, T.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N. Engl. J Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef]
- Diaz, L.A., Jr.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): Final analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 2022, 23, 659–670. [Google Scholar] [CrossRef]
- Le, D.T.; Kim, T.W.; Van Cutsem, E.; Geva, R.; Jäger, D.; Hara, H.; Burge, M.; O’Neil, B.; Kavan, P.; Yoshino, T.; et al. Phase II open-label study of pembrolizumab in treatment-refractory, microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: KEYNOTE-164. J. Clin. Oncol. 2020, 38, 11–19. [Google Scholar] [CrossRef]
- Le, D.T.; Diaz, L.A., Jr.; Kim, T.W.; Van Cutsem, E.; Geva, R.; Jäger, D.; Hara, H.; Burge, M.; O’Neil, B.H.; Kavan, P.; et al. Pembrolizumab for previously treated, microsatellite instability-high/mismatch repair-deficient advanced colorectal cancer: Final analysis of KEYNOTE-164. Eur. J. Cancer 2023, 186, 185–195. [Google Scholar] [CrossRef]
- Ludford, K.; Cohen, R.; Svrcek, M.; Foo, W.C.; Colle, R.; Parc, Y.; Thomas, J.V.; Morris, V.K.; Kopetz, S.; Chang, G.J.; et al. Pathological tumor response following immune checkpoint blockade for deficient mismatch repair advanced colorectal cancer. J. Natl. Cancer Inst. 2021, 113, 208–211. [Google Scholar] [CrossRef]
- Pei, F.; Wu, J.; Zhao, Y.; He, W.; Yao, Q.; Huang, M.; Huang, J. Single-agent neoadjuvant immunotherapy with a PD-1 antibody in locally advanced mismatch repair-deficient or microsatellite instability-high colorectal cancer. Clin. Color. Cancer 2023, 22, 85–91. [Google Scholar] [CrossRef]
- Chalabi, M.; Fanchi, L.F.; Dijkstra, K.K.; Van Den Berg, J.G.; Aalbers, A.G.; Sikorska, K.; Lopez-Yurda, M.; Grootscholten, C.; Beets, G.L.; Snaebjornsson, P.; et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat. Med. 2020, 26, 566–576. [Google Scholar] [CrossRef]
- Kothari, A.; White, M.G.; Peacock, O.; Kaur, H.; Palmquist, S.M.; You, N.; Taggart, M.; Salem, U.; Overman, M.; Kopetz, S.; et al. Pathological response following neoadjuvant immunotherapy in mismatch repair-deficient/microsatellite instability-high locally advanced, non-metastatic colorectal cancer. Br. J. Surg. 2022, 109, 489–492. [Google Scholar] [CrossRef]
- Xiao, B.Y.; Zhang, X.; Cao, T.Y.; Li, D.-D.; Jiang, W.; Kong, L.-H.; Tang, J.-H.; Han, K.; Zhang, C.-Z.; Mei, W.-J.; et al. Neoadjuvant immunotherapy leads to major response and low recurrence in localized mismatch repair-deficient colorectal cancer. J. Natl. Compr. Cancer Netw. 2023, 21, 60–66.e5. [Google Scholar] [CrossRef]
- Seymour, L.; Bogaerts, J.; Perrone, A.; Ford, R.; Schwartz, L.H.; Mandrekar, S.; Lin, N.U.; Litière, S.; Dancey, J.; Chen, A.; et al. iRECIST: Guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017, 18, e143–e152. [Google Scholar] [CrossRef]
- Overman, M.J.; McDermott, R.; Leach, J.L.; Lonardi, S.; Lenz, H.-J.; Morse, M.A.; Desai, J.; Hill, A.; Axelson, M.; Moss, R.A.; et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): An open-label, multicentre, phase 2 study. Lancet Oncol. 2017, 18, 1182–1191. [Google Scholar] [CrossRef]
- Overman, M.J.; Lonardi, S.; Wong, K.Y.M.; Lenz, H.J.; Gelsomino, F.; Aglietta, M.; Morse, M.A.; Van Cutsem, E.; McDermott, R.; Hill, A.; et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J. Clin. Oncol. 2018, 36, 773–779. [Google Scholar] [CrossRef]
- Cercek, A.; Lumish, M.; Sinopoli, J.; Weiss, J.; Shia, J.; Lamendola-Essel, M.; El Dika, I.H.; Segal, N.; Shcherba, M.; Sugarman, R.; et al. PD-1 blockade in mismatch repair-deficient, locally advanced rectal cancer. N. Engl. J. Med. 2022, 386, 2363–2376. [Google Scholar] [CrossRef]
- Fukuoka, S.; Hara, H.; Takahashi, N.; Kojima, T.; Kawazoe, A.; Asayama, M.; Yoshii, T.; Kotani, D.; Tamura, H.; Mikamoto, Y.; et al. Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: An open-label, dose-escalation, and dose-expansion phase Ib trial (REGONIVO, EPOC1603). J. Clin. Oncol. 2020, 38, 2053–2061. [Google Scholar] [CrossRef]
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. ESMO Guidelines Committee. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 10–32. [Google Scholar] [CrossRef]
- Hiroi, S.; Kawahara, M.; Tonoike, Y.; Kobayashi, Y.; Ikarashi, T.; Nikkuni, K. A case of metastatic colon cancer with high microsatellite instability achieving a complete pathological response to pembrolizumab therapy. Nihon Shokakibyo Gakkai Zasshi 2022, 119, 580–585. (In Japanese) [Google Scholar]
- Matsumoto, A.; Shimada, Y.; Nakano, M.; Ozeki, H.; Yamai, D.; Murata, M.; Ishizaki, F.; Nyuzuki, H.; Ikeuchi, T.; Wakai, T. Conversion therapy with pembrolizumab for a peritoneal metastasis of rectal cancer causing hydronephrosis in a patient with Lynch syndrome. Clin. J. Gastroenterol. 2024, 17, 451–456. [Google Scholar] [CrossRef]
- Marolleau, P.; Tougeron, D.; Allignet, B.; Cohen, R.; Sefrioui, D.; Gallet, B.; Dumont, F.; Guimbaud, R.; Alouani, E.; Passot, G.; et al. Complete pathological response after chemotherapy or immune checkpoint inhibitors in deficient MMR metastatic colorectal cancer: Results of a retrospective multicenter study. Int. J. Cancer 2023, 153, 1376–1385. [Google Scholar] [CrossRef]
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef]
- Li, B.; Chan, H.L.; Chen, P. Immune checkpoint inhibitors: Basics and challenges. Curr. Med. Chem. 2019, 26, 3009–3025. [Google Scholar] [CrossRef]
- Goleva, E.; Lyubchenko, T.; Kraehenbuehl, L.; Lacouture, M.E.; Leung, D.Y.; Kern, J.A. Our current understanding of checkpoint inhibitor therapy in cancer immunotherapy. Ann. Allergy Asthma Immunol. 2021, 126, 630–638. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Mittra, A.; Naqash, A.R.; Takebe, N. A review of mechanisms of resistance to immune checkpoint inhibitors and potential strategies for therapy. Cancer Drug Resist. 2020, 3, 252–275. [Google Scholar] [CrossRef]
- Yan, S.; Wang, W.; Feng, Z.; Xue, J.; Liang, W.; Wu, X.; Tan, Z.; Zhang, X.; Zhang, S.; Li, X.; et al. Immune checkpoint inhibitors in colorectal cancer: Limitation and challenges. Front. Immunol. 2024, 15, 1403533. [Google Scholar] [CrossRef]
- Amaria, R.N.; Reddy, S.M.; Tawbi, H.A.; Davies, M.A.; Ross, M.I.; Glitza, I.C.; Cormier, J.N.; Lewis, C.; Hwu, W.-J.; Hanna, E.; et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat. Med. 2018, 24, 1649–1654. [Google Scholar] [CrossRef] [PubMed]
- Forde, P.M.; Chaft, J.E.; Smith, K.N.; Anagnostou, V.; Cottrell, T.R.; Hellmann, M.D.; Zahurak, M.; Yang, S.C.; Jones, D.R.; Broderick, S.; et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N. Engl. J. Med. 2018, 378, 1976–1986. [Google Scholar] [CrossRef] [PubMed]
- Siravegna, G.; Marsoni, S.; Siena, S.; Bardelli, A. Integrating liquid biopsies into the management of cancer. Nat. Rev. Clin. Oncol. 2017, 14, 531–548. [Google Scholar] [CrossRef] [PubMed]
- Tie, J.; Wang, Y.; Tomasetti, C.; Li, L.; Springer, S.; Kinde, I.; Silliman, N.; Tacey, M.; Wong, H.-L.; Christie, M.; et al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci. Transl. Med. 2016, 8, 346ra92. [Google Scholar] [CrossRef]
- Nakamura, Y.; Watanabe, J.; Akazawa, N.; Hirata, K.; Kataoka, K.; Yokota, M.; Kato, K.; Kotaka, M.; Kagawa, Y.; Yeh, K.-H.; et al. ctDNA-based molecular residual disease and survival in resectable colorectal cancer. Nat. Med. 2024, 30, 3272–3283. [Google Scholar] [CrossRef]
- Willis, J.; Lefterova, M.I.; Artyomenko, A.; Kasi, P.M.; Nakamura, Y.; Mody, K.; Catenacci, D.V.; Fakih, M.; Barbacioru, C.; Zhao, J.; et al. Validation of microsatellite instability detection using a comprehensive plasma-based genotyping panel. Clin. Cancer Res. 2019, 25, 7035–7045. [Google Scholar] [CrossRef]
- Zhang, Q.; Luo, J.; Wu, S.; Si, H.; Gao, C.; Xu, W.; Abdullah, S.E.; Higgs, B.W.; Dennis, P.A.; van der Heijden, M.S.; et al. Prognostic and predictive impact of circulating tumor DNA in patients with advanced cancers treated with immune checkpoint blockade. Cancer Discov. 2020, 10, 1842–1853. [Google Scholar] [CrossRef]

| Characteristic | n (%) | |
|---|---|---|
| Age, median (range), y | 69 (37–81) | |
| Sex | Male | 14 (52) |
| Female | 13 (48) | |
| Primary tumor location | Cecum | 7 (26) |
| Ascending | 9 (33) | |
| Transverse | 3 (11) | |
| Sigmoid | 4 (15) | |
| Rectum | 4 (15) | |
| KRAS status | Wild type | 23 (85) |
| Mutant | 4 (15) | |
| NRAS status | Wild type | 26 (96) |
| Mutant | 1 (4) | |
| BRAF V600E | Wild type | 17 (63) |
| Mutant | 9 (33) | |
| Unknown | 1 (4) | |
| Initial stage | II | 4 (15) |
| III | 9 (33) | |
| IV | 14 (52) | |
| Type of metastases | Synchronous | 14 (52) |
| Metachronous | 13 (48) | |
| Number of metastatic organs | One | 19 (70) |
| Two or more | 8 (30) | |
| Lynch syndrome | Yes | 3 (11) |
| No | 4 (15) | |
| Unknown | 20 (74) | |
| Timing of PEM | First line | 12 (44) |
| Second line and beyond | 15 (56) | |
| Metastasectomy after PEM | Absence | 22 (81) |
| Presence | 5 (19) |
| Characteristic | n (%) | |
|---|---|---|
| Objective response | 13 (48) | |
| Radiologic response | CR | 3 (11) |
| PR | 10 (37) | |
| SD | 9 (33) | |
| PD | 5 (19) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimada, Y.; Nakano, M.; Matsumoto, A.; Ozeki, H.; Abe, K.; Tajima, Y.; Yamai, D.; Nogami, H.; Nakano, M.; Tani, T.; et al. Discordance Between Radiological and Pathological Responses to Pembrolizumab in Mismatch Repair-Deficient Metastatic Colorectal Cancer: Implications for Precision Oncology. Cancers 2025, 17, 2233. https://doi.org/10.3390/cancers17132233
Shimada Y, Nakano M, Matsumoto A, Ozeki H, Abe K, Tajima Y, Yamai D, Nogami H, Nakano M, Tani T, et al. Discordance Between Radiological and Pathological Responses to Pembrolizumab in Mismatch Repair-Deficient Metastatic Colorectal Cancer: Implications for Precision Oncology. Cancers. 2025; 17(13):2233. https://doi.org/10.3390/cancers17132233
Chicago/Turabian StyleShimada, Yoshifumi, Mae Nakano, Akio Matsumoto, Hikaru Ozeki, Kaoru Abe, Yosuke Tajima, Daisuke Yamai, Hitoshi Nogami, Masato Nakano, Tatsuo Tani, and et al. 2025. "Discordance Between Radiological and Pathological Responses to Pembrolizumab in Mismatch Repair-Deficient Metastatic Colorectal Cancer: Implications for Precision Oncology" Cancers 17, no. 13: 2233. https://doi.org/10.3390/cancers17132233
APA StyleShimada, Y., Nakano, M., Matsumoto, A., Ozeki, H., Abe, K., Tajima, Y., Yamai, D., Nogami, H., Nakano, M., Tani, T., Kawahara, M., Nishimura, A., Kobayashi, Y., Bamba, Y., Suzuki, S., Oyanagi, H., Ohashi, T., Kameyama, H., Iwaya, A., ... Wakai, T. (2025). Discordance Between Radiological and Pathological Responses to Pembrolizumab in Mismatch Repair-Deficient Metastatic Colorectal Cancer: Implications for Precision Oncology. Cancers, 17(13), 2233. https://doi.org/10.3390/cancers17132233






