A Novel Treatment Strategy for Unresectable Locally Recurrent Rectal Cancer—Upfront Carbon-Ion Radiotherapy Followed by Surgical Resection of the Irradiated Intestines
Simple Summary
Abstract
1. Introduction
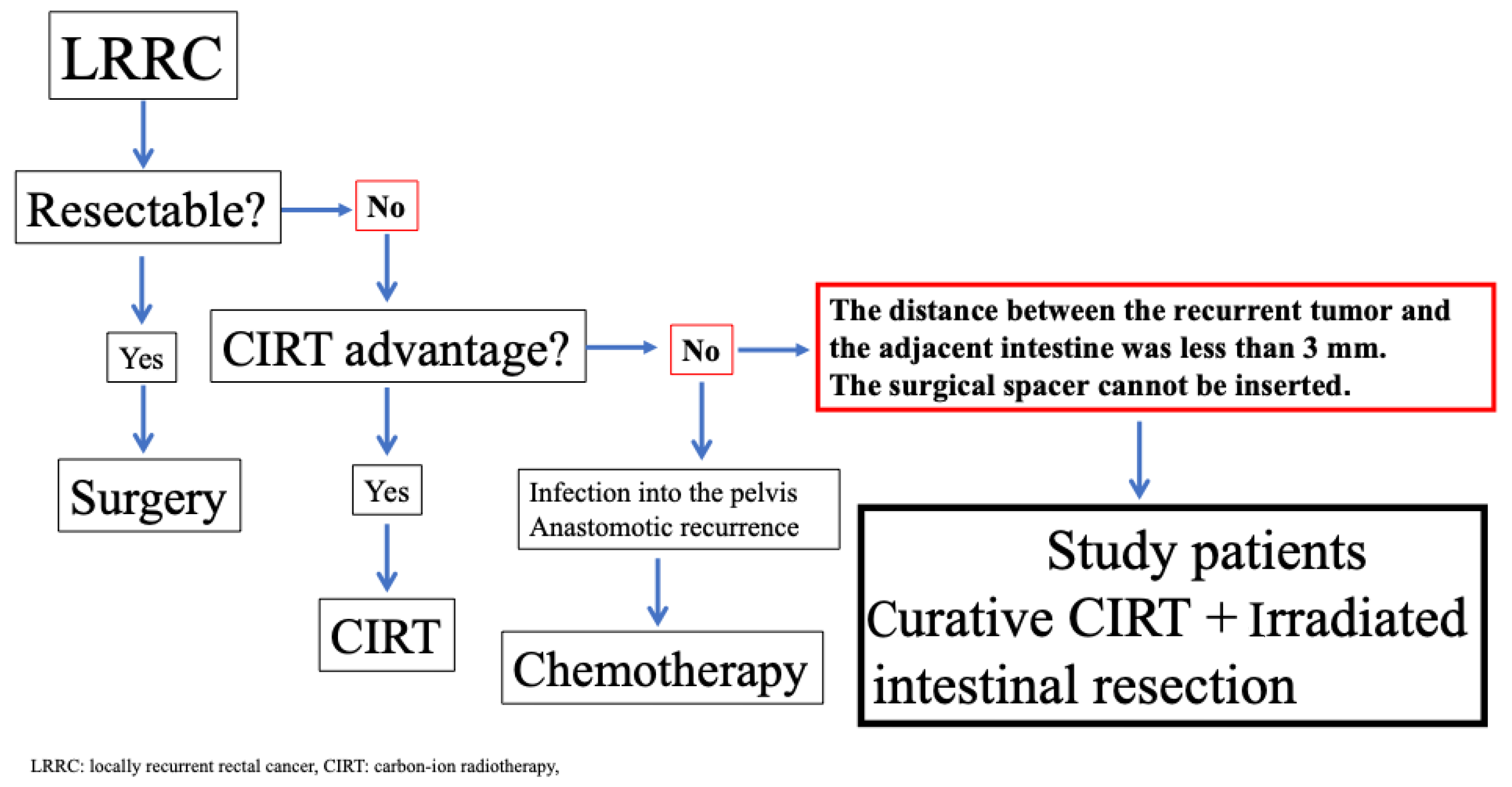
2. Materials and Methods
2.1. Patients
2.2. Treatment
2.3. Follow-Up
3. Results
| Surgery Type | Operating Time (Minutes) | Blood Loss (mL) | Postoperative Hospitalization (Days) | Surgical Complications (C/D Grade) | Surgical Complications | |
|---|---|---|---|---|---|---|
| 1 | LAR Small intestine resection | 457 | 70 | 21 | II | Bleeding from anastomosis |
| 2 | Small intestine resection | 247 | 0 | 8 | 0 | − |
| 3 | LAR | 359 | 200 | 18 | 0 | − |
| 4 | Small intestine resection | 162 | 15 | 7 | 0 | − |
| 5 | LAR | 613 | 152 | 22 | II | Urinary dysfunction |
| 6 | LAR | 425 | 50 | 26 | II | Ileus |
| 7 | Small intestine resection | 245 | 1015 | 10 | 0 | − |
| 8 | LAR | 470 | 100 | 28 | 0 | |
| 9 | LAR | 240 | 0 | 14 | 0 | |
| 10 | Small intestine resection | 245 | 60 | 13 | 0 | |
| 11 | LAR | 267 | 0 | 30 | II | Ileus |
| 12 | LAR | 523 | 385 | 38 | IIIa | Pancreatitis |
| Patients | In-Field Recurrence | Time to Recurrence (Months) | Out-of-Field Recurrence | Time to Recurrence (Months) | Distant Recurrence | Time to Recurrence (Months) | Recurrence Site | Mortality | Follow-Up Duration (Months) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | − | + | 12 | + | 17 | Lung | Yes | 60 | |
| 2 | − | − | − | − | No | 60 | |||
| 3 | − | − | − | No | 60 | ||||
| 4 | − | + | 25 | No | 57 | ||||
| 5 | − | − | + | 6 | Lung | No | 36 | ||
| 6 | − | − | − | − | No | 39 | |||
| 7 | − | − | − | − | No | 36 | |||
| 8 | − | + | 8 | + | 28 | Lung | No | 46 | |
| 9 | + | 12 | − | + | 20 | Lung | No | 28 | |
| 10 | − | − | No | 36 | |||||
| 11 | − | − | − | No | 20 | ||||
| 12 | − | − | − | No | 27 |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CIRT | Carbon-ion radiotherapy |
| LRRC | Locally recurrent rectal cancer |
| RBE | Relative biologic effectiveness |
| AEs | Adverse events |
| QST | Quantum Science and Technology |
| CEA | Carcinoembryonic antigen |
| CA19-9 | Carbohydrate antigen |
| CT | Computed tomography |
| MRI | Magnetic resonance imaging |
| LAR | Low anterior resection |
References
- Moriya, Y.; Akasu, T.; Fujita, S.; Yamamoto, S. Total pelvic exenteration with distal sacrectomy for fixed recurrent rectal cancer in the pelvis. Dis. Colon Rectum 2004, 47, 2047–2053, discussion 2044–2053. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.E.; Aalbers, A.G.; Aziz, N.A.; Abraham-nordling, M.; Alberda, W.; Antoniou, A.; Austin, K.K.; Baker, R.; Bali, M.; Baseckas, G.; et al. Changing outcomes following pelvic exenteration for locally advanced and recurrent rectal cancer. BJS Open 2019, 3, 516–520. [Google Scholar]
- Solomon, M.J.; Brown, K.G.M. Extended Radical Resection: The Standard of Care for Patients with Advanced Pelvic Malignancy. Ann. Surg. Oncol. 2020, 27, 323–324. [Google Scholar] [CrossRef] [PubMed]
- Platt, E.; Dovell, G.; Smolarek, S. Systematic review of outcomes following pelvic exenteration for the treatment of primary and recurrent locally advanced rectal cancer. Tech. Coloproctol. 2018, 22, 835–845. [Google Scholar] [CrossRef]
- Aiba, T.; Uehara, K.; Tsuyuki, Y.; Ogura, A.; Murata, Y.; Mizuno, T.; Yamaguchi, J.; Kokuryo, T.; Yokoyama, Y.; Ebata, T. Minimum radial margin in pelvic exenteration for locally advanced or recurrent rectal cancer. Eur. J. Surg. Oncol. 2022, 48, 2502–2508. [Google Scholar] [CrossRef]
- Boyle, K.M.; Sagar, P.M.; Chalmers, A.G.; Sebag-Montefiore, D.; Cairns, A.; Eardley, I. Surgery for locally recurrent rectal cancer. Dis. Colon Rectum 2005, 48, 929–937. [Google Scholar] [CrossRef]
- Rokan, Z.; Simillis, C.; Kontovounisios, C.; Moran, B.J.; Tekkis, P.; Brown, G. Systematic review of classification systems for locally recurrent rectal cancer. BJS Open 2021, 5, zrab024. [Google Scholar] [CrossRef]
- Sakata, S.; Karim, S.M.; Mathis, K.L.; Kelley, S.R.; Rose, P.S.; Dozois, E.J. Posterior-First Two-Stage Approach to En Bloc Resection of Locally Recurrent Rectal Cancer Involving the Pelvic Sidewall. Dis. Colon Rectum 2021, 64, e465–e470. [Google Scholar] [CrossRef] [PubMed]
- Shiba, S.; Okamoto, M.; Shibuya, K.; Kobayashi, D.; Miyasaka, Y.; Ohno, T. Five-year outcomes in carbon-ion radiotherapy for postoperative pelvic recurrence of rectal cancer: A prospective clinical trial (GUNMA 0801). Clin. Transl. Radiat. Oncol. 2024, 44, 100701. [Google Scholar] [CrossRef]
- Yamada, S.; Kamada, T.; Ebner, D.K.; Shinoto, M.; Terashima, K.; Isozaki, Y.; Yasuda, S.; Makishima, H.; Tsuji, H.; Tsujii, H.; et al. Carbon-Ion Radiation Therapy for Pelvic Recurrence of Rectal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 93–101. [Google Scholar] [CrossRef]
- Bin Sumaida, A.; Shanbhag, N.M.; AlKaabi, K.; Balaraj, K. Cost-Effectiveness of Carbon Ion Radiotherapy in Oncology: A Systematic Review. Cureus 2025, 17, e84008. [Google Scholar] [CrossRef] [PubMed]
- Jeans, E.B.; Ebner, D.K.; Takiyama, H.; Qualls, K.; Cunningham, D.A.; Waddle, M.R.; Jethwa, K.R.; Harmsen, W.S.; Hubbard, J.M.; Dozois, E.J.; et al. Comparing Oncologic Outcomes and Toxicity for Combined Modality Therapy vs. Carbon-Ion Radiotherapy for Previously Irradiated Locally Recurrent Rectal Cancer. Cancers 2023, 15, 3057. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Ohno, T.; Tsujii, H.; Nakano, T.; Mizoe, J.E.; Kamada, T.; Miyamoto, T.; Tsuji, H.; Kato, H.; Yamada, S.; et al. Dose escalation study of carbon ion radiotherapy for locally advanced carcinoma of the uterine cervix. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 388–397. [Google Scholar] [CrossRef]
- Cai, X.; Du, Y.; Wang, Z.; Li, P.; Yu, Z.; Zhang, Q.; Zhang, Z. The role of carbon ion radiotherapy for unresectable locally recurrent rectal cancer: A single institutional experience. Radiat. Oncol. 2020, 15, 209. [Google Scholar] [CrossRef]
- Kobayashi, T.; Sekimoto, M.; Miki, H.; Yamamoto, N.; Harino, T.; Yagyu, T.; Hori, S.; Hatta, M.; Hashimoto, Y.; Kotsuka, M.; et al. Laparoscopic polyglycolic acid spacer placement for locally recurrent rectal cancer. Color. Dis. 2024, 26, 760–765. [Google Scholar] [CrossRef]
- Skwarchuk, M.W.; Jackson, A.; Zelefsky, M.J.; Venkatraman, E.S.; Cowen, D.M.; Levegrün, S.; Burman, C.M.; Fuks, Z.; Leibel, S.A.; Ling, C.C. Late rectal toxicity after conformal radiotherapy of prostate cancer (I): Multivariate analysis and dose-response. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Ono, T.; Sato, H.; Miyasaka, Y.; Hagiwara, Y.; Yano, N.; Akamatsu, H.; Harada, M.; Ichikawa, M. Correlation between dose-volume parameters and rectal bleeding after 12 fractions of carbon ion radiotherapy for prostate cancer. World J. Radiol. 2024, 16, 256–264. [Google Scholar] [CrossRef]
- Jackson, A.; Skwarchuk, M.W.; Zelefsky, M.J.; Cowen, D.M.; Venkatraman, E.S.; Levegrun, S.; Burman, C.M.; Kutcher, G.J.; Fuks, Z.; Liebel, S.A.; et al. Late rectal bleeding after conformal radiotherapy of prostate cancer. II. Volume effects and dose-volume histograms. Int. J. Radiat. Oncol. Biol. Phys. 2001, 49, 685–698. [Google Scholar] [CrossRef]
- Susil, R.C.; McNutt, T.R.; DeWeese, T.L.; Song, D. Effects of prostate-rectum separation on rectal dose from external beam radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 1251–1258. [Google Scholar] [CrossRef]
- Ozaki, K.; Mukai, T.; Ando, Y.; Noguchi, T.; Sakamoto, T.; Matsui, S.; Yamaguchi, T.; Akiyoshi, T. Evaluating the short-term outcome of laparoscopic pelvic exenteration in locally advanced and recurrent rectal cancer. Surg. Today 2025, 1–8. [Google Scholar] [CrossRef]
- Takiyama, H.; Yamada, S.; Isozaki, T.; Ikawa, H.; Shinoto, M.; Imai, R.; Koto, M. Carbon-Ion Radiation Therapy for Unresectable Locally Recurrent Colorectal Cancer: A Promising Curative Treatment for Both Radiation Therapy: Naïve Cases and Reirradiation Cases. Int. J. Radiat. Oncol. Biol. Phys. 2024, 118, 734–742. [Google Scholar] [CrossRef]
- Yamada, S.; Takiyama, H.; Isozaki, Y.; Shinoto, M.; Ebner, D.K.; Koto, M.; Tsuji, H.; Miyauchi, H.; Sekimoto, M.; Ueno, H.; et al. Carbon Ion Radiotherapy for Locally Recurrent Rectal Cancer of Patients with Prior Pelvic Irradiation. Ann. Surg. Oncol. 2022, 29, 99–106. [Google Scholar] [CrossRef]
- Fukahori, M.; Matsufuji, N.; Himukai, T.; Kanematsu, N.; Mizuno, H.; Fukumura, A.; Tsuji, H.; Kamada, T. Estimation of late rectal normal tissue complication probability parameters in carbon ion therapy for prostate cancer. Radiother. Oncol. 2016, 118, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Barcellini, A.; Mirandola, A.; Fiore, M.R.; Orlandi, E.; Cobianchi, L. Omentum flap as a spacer before carbon ion radiotherapy for gynecological recurrences. A technical note. Cancer Radiother. 2022, 26, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Sagebiel, T.L.; Faria, S.C.; Aparna, B.; Sacks, J.M.; You, Y.N.; Bhosale, P.R. Pelvic reconstruction with omental and VRAM flaps: Anatomy, surgical technique, normal postoperative findings, and complications. Radiographics 2011, 31, 2005–2019. [Google Scholar] [CrossRef]
- Shiraishi, S.; Horikawa, Y.; Umeda, R.; Matsumoto, K.; Yamano, A.; Yamanaka, M.; Shimo, T.; Murai, T.; Miura, I.; Tokuuye, K. Efficacy and Safety of Newly Installed Spot Scanning Proton Beam Therapy for Prostate Cancer. Vivo 2025, 39, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Neu, M.; Wöhrl, C.M.; Walter, R.; Balagiannis, N.; Poettgen, C.; Käsmann, L.; Stuschke, M.; Dannecker, C.; Stüben, G.; Kahl, K.H. Multimodal chemoradiotherapy including interstitial brachytherapy enhances outcomes in FIGO stage IVA cervical cancer: A focus on tumor control and quality of life. Strahlenther. Onkol. 2025, 1–13. [Google Scholar] [CrossRef]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef]
- Kamada, T.; Tsujii, H.; Blakely, E.A.; Debus, J.; De Neve, W.; Durante, M.; Jäkel, O.; Mayer, R.; Orecchia, R.; Pötter, R.; et al. Carbon ion radiotherapy in Japan: An assessment of 20 years of clinical experience. Lancet Oncol. 2015, 16, e93–e100. [Google Scholar] [CrossRef]
- Wakatsuki, M.; Kato, S.; Ohno, T.; Karasawa, K.; Kiyohara, H.; Tamaki, T.; Ando, K.; Tsujii, H.; Nakano, T.; Kamada, T.; et al. Clinical outcomes of carbon ion radiotherapy for locally advanced adenocarcinoma of the uterine cervix in phase 1/2 clinical trial (protocol 9704). Cancer 2014, 120, 1663–1669. [Google Scholar] [CrossRef]
- Ahmadinejad, M.; Parvizi, A.; Sheikhi, S.; Eghbal, F.; Navabian, S.; Chaboki, F.; Bahri, M.H.; Bozorgmehr, R.; Bagherpour, J.Z.; Ziaie, S. Optimal timing of surgery after neoadjuvant chemoradiotherapy in rectal cancer: A retrospective analysis. Eur. J. Surg. Oncol. 2025, 51, 109702. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Su, Z.; Wang, D.; Liu, W.; Wei, Z. Optimal Interval to Surgery After Neoadjuvant Chemoradiotherapy in Rectal Cancer: A Systematic Review and Meta-analysis. Clin. Color. Cancer. 2018, 17, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.D.; Jones, E.L.; Falk, S.; Cooper, E.J.; Francis, N.K. Timing of surgery after long-course neoadjuvant chemoradiotherapy for rectal cancer: A systematic review of the literature. Dis. Colon Rectum 2013, 56, 921–930. [Google Scholar] [CrossRef] [PubMed]

| Patients | Sex | Age | RT for Primary Surgery | Primary Surgery | Histology | CRM | TNM Stage | Adjuvant CT for Primary Surgery | Recurrence Site | Reason for Nonresectability | Size of the Recurrent Tumor (mm) | Interval from Primary Surgery to Diagnosis of LRRC (Months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 62 | − | LAR | tub1 | − | IIIb | CapOX | Lateral | Need to resect the S1 nerve | 19 | 54 |
| 2 | Male | 68 | − | APR LLND | tub1 | + | IIIb | CapOX | Posterior | Need to resect the S1 nerve | 16 | 8 |
| 3 | Male | 59 | − | LAR | tub2 | − | IIIc | CapOX | Posterior | Need to resect the S1 nerve | 18 | 22 |
| 4 | Male | 38 | + | LAR | tub2 | + | IVc | SOX | Lateral | Need to resect the S2 nerve No consent provided for surgery | 17 | 35 |
| 5 | Male | 78 | − | LAR | tub2 | + | IIIc | CapOX | Posterior | Need to resect the S1 nerve | 29 | 20 |
| 6 | Male | 78 | + | LAR | tub2 | + | IIIc | Cape | Posterior | Need to resect the S1 nerve | 26 | 16 |
| 7 | Male | 66 | + | APR LLND | tub1 | − | I | − | Lateral | Difficult to secure the lateral margin | 30 | 9 |
| 8 | Female | 51 | − | LAR | tub2 | − | IV | CapOX | Posterior | Need to resect the S1 nerve | 37 | 34 |
| 9 | Male | 69 | − | LAR | tub2 | − | IIIb | Cape | Lateral | Need to resect the S1 nerve | 22 | 36 |
| 10 | Male | 44 | + | LAR | tub2 | − | I | − | Lateral | Need to resect the S1 nerve | 22 | 12 |
| 11 | Female | 67 | − | LAR | tub2 | − | IIIc | CapOX | Posterior | Need to resect the S1 nerve | 29 | 93 |
| 12 | Male | 50 | − | LAR | por2 | + | IIIc | CapOX | Posterior | Need to resect the S1 nerve | 25 | 23 |
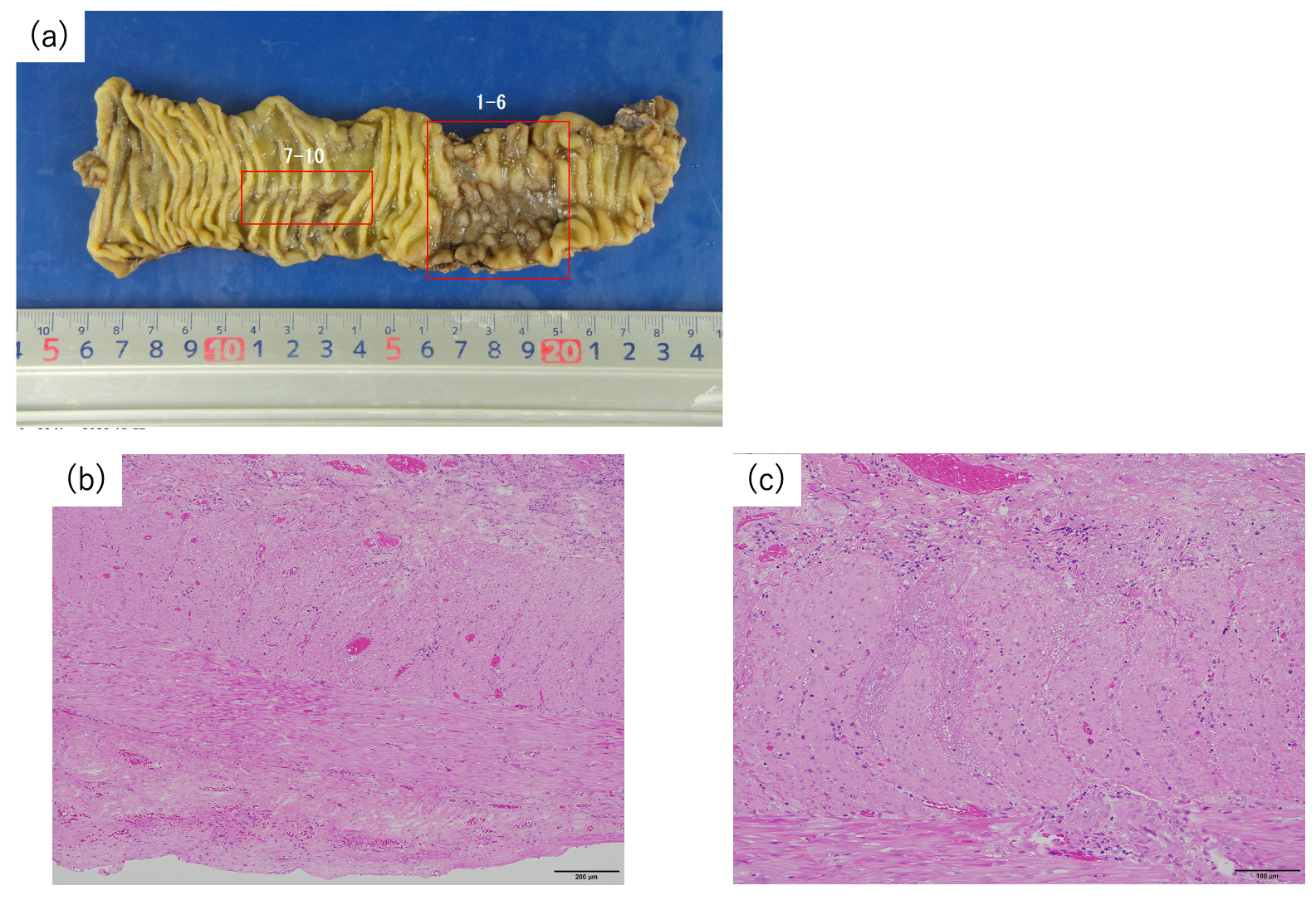
| Patients | Dose (Gy) | Waiting Time Before Surgery (Weeks) | Acute AEs | Late AEs | Time to Onset (Months) | Histological Findings |
|---|---|---|---|---|---|---|
| 1 | 73.6 | 8 | None | Sciatic nerve pain Grade 3 | 9 | No presence of cancer cells |
| 2 | 73.6 | 5 | None | None | No presence of cancer cells | |
| 3 | 73.6 | 8 | None | None | No presence of cancer cells | |
| 4 | 70.4 | 4 | None | Sciatic nerve pain Grade 1 | 6 | No presence of cancer cells |
| 5 | 73.6 | 6 | None | Sciatic nerve pain Grade 1 | 15 | No presence of cancer cells |
| 6 | 73.6 | 4 | None | None | No presence of cancer cells | |
| 7 | 70.4 | 2 | None | None | No presence of cancer cells | |
| 8 | 73.6 | 3 | None | None | No presence of cancer cells | |
| 9 | 73.6 | 3 | None | None | No presence of cancer cells | |
| 10 | 70.4 | 3 | None | None | No presence of cancer cells | |
| 11 | 73.6 | 8 | None | None | No presence of cancer cells | |
| 12 | 73.6 | 5 | None | None | No presence of cancer cells |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kimura, K.; Takiyama, H.; Yamada, S.; Ito, K.; Koba, M.; Imada, A.; Song, J.; Kataoka, K.; Kihara, T.; Matsuda, I.; et al. A Novel Treatment Strategy for Unresectable Locally Recurrent Rectal Cancer—Upfront Carbon-Ion Radiotherapy Followed by Surgical Resection of the Irradiated Intestines. Cancers 2025, 17, 2230. https://doi.org/10.3390/cancers17132230
Kimura K, Takiyama H, Yamada S, Ito K, Koba M, Imada A, Song J, Kataoka K, Kihara T, Matsuda I, et al. A Novel Treatment Strategy for Unresectable Locally Recurrent Rectal Cancer—Upfront Carbon-Ion Radiotherapy Followed by Surgical Resection of the Irradiated Intestines. Cancers. 2025; 17(13):2230. https://doi.org/10.3390/cancers17132230
Chicago/Turabian StyleKimura, Kei, Hirotoshi Takiyama, Shigeru Yamada, Kazuma Ito, Mizuki Koba, Ayako Imada, Jihyung Song, Kozo Kataoka, Takako Kihara, Ikuo Matsuda, and et al. 2025. "A Novel Treatment Strategy for Unresectable Locally Recurrent Rectal Cancer—Upfront Carbon-Ion Radiotherapy Followed by Surgical Resection of the Irradiated Intestines" Cancers 17, no. 13: 2230. https://doi.org/10.3390/cancers17132230
APA StyleKimura, K., Takiyama, H., Yamada, S., Ito, K., Koba, M., Imada, A., Song, J., Kataoka, K., Kihara, T., Matsuda, I., Beppu, N., Horio, Y., Kitajima, K., Uchino, M., Ikeuchi, H., & Ikeda, M. (2025). A Novel Treatment Strategy for Unresectable Locally Recurrent Rectal Cancer—Upfront Carbon-Ion Radiotherapy Followed by Surgical Resection of the Irradiated Intestines. Cancers, 17(13), 2230. https://doi.org/10.3390/cancers17132230







