Simple Summary
Gastric neoplasms are a prevalent malignant pathology with high incidence and prevalence, necessitating precise histopathological characterization for optimal treatment planning. A retrospective analysis of 67 gastric neoplasia subjects from January 2020 to December 2021 revealed that adenocarcinoma was the most common histologic type (91.0%), with most tumors diagnosed as Stage III (40.3%). The intestinal type was the most common (49.2%), followed by diffuse (36.1%) and mixed (14.8%). Poorly differentiated tumors (G3) accounted for 53.7% of cases. The surgical team performed curative resection in 75% of patients, achieving R0 margins in 88% of cases. Subtotal gastrectomy with D2 lymphadenectomy yielded the highest curative success rate (96.6% R0 re-section). Two significant correlations were found between age and tumor grade and between the number of lymph nodes examined and the number of lymph nodes invaded. Adenocarcinomas showed higher rates of lymph node invasion than other tumor types. The analysis of patients with gastric neoplasms is crucial for appropriate therapeutic management, and maintaining oncologic standards during medical crises is essential.
Abstract
Background: Gastric neoplasms remain pathologies of the malignant spectrum with high incidence and prevalence, with their management requiring a precise histopathological characterization for optimal treatment planning. Methods: The present study is a retrospective analysis that included 67 histopathologically confirmed gastric neoplasia subjects and was performed at a single surgical center from January 2020 to December 2021. Demographics, tumor characteristics, surgical procedures, and oncologic outcomes were included, filtered, and subsequently analyzed using SPSS Statistics 29.0. Results: This study involved 67 patients (mean age 65.7 years, 56.7% men), with adenocarcinoma being the most common histologic type (91.0%) and most tumors being diagnosed directly as Stage III (40.3%). Lauren classification revealed the intestinal type as the most common (49.2%), followed by diffuse (36.1%) and mixed (14.8%). Poorly differentiated tumors (G3) accounted for 53.7% of cases. The surgical team performed curative resection in 75% (n = 50) of patients, achieving R0 margins in 88% of these cases. Subtotal gastrectomy with D2 lymphadenectomy yielded the highest curative success rate with 96.6% R0 resection. Statistically, we identified two significant correlations between age and tumor grade (rho = 0.28; p = 0.021) and between the number of lymph nodes examined and the number of lymph nodes invaded (rho = 0.65, p < 0.001). This study again revealed that adenocarcinomas showed higher rates of lymph node invasion than other tumor types (p = 0.017). Conclusions: The analysis of patients with gastric neoplasms is vital for appropriate therapeutic management. Even though the study period included a pandemic, the analysis remained a complex one with high-quality surgical outcomes, confirming the importance of maintaining oncologic standards during medical crises.
1. Introduction
Despite surgical advances, gastric neoplasia represents a major global health problem with significant incidence in many regions of the world, particularly in Asia and Latin America [1,2,3,4]. According to statistics from 2020, more than 1,089,000 new cases of gastric neoplasia and 769,000 associated deaths were reported worldwide [5,6,7,8].
Knowledge of histopathologic features is crucial for accurate diagnosis and prognosis determination [9,10]. Lauren’s histologic classification divides gastric adenocarcinomas into the intestinal type (associated with environmental factors like H. pylori, more common in men) and diffuse type (more frequent in women, associated with poorer prognosis) [10,11,12].
In 2019, the World Health Organization established the classification of gastric tumors. It provides a detailed framework for accurate diagnosis, dividing them into epithelial, mesenchymal, lymphoid, and neuroendocrine tumors. The most common subtype is gastric adenocarcinoma, further divided into the following variants: tubular adenocarcinoma, papillary adenocarcinoma, mucinous adenocarcinoma, pitted ring cell adenocarcinoma, and cribriform adenocarcinoma [1,13,14,15,16,17,18].
Gastric neoplasia treatment has evolved into a multimodal and personalized treatment approach [19,20]. While involving radiotherapy, chemotherapy, and immunotherapy, elective surgery with lymphadenectomy remains the only curative treatment [12,17,21,22].
The surgical interventions performed for malignant gastric tumors, depending on the anatomical location of the tumor (fundus, body, or antrum), are as follows: upper polar gastrectomy, total gastrectomy, and distal subtotal gastrectomy [21,22]. Lymphadenectomy is essential for staging and oncological outcomes and is classified as the D1, D2, or D3 type, with D2 lymphadenectomy combined with appropriate gastrectomy representing the “gold standard” in high-volume centers [23,24].
This article makes an original contribution to the existing literature by focusing on gastric neoplasia treatment during the COVID-19 pandemic. The COVID-19 pandemic has also put its “stamp” on the delay of this diagnosis, which brings with it a limitation on the number of curative interventions and an increase in the number of patients at a more advanced stage, disrupting health systems worldwide.
The aim of this article is to analyze the histopathological and surgical features observed in gastric neoplasia cases in a single surgical center from 2020 to 2021. This article aims to identify the predominant histological types and subtypes of gastric neoplasia, to highlight the relevant prognostic factors, to quantify the type of surgeries and the indices of curability (lymphadenectomy, resection margins), and to analyze the implications and possible correlations of these parameters as impact factors in the management of patients with gastric neoplasms.
2. Materials and Methods
This retrospective observational study was conducted at the Surgery II Clinic of “Pius Brînzeu” County Emergency Hospital, Timișoara. A total of 67 patients hospitalized between 1 January 2020 and 31 December 2021 with histopathologically confirmed gastric neoplasia were included. Data were extracted from the hospital’s InfoWorld electronic medical record system to identify associations between histopathologic parameters, surgical interventions, and patient prognosis.
Inclusion criteria were as follows: age ≥18 years, histopathologically confirmed gastric neoplasia, complete medical records, and patient consent.
Three data sources were analyzed in this paper: demographic information (age, sex), clinical records (diagnosis, surgical procedures), and histopathological findings (stage, grade, tumor characteristics). Histopathologic evaluation was based on the WHO 2019 classification criteria with the Lauren classification applied to adenocarcinomas. All slides were independently reviewed by two pathologists. Ki-67 assessment was performed in 6 cases, mainly GIST cases (n = 5), according to standard diagnostic protocols for risk stratification. Ki-67 assessment was limited due to institutional protocol constraints during the COVID-19 pandemic period.
The statistical analysis part was performed using SPSS Statistics version 29.0, with p < 0.05 considered significant. Descriptive statistics were used to assess normality using the Shapiro–Wilk test for continuous variables and frequencies/percentages for categorical variables. Spearman’s rank correlation coefficient was used for correlation analysis due to the non-normal distribution of data and sample size considerations.
Lymph node harvest variability varies based on clinical circumstances, including palliative interventions, local infiltration, and poor biological status, and depends on gastrectomy type, degree, patient status, and pandemic conditions.
This study was approved by the Ethics Committee of the County Emergency Hospital “Pius Brînzeu”, Timisoara. Patient data were coded to ensure anonymity and handled in accordance with GDPR regulations. All data uses respected patient privacy standards. The retrospective design introduces potential selection bias and relies on existing medical records, which may be incomplete. Non-randomized patient selection is a limitation in the study methodology.
This study has limitations, including its potential selection bias, incomplete medical records, small sample sizes, single-center design, missing Ki-67 data, lack of long-term follow-up outcomes, and high variability in lymph node harvest.
3. Results
3.1. General Characteristics of Study Population
This study included 67 patients with gastric neoplasia treated between January 2020 and December 2021 (Table 1). Adenocarcinoma was the predominant histological type (91.0%, n = 61), followed by GIST (7.5%, n = 5) and lymphoma (1.5%, n = 1). According to the Lauren classification, the intestinal type was the most common (49.2%), followed by the diffuse (36.1%) and mixed types (14.8%).

Table 1.
General characteristics of study population.
An analysis of continuous variables with respect to clinical characteristics (Table 2) revealed that advanced tumor stages were associated with increased lymph node involvement, with Stage III disease showing significantly more invaded nodes than early-stage tumors (p = 0.04). No significant differences were observed in age distribution or nodal sampling across Lauren classification subtypes (p = 0.73) or surgical approaches (p = 0.32), indicating consistent treatment approaches regardless of histological subtype.

Table 2.
Comparison of continuous variables in terms of clinical characteristics.
Tumor staging, based on the TNM classification (AJCC, 8th edition), revealed a heterogeneous distribution [25]. As illustrated in Figure 1A, tumor staging revealed a concerning predominance of advanced disease, with Stage III being the most frequent (40.3%, n = 27), substantially exceeding Stage I presentations (20.9%, n = 14). This distribution suggests delayed diagnosis patterns, particularly notable during the pandemic period. Stage II represented 26.87% (n = 18), while Stage IV accounted for 11.94% (n = 8) of cases. Histological grading (Figure 1B) demonstrated aggressive tumor biology, with poorly differentiated (G3) tumors predominating at 53.7% (n = 36), more than double the rate of moderately differentiated tumors (G2, 26.87%, n = 18). Well-differentiated tumors (G1) were rare at 5.97% (n = 4), while undifferentiated (G4) and undetermined grade (Gx) represented 2.99% and 10.45%, respectively. Lymph node involvement was observed in 61.2% (n = 41) of patients, with a mean of 19.2 ± 12.8 lymph nodes examined (median 18, IQR 12–26, range 0–49) and a mean of 4.1 ± 6.7 lymph nodes invaded (median 3, IQR 0–6, range 0–38). Distant metastases (M1) were present in 11.94% (n = 8) of the cohort.
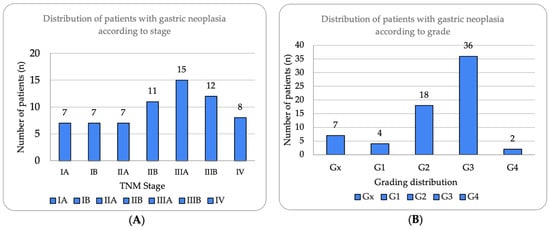
Figure 1.
Distribution of patients with gastric neoplasia according to stage and histological grade. (A) Tumor stage distribution according to TNM classification (8th edition), showing Stage III as most prevalent (n = 27, 40.3%), followed by Stage II (n = 18, 26.87%), Stage I (n = 14, 20.90%), and Stage IV (n = 8, 11.94%). Substages IIIA and IIIB represented 22.39% and 17.91%, respectively. (B) Histological grade distribution demonstrating predominance of poorly differentiated tumors (G3, n = 36, 53.7%), followed by moderately differentiated (G2, n = 18, 26.87%), well-differentiated (G1, n = 4, 5.97%), undifferentiated (G4, n = 2, 2.99%), and undetermined grade (Gx, n = 7, 10.45%). High prevalence of G3 tumors correlates with advanced stage presentation.
Lymphatic invasion (LV1) was identified in 58.2% (n = 39) of patients, vascular invasion (VI) in 10.4% (n = 7), and perineural invasion (PnI) in 58.2% (n = 39). The Ki67 proliferation marker was assessed in 9.0% (n = 6) of cases, with a median value of 45% (range: 10–70%), predominantly in GIST and advanced adenocarcinomas. These results are presented in Table 1.
The WHO classification revealed tubular adenocarcinoma as the most common (36%), followed by the mixed subtype (27%) and poorly cohesive type (13%). Next are the following subtypes: signet ring adenocarcinoma—11%; mucinous adenocarcinoma—7%; adenocarcinoma with special subtypes—4%; cribriform adenocarcinoma—2%; and papillary adenocarcinoma—2%.
Hematoxylin–eosin staining was used for the direct exposure of the histopathologic subtypes (according to the WHO classification) present in the studied group, as shown in optical microscopy images.
Representative histopathological images of different tumor types are shown in Figure 2, Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7, illustrating the histological subtypes observed in our cohort.
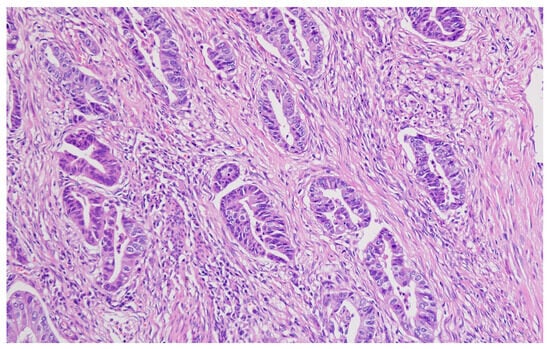
Figure 2.
Tubular adenocarcinoma showing well-formed glandular structures with moderate nuclear atypia (H&E, ×200, scale bar = 100 μm).
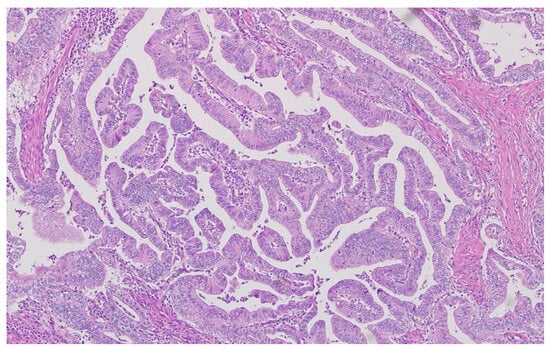
Figure 3.
Poorly cohesive adenocarcinoma with signet ring cell morphology infiltrating the stroma (H&E, ×400, scale bar = 50 μm).
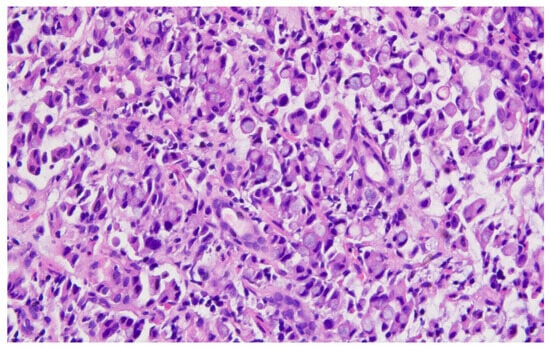
Figure 4.
Mucinous adenocarcinoma with abundant extracellular mucin pools surrounding malignant glands (H&E, ×100, scale bar = 200 μm).
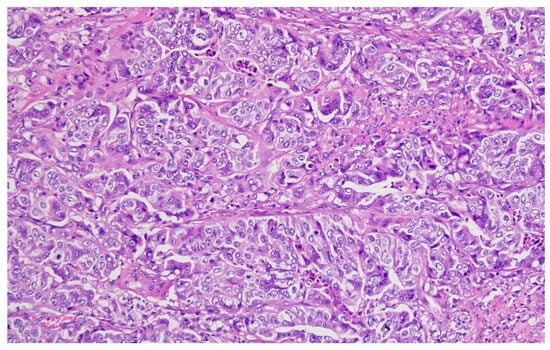
Figure 5.
Moderately differentiated (G2) adenocarcinoma with irregular glandular architecture and moderate nuclear pleomorphism (H&E, ×200, scale bar = 100 μm).
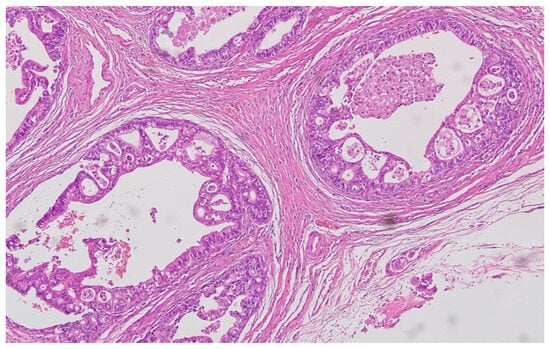
Figure 6.
Poorly differentiated (G3) adenocarcinoma showing solid growth pattern with high nuclear pleomorphism and increased mitotic activity (H&E, ×200, scale bar = 100 μm).
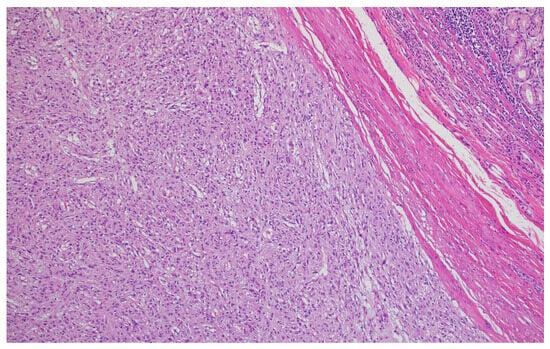
Figure 7.
Lymphatic invasion demonstrating tumor cells within lymphatic vessels, supporting our finding of 58.2% lymphatic invasion rate (H&E, ×400, scale bar = 50 μm).
3.2. The Curability Rate of Surgical Interventions in the Study Group
In the analyzed cohort, according to Figure 8, the surgical resection margins were negative (R0) in 88% of cases (n = 44), indicating complete tumor excision without residual microscopic invasion at the specimen margins. This result reflects an optimal surgical quality and is associated with better overall survival and a lower risk of local recurrence in the literature.
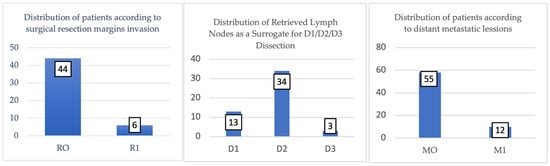
Figure 8.
Surgical resection margin status in study cohort (n = 50 curative resections). R0 (negative margins) achieved in 44 cases (88%), indicating complete tumor excision without residual microscopic disease at specimen margins. R1 (positive microscopic margins) found in 6 cases (12%), suggesting remaining tumor cells with implications for adjuvant therapy and prognosis. Chart shows absolute numbers and percentages for each category.
On the other hand, in 12% of cases (n = 6), positive margins (R1) were recorded, suggesting the presence of remaining tumor cells at the microscopic level, with unfavorable prognostic implications.
These cases may require additional adjuvant treatments and close oncological monitoring. The relatively low rate of R1 margins emphasizes the efficiency of the operative action in most procedures and may be an indirect indicator of good preoperative selection and correct surgical technique.
3.3. Analyzing the Batch According to the Type of Surgical Interventions
Of the 67 interventions performed, 50 (75%) were curative, and 17 (25%) were palliative. Palliative interventions were indicated in the presence of metastases, extensive tumor invasion with the impossibility of complete resection, or poor overall biological status.
The most common interventions were total subtotal distal gastrectomies (n = 29), followed by total gastrectomies (n = 11) and superior polar gastrectomies (n = 10).
The analyzed table highlights the distribution of the types of gastrectomy (subtotal, total, and upper polar) according to the degree of lymphadenectomy performed (D1, D2, D3), providing a clear perspective on current surgical practice.
Subtotal gastrectomy is predominantly performed with D2 lymph node dissection (19%) but also in a relevant proportion with D1 (8%) and, marginally, D3 (2%), suggesting that this intervention is preferred in cases where the tumor is distally located and the oncological stage allows for a conservative approach while maintaining the curative intent.
The distribution of resection margin status (R0/R1) across the three types of gastrectomy provides valuable insight into the oncological effectiveness and curability of different surgical approaches in gastric neoplasia treatment.
In patients who underwent subtotal distal gastrectomy, 28 cases of R0 resection (96.6%) and only 1 case of R1 resection (3.4%) were identified, giving this procedure the highest curative potential in the group studied. This suggests that, when correctly selected, this procedure can achieve complete resection in the majority of patients, especially when the tumor is located distal to the stomach. The low R1 resection rate further supports the suitability of this procedure for less invasive, early-stage gastric neoplasias, where a conservative approach combined with a D2 lymphadenectomy (as seen in many cases) can provide optimal results.
In the subgroup of patients in whom total gastrectomy was performed, an R0 rate of 72.7% and R1 rate of 27.3% indicate a relatively lower curative success compared to the subgroup in whom subtotal gastrectomy was performed. This result may be related to the higher complexity of the procedure, especially when tumors are located proximally or in advanced stages. The higher proportion of R1 resections suggests that some patients may not benefit fully from surgical resection alone, and adjuvant therapy may be required for these cases.
In the third subgroup, upper polar gastrectomy was practiced, which is accompanied by an R0 resection in 80% of cases (n = 8) and an R1 resection in 20% (n = 2). The slightly higher R1 rate suggests that obtaining clear margins in this region may be more difficult, and as with total gastrectomy, multimodality therapy may be an essential part of the treatment strategy.
The classification of surgical interventions according to type of lymphadenectomy and status of resectinal margins is illustrated in Figure 9.
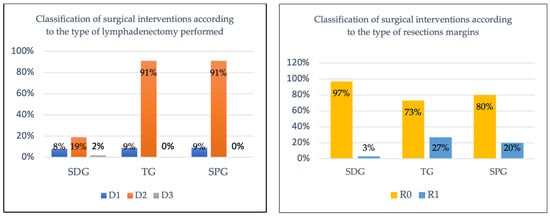
Figure 9.
Classification of surgical interventions according to type of lymphadenectomy and status of resectional margins.
The observed differences in R0 rates between surgical approaches should be interpreted cautiously given the wide confidence intervals, particularly for total gastrectomy (CI: 39.0–94.0%) and superior polar gastrectomy (CI: 44.4–97.5%), reflecting the limited statistical precision inherent in smaller subgroups.
3.4. Correlations Between Clinical and Pathological Variables
The relationship between continuous and ordinal clinical and pathological variables was examined using Spearman’s rank correlation coefficient (rho). Three specific correlations were analyzed to explore potential correlations relevant to disease progression and patient characteristics (Table 3).

Table 3.
Evaluating the oncological effectiveness of surgical procedures based on lymphadenectomy level (D1, D2, D3) and resection status (R0, R1).
First, a statistically significant positive correlation was observed between patient age and histological grade (rho = 0.28; p = 0.021). This finding suggests that older patients were more likely to present with higher-grade tumors (G3), indicating a potential correlation between age and tumor aggressiveness. Patients with poorly differentiated tumors (G3) had a mean age of 67.3 years, compared to 62.8 years for those with well- or moderately differentiated tumors (G1 or G2).
Second, according to Figure 10, a strong and highly significant positive correlation was identified between the number of lymph nodes examined and the number of lymph nodes invaded (rho = 0.65, p < 0.001; Figure 5). This result indicates that a greater extent of lymph node sampling was associated with an increased likelihood of detecting lymph node metastases, reflecting the extent of disease spread in surgically resected specimens. Among patients with more than 20 lymph nodes examined (n = 25), 76.0% (n = 19) had lymph node involvement, compared to 48.8% (n = 20) in those with fewer lymph nodes examined (n = 41).
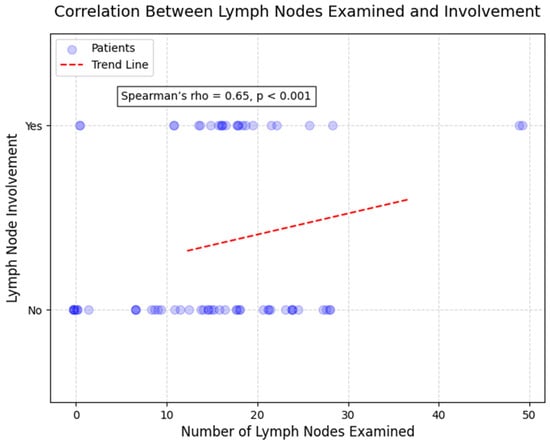
Figure 10.
Scatterplot correlation between number of lymph nodes examined and number of lymph nodes invaded (Spearman’s rho = 0.65, p < 0.001). Each point represents one patient. Strong positive correlation demonstrates that more extensive lymph node sampling increases detection of nodal metastases. Patients with >20 nodes examined showed 76.0% lymph node involvement versus 48.8% in those with <20 nodes examined. Line represents best-fit trend.
Finally, in concordance with Table 4, the correlation between patient age and TNM stage was assessed but found to be non-significant (rho = 0.14; p = 0.27). This suggests that, in this cohort, age did not strongly influence the overall stage at diagnosis, with advanced stages (III and IV) distributed relatively evenly across age groups. For instance, Stage III was observed in 42.9% (n = 15) of patients under 65 years and 46.9% (n = 15) of those 65 years or older.

Table 4.
Spearman correlation coefficients for clinical and pathological variables.
These findings highlight key relationships between clinical and pathological features, which are further summarized in Table 4.
3.5. Associations Between Tumor Characteristics and Invasive Features
The distribution of invasive features across key clinical subgroups is summarized in Figure 11. The associations between tumor characteristics and invasive features in this cohort was investigated using the Chi-square test. No significant association was found between patient age (categorized as <65 years vs. ≥65 years) and vascular invasion (χ2 = 0.92; df = 1; p = 0.34). Vascular invasion was observed in 62.9% (n = 22) of patients under 65 years and 53.1% (n = 17) of those 65 years or older, suggesting that age does not substantially influence this feature in our cohort. Similarly, the association between age and lymphatic invasion was not statistically significant (χ2 = 1.15; df = 1; p = 0.28), with lymphatic invasion present in 65.7% (n = 23) of younger patients and 50.0% (n = 16) of older patients.
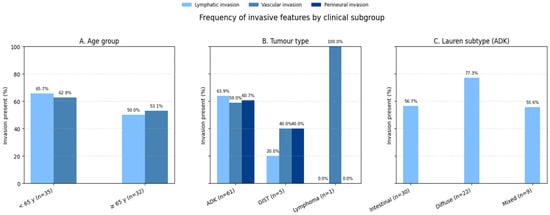
Figure 11.
Distribution of invasive features across clinical subgroups. Bar chart comparing lymphatic invasion, vascular invasion, and perineural invasion rates between (A) age groups (<65 vs. ≥65 years), (B) tumor types (adenocarcinoma vs. GIST vs. lymphoma), and (C) Lauren classification subtypes (intestinal vs. diffuse vs. mixed). Error bars represent 95% confidence intervals. Adenocarcinomas showed significantly higher lymphatic invasion rates (63.9%) compared to GIST (20.0%, p = 0.017).
According to Figure 11, Tumor type (adenocarcinoma [ADK], gastrointestinal stromal tumor [GIST], and lymphoma) showed a borderline association with vascular invasion (χ2 = 4.87; df = 2; p = 0.087). ADK patients exhibited vascular invasion in 59.0% (n = 36) of cases, compared to 40.0% (n = 2) in GIST and 100% (n = 1) in lymphoma; however, this trend did not reach statistical significance, likely due to the small number of GIST and lymphoma cases. In contrast, a significant association was observed between tumor type and lymphatic invasion (χ2 = 8.12; df = 2; p = 0.017). ADK patients had a higher prevalence of lymphatic invasion (63.9%, n = 39) compared to GIST (20.0%, n = 1), with the single lymphoma case showing no lymphatic invasion (0%). This suggests that adenocarcinomas are more likely to exhibit lymphatic spread than GISTs.
The association between tumor type and perineural invasion was not significant (χ2 = 3.45; df = 2; p = 0.18). Perineural invasion was present in 60.7% (n = 37) of ADK cases and 40.0% (n = 2) of GIST cases and absent in the lymphoma case, indicating no clear differential pattern across tumor types in this cohort.
In concordance with Figure 11, among adenocarcinomas, the Lauren classification (intestinal, diffuse, or mixed) was significantly associated with lymphatic invasion (χ2 = 6.23; df = 2; p = 0.044). Diffuse-type adenocarcinomas showed the highest rate of lymphatic invasion (77.3%, n = 17), followed by mixed-type (55.6%, n = 5) and intestinal-type (56.7%, n = 17), highlighting a greater propensity for lymphatic spread in the diffuse subtype.
These results, summarized in Table 5, provide insights into the invasive behavior of gastric neoplasia in relation to patient and tumor characteristics.

Table 5.
Associations between tumor characteristics and invasive features.
4. Discussion
4.1. Interpretation of Main Findings
Gastric neoplasia remains a formidable challenge that requires early detection and personalized therapeutic approaches based on multidisciplinary expertise. Current knowledge highlights the importance of understanding the histopathological forms of gastric neoplasia (the main types and subtypes and recognized classifications such as the Lauren classification), due to the desire to accurately categorize patients.
This single-center study during the COVID-19 pandemic provides important insights into gastric neoplasia histopathology and surgical outcomes. The present analysis identified three major tumor types, adenocarcinomas (91.0%), gastrointestinal stromal tumors (7.5%), and lymphomas (1.5%), with Stage III disease being the most common (40.3%) and poorly differentiated (G3) tumors predominating (53.7%). The most significant finding was the strong association between tumor type and lymphatic invasion (p = 0.017), with adenocarcinomas demonstrating much higher rates of lymphatic spread compared to GISTs, highlighting their more aggressive biological behavior.
4.2. Pandemic Context and Clinical Impact
This study, conducted during COVID-19, maintained high surgical standards with an 88% R0 resection rate despite pandemic challenges. Specifically, the surgical center where the patients were treated maintained high surgical standards, with an R0 resection rate of 88%. From a morphopathological point of view, there is a high incidence of the presentation of patients in Stage III, which can also be justified by the phenomenon of “stage delay” due to the pandemic.
4.3. Balanced Assessment of Clinical Outcomes
While maintaining an 88% R0 resection rate during the pandemic demonstrates surgical quality preservation, the predominance of Stage III disease (40.3%) versus Stage I (20.9%) represents a concerning failure in early detection. This “stage migration” significantly undermines the clinical impact of excellent surgical technique, as advanced-stage presentations inherently limit curative potential regardless of procedural success.
The high rates of lymph node involvement (61.2%) and lymphatic invasion (58.2%) reflect the consequences of delayed diagnosis that surgical excellence cannot compensate for. Our 25% palliative intervention rate illustrates cases where optimal surgical capability could not achieve cure due to late presentation.
These findings highlight that future quality improvement should prioritize early detection over further surgical refinement. Strategies should include enhanced screening protocols for high-risk populations, pandemic-resistant diagnostic pathways, and expedited referral systems for alarm symptoms.
4.4. Histopathological Validation
Our histopathological findings align with the established literature, with the adenocarcinoma distribution being consistent with Werner et al.’s findings [10].
The distribution of our patients according to the Lauren classification (intestinal 49.2%, diffuse 36.1%, mixed 14.8%) shows information about tumor biology and tumor invasion patterns. For example, diffuse adenocarcinomas had the highest rates of lymphatic invasion (77.3%), confirming their more aggressive character.
In our group, tumors with lower incidence such as GIST (7.5%) and gastric lymphoma (1.5%) were highlighted, which clearly required personalized therapeutic approaches. As highlighted in the current study, the low incidence of these tumors was evident even during the pandemic period. ESMO guidelines recommend immunohistochemical markers (CD-117, DOG1, CD34, SMA) for GIST characterization, highlighting the need for specialized management strategies [26].
4.5. Surgical Approach and D2 Lymphadenectomy
Concerning the type of gastrectomy performed and the associated lymphadenectomy, in the present study, the distribution of the types of gastrectomy (subtotal, total, and upper polar) according to the degree of lymphadenectomy performed was analyzed. Subtotal gastrectomy is predominantly performed with D2 lymph node dissection (19%), indicating compliance with current therapeutic standards. D2 lymphadenectomy is the gold standard in the treatment of gastric neoplasia. The majority of cases (66–91% depending on gastrectomy type) chose D2 lymphadenectomy, which brings superior benefits in terms of correct staging and a reduction in the risk of local recurrence.
One of the most representative studies in the literature, led by Songun et al., concludes that after a 15-year follow-up, D2 lymphadenectomies are associated with a lower loco-regional recurrence rate and lower gastric neoplasia mortality compared to D1 lymphadenectomies [27]. These results support the growing consensus that D2 lymphadenectomy represents the preferred standard in the surgical treatment of gastric neoplasia in specialized centers.
4.6. RO Index
An additional index of curability in this study is the assessment of the status of the resection margins in the study group and their correlation with the type of surgery performed. Specifically, distal subtotal gastrectomy demonstrates the highest curative potential, with 28 cases of R0 resection (96.6%) and only 1 case of R1 resection (3.4%). Total gastrectomy has a lower curative success rate (72.7%) and a higher R1 rate (27.3%), suggesting that some patients may not fully benefit from surgical resection alone. Upper polar gastrectomy has an intermediate level of curative success (80.0%) and a higher R1 rate (20.0%), suggesting the need for multimodal therapy.
The lower R1 index is a beneficial outcome, being associated with lower distant recurrence rates and lower morbidity and mortality, with the ultimate goal or ultimate aim being that all gastrectomies should be R0 in the future. Multiple studies in the literature report major benefits associated with RO-type resections.
For example, a study by Biondi et al. states that the direction of all therapeutic strategies in gastric neoplasia is to achieve a curative resection (RO), which is associated with significant improvement in the quality of life, decreased morbidity and mortality, and improved survival [28].
Two other specialty studies, one led by Ridwelski et al. and the other by Figueiredo et al., describing outcome data of R1 gastrectomized patients, show that an R1 situation worsens long-term survival, particularly for low T-stage and lymph node metastases [29,30].
Another study, led by Cordero-García et al., supports the impact of a set of parameters on improving survival and reducing recurrence. This set consists of the following: the status of resection margins, tumor type, and the degree of differentiation [31].
In this context, according to Figure 12, we propose naming a set of indices (resection margin status, type of lymphadenectomy performed, tumor type, degree of differentiation) as curability indices of gastric neoplasia and to present the results obtained for our group.
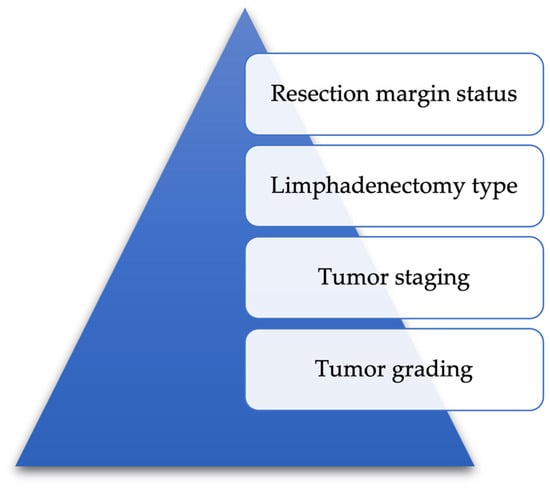
Figure 12.
Establishing a set of curability indicators for gastric cancer surgery.
4.7. Adjuvant Therapy
The place of adjuvant therapy in gastric neoplasms remains a subject of ongoing debate in the literature, with significant geographical variations in treatment approaches [19,32,33,34,35,36,37].
Meta-analyses demonstrate that adjuvant fluorouracil-based chemotherapy provides a 6% absolute survival benefit compared to surgery alone (HR 0.82; 95% CI 0.76–0.90; p < 0.001) [38]. Recent studies on cytoreductive surgery with HIPEC show survival benefits particularly relevant for our cohort, given that 40.3% were Stage III with 58.2% lymphatic invasion [39].
4.8. Study Limitations
This study has several important limitations that must be acknowledged when interpreting the results. The relatively small sample size (n = 67) significantly limits statistical power for subgroup analyses, particularly for comparisons between rare tumor types such as GIST (n = 5) and lymphoma (n = 1) versus adenocarcinomas.
Additionally, the limited sample size precluded meaningful multivariable analysis to control for potential confounding factors such as age, tumor stage, and comorbidities simultaneously.
The retrospective observational design introduces inherent selection bias through the inclusion of only patients with complete medical documentation, potentially excluding cases representing different disease severity or management approaches, while information bias may arise from inconsistencies in data recording across different time periods and healthcare providers.
The study period (2020–2021) coinciding with the COVID-19 pandemic significantly impacts generalizability, likely contributing to delayed diagnoses, as evidenced by the high proportion of Stage III presentations (40.3%) and potential “stage migration” effects.
Resource limitations during the pandemic affected diagnostic comprehensiveness, including limited Ki-67 assessment and variations in lymph node harvesting techniques that may not reflect standard care capabilities.
Most critically, the absence of long-term follow-up data prevents the assessment of crucial oncological outcomes such as overall survival, disease-free survival, and recurrence patterns, limiting our ability to correlate the histopathological findings with clinical prognosis or validate surgical outcome classifications through real-world results.
As a single-center study conducted in Romania, the findings may not be generalizable to other healthcare systems, geographic regions, or patient populations with different characteristics, while the observational design precludes establishing causality between identified associations, representing correlations rather than causal relationships.
This study’s limitations include a small sample size in certain surgical subgroups, particularly total gastrectomy and superior polar gastrectomy, which limits the statistical power and reliability of comparisons, and the results should be interpreted cautiously due to wide confidence intervals and unstable estimates.
Despite these limitations presented by the pandemic context, our findings demonstrate the maintenance of surgical quality during the pandemic, with an R0 resection rate of 88%.
5. Conclusions
Gastric neoplasia is a neoplasm that continues to amaze researchers with its large number of new cases and systemic involvement and is often labeled as a global burden in terms of associated long-term care costs for these patients. This single-center study of 67 patients with gastric neoplasia during the COVID-19 pandemic presents important insights into histopathologic patterns and surgical outcomes.
This study on the histopathologic aspects of gastric neoplasia identified three major tumor types, adenocarcinomas (91.0%), gastrointestinal stromal tumors (GISTs, 7.5%), and lymphomas (1.5%), with adenocarcinomas being the most common. Stage III disease was the most frequent (40.3%), predominantly poorly differentiated (G3, 53.7%). The most significant finding was the association between tumor type and lymphatic invasion (p = 0.017), with adenocarcinomas showing a higher tendency for lymphatic dissemination compared to GIST and lymphomas, highlighting their more aggressive biological behavior.
From a surgical point of view, it can be concluded that curative interventions remain the most common (75%), with distal subtotal gastrectomy with D2 lymphadenectomy presenting the highest curative success (96.6% R0), emphasizing the importance of the type of surgery and the extent of lymphadenectomy. These findings have direct clinical implications: the strong association between adenocarcinomas and lymphatic invasion (p = 0.017) should guide staging protocols and treatment decisions, while the superior R0 rates obtained with subtotal gastrectomy plus D2 lymphadenectomy (96.6%) support this approach as the preferred one.
This study demonstrates the surgical quality maintained during pandemic conditions (88% R0 rate) while revealing concerning patterns of delayed presentation (40.3% Stage III). The findings provide a regional validation of international classification systems and confirm established associations between tumor characteristics and biological behavior. While technical surgical outcomes remained robust, the clinical impact is limited by late-stage presentations that surgical excellence cannot overcome.
Future improvements must prioritize early detection infrastructure alongside continued surgical standard maintenance. This study’s contributions are primarily confirmatory, highlighting the need for systematic approaches to earlier diagnosis in gastric neoplasia management.
Author Contributions
Conceptualization, C.P.-B. and S.B.; methodology, S.B.; software, S.B.; validation, F.I.F., R.H., P.P. and D.B.; formal analysis, F.I.F., R.H., P.P. and D.B.; investigation, F.I.F., R.H., P.P. and D.B. resources, A.D. (Alis Dema), C.D.; data curation, A.D. (Alis Dema). and C.D.; writing—original draft preparation, C.P.-B., A.D. (Amadeus Dobrescu) and C.D.; writing—review and editing, C.P.-B., A.D. (Amadeus Dobrescu) and C.D.; visualization, N.-I.V., N.F., C.I.V.F. and L.-A.G.; supervision, N.-I.V., N.F., C.I.V.F. and L.-A.G.; project administration, C.P.-B., C.I.V.F. and L.-A.G.; funding acquisition, N.-I.V. and N.F. All authors have read and agreed to the published version of the manuscript.
Funding
The Article Processing Charge was paid by “Victor Babes” University of Medicine and Pharmacy, Timisoara, Romania.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Scientific Research Ethics Committee of the County Emergency Hospital “Pius Brînzeu”, Timisoara.
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The original contributions presented in this study are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ADK | Adenocarcinoma |
| AJCC | American Joint Committee on Cancer |
| EBV | Epstein–Barr Virus |
| ESMO | European Society for Medical Oncology |
| GIST | Gastrointestinal Stromal Tumor |
| GS | Genomically Stable |
| CI | Chromosomal Instability |
| LV | Lymphatic Invasion |
| M | Magnification |
| MSI | Microsatellite Instability |
| PnI | Perineural Invasion |
| R0 | Resection with Negative Margins |
| R1 | Resection with Positive Microscopic Margins |
| TNM | Tumor, Node, Metastasis |
| VI | Vascular Invasion |
| WHO | World Health Organization |
References
- Norton, E.J.; Bateman, A.C. Intestinal Type Adenocarcinoma. PathologyOutlines.com Website. Available online: https://www.pathologyoutlines.com/topic/stomachintestinal.html (accessed on 25 April 2025).
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; Van Grieken, N.C.; Lordick, F. Gastric Cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef]
- Sharma, R. Burden of Stomach Cancer Incidence, Mortality, Disability-Adjusted Life Years, and Risk Factors in 204 Countries, 1990–2019: An Examination of Global Burden of Disease 2019. J. Gastrointest. Cancer 2024, 55, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Lin, L.; Su, X. Global Burden of Depression or Depressive Symptoms in Children and Adolescents: A Systematic Review and Meta-Analysis. J. Affect. Disord. 2024, 354, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-L.; Lin, J.-X.; Lin, G.-T.; Huang, C.-M.; Zheng, C.-H.; Xie, J.-W.; Wang, J.; Lu, J.; Chen, Q.-Y.; Li, P. Global Incidence and Mortality Trends of Gastric Cancer and Predicted Mortality of Gastric Cancer by 2035. BMC Public Health 2024, 24, 1763. [Google Scholar] [CrossRef] [PubMed]
- Ilic, M.; Ilic, I. Epidemiology of Stomach Cancer. World J. Gastroenterol. 2022, 28, 1187–1203. [Google Scholar] [CrossRef]
- Rawla, P.; Barsouk, A. Epidemiology of Gastric Cancer: Global Trends, Risk Factors and Prevention. Gastroenterol. Rev. 2019, 14, 26–38. [Google Scholar] [CrossRef]
- Wong, M.C.S.; Huang, J.; Chan, P.S.F.; Choi, P.; Lao, X.Q.; Chan, S.M.; Teoh, A.; Liang, P. Global Incidence and Mortality of Gastric Cancer, 1980–2018. JAMA Netw. Open 2021, 4, e2118457. [Google Scholar] [CrossRef]
- Yakirevich, E.; Resnick, M.B. Pathology of Gastric Cancer and Its Precursor Lesions. Gastroenterol. Clin. N. Am. 2013, 42, 261–284. [Google Scholar] [CrossRef]
- Werner, M.; Becker, K.F.; Keller, G.; Höfler, H. Gastric Adenocarcinoma: Pathomorphology and Molecular Pathology. J. Cancer Res. Clin. Oncol. 2001, 127, 207–216. [Google Scholar] [CrossRef]
- Li, R.; Zhang, H.; Cao, Y.; Liu, X.; Chen, Y.; Qi, Y.; Wang, J.; Yu, K.; Lin, C.; Liu, H.; et al. Lauren Classification Identifies Distinct Prognostic Value and Functional Status of Intratumoral CD8+ T Cells in Gastric Cancer. Cancer Immunol. Immunother. 2020, 69, 1327–1336. [Google Scholar] [CrossRef]
- Vauhkonen, M.; Vauhkonen, H.; Sipponen, P. Pathology and Molecular Biology of Gastric Cancer. Best Pract. Res. Clin. Gastroenterol. 2006, 20, 651–674. [Google Scholar] [CrossRef] [PubMed]
- Gray, H. Gray’s Anatomy: With Original Illustrations by Henry Carter; Arcturus Publishing: London, UK, 2013. [Google Scholar]
- Hajar, R. Medicine from Galen to the Present: A Short History. Heart Views 2021, 22, 307–308. [Google Scholar] [CrossRef]
- Fernicola, A.; Calogero, A.; Santangelo, M. Theodor Billroth: The Pioneer Gastrectomy Surgeon and His Contributions to the Evolution of General Surgery. Cureus 2024, 16, e68861. [Google Scholar] [CrossRef] [PubMed]
- Waldum, H.L.; Fossmark, R. Types of Gastric Carcinomas. Int. J. Mol. Sci. 2018, 19, 4109. [Google Scholar] [CrossRef]
- Hu, B.; El Hajj, N.; Sittler, S.; Lammert, N.; Barnes, R.; Meloni-Ehrig, A. Gastric Cancer: Classification, Histology and Application of Molecular Pathology. J. Gastrointest. Oncol. 2012, 3, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A.; the WHO Classification of Tumours Editorial Board. The 2019 WHO Classification of Tumours of the Digestive System. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef]
- Guan, W.-L.; He, Y.; Xu, R.-H. Gastric Cancer Treatment: Recent Progress and Future Perspectives. J. Hematol. Oncol. 2023, 16, 57. [Google Scholar] [CrossRef]
- Orditura, M. Treatment of Gastric Cancer. World J. Gastroenterol. 2014, 20, 1635. [Google Scholar] [CrossRef]
- Kaslow, S.R.; He, Y.; Sacks, G.D.; Berman, R.S.; Lee, A.Y.; Correa-Gallego, C. Time to Curative-Intent Surgery in Gastric Cancer Shows a Bimodal Relationship with Overall Survival. J. Gastrointest. Surg. 2023, 27, 855–865. [Google Scholar] [CrossRef]
- Mendes De Almeida, J.C.; Bettencourt, A.; Santos Costa, C.; Mendes De Almeida, J.M. Curative Surgery for Gastric Cancer: Study of 166 Consecutive Patients. World J. Surg. 1994, 18, 889–894. [Google Scholar] [CrossRef]
- Monrabal Lezama, M.; Murdoch Duncan, N.S.; Bertona, S.; Schlottmann, F. Current Standards of Lymphadenectomy in Gastric Cancer. Updat. Surg. 2023, 75, 1751–1758. [Google Scholar] [CrossRef] [PubMed]
- Dehal, A.; Woo, Y.; Glazer, E.S.; Davis, J.L.; Strong, V.E.; Society of Surgical Oncology Gastrointestinal Disease Site Workgroup; Chai, C.; Ward, E.; Nunns, G.; Allenson, K.; et al. D2 Lymphadenectomy for Gastric Cancer: Advancements and Technical Considerations. Ann. Surg. Oncol. 2025, 32, 2129–2140. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Bu, Z.-D.; Yan, Y.; Li, Z.-Y.; Wu, A.-W.; Zhang, L.-H.; Zhang, J.; Wu, X.-J.; Zong, X.-L.; Li, S.-X.; et al. The 8th Edition of the American Joint Committee on Cancer Tumor-Node-Metastasis Staging System for Gastric Cancer Is Superior to the 7th Edition: Results from a Chinese Mono-Institutional Study of 1663 Patients. Gastric Cancer 2018, 21, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Casali, P.G.; Blay, J.Y.; Abecassis, N.; Bajpai, J.; Bauer, S.; Biagini, R.; Bielack, S.; Bonvalot, S.; Boukovinas, I.; Bovee, J.V.M.G.; et al. Gastrointestinal Stromal Tumours: ESMO-EURACAN-GENTURIS Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2022, 33, 20–33. [Google Scholar] [CrossRef]
- Songun, I.; Putter, H.; Kranenbarg, E.M.-K.; Sasako, M.; Van De Velde, C.J. Surgical Treatment of Gastric Cancer: 15-Year Follow-up Results of the Randomised Nationwide Dutch D1D2 Trial. Lancet Oncol. 2010, 11, 439–449. [Google Scholar] [CrossRef]
- Biondi, A. R0 Resection in the Treatment of Gastric Cancer: Room for Improvement. World J. Gastroenterol. 2010, 16, 3358. [Google Scholar] [CrossRef]
- Ridwelski, K.; Fahlke, J.; Huß, M.; Otto, R.; Wolff, S. R1-Resektion beim Magenkarzinom. Der Chirurg 2017, 88, 756–763. [Google Scholar] [CrossRef]
- Gaspar-Figueiredo, S.; Allemann, P.; Borgstein, A.B.J.; Joliat, G.-R.; Luzuy-Guarnero, V.; Brunel, C.; Sempoux, C.; Gisbertz, S.S.; Demartines, N.; Van Berge Henegouwen, M.I.; et al. Impact of Positive Microscopic Resection Margins (R1) after Gastrectomy in Diffuse-Type Gastric Cancer. J. Cancer Res. Clin. Oncol. 2023, 149, 11105–11115. [Google Scholar] [CrossRef]
- Cordero-García, E.; Ramos-Esquivel, A.; Alpízar-Alpízar, W. Predictors of Overall Survival after Surgery in Gastric Cancer Patients from a Latin-American Country. J. Gastrointest. Oncol. 2018, 9, 64–72. [Google Scholar] [CrossRef]
- Kim, I.-H. Current Status of Adjuvant Chemotherapy for Gastric Cancer. World J. Gastrointest. Oncol. 2019, 11, 679–687. [Google Scholar] [CrossRef]
- Takayama, T.; Tsuji, Y. Updated Adjuvant Chemotherapy for Gastric Cancer. J. Clin. Med. 2023, 12, 6727. [Google Scholar] [CrossRef] [PubMed]
- Foo, M. Adjuvant Therapy for Gastric Cancer: Current and Future Directions. World J. Gastroenterol. 2014, 20, 13718. [Google Scholar] [CrossRef]
- Lordick, F.; Mauer, M.E.; Stocker, G.; Cella, C.A.; Ben-Aharon, I.; Piessen, G.; Wyrwicz, L.; Al-Haidari, G.; Fleitas-Kanonnikoff, T.; Boige, V.; et al. Adjuvant Immunotherapy in Patients with Resected Gastric and Oesophagogastric Junction Cancer Following Preoperative Chemotherapy with High Risk for Recurrence (ypN+ and/or R1): European Organisation of Research and Treatment of Cancer (EORTC) 1707 VESTIGE Study. Ann. Oncol. 2025, 36, 197–207. [Google Scholar] [CrossRef]
- Fong, C.; Johnston, E.; Starling, N. Neoadjuvant and Adjuvant Therapy Approaches to Gastric Cancer. Curr. Treat. Options Oncol. 2022, 23, 1247–1268. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-X.; Tang, Y.-H.; Lin, G.-J.; Ma, Y.-B.; Desiderio, J.; Li, P.; Xie, J.-W.; Wang, J.-B.; Lu, J.; Chen, Q.-Y.; et al. Association of Adjuvant Chemotherapy With Overall Survival Among Patients With Locally Advanced Gastric Cancer After Neoadjuvant Chemotherapy. JAMA Netw. Open 2022, 5, e225557. [Google Scholar] [CrossRef] [PubMed]
- Miceli, R. Adjuvant Chemotherapy for Gastric Cancer: Current Evidence and Future Challenges. World J. Gastroenterol. 2014, 20, 4516. [Google Scholar] [CrossRef]
- Granieri, S.; Bonomi, A.; Frassini, S.; Chierici, A.P.; Bruno, F.; Paleino, S.; Kusamura, S.; Germini, A.; Facciorusso, A.; Deraco, M.; et al. Prognostic Impact of Cytoreductive Surgery (CRS) with Hypertermic Intraperitoneal Chemotherapy (HIPEC) in Gastric Cancer Patients: A Meta-Analysis of Randomized Controlled Trials. Eur. J. Surg. Oncol. 2021, 47, 2757–2767. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).