Expression Profiles of Co-Inhibitory Receptors in Non-Urothelial Bladder Cancer: Preclinical Evidence for the Next Generation of Immune Checkpoint Inhibitors
Simple Summary
Abstract
1. Introduction
2. Methods
3. Results
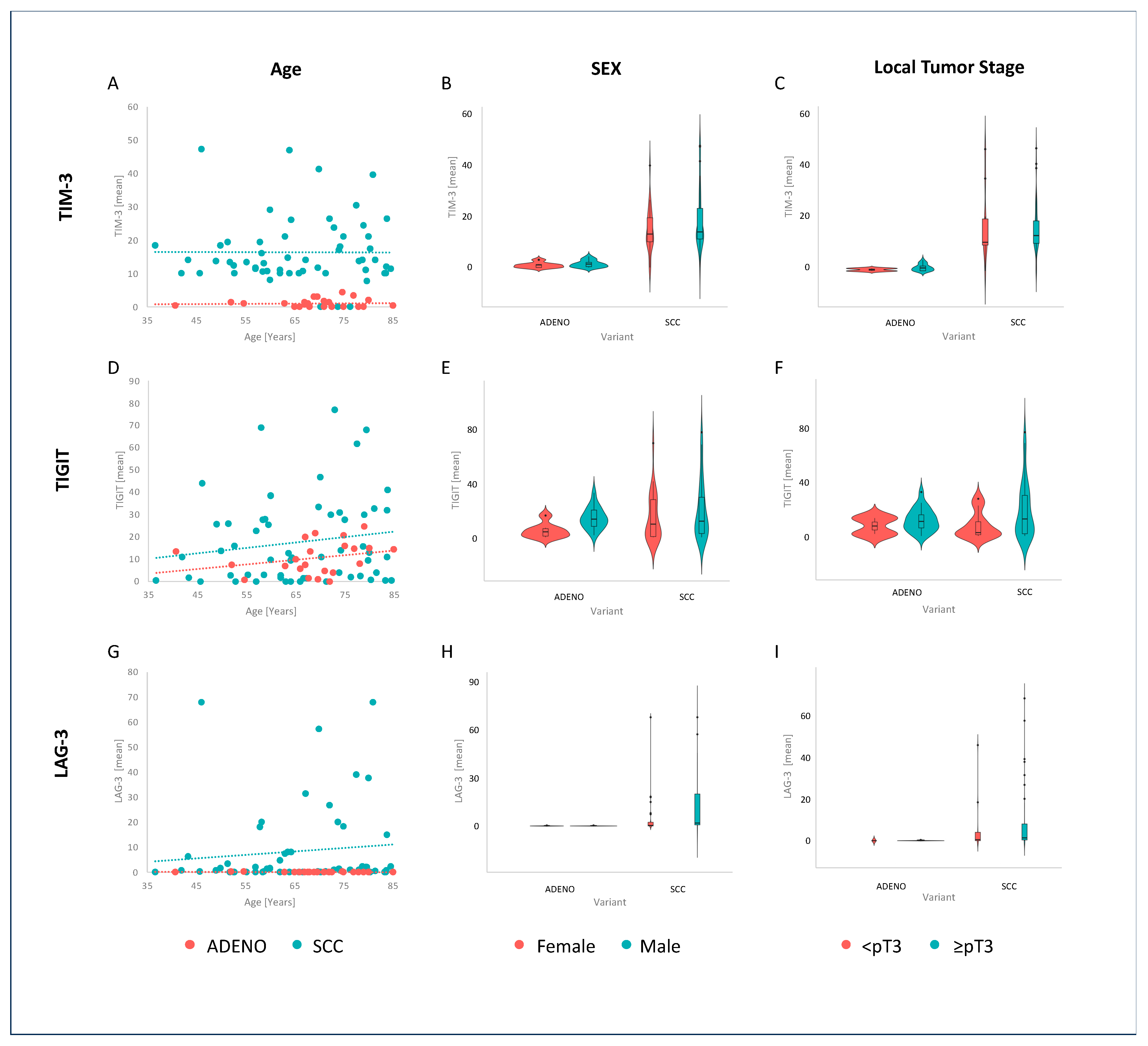
3.1. Expression Patterns
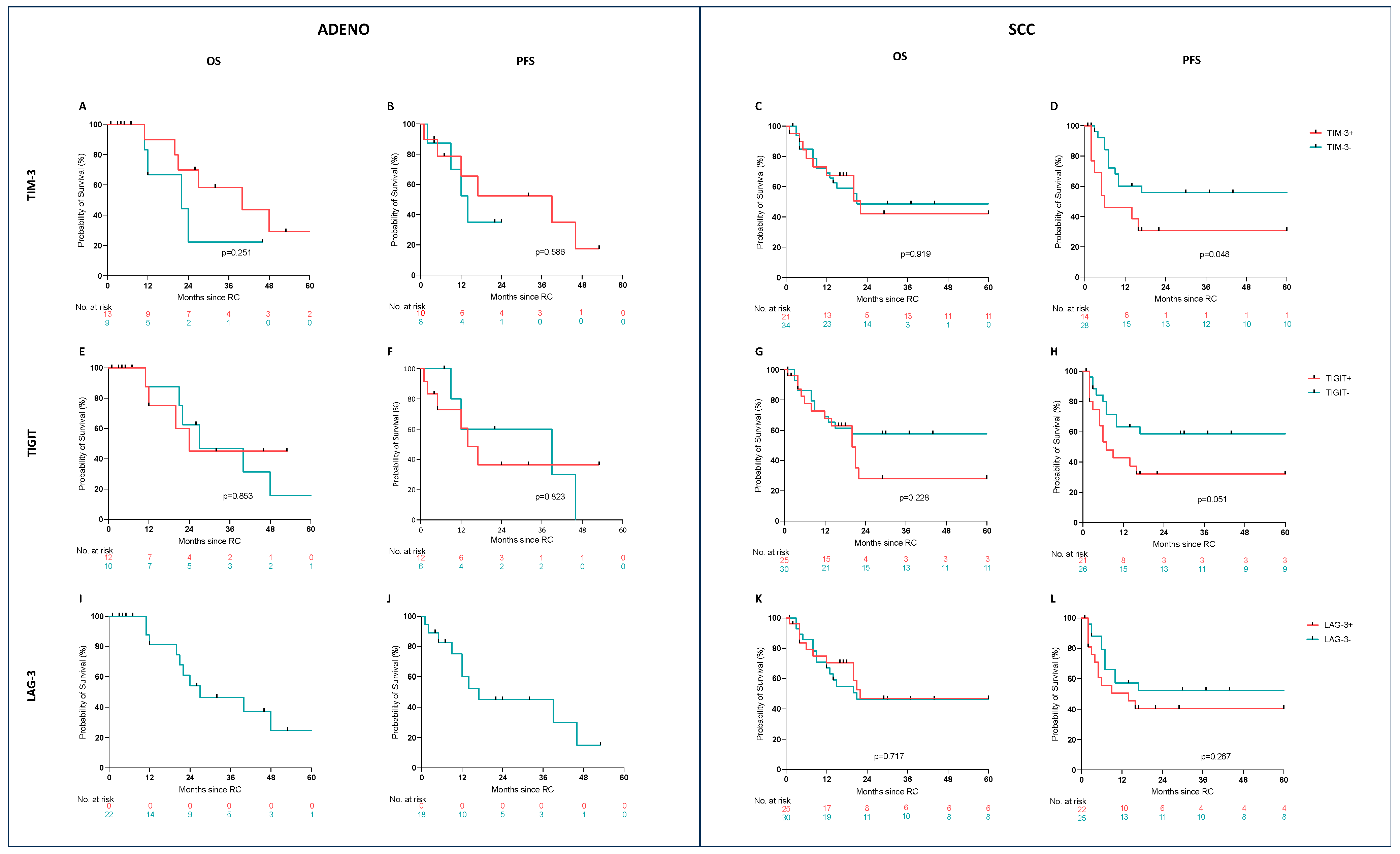
3.2. Prognostic Value
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Daneshmand, S.; Nazemi, A. Neoadjuvant Chemotherapy in Variant Histology Bladder Cancer: Current Evidence. Eur. Urol. Focus 2020, 6, 639–641. [Google Scholar] [CrossRef] [PubMed]
- Grilo, I.; Rodrigues, C.; Soares, A.; Grande, E. Facing treatment of non-urothelial bladder cancers in the immunotherapy era. Crit. Rev. Oncol./Hematol. 2020, 153, 103034. [Google Scholar] [CrossRef]
- Chu, C.E.; Chen, Z.; Whiting, K.; Ostrovnaya, I.; Lenis, A.T.; Clinton, T.N.; Rammal, R.; Ozcan, G.G.; Akbulut, D.; Basar, M.; et al. Clinical Outcomes, Genomic Heterogeneity, and Therapeutic Considerations Across Histologic Subtypes of Urothelial Carcinoma. Eur. Urol. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Drouaud, A.; Xu, V.; Velasquez, A.; Antar, R.; Boyarsky, B.; Weiss, J.; Gonzalez, D.; Silverman, R.; Whalen, M.J. Metastatic Tropism in Urothelial Carcinoma with Variant Histology: A Comprehensive NCDB Analysis. Clin. Genitourin. Cancer 2024, 22, 102179. [Google Scholar] [CrossRef]
- Kwon, W.A.; Seo, H.K.; Song, G.; Lee, M.K.; Park, W.S. Advances in Therapy for Urothelial and Non-Urothelial Subtype Histologies of Advanced Bladder Cancer: From Etiology to Current Development. Biomedicines 2025, 13, 86. [Google Scholar] [CrossRef] [PubMed]
- Johnson Iii, B.A.; Teply, B.A.; Kagemann, C.; McGuire, B.; Lombardo, K.; Jing, Y.; Langbo, W.; Epstein, J.I.; Netto, G.J.; Baras, A.S.; et al. Neoadjuvant Cisplatin, Gemcitabine, and Docetaxel in Sarcomatoid Bladder Cancer: Clinical Activity and Whole Transcriptome Analysis. Bladder Cancer 2024, 10, 133–143. [Google Scholar] [CrossRef]
- Hong, J.Y.; Choi, M.K.; Uhm, J.E.; Park, M.J.; Lee, J.; Park, S.H.; Park, J.O.; Kim, W.S.; Kang, W.K.; Lee, H.M.; et al. Palliative chemotherapy for non-transitional cell carcinomas of the urothelial tract. Med. Oncol. 2009, 26, 186–192. [Google Scholar] [CrossRef]
- Vetterlein, M.W.; Wankowicz, S.A.M.; Seisen, T.; Lander, R.; Löppenberg, B.; Chun, F.K.; Menon, M.; Sun, M.; Barletta, J.A.; Choueiri, T.K.; et al. Neoadjuvant chemotherapy prior to radical cystectomy for muscle-invasive bladder cancer with variant histology. Cancer 2017, 123, 4346–4355. [Google Scholar] [CrossRef] [PubMed]
- Necchi, A.; Raggi, D.; Gallina, A.; Madison, R.; Colecchia, M.; Lucianò, R.; Montironi, R.; Giannatempo, P.; Farè, E.; Pederzoli, F.; et al. Updated Results of PURE-01 with Preliminary Activity of Neoadjuvant Pembrolizumab in Patients with Muscle-invasive Bladder Carcinoma with Variant Histologies. Eur. Urol. 2020, 77, 439–446. [Google Scholar] [CrossRef]
- Raychaudhuri, R.; Khaki, A.R.; Redman, M.W.; Baker, K.K.; Lin, A.; Woo, B.; Hannochka, A.; Conrad, N.; Vakar-Lopez, F.; Yezefski, T.; et al. Neoadjuvant pembrolizumab (pembro) and accelerated methotrexate, vinblastine, doxorubicin, cisplatin (aMVAC) in non-urothelial (non-UC) histologic subtype muscle invasive bladder cancer (MIBC): A phase 2 trial. J. Clin. Oncol. 2025, 43 (Suppl. S5), 769. [Google Scholar] [CrossRef]
- Principe, N.; Phung, A.L.; Stevens, K.L.P.; Elaskalani, O.; Wylie, B.; Tilsed, C.M.; Sheikh, F.; Morales, M.L.O.; Kidman, J.; Marcq, E.; et al. Anti-metabolite chemotherapy increases LAG-3 expressing tumor-infiltrating lymphocytes which can be targeted by combination immune checkpoint blockade. J. Immunother. Cancer 2024, 12, e008568. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-R.; Wu, X.-L.; Sun, Y.-L. Therapeutic targets and biomarkers of tumor immunotherapy: Response versus non-response. Signal Transduct. Target. Ther. 2022, 7, 331. [Google Scholar] [CrossRef] [PubMed]
- Mellman, I.; Chen, D.S.; Powles, T.; Turley, S.J. The cancer-immunity cycle: Indication, genotype, and immunotype. Immunity 2023, 56, 2188–2205. [Google Scholar] [CrossRef]
- Humphrey, P.A.; Moch, H.; Cubilla, A.L.; Ulbright, T.M.; Reuter, V.E. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part B: Prostate and Bladder Tumours. Eur. Urol. 2016, 70, 106–119. [Google Scholar] [CrossRef]
- Moschini, M.; Shariat, S.F.; Lucianò, R.; D’Andrea, D.; Foerster, B.; Abufaraj, M.; Bandini, M.; Dell, P.; Damiano, R.; Salonia, A.; et al. Pure but Not Mixed Histologic Variants Are Associated with Poor Survival at Radical Cystectomy in Bladder Cancer Patients. Clin. Genitourin. Cancer 2017, 15, e603–e607. [Google Scholar] [CrossRef]
- Agrawal, P.; Rostom, M.; Alam, R.; Florissi, I.; Biles, M.; Rodriguez, K.; Hahn, N.M.; Johnson, B.A.; Matoso, A.; Smith, A.; et al. Clinicopathologic and Survival After Cystectomy Outcomes in Squamous Cell Carcinoma of the Bladder. Clin. Genitourin. Cancer 2023, 21, 631–638.e1. [Google Scholar] [CrossRef]
- Santa, F.; Akgul, M.; Tannous, E.; Pacheco, R.R.; Lightle, A.R.; Mohanty, S.K.; Cheng, L. Primary adenocarcinoma of the urinary tract and its precursors: Diagnostic criteria and classification. Hum. Pathol. 2025, 155, 105734. [Google Scholar] [CrossRef] [PubMed]
- Natale, C.; Leinwand, G.Z.; Chiang, J.; Silberstein, J.L.; Krane, L.S. Reviewing the Demographic, Prognostic, and Treatment Factors of Primary Adenocarcinoma of the Bladder: A SEER Population-based Study. Clin. Genitourin. Cancer 2019, 17, 380–388. [Google Scholar] [CrossRef]
- Tappero, S.; Barletta, F.; Piccinelli, M.L.; Garcia, C.C.; Incesu, R.-B.; Morra, S.; Scheipner, L.; Tian, Z.; Parodi, S.; Dell, P.; et al. Adenocarcinoma of the Bladder: Assessment of Survival Advantage Associated with Radical Cystectomy and Comparison with Urothelial Bladder Cancer. Urol. Oncol. Semin. Orig. Investig. 2023, 41, 326.e9–326.e16. [Google Scholar] [CrossRef]
- Deuker, M.; Martin, T.; Stolzenbach, F.; Rosiello, G.; Ruvolo, C.C.; Nocera, L.; Tian, Z.; Becker, A.; Kluth, L.; Roos, F.C.; et al. Bladder Cancer: A Comparison Between Non-urothelial Variant Histology and Urothelial Carcinoma Across All Stages and Treatment Modalities. Clin. Genitourin. Cancer 2021, 19, 60–68.e1. [Google Scholar] [CrossRef]
- Jubber, I.; Ong, S.; Bukavina, L.; Black, P.C.; Compérat, E.; Kamat, A.M.; Kiemeney, L.; Lawrentschuk, N.; Lerner, S.P.; Meeks, J.J.; et al. Epidemiology of Bladder Cancer in 2023: A Systematic Review of Risk Factors. Eur. Urol. 2023, 84, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Dobruch, J.; Daneshmand, S.; Fisch, M.; Lotan, Y.; Noon, A.P.; Resnick, M.J.; Shariat, S.F.; Zlotta, A.R.; Boorjian, S.A. Gender and Bladder Cancer: A Collaborative Review of Etiology, Biology, and Outcomes. Eur. Urol. 2016, 69, 300–310. [Google Scholar] [CrossRef]
- Banek, S.; Hoeh, B.; Garcia, C.C.; Koll, F.; Köllermann, J.; Chun, F.K.H.; Mandel, P.; Kluth, L.A.; Wenzel, M. Metastatic Squamous Cell Carcinoma of the Urinary Bladder: Urgent Call for New Therapies. Urol. Int. 2023, 108, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Acharya, N.; Sabatos-Peyton, C.; Anderson, A.C. Tim-3 finds its place in the cancer immunotherapy landscape. J. Immunother. Cancer 2020, 8, e000911. [Google Scholar] [CrossRef]
- Luke, J.J.; Patel, M.R.; Blumenschein, G.R.; Hamilton, E.; Chmielowski, B.; Ulahannan, S.V.; Connolly, R.M.; Santa-Maria, C.A.; Wang, J.; Bahadur, S.W.; et al. The PD-1- and LAG-3-targeting bispecific molecule tebotelimab in solid tumors and hematologic cancers: A phase 1 trial. Nat. Med. 2023, 29, 2814–2824. [Google Scholar] [CrossRef]
- Rousseau, A.; Parisi, C.; Barlesi, F. Anti-TIGIT therapies for solid tumors: A systematic review. ESMO Open 2023, 8, 101184. [Google Scholar] [CrossRef]
- Hu, C.; Dignam, J.J. Biomarker-Driven Oncology Clinical Trials: Key Design Elements, Types, Features, and Practical Considerations. JCO Precis. Oncol. 2019, 3, 1–12. [Google Scholar] [CrossRef]
- Curigliano, G.; Gelderblom, H.; Mach, N.; Doi, T.; Tai, D.; Forde, P.M.; Sarantopoulos, J.; Bedard, P.L.; Lin, C.-C.; Hodi, F.S.; et al. Phase I/Ib Clinical Trial of Sabatolimab, an Anti–TIM-3 Antibody, Alone and in Combination with Spartalizumab, an Anti–PD-1 Antibody, in Advanced Solid Tumors. Clin. Cancer Res. 2021, 27, 3620–3629. [Google Scholar] [CrossRef] [PubMed]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Gutiérrez, E.C.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; Menezes, J.J.D.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef]
- Cho, B.C.; Abreu, D.R.; Hussein, M.; Cobo, M.; Patel, A.J.; Secen, N.; Lee, K.H.; Massuti, B.; Hiret, S.; Yang, J.C.H.; et al. Tiragolumab plus atezolizumab versus placebo plus atezolizumab as a first-line treatment for PD-L1-selected non-small-cell lung cancer (CITYSCAPE): Primary and follow-up analyses of a randomised, double-blind, phase 2 study. Lancet Oncol. 2022, 23, 781–792. [Google Scholar] [CrossRef]
- Li, H.; van der Merwe, P.A.; Sivakumar, S. Biomarkers of response to PD-1 pathway blockade. Br. J. Cancer 2022, 126, 1663–1675. [Google Scholar] [CrossRef] [PubMed]
- Guégan, J.-P.; Peyraud, F.; Dadone-Montaudie, B.; Teyssonneau, D.; Palmieri, L.-J.; Clot, E.; Cousin, S.; Roubaud, G.; Cabart, M.; Leroy, L.; et al. Analysis of PD1, LAG3, TIGIT, and TIM3 expression in human lung adenocarcinoma reveals a 25-gene signature predicting immunotherapy response. Cell Rep. Med. 2024, 5, 101831. [Google Scholar] [CrossRef] [PubMed]
- Rodler, S.; Jung, A.; Greif, P.A.; Rühlmann, K.; Apfelbeck, M.; Tamalunas, A.; Kretschmer, A.; Schulz, G.B.; Szabados, B.; Stief, C.; et al. Routine application of next-generation sequencing testing in uro-oncology-Are we ready for the next step of personalised medicine? Eur. J. Cancer 2021, 146, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Debatin, N.F.; Bady, E.; Mandelkow, T.; Huang, Z.; Lurati, M.C.J.; Raedler, J.B.; Müller, J.H.; Vettorazzi, E.; Plage, H.; Samtleben, H.; et al. Prognostic Impact and Spatial Interplay of Immune Cells in Urothelial Cancer. Eur. Urol. 2024, 86, 42–51. [Google Scholar] [CrossRef]
- Moschini, M.; D’Andrea, D.; Korn, S.; Irmak, Y.; Soria, F.; Compérat, E.; Shariat, S.F. Characteristics and clinical significance of histological variants of bladder cancer. Nat. Rev. Urol. 2017, 14, 651–668. [Google Scholar] [CrossRef]
- Eismann, L.; Rodler, S.; Buchner, A.; Schulz, G.B.; Volz, Y.; Bischoff, R.; Ebner, B.; Westhofen, T.; Casuscelli, J.; Waidelich, R.; et al. Identification of the Tumor Infiltrating Lymphocytes (TILs) Landscape in Pure Squamous Cell Carcinoma of the Bladder. Cancers 2022, 14, 3999. [Google Scholar] [CrossRef]
- Rodler, S.; Eismann, L.; Schlenker, B.; Casuscelli, J.; Brinkmann, I.; Sendelhofert, A.; Waidelich, R.; Buchner, A.; Stief, C.; Schulz, G.B.; et al. Expression of Nectin-4 in Variant Histologies of Bladder Cancer and Its Prognostic Value-Need for Biomarker Testing in High-Risk Patients? Cancers 2022, 14, 4411. [Google Scholar] [CrossRef]
- Powles, T.; Begoña, P.V.; Gupta, S.; Bedke, J.; Kikuchi, E.; Hoffman-Censits, J.; Iyer, G.; Vulsteke, C.; Se, H.P.; Sang, J.S.; et al. Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. N. Engl. J. Med. 2024, 390, 875–888. [Google Scholar] [CrossRef]
| Characteristic | ADENO, N = 27 1 | SCC, N = 61 1 | p-Value 2 |
|---|---|---|---|
| Age | 71 (67, 75) | 66 (58, 77) | 0.2 |
| Sex | 0.088 | ||
| female | 8 (30%) | 30 (49%) | |
| male | 19 (70%) | 31 (51%) | |
| T-stage | 0.3 | ||
| <pT3 | 2 (7.4%) | 11 (18%) | |
| ≥pT3 | 25 (93%) | 50 (82%) | |
| TIM-3 (median) | 1 (0, 2) | 14 (10, 20) | <0.001 |
| TIGIT (median) | 11 (5, 15) | 11 (2, 28) | 0.9 |
| LAG-3 (median) | 0 (0, 0) | 1 (0, 8) | <0.001 |
| Unknown | 3 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodler, S.; Ledderose, S.T.; Waidelich, R.; Kohler, J.; Sendelhofert, A.; Casuscelli, J.; Schulz, G.; Stief, C.G.; Eismann, L. Expression Profiles of Co-Inhibitory Receptors in Non-Urothelial Bladder Cancer: Preclinical Evidence for the Next Generation of Immune Checkpoint Inhibitors. Cancers 2025, 17, 2210. https://doi.org/10.3390/cancers17132210
Rodler S, Ledderose ST, Waidelich R, Kohler J, Sendelhofert A, Casuscelli J, Schulz G, Stief CG, Eismann L. Expression Profiles of Co-Inhibitory Receptors in Non-Urothelial Bladder Cancer: Preclinical Evidence for the Next Generation of Immune Checkpoint Inhibitors. Cancers. 2025; 17(13):2210. https://doi.org/10.3390/cancers17132210
Chicago/Turabian StyleRodler, Severin, Stephan T. Ledderose, Raphaela Waidelich, Jakob Kohler, Andrea Sendelhofert, Jozefina Casuscelli, Gerald Schulz, Christian G. Stief, and Lennert Eismann. 2025. "Expression Profiles of Co-Inhibitory Receptors in Non-Urothelial Bladder Cancer: Preclinical Evidence for the Next Generation of Immune Checkpoint Inhibitors" Cancers 17, no. 13: 2210. https://doi.org/10.3390/cancers17132210
APA StyleRodler, S., Ledderose, S. T., Waidelich, R., Kohler, J., Sendelhofert, A., Casuscelli, J., Schulz, G., Stief, C. G., & Eismann, L. (2025). Expression Profiles of Co-Inhibitory Receptors in Non-Urothelial Bladder Cancer: Preclinical Evidence for the Next Generation of Immune Checkpoint Inhibitors. Cancers, 17(13), 2210. https://doi.org/10.3390/cancers17132210







