Why Do Radiologists Disown Breast Thermography? A Critical Review of Recent Studies and Recommendations
Simple Summary
Abstract
1. Introduction
2. Clinical Evidence
2.1. Rise and Fall of Thermography in the 20th Century
2.2. Resurgence of Breast Thermography in the 21st Century
- Parisky et al. [47] tested a system developed by Computerized Thermal Imaging Inc. (Ogden, UT, USA), called BCS 2100, which was intended as an adjunct to mammography to avoid biopsying benign breast masses. These authors examined 769 subjects with 875 lesions biopsied due to abnormal mammographic and/or clinical findings. A series of images were acquired while the patient lay prone with both breasts suspended through openings in the imaging bed and cool air was blown over them. After manually defining the location of the lesions in the mammogram, the proprietary software determined an index of suspicion for the thermogram, which resulted in a sensitivity of 97% and a specificity of 14%. This implies that limiting biopsies to the lesions with a positive thermogram would avoid 14% of unnecessary biopsies of benign lesions, at the expense of missing 3% of the cancers. All the lesions that were assessed as false negative were microcalcifications; excluding these lesions (which were 45% of the total), sensitivity increased to 99% and specificity to 18%. In reply to Moskowitz, who criticized the dependency on mammography to localize the suspicious area in the infrared image, Parisky emphasized that the system was intended to be used in conjunction with mammography to avoid unnecessary biopsies of benign lesions, not to replace it [48]. In 2002, the US Food and Drug Administration (FDA) denied clearance for the BCS 2100 system, and the company became involved in multiple shareholder class-action lawsuits [49].
- Arora et al. [50] assessed the effectiveness of another system, Sentinel BreastScan (SBS), by Infrared Sciences Corp. (Bohemia, NY, USA), in a group of 92 women with suspicious findings on mammography or ultrasound. Among three different analysis modes compared, the one using an artificial neural network obtained the highest sensitivity (96.7%), with a specificity of 26.5%. Although the SBS was cleared by the FDA for adjunctive breast cancer screening in 2004 and obtained the CE Mark, the company seems to have gone out of business.
- In 2010, Wishart et al. [51] evaluated the same SBS system analyzing the images with new software called NoTouch BreastScan (NTBS), by UE Life Sciences Inc. (Philadelphia, PA, USA), which uses AI to detect areas of increased heat. After examining 100 women with 106 lesions biopsied, NTBS obtained a higher sensitivity than the SBS neural network (70% vs. 48%), whereas specificity was lower (48% vs. 74%). In women under 50, the sensitivity of NTBS increased to 78% (the same as mammography) and the specificity to 75%, suggesting that thermography could be particularly helpful for this specific population. In fact, the combination of both techniques raised the sensitivity to 89% for this age group.
- Later, UE Life Sciences Inc. developed a new system, similar to SBS, by incorporating two high-resolution thermal cameras to their NTBS software, each pointing at one breast. Collet et al. [52] independently evaluated it by examining 99 patients prior to scheduled breast biopsy. Depending on the purpose of the examination, NTBS offered two types of analysis: a high specificity screening mode, in which the objective is to minimize false positives at the expense of a lower sensitivity, and a high sensitivity diagnostic mode, which aims to correctly identify all malignant cases at the cost of a higher false-positive rate. Although the sensitivity (78.8%) and specificity (48.6%) obtained with the diagnostic mode were similar to Wishart’s, Collet et al. did not consider these results good enough to recommend thermography as a screening modality, not even as an adjunct to mammography. In spite of the FDA’s clearance of NTBS as an adjunct to other screening modalities in 2012, UE Life Sciences subsequently switched its strategy to tactile technology for breast cancer detection (their iBreastExam device, https://www.uelifesciences.com/ibreastexam) and the website for NoTouch BreastScan is no longer available.
- A prototype 3D infrared imaging system by Real Imaging Ltd. (Airport City, Israel) was evaluated in 2013 by Sella et al. [53] in 256 healthy women—with BI-RADS-1 results in routine mammography screening—and 178 women with newly diagnosed breast cancer (it is not specified whether they were symptomatic, or cancer was discovered in screening). The device, which has received different names (3DIRI, Real Imager 8, or MIRA), consists of two infrared cameras placed at a 60° angle between them, which produce a 3D thermal map of the breast. AI was used to output a score between −100 (healthy) and 100 (suspicious), with a sensitivity of 90.9% and a specificity of 72.5%. Real Imaging Ltd. sponsored two other studies in which women with a genetic predisposition for breast cancer and women with dense breasts were examined by mammography, ultrasound, and 3DIRI. Patients with negative mammography or ultrasound but an abnormal thermogram were referred to MRI. In the first study [54], 8 cancers were found among the 226 patients at high risk due to genetic predisposition: 7 of them were correctly diagnosed by 3DIRI (87.5% sensitivity), whereas mammography and ultrasound missed 3 of them (60% sensitivity). The specificity for 3DIRI was also high (84.32%). Among 1727 women with dense breasts, thermography detected 6 cancers in 5 women in addition to the 7 cases identified by mammography, thus increasing the detection rate from 4.05 to 6.95 per 1000 [55]. However, 5 of the cancers visible in mammography were missed by 3DIRI. Although the individual sensitivity was low for both mammography and 3DIRI (7 cancers out of 12 detected by each technique, i.e., 58.3%), the combined sensitivity reached 100% (all the cancers were detected), with a specificity of 86% (87% 3DIRI, 98% mammography).
- Thermalytix, a computer-aided diagnostic solution by the start-up Niramai Health Analytix (Bangalore, India), uses AI to compute a risk score based on the presence of asymmetric vascular and thermal patterns (https://www.niramai.com/about/thermalytix). The company funded three studies to evaluate it. Kakileti et al. [56] examined 470 women, 50.6% of which were symptomatic, obtaining a sensitivity of 91.02% (89.85% for symptomatic; 100% for asymptomatic) and a specificity of 82.39% (69.04% for symptomatic; 92.41% for asymptomatic). Singh et al. [57] reported a sensitivity of 82.5% and a specificity of 80.5% after imaging 258 symptomatic women. Bansal et al. [58] used Thermalytix to examine 459 women; 85% were asymptomatic and 36.6% had dense breasts. The overall sensitivity was 95.24% and the specificity 88.58%. Three of the biopsy-proven malignancies had an inconclusive mammography (BI-RADS 0) but an abnormal thermogram. Among the women with dense breasts, which accounted for 57% of women under 45 years, the sensitivity was 100% (the same as mammography) and the specificity 81.65% (superior to that of mammography). Thermalytix obtained CE marking in 2021 and is now commercially available in 22 countries. The following year their SMILE-100 system, which uses a subset of features of Thermalytix, was cleared by the FDA.
- Gutierrez-Delgado and Vazquez-Luna published the results obtained in a study conducted in Mexico that included 911 women attending cancer screening programs, with physical examination, breast thermography and mammography [59]. Among the 17 biopsy-proven cancers (a higher-than-normal incidence), 16 had an abnormal thermogram (94.12% sensitivity). More importantly, three of the cancers were detected in women under 40 years—a population for which guidelines do not recommend mammography—with a positive thermogram. This detection rate is surprisingly high, considering that images were interpreted with obsolete visual criteria. Unfortunately, the number of false positives was not reported.
- Rassiwala et al. [60] conducted a study, also in a screening setting, in India. Among the 1008 asymptomatic women, 49 had an abnormal thermogram. All of them had a palpable lump in the clinical examination and were subjected to mammography and histopathological examination. In this group, 41 cancers were found, which is a very high incidence; 3 of them were missed by mammography. With only one false-negative, the sensitivity of thermography was 97.62% and the specificity 99.17%. Such good results are unexpected given the low resolution of the camera and the simple interpretation criterion, as the diagnosis was based solely on temperature differences between the breasts.
- Yao et al. [61] examined 2036 women in China who required a biopsy or surgical excision because of abnormal mammographic or ultrasound findings. The sensitivity of thermography (84.4%) was superior to that of mammography (78.3%) and ultrasound (83.1%), whereas specificity (94.0%) was inferior to mammography (98.3%) but superior to ultrasound (93.1%). For tumors smaller than 2 cm in diameter, the sensitivity and specificity of thermography increased to 90.4% and 97.8%, respectively, outperforming mammography (80.8% and 97.6%).
- In Mexico, Garduño-Ramón et al. [62] obtained a sensitivity of 86.84% and a specificity of 89.65% when examining 454 voluntary women; it is not specified how they were recruited. In contrast with the sophisticated algorithms used in other contemporary publications, this study classified thermal images as healthy or sick by simply comparing the average temperature of the hottest region in each breast.
- Wang et al. proposed using a thermal camera attached to a mobile phone for in-home pre-screening [63]. By applying AI, they obtained an accuracy of 86.27%, sensitivity of 84.51%, and a specificity of 83.87% in 2202 patients recruited from 20 health centers in 10 regions of China.
3. Recommendations and Position Statements
4. Discussion
A Proposal for the Standardization of Breast Thermography Studies
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MRI | Magnetic resonance imaging |
| BI-RADS | Breast Imaging Reporting and Data System |
| BCDDP | Breast Cancer Detection and Demonstration Project |
| NCI | National Cancer Institute |
| AI | Artificial intelligence |
| DMR | Database for Mastology Research |
| FDA | Food and Drug Administration |
| SBS | Sentinel BreastScan |
| NTBS | NoTouch BreastScan |
| 3DIRI | 3D Infrared Imaging |
| SBI | Society of Breast Imaging |
| EUSOBI | European Society of Breast Imaging |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Pötsch, N.; Vatteroni, G.; Clauser, P.; Helbich, T.H.; Baltzer, P.A.T. Contrast-Enhanced Mammography versus Contrast-Enhanced Breast MRI: A Systematic Review and Meta-Analysis. Radiology 2022, 305, 94–103. [Google Scholar] [CrossRef]
- Melnikow, J.; Fenton, J.J.; Whitlock, E.P.; Miglioretti, D.L.; Weyrich, M.S.; Thompson, J.H.; Shah, K. Supplemental Screening for Breast Cancer in Women With Dense Breasts: A Systematic Review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2016, 164, 268. [Google Scholar] [CrossRef]
- Lawson, R. Implications of Surface Temperatures in the Diagnosis of Breast Cancer. Can. Med. Assoc. 1956, 75, 309–311. [Google Scholar]
- Haberman, J.D. The Present Status of Mammary Thermography. CA Cancer J. Clin. 1968, 18, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, M. Screening for Breast Cancer: How Effective Are Our Tests? A Critical Review. CA Cancer J. Clin. 1983, 33, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Keyserlingk, J.R.; Ahlgren, P.D.; Yu, E.; Belliveau, N.; Yassa, M. Functional Infrared Imaging of the Breast. IEEE Eng. Med. Biol. Mag. 2000, 19, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Amalu, W.C.; Hobbins, W.B.; Head, J.F.; Elliot, R.L. Infrared Imaging of the Breast—An Overview. In The Biomedical Engineering Handbook, Medical Devices and Systems; Bronzino, J.D., Ed.; CRC Press: Boca Raton, FL, USA, 2006; pp. 1–20. [Google Scholar]
- Hoffman, R.L. Thermography in the Detection of Breast Malignancy. Am. J. Obs. Gynecol. 1967, 98, 681–686. [Google Scholar] [CrossRef]
- Wallace, J.D.; Dodd, G.D. Thermography in the Diagnosis of Breast Cancer. Radiology 1968, 91, 679–685. [Google Scholar] [CrossRef]
- Lilienfeld, A.M.; Barnes, J.M.; Barnes, R.B.; Brasfield, R.; Connell, J.F.; Diamond, E.; Gershon-Cohen, J.; Haberman, J.; Isard, H.J.; Lane, W.Z.; et al. An Evaluation of Thermography in the Detection of Breast Cancer. A Cooperative Pilot Study. Cancer 1969, 24, 1206–1211. [Google Scholar] [CrossRef]
- Isard, H.J.; Becker, W.; Shilo, R.; Ostrum, B.J. Breast Thermography after Four Years and 10,000 Studies. Am. J. Roentgenol. 1972, 115, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Stark, A.M.; Way, S. The Screening of Well Women for the Early Detection of Breast Cancer Using Clinical Examination with Thermography and Mammography. Cancer 1974, 33, 1671–1679. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.H.; Greening, W.P.; Davey, J.B.; McKinna, J.A.; Greeves, V.J. Thermography of the Female Breast: A Five-Year Study in Relation to the Detection and Prognosis of Cancer. Br. J. Radiol. 1975, 48, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Raskin, M.M.; Martinez-Lopez, M. Thermographic Patterns of the Breast: A Critical Analysis of Interpretation. Radiology 1976, 121, 553–555. [Google Scholar] [CrossRef]
- Haberman, J.D.; Love, T.J.; Francis, J.E. Screening a Rural Population for Breast Cancer Using Thermography and Physical Examination Techniques: Methods and Results-a Preliminary Report. Ann. N. Y. Acad. Sci. 1980, 335, 492–500. [Google Scholar] [CrossRef]
- Gautherie, M.; Gros, C.M. Breast Thermography and Cancer Risk Prediction. Cancer 1980, 45, 51–56. [Google Scholar] [CrossRef]
- Nyirjesy, I. Breast Thermography. Clin. Obs. Gynecol. 1982, 25, 401–408. [Google Scholar] [CrossRef]
- Amalric, R.; Giraud, D.; Thomassin, L.; Altschuler, C.; Spitalier, J.M. Detection of Subclinical Breast Cancers by Infrared Thermography. In Recent Advances in Medical Thermology; Ring, E.F.J., Phillips, B., Eds.; Springer: New York, NY, USA, 1984; pp. 575–579. [Google Scholar]
- Hobbins, W.B. Abnormal Thermogram. Significance in Breast Cancer. Interamer. J. Rad. 1987, 12, 337–343. [Google Scholar][Green Version]
- Hitchcock, C.R. Thermography in Mass Screening for Occult Breast Cancer. JAMA J. Am. Med. Assoc. 1968, 204, 419. [Google Scholar] [CrossRef]
- Furnival, I.G.; Stewart, H.J.; Weddell, J.M.; Dovey, P.; Gravelle, I.H.; Evans, K.T.; Forrest, A.P.M. Accuracy of Screening Methods for the Diagnosis of Breast Disease. BMJ 1970, 4, 461–463. [Google Scholar] [CrossRef]
- Nathan, B.E.; Burn, J.I.; MacErlean, D.P. Value of Mammary Thermography in Differential Diagnosis. BMJ 1972, 2, 316–317. [Google Scholar] [CrossRef] [PubMed]
- Egan, R.L.; Goldstein, G.T.; McSweeney, M.M. Conventional Mammography, Physical Examination, Thermography and Xeroradiography in the Detection of Breast Cancer. Cancer 1977, 39, 1984–1992. [Google Scholar] [CrossRef]
- Sterns, E.E.; Curtis, A.C.; Miller, S.; Hancock, J.R. Thermography in Breast Diagnosis. Cancer 1982, 50, 323–325. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, M.; Milbrath, J.; Gartside, P.; Zermeno, A.; Mandel, D. Lack of Efficacy of Thermography as a Screening Tool for Minimal and Stage I Breast Cancer. N. Engl. J. Med. 1976, 295, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Strax, P. Results of Mass Screening for Breast Cancer in 50,000 Examinations. Cancer 1976, 37, 30–35. [Google Scholar] [CrossRef]
- Baker, L.H. Breast Cancer Detection Demonstration Project: Five-Year Summary Report. CA Cancer J. Clin. 1982, 32, 194–225. [Google Scholar] [CrossRef]
- Goldsmith, M.F. In the Hot Seat: Thermography for Breast Cancer Diagnosis. JAMA 1984, 251, 693–695. [Google Scholar] [CrossRef]
- Feig, S.A.; Shaber, G.S.; Schwartz, G.F.; Patchefsky, A.; Libshitz, H.I.; Edeiken, J.; Nerlinger, R.; Curley, R.F.; Wallace, J.D. Thermography, Mammography, and Clinical Examination in Breast Cancer Screening. Radiology 1977, 122, 123–127. [Google Scholar] [CrossRef]
- Threatt, B.; Norbeck, J.M.; Ullman, N.S.; Kummer, R.; Roselle, P.F. Thermography and Breast Cancer: An Analysis of a Blind Reading. Ann. N. Y. Acad. Sci. 1980, 335, 501–519. [Google Scholar] [CrossRef]
- Lapayowker, M.S.; Barash, I.; Byrne, R.; Chang, C.H.J.; Dodd, G.; Farrell, C.; Haberman, J.D.; Isard, H.J.; Threatt, B. Criteria for Obtaining and Interpreting Breast Thermograms. Cancer 1976, 38, 1931–1935. [Google Scholar] [CrossRef]
- Barnes, R.B.; Gershon-Cohen, J. Clinical Thermography. JAMA J. Am. Med. Assoc. 1963, 185, 949. [Google Scholar] [CrossRef] [PubMed]
- Ring, E.F.J. The Historical Development of Thermometry and Thermal Imagingin Medicine. J. Med. Eng. Technol. 2006, 30, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Singh, A.K. Role of Image Thermography in Early Breast Cancer Detection-Past, Present and Future. Comput. Methods Programs Biomed. 2020, 183, 105074. [Google Scholar] [CrossRef]
- Hakim, A.; Awale, R.N. Thermal Imaging-An Emerging Modality for Breast Cancer Detection: A Comprehensive Review. J. Med. Syst. 2020, 44, 136. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Arora, A.S. Automated Approaches for ROIs Extraction in Medical Thermography: A Review and Future Directions. Multimed. Tools Appl. 2020, 79, 15273–15296. [Google Scholar] [CrossRef]
- Saniei, E.; Setayeshi, S.; Akbari, M.E.; Navid, M. A Vascular Network Matching in Dynamic Thermography for Breast Cancer Detection. Quant. Infrared Thermogr. J. 2015, 12, 24–36. [Google Scholar] [CrossRef]
- Kakileti, S.T.; Venkataramani, K. Automated Blood Vessel Extraction in Two-Dimensional Breast Thermography. In Proceedings of the 2016 IEEE International Conference on Image Processing (ICIP), Phoenix, AZ, USA, 25–28 September 2016; IEEE: Piscataway, NJ, USA, 2016; pp. 380–384. [Google Scholar]
- Raghavendra, U.; Gudigar, A.; Rao, T.N.; Ciaccio, E.J.; Ng, E.Y.K.; Rajendra Acharya, U. Computer-Aided Diagnosis for the Identification of Breast Cancer Using Thermogram Images: A Comprehensive Review. Infrared Phys. Technol. 2019, 102, 103041. [Google Scholar] [CrossRef]
- ud din, N.M.; Dar, R.A.; Rasool, M.; Assad, A. Breast Cancer Detection Using Deep Learning: Datasets, Methods, and Challenges Ahead. Comput. Biol. Med. 2022, 149, 106073. [Google Scholar] [CrossRef]
- Zuluaga-Gomez, J.; Zerhouni, N.; Al Masry, Z.; Devalland, C.; Varnier, C. A Survey of Breast Cancer Screening Techniques: Thermography and Electrical Impedance Tomography. J. Med. Eng. Technol. 2019, 43, 305–322. [Google Scholar] [CrossRef]
- Roslidar, R.; Rahman, A.; Muharar, R.; Syahputra, M.R.; Arnia, F.; Syukri, M.; Pradhan, B.; Munadi, K. A Review on Recent Progress in Thermal Imaging and Deep Learning Approaches for Breast Cancer Detection. IEEE Access 2020, 8, 116176–116194. [Google Scholar] [CrossRef]
- Al Husaini, M.A.S.; Habaebi, M.H.; Hameed, S.A.; Islam, M.R.; Gunawan, T.S. A Systematic Review of Breast Cancer Detection Using Thermography and Neural Networks. IEEE Access 2020, 8, 208922–208937. [Google Scholar] [CrossRef]
- Mammoottil, M.J.; Kulangara, L.J.; Cherian, A.S.; Mohandas, P.; Hasikin, K.; Mahmud, M. Detection of Breast Cancer from Five-View Thermal Images Using Convolutional Neural Networks. J. Healthc. Eng. 2022, 2022, 295221. [Google Scholar] [CrossRef]
- Pérez-Martín, J.; Sánchez-Cauce, R. Quality Analysis of a Breast Thermal Images Database. Health Inform. J. 2023, 29, 146045822311537. [Google Scholar] [CrossRef] [PubMed]
- Parisky, Y.R.; Sardi, A.; Hamm, R.; Hughes, K.; Esserman, L.; Rust, S.; Callahan, K. Efficacy of Computerized Infrared Imaging Analysis to Evaluate Mammographically Suspicious Lesions. Am. J. Roentgenol. 2003, 180, 263–269. [Google Scholar] [CrossRef]
- Moskowitz, M. Efficacy of Computerized Infrared Imaging. Am. J. Roentgenol. 2003, 181, 596. [Google Scholar] [CrossRef]
- Moody, R.J. Auditor Drops Computerized Thermal Imaging. Portland Bus. J. 2003. Available online: https://www.bizjournals.com/portland/stories/2003/02/10/daily24.html (accessed on 26 June 2025).
- Arora, N.; Martins, D.; Ruggerio, D.; Tousimis, E.; Swistel, A.J.; Osborne, M.P.; Simmons, R.M. Effectiveness of a Noninvasive Digital Infrared Thermal Imaging System in the Detection of Breast Cancer. Am. J. Surg. 2008, 196, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Wishart, G.C.; Campisi, M.; Boswell, M.; Chapman, D.; Shackleton, V.; Iddles, S.; Hallett, A.; Britton, P.D. The Accuracy of Digital Infrared Imaging for Breast Cancer Detection in Women Undergoing Breast Biopsy. Eur. J. Surg. Oncol. (EJSO) 2010, 36, 535–540. [Google Scholar] [CrossRef]
- Collett, A.E.; Guilfoyle, C.; Gracely, E.J.; Frazier, T.G.; Barrio, A.V. Infrared Imaging Does Not Predict the Presence of Malignancy in Patients with Suspicious Radiologic Breast Abnormalities. Breast J. 2014, 20, 375–380. [Google Scholar] [CrossRef]
- Sella, T.; Sklair-Levy, M.; Cohen, M.; Rozin, M.; Shapiro-Feinberg, M.; Allweis, T.M.; Libson, E.; Izhaky, D. A Novel Functional Infrared Imaging System Coupled with Multiparametric Computerised Analysis for Risk Assessment of Breast Cancer. Eur. Radiol. 2013, 23, 1191–1198. [Google Scholar] [CrossRef]
- Sklair-Levy, M.; Friedman, E.; Shalmon, A.; Rudnstein, A. A Prospective Blind Evaluation of a 3D Functional Infrared Imaging for Risk Assessment in Women at High Risk for Breast Cancer. In Proceedings of the RSNA 2016: Beyond Imaging, Chicago, IL, USA, 27 November–2 December 2016. [Google Scholar]
- Hellgren, R.J.; Sundbom, A.E.; Czene, K.; Izhaky, D.; Hall, P.; Dickman, P.W. Does Three-Dimensional Functional Infrared Imaging Improve Breast Cancer Detection Based on Digital Mammography in Women with Dense Breasts? Eur. Radiol. 2019, 29, 6227–6235. [Google Scholar] [CrossRef] [PubMed]
- Kakileti, S.T.; Madhu, H.J.; Krishnan, L.; Manjunath, G.; Sampangi, S.; Ramprakash, H.V. Observational Study to Evaluate the Clinical Efficacy of Thermalytix for Detecting Breast Cancer in Symptomatic and Asymptomatic Women. JCO Glob. Oncol. 2020, 6, 1472–1480. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Bhat, V.; Sudhakar, S.; Namachivayam, A.; Gangadharan, C.; Pulchan, C.; Sigamani, A. Multicentric Study to Evaluate the Effectiveness of Thermalytix as Compared with Standard Screening Modalities in Subjects Who Show Possible Symptoms of Suspected Breast Cancer. BMJ Open 2021, 11, e052098. [Google Scholar] [CrossRef]
- Bansal, R.; Collison, S.; Krishnan, L.; Aggarwal, B.; Vidyasagar, M.; Kakileti, S.T.; Manjunath, G. A Prospective Evaluation of Breast Thermography Enhanced by a Novel Machine Learning Technique for Screening Breast Abnormalities in a General Population of Women Presenting to a Secondary Care Hospital. Front. Artif. Intell. 2023, 5, 1050803. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Delgado, F.; Vázquez-Luna, J.G. Feasibility of New-Generation Infrared Imaging Screening for Breast Cancer in Rural Communities. Oncol. Hematol. Rev. 2010, 6, 60–64. [Google Scholar] [CrossRef][Green Version]
- Rassiwala, M.; Mathur, P.; Mathur, R.; Farid, K.; Shukla, S.; Gupta, P.K.; Jain, B. Evaluation of Digital Infra–Red Thermal Imaging as an Adjunctive Screening Method for Breast Carcinoma: A Pilot Study. Int. J. Surg. 2014, 12, 1439–1443. [Google Scholar] [CrossRef]
- Yao, X.; Wei, W.; Li, J.; Wang, L.; Xu, Z.; Wan, Y.; Li, K.; Sun, S. A Comparison of Mammography, Ultrasonography, and Far-Infrared Thermography with Pathological Results in Screening and Early Diagnosis of Breast Cancer. Asian Biomed. 2014, 8, 11–19. [Google Scholar] [CrossRef]
- Garduño-Ramón, M.; Vega-Mancilla, S.; Morales-Henández, L.; Osornio-Rios, R. Supportive Noninvasive Tool for the Diagnosis of Breast Cancer Using a Thermographic Camera as Sensor. Sensors 2017, 17, 497. [Google Scholar] [CrossRef]
- Wang, X.; Chou, K.; Zhang, G.; Zuo, Z.; Zhang, T.; Zhou, Y.; Mao, F.; Lin, Y.; Shen, S.; Zhang, X.; et al. Breast Cancer Pre-Clinical Screening Using Infrared Thermography and Artificial Intelligence: A Prospective, Multicentre, Diagnostic Accuracy Cohort Study. Int. J. Surg. 2023, 109, 3021–3031. [Google Scholar] [CrossRef]
- Goñi-Arana, A.; Pérez-Martín, J.; Díez, F.J. Breast Thermography: A Systematic Review and Meta-Analysis. Syst. Rev. 2024, 13, 295. [Google Scholar] [CrossRef]
- FDA Breast Cancer Screening: Thermogram No Substitute for Mammogram. Available online: https://www.fda.gov/consumers/consumer-updates/breast-cancer-screening-thermogram-no-substitute-mammogram (accessed on 18 April 2023).
- Sardanelli, F.; Aase, H.S.; Álvarez, M.; Azavedo, E.; Baarslag, H.J.; Balleyguier, C.; Baltzer, P.A.; Beslagic, V.; Bick, U.; Bogdanovic-Stojanovic, D.; et al. Position Paper on Screening for Breast Cancer by the European Society of Breast Imaging (EUSOBI) and 30 National Breast Radiology Bodies from Austria, Belgium, Bosnia and Herzegovina, Bulgaria, Croatia, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Israel, Lithuania, Moldova, The Netherlands, Norway, Poland, Portugal, Romania, Serbia, Slovakia, Spain, Sweden, Switzerland and Turkey. Eur. Radiol. 2017, 27, 2737–2743. [Google Scholar] [CrossRef] [PubMed]
- Brkljacić, B.; Miletić, D.; Sardanelli, F. Thermography Is Not a Feasible Method for Breast Cancer Screening. Coll. Antropol. 2013, 37, 589–593. [Google Scholar]
- Kolarić, D.; Herceg, Z.; Nola, I.A.; Ramljak, V.; Kulis, T.; Holjevac, J.K.; Deutsch, J.A.; Antonini, S. Thermography—A Feasible Method for Screening Breast Cancer? Coll. Antropol. 2013, 37, 583–588. [Google Scholar]
- Kontos, M.; Wilson, R.; Fentiman, I. Digital Infrared Thermal Imaging (DITI) of Breast Lesions: Sensitivity and Specificity of Detection of Primary Breast Cancers. Clin. Radiol. 2011, 66, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Australian Government Statement on Use of Thermography to Detect Breast Cancer. Available online: https://www.health.gov.au/sites/default/files/documents/2019/11/breastscreen-australia-statement-on-use-of-thermography-to-detect-breast-cancer.pdf (accessed on 18 April 2023).
- Martin, J.E. Breast Imaging Techniques. Radiol. Clin. N. Am. 1983, 21, 149–153. [Google Scholar] [CrossRef]
- Homer, M.J. Breast Imaging: Pitfalls, Controversies, and Some Practical Thoughts. Radiol. Clin. N. Am. 1985, 23, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Beresford, S.; Cording, J.; Gribble, A.; Haddow, J.; Scanlan, A.; White, E. The Future of Breast Screening: A Literature Review of Emerging Technologies in Breast Cancer Screening. 2018. Available online: https://www.health.gov.au/sites/default/files/documents/2019/09/the-future-of-breast-screening-the-future-of-breast-screening-literature-review.pdf (accessed on 26 June 2025).
- Fitzgerald, A.; Berentson-Shaw, J. Thermography as a Screening and Diagnostic Tool: A Systematic Review. N. Z. Med. J. 2012, 125, 80–91. [Google Scholar]
- Brennan, M.; Houssami, N. Thermography in Breast Cancer Diagnosis, Screening and Risk Assessment: Systematic Review. Breast Cancer Manag. 2013, 2, 163–172. [Google Scholar] [CrossRef]
- Vreugdenburg, T.D.; Willis, C.D.; Mundy, L.; Hiller, J.E. A Systematic Review of Elastography, Electrical Impedance Scanning, and Digital Infrared Thermography for Breast Cancer Screening and Diagnosis. Breast Cancer Res. Treat. 2013, 137, 665–676. [Google Scholar] [CrossRef]
- Yussof, N.A. Infrared Regulation Thermography for Cancer; Ministry of Health in Malaysia: Putrajaya, Malaysia, 2014.
- Cohen, J.F.; Korevaar, D.A.; Altman, D.G.; Bruns, D.E.; Gatsonis, C.A.; Hooft, L.; Irwig, L.; Levine, D.; Reitsma, J.B.; de Vet, H.C.W.; et al. STARD 2015 Guidelines for Reporting Diagnostic Accuracy Studies: Explanation and Elaboration. BMJ Open 2016, 6, e012799. [Google Scholar] [CrossRef]
- Collins, G.S.; Moons, K.G.M.; Dhiman, P.; Riley, R.D.; Beam, A.L.; Van Calster, B.; Ghassemi, M.; Liu, X.; Reitsma, J.B.; van Smeden, M.; et al. TRIPOD+AI Statement: Updated Guidance for Reporting Clinical Prediction Models That Use Regression or Machine Learning Methods. BMJ 2024, 385, e078378. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Hernandez, J.-L.; Recinella, A.N.; Kandlikar, S.G.; Dabydeen, D.; Medeiros, L.; Phatak, P. An Inverse Heat Transfer Approach for Patient-Specific Breast Cancer Detection and Tumor Localization Using Surface Thermal Images in the Prone Position. Infrared Phys. Technol. 2020, 105, 103202. [Google Scholar] [CrossRef]
- Sarigoz, T.; Ertan, T. Role of Dynamic Thermography in Diagnosis of Nodal Involvement in Patients with Breast Cancer: A Pilot Study. Infrared Phys. Technol. 2020, 108, 103336. [Google Scholar] [CrossRef]
- Gershenson, M.; Gershenson, J. Dynamic Vascular Imaging Using Active Breast Thermography. Sensors 2023, 23, 3012. [Google Scholar] [CrossRef]
- Jacob, G.; Jose, I.; Sujatha, S. Breast Cancer Detection: A Comparative Review on Passive and Active Thermography. Infrared Phys. Technol. 2023, 134, 104932. [Google Scholar] [CrossRef]
- Tsietso, D.; Yahya, A.; Samikannu, R. A Review on Thermal Imaging-Based Breast Cancer Detection Using Deep Learning. Mob. Inf. Syst. 2022, 2022, 952849. [Google Scholar] [CrossRef]
- Jalloul, R.; Krishnappa, C.H.; Agughasi, V.I.; Alkhatib, R. Enhancing Early Breast Cancer Detection with Infrared Thermography: A Comparative Evaluation of Deep Learning and Machine Learning Models. Technologies 2024, 13, 7. [Google Scholar] [CrossRef]
- Iyadurai, J.; Chandrasekharan, M.; Muthusamy, S.; Panchal, H. An Extensive Review on Emerging Advancements in Thermography and Convolutional Neural Networks for Breast Cancer Detection. Wirel. Pers. Commun. 2024, 137, 1797–1821. [Google Scholar] [CrossRef]
- Teach, R.L.; Shortliffe, E.H. An Analysis of Physician Attitudes Regarding Computer-Based Clinical Consultation Systems. Comput. Biomed. Res. 1981, 14, 542–558. [Google Scholar] [CrossRef]
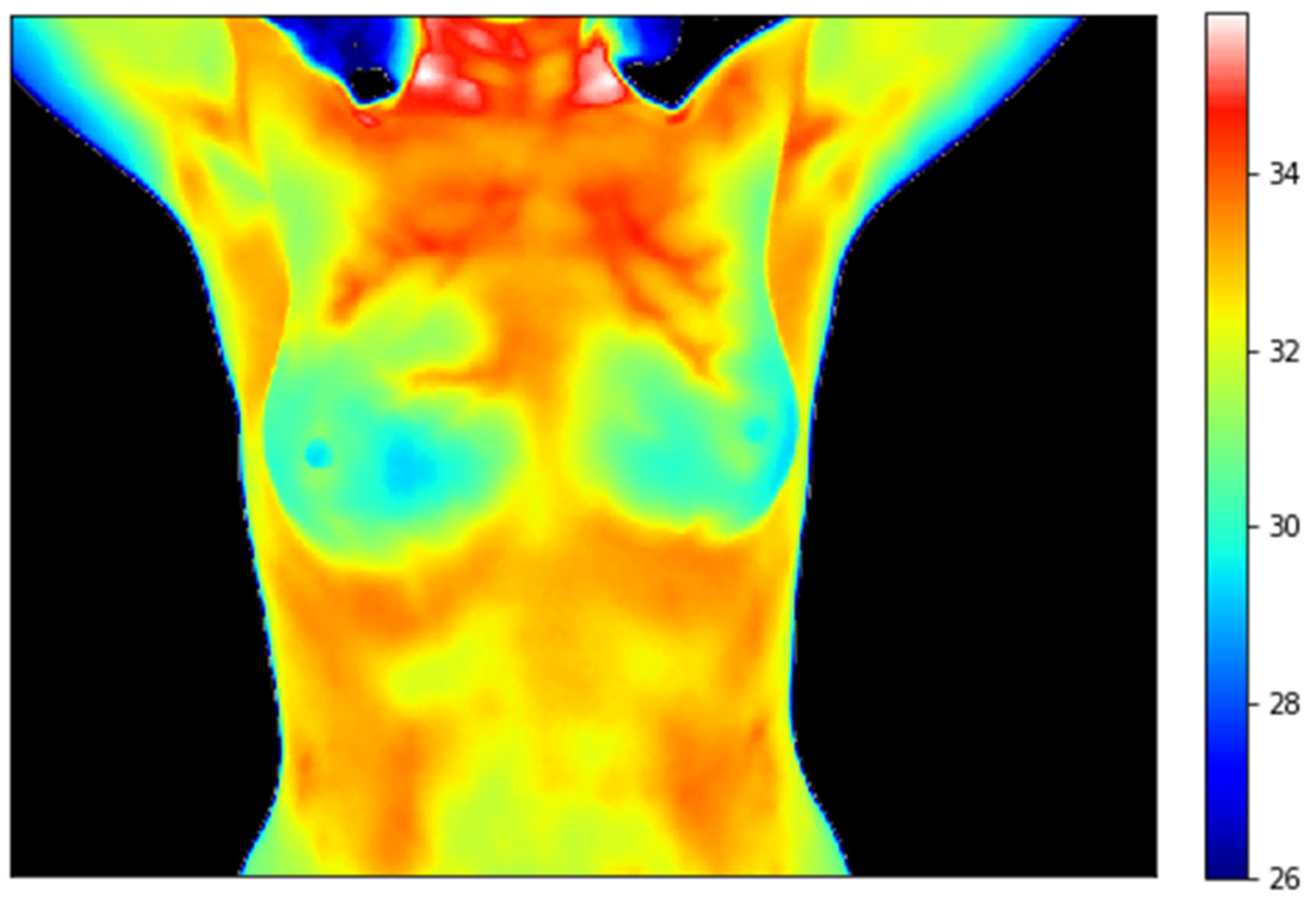
| Study | Year | Device | Population | Sensitivity | Specificity | |
|---|---|---|---|---|---|---|
| Studies to evaluate commercial systems | Parisky et al. [47] | 2003 | BCS 2100 (Computerized Thermal Imaging Inc., Ogden, UT, USA) | 769 patients scheduled for biopsy | 97% | 14% |
| Arora et al. [50] | 2008 | Sentinel BreastScan (SBS) (Infrared Sciences Corp., Bohemia, NY, USA) | 92 women scheduled for biopsy | 96.7% | 26.5% | |
| Wishart et al. [51] | 2010 | -NoTouch BreastScan (NTBS) (UE Life Sciences Inc., Philadelphia, PA, USA) -SBS | 100 patients scheduled for biopsy | 70% 48% | 48% 74% | |
| Collet et al. [52] | 2014 | NTBS | 99 patients scheduled for biopsy | 78.8% | 48.6% | |
| Sella et al. [53] | 2013 | 3DIRI (Real Imaging Ltd., Airport City, Israel) | 256 healthy asymptomatic women and 178 breast cancer patients | 90.9% | 72.5% | |
| Sklair-Levy et al. [54] | 2016 | 3DIRI | 226 patients at high risk due to genetic predisposition | 87.5% | 84.32% | |
| Hellgren et al. [55] | 2019 | 3DIRI | 1727 asymptomatic women with dense breasts | 58.3% | 87% | |
| Kakileti et al. [56] | 2020 | Thermalytix (Niramai Health Analytix, Bangalore, India) | 470 women -238 symptomatic -232 asymptomatic | 91.02% 89.85% 100% | 82.39% 69.04% 92.41% | |
| Singh et al. [57] | 2021 | Thermalytix | 258 symptomatic women | 82.5% | 80.5% | |
| Bansal et al. [58] | 2023 | Thermalytix | 459 women (symptomatic and asymptomatic) -168 with dense breasts | 95.24% 100% | 88.58% 81.65% | |
| Other studies | Gutierrez-Delgado and Vazquez-Luna [59] | 2010 | DL-700 (Zhejiang Dali Technology Co., Hangzhou, China) | 911 women attending cancer screening programs | 94.12% | unknown |
| Rassiwala et al. [60] | 2014 | FLIR ThermoVision A-20 (Teledyne Technologies Inc., Thousand Oaks, CA, USA) | 1008 asymptomatic women | 97.62% | 99.17% | |
| Yao et al. [61] | 2014 | Wuhan Hao Technology Co. (Wuhan, China) | 2036 women with abnormal findings in mammography or ultrasound | 84.4% | 94.0% | |
| Garduño-Ramón et al. [62] | 2017 | FLIR A-300 (Teledyne Technologies Inc., Thousand Oaks, CA, USA) | 454 women | 86.84% | 89.65% | |
| Wang et al. [63] | 2023 | InfiRay (IRay Technology Co., Yantai, China) | 2202 screening patients | 84.51% | 83.87% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goñi-Arana, A.; Pérez-Martín, J.; Díez, F.J. Why Do Radiologists Disown Breast Thermography? A Critical Review of Recent Studies and Recommendations. Cancers 2025, 17, 2195. https://doi.org/10.3390/cancers17132195
Goñi-Arana A, Pérez-Martín J, Díez FJ. Why Do Radiologists Disown Breast Thermography? A Critical Review of Recent Studies and Recommendations. Cancers. 2025; 17(13):2195. https://doi.org/10.3390/cancers17132195
Chicago/Turabian StyleGoñi-Arana, Ane, Jorge Pérez-Martín, and Francisco Javier Díez. 2025. "Why Do Radiologists Disown Breast Thermography? A Critical Review of Recent Studies and Recommendations" Cancers 17, no. 13: 2195. https://doi.org/10.3390/cancers17132195
APA StyleGoñi-Arana, A., Pérez-Martín, J., & Díez, F. J. (2025). Why Do Radiologists Disown Breast Thermography? A Critical Review of Recent Studies and Recommendations. Cancers, 17(13), 2195. https://doi.org/10.3390/cancers17132195






