The Efficacy of First-Line Pembrolizumab Monotherapy in Patients with Metastatic NSCLC Aged ≥70 Years with High PD-L1 (TPS ≥ 50%) Expression: A Multicenter Real-World Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Ethics Approval
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Lung Cancer Statistics. How Common Is Lung Cancer? American Cancer Society. Available online: https://www.cancer.org/cancer/types/lung-cancer/about/key-statistics.html (accessed on 11 December 2024).
- Lung and Bronchus Cancer—Cancer Stat Facts. Available online: https://seer.cancer.gov/statfacts/html/lungb.html (accessed on 11 December 2024).
- Montrone, M.; Rosati, G.; Longo, V.; Catino, A.; Massafra, R.; Nardone, A.; Pesola, F.; Montagna, E.S.; Marech, I.; Pizzutilo, P.; et al. Immunotherapy in Elderly Patients Affected by Non-Small Cell Lung Cancer: A Narrative Review. J. Clin. Med. 2023, 12, 1833. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Five-Year Outcomes with Pembrolizumab Versus Chemotherapy for Metastatic Non–Small-Cell Lung Cancer with PD-L1 Tumor Proportion Score ≥ 50%. J. Clin. Oncol. 2021, 39, 2339–2349. [Google Scholar] [CrossRef]
- Hendriks, L.; Kerr, K.; Menis, J.; Mok, T.; Nestle, U.; Passaro, A.; Peters, S.; Planchard, D.; Smit, E.; Solomon, B.; et al. Non-oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 358–376. [Google Scholar] [CrossRef]
- Hou, C.; Wang, Z.; Lu, X. Impact of immunosenescence and inflammaging on the effects of immune checkpoint inhibitors. Cancer Pathog. Ther. 2024, 2, 24–30. [Google Scholar] [CrossRef]
- Grosjean, H.A.I.; Dolter, S.; Meyers, D.E.; Ding, P.Q.; Stukalin, I.; Goutam, S.; Kong, S.; Chu, Q.; Heng, D.Y.C.; Bebb, D.G.; et al. Effectiveness and Safety of First-Line Pembrolizumab in Older Adults with PD-L1 Positive Non-Small Cell Lung Cancer: A Retrospective Cohort Study of the Alberta Immunotherapy Database. Curr. Oncol. 2021, 28, 4213–4222. [Google Scholar] [CrossRef] [PubMed]
- Galli, G.; De Toma, A.; Pagani, F.; Randon, G.; Trevisan, B.; Prelaj, A.; Ferrara, R.; Proto, C.; Signorelli, D.; Ganzinelli, M.; et al. Efficacy and safety of immunotherapy in elderly patients with non-small cell lung cancer. Lung Cancer 2019, 137, 38–42. [Google Scholar] [CrossRef]
- Alessi, J.V.; Ricciuti, B.; Jiménez-Aguilar, E.; Hong, F.; Wei, Z.; Nishino, M.; Plodkowski, A.J.; Sawan, P.; Luo, J.; Rizvi, H.; et al. Outcomes to first-line pembrolizumab in patients with PD-L1-high (≥50%) non–small cell lung cancer and a poor performance status. J. Immunother. Cancer 2020, 8, e001007. [Google Scholar] [CrossRef]
- Kontić, M.; Marković, F.; Nikolić, N.; Samardžić, N.; Stojanović, G.; Simurdić, P.; Petkov, S.; Bursać, D.; Zarić, B.; Stjepanović, M. Efficacy of Atezolizumab in Subsequent Lines of Therapy for NSCLC Patients: Insights from Real-World Data. Cancers 2024, 16, 3696. [Google Scholar] [CrossRef]
- Sun, L.; Cen, W.; Tang, W.; Long, Y.; Yang, X.; Ji, X.; Yang, J.; Zhang, R.; Wang, F.; Shao, J.; et al. Smoking status combined with tumor mutational burden as a prognosis predictor for combination immune checkpoint inhibitor therapy in non-small cell lung cancer. Cancer Med. 2021, 10, 6610–6617. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, Q.; Shen, J.; Wei, T.; Shen, W.; Zhang, N.; Luo, P.; Zhang, J. The Effect of Smoking on the Immune Microenvironment and Immunogenicity and Its Relationship with the Prognosis of Immune Checkpoint Inhibitors in Non-small Cell Lung Cancer. Front. Cell Dev. Biol. 2021, 9, 745859. [Google Scholar] [CrossRef] [PubMed]
- Popat, S.; Liu, S.V.; Scheuer, N.; Gupta, A.; Hsu, G.G.; Ramagopalan, S.V.; Griesinger, F.; Subbiah, V. Association Between Smoking History and Overall Survival in Patients Receiving Pembrolizumab for First-Line Treatment of Advanced Non–Small Cell Lung Cancer. JAMA Netw. Open 2022, 5, e2214046. [Google Scholar] [CrossRef]
- Cortellini, A.; De Giglio, A.; Cannita, K.; Cortinovis, D.L.; Cornelissen, R.; Baldessari, C.; Giusti, R.; D’Argento, E.; Grossi, F.; Santoni, M.; et al. Smoking status during first-line immunotherapy and chemotherapy in NSCLC patients: A case–control matched analysis from a large multicenter study. Thorac. Cancer 2021, 12, 880–889. [Google Scholar] [CrossRef]
- Luo, D.; Yang, D.; Cao, D.; Gong, Z.; He, F.; Hou, Y.; Lin, S. Effect of smoking status on immunotherapy for lung cancer: A systematic review and meta-analysis. Front. Oncol. 2024, 14, 1422160. [Google Scholar] [CrossRef]
- Sehgal, K.; Gill, R.R.; Widick, P.; Bindal, P.; McDonald, D.C.; Shea, M.; Rangachari, D.; Costa, D.B. Association of Performance Status with Survival in Patients with Advanced Non–Small Cell Lung Cancer Treated with Pembrolizumab Monotherapy. JAMA Netw. Open 2021, 4, e2037120. [Google Scholar] [CrossRef]
- Passaro, A.; Spitaleri, G.; Gyawali, B.; de Marinis, F. Immunotherapy in Non–Small-Cell Lung Cancer Patients with Performance Status 2: Clinical Decision Making with Scant Evidence. J. Clin. Oncol. 2019, 37, 1863–1867. [Google Scholar] [CrossRef]
- Lee, S.M.; Schulz, C.; Prabhash, K.; Kowalski, D.; Szczesna, A.; Han, B.; Rittmeyer, A.; Talbot, T.; Vicente, D.; Califano, R.; et al. First-line atezolizumab monotherapy versus single-agent chemotherapy in patients with non-small-cell lung cancer ineligible for treatment with a platinum-containing regimen (IPSOS): A phase 3, global, multicentre, open-label, randomised controlled study. Lancet 2023, 402, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Meyers, D.E.; Pasternak, M.; Dolter, S.; Grosjean, H.A.; Lim, C.A.; Stukalin, I.; Goutam, S.; Navani, V.; Heng, D.Y.; Cheung, W.Y.; et al. Impact of Performance Status on Survival Outcomes and Health Care Utilization in Patients with Advanced NSCLC Treated with Immune Checkpoint Inhibitors. JTO Clin. Res. Rep. 2023, 4, 100482. [Google Scholar] [CrossRef] [PubMed]
- Descourt, R.; Greillier, L.; Perol, M.; Ricordel, C.; Auliac, J.-B.; Falchero, L.; Gervais, R.; Veillon, R.; Vieillot, S.; Guisier, F.; et al. First-line single-agent pembrolizumab for PD-L1-positive (tumor proportion score ≥ 50%) advanced non-small cell lung cancer in the real world: Impact in brain metastasis: A national French multicentric cohort (ESCKEYP GFPC study). Cancer Immunol. Immunother. 2023, 72, 91–99. [Google Scholar] [CrossRef]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Giaccone, G.; de Marinis, F.; Reinmuth, N.; Vergnenegre, A.; Barrios, C.H.; Morise, M.; Felip, E.; Andric, Z.; Geater, S.; et al. Atezolizumab for First-Line Treatment of PD-L1–Selected Patients with NSCLC. N. Engl. J. Med. 2020, 383, 1328–1339. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gümüş, M.; Mazières, J.; Hermes, B.; Çay Şenler, F.; Csőszi, T.; Fülöp, A.; et al. Pembrolizumab plus Chemotherapy for Squamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef]
- Tomasik, B.; Bieńkowski, M.; Braun, M.; Popat, S.; Dziadziuszko, R. Effectiveness and safety of immunotherapy in NSCLC patients with ECOG PS score ≥2—Systematic review and meta-analysis. Lung Cancer 2021, 158, 97–106. [Google Scholar] [CrossRef]
- Ahmed, T.; Lycan, T.; Dothard, A.; Ehrlichman, P.; Ruiz, J.; Farris, M.; Topaloglu, U.; Levine, B.; Grant, S.; Klepin, H.D.; et al. Performance Status and Age as Predictors of Immunotherapy Outcomes in Advanced Non–Small-Cell Lung Cancer. Clin. Lung Cancer 2020, 21, e286–e293. [Google Scholar] [CrossRef]
- Marković, F.; Stjepanović, M.; Samardžić, N.; Kontić, M. The Association of Immune-Related Adverse Events with the Efficacy of Atezolizumab in Previously Treated Advanced Non-Small-Cell Lung Cancer Patients: A Single-Center Experience. Cancers 2024, 16, 2995. [Google Scholar] [CrossRef]
- Marković, F.; Stjepanović, M.; Rančić, M.; Cekić, M.; Kontić, M. Real-World Outcomes of First-Line Pembrolizumab Monotherapy in Metastatic NSCLC with High PD-L1 Expression (TPS ≥ 50%): A Multicenter Study from Serbia. Biomedicines 2025, 13, 1175. [Google Scholar] [CrossRef]
- Yao, J.; Li, S.; Bai, L.; Chen, J.; Ren, C.; Liu, T.; Qiu, J.; Dang, J. Efficacy and safety of immune checkpoint inhibitors in elderly patients with advanced non-small cell lung cancer: A systematic review and meta-analysis. eClinicalMedicine 2025, 81, 103081. [Google Scholar] [CrossRef]
- Tateishi, K.; Mizugaki, H.; Ikezawa, Y.; Morita, R.; Yokoo, K.; Sumi, T.; Aso, M.; Kikuchi, H.; Nakamura, A.; Sekikawa, M.; et al. Real-world data of first-line treatment with pembrolizumab for NSCLC with high PD-L1 expression in elderly patients: A subgroup analysis of HOT/NJLCG2001. Ultrasound Med. Biol. 2025, 55, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Middleton, G.; Brock, K.; Savage, J.; Mant, R.; Summers, Y.; Connibear, J.; Shah, R.; Ottensmeier, C.; Shaw, P.; Lee, S.-M.; et al. Pembrolizumab in patients with non-small-cell lung cancer of performance status 2 (PePS2): A single arm, phase 2 trial. Lancet Respir. Med. 2020, 8, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Seegobin, K.; Majeed, U.; Zhou, K.; Shi, H.; Lou, Y.; Zhao, Y.; Manochakian, R. P40.18 Second Line Immunotherapy After Progression on a Different First Line Immunotherapy in Advanced Non-Small Cell Lung Cancer with Focus on Elderly. J. Thorac. Oncol. 2021, 16, S1077–S1078. [Google Scholar] [CrossRef]
- Liguori, L.; Giorgio, G.; Polcaro, G.; Pagliara, V.; Malandrino, D.; Perri, F.; Cascella, M.; Ottaiano, A.; Conti, V.; Servetto, A.; et al. Checkpoint based immunotherapy in non-small cell lung cancer: A real-world retrospective study. Front. Immunol. 2024, 15, 1419544. [Google Scholar] [CrossRef]

| N = 381 | N (%) | ||
|---|---|---|---|
| Mean age at treatment start (range) [years] | 66.21 (35–90) | ||
| Aged ≥70 years | |||
| Yes | 149 (39.1) | ||
| No | 232 (60.8) | ||
| Patients ≥ 70 (N = 149) | Patients < 70 (N = 232) | p value | |
| Sex | 0.54 | ||
| Male | 84 (56.4%) | 138 (59.5%) | |
| Female | 65 (43.6%) | 94 (40.5%) | |
| Smoking status | 0.01 | ||
| Current or former | 123 (84.5%) | 220 (94.8%) | |
| Never | 26 (15.5%) | 12 (5.2%) | |
| ECOG PS | 0.35 | ||
| 0–1 | 123 (82.5%) | 180 (77.6%) | |
| ≥2 | 26 (17.5%) | 52 (22.4%) | |
| Histology | 0.88 | ||
| Non-squamous | 114 (76.5%) | 179 (77.3%) | |
| Squamous | 35 (23.5%) | 53 (22.8%) | |
| PD-L1 status | 0.37 | ||
| 50–79% | 72 (48.3%) | 123 (53%) | |
| 80–100% | 77 (51.7%) | 109 (47%) | |
| CNS metastasis at baseline | 0.07 | ||
| Yes | 23 (15.4%) | 53 (22.8%) | |
| No | 126 (84.6%) | 179 (77.2) | |
| Radiotherapy | 0.52 | ||
| Yes | 38 (25.5%) | 66 (28.5%) | |
| No | 111 (74.5) | 166 (71.5%) | |
| PD as per RECIST | |||
| Yes | 215 (56.4) | ||
| Received systemic treatment in 2nd line of therapy | 36 (16.7) | ||
| Did not receive systemic treatment in 2nd line of therapy | 179 (83.3) | ||
| No | 166 (43.6) | ||
| Best response to pembrolizumab (RECIST) | |||
| PD | 101 (26.6) | ||
| SD | 141 (37.0) | ||
| PR | 127 (33.3) | ||
| CR | 12 (3.1) | ||
| Real-world DCR | 279 (73.4) | ||
| Real-world ORR | 138 (36.4) | ||
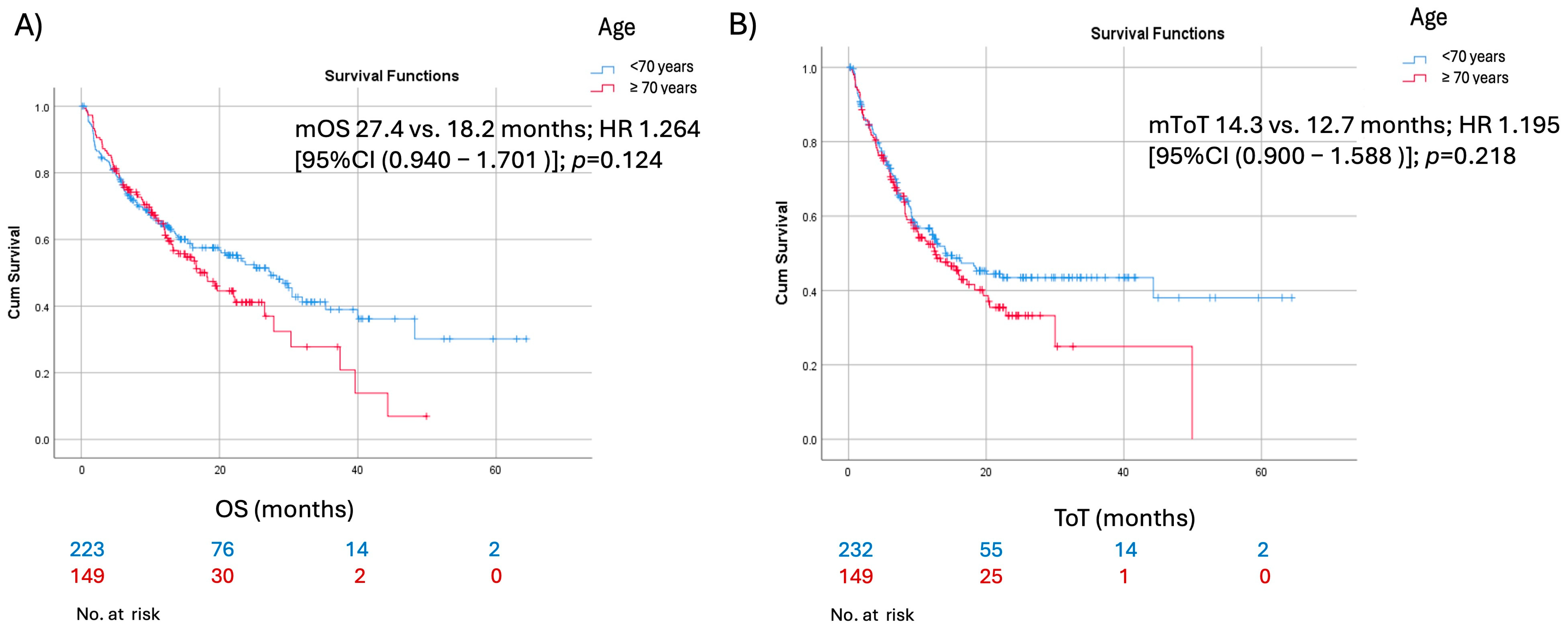
| Median ToT (95% confidence interval) [months] | 14.0 (10.5–17.5) | ||
| Median overall survival (95% confidence interval) [months] | 22.63 (16.7–28.6) | ||
| Discontinuation Reason | <70 Years (n = 167) | ≥70 Years (n = 102) |
|---|---|---|
| Disease progression | 129 (77.2%) | 86 (84.3%) |
| High-grade irAEs | 22 (13.2%) | 9 (8.8%) |
| Decline in ECOG PS/comorbidities | 16 (9.6%) | 7 (6.9%) |
| Age ≥70 Years (N = 149)-N (%) | Age <70 Years (N = 232)-N (%) | |
|---|---|---|
| CR | 6 (4.0%) | 6 (2.6%) |
| PR | 53 (35.6%) | 74 (31.9%) |
| SD | 51 (34.2%) | 91 (39.2%) |
| PD | 39 (26.2%) | 61 (26.3%) |
| Univariate Regression Analysis | Multivariate Regression Analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Radiotherapy (yes vs. no) | 0.677 | 0.423–1.143 | 0.134 | |||
| Sex (male vs. female) | 1.393 | 0.926–2.094 | 0.111 | |||
| Histology (non-squamous vs. squamous) | 1.085 | 0.707–1.665 | 0.708 | |||
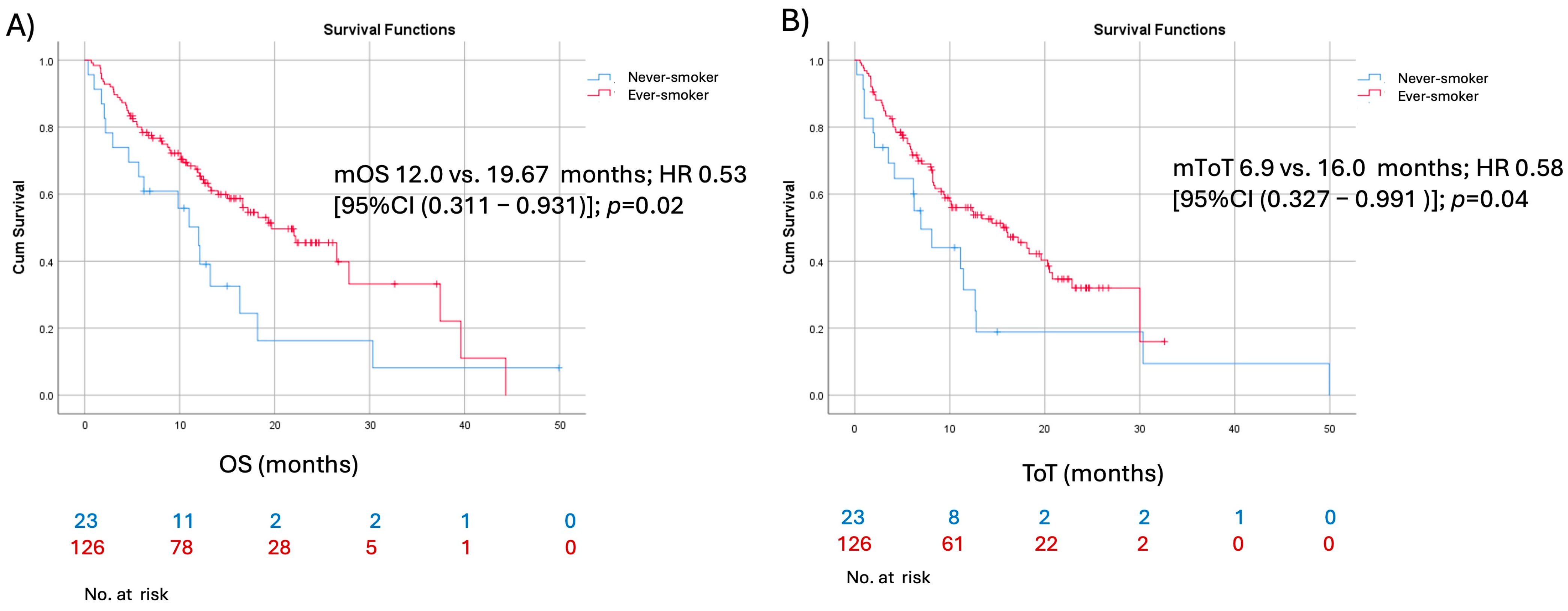
| Smoking status (current or former smoker vs. never-smoker) | 0.581 | 0.327–0.991 | 0.048 | 0.645 | 0.379–1.098 | 0.108 |
| PD-L1 (50–79 vs. 80%+) | 0.979 | 0.648–1.480 | 0.920 | |||
| ECOG PS (0–1 vs. ≥2) | 2.364 | 1.445–3.867 | 0.001 | 2.266 | 1.380–3.720 | 0.001 |
| CNS mets (yes vs. no) | 1.024 | 0.568–1.847 | 0.937 | |||
| Univariate Regression Analysis | Multivariate Regression Analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Radiotherapy (yes vs. no) | 0.685 | 0.416–1.214 | 0.134 | |||
| Sex (male vs. female) | 1.308 | 0.834–2.051 | 0.242 | |||
| Histology (non-squamous vs. squamous) | 1.050 | 0.610–1.805 | 0.861 | |||
| Smoking status (current or former smoker vs. never-smoker) | 0.538 | 0.311–0.931 | 0.027 | 1.104 | 0.8–1.524 | 0.546 |
| PD-L1 (50–79 vs. 80%+) | 0.992 | 0.644–1.530 | 0.973 | |||
| ECOG PS (0–1 vs. ≥2) | 2.867 | 1.718–4.782 | 0.001 | 2.789 | 1.659–4.690 | 0.001 |
| CNS mets (yes vs. no) | 1.189 | 0.638–2.216 | 0.585 | |||
| Patients Receiving Second-Line Systemic Therapy Following PD as per RECIST (N = 215; 100%) | ||
|---|---|---|
| Yes (N = 36; 16.7%) | No (N = 179; 83.3%) | |
| Age < 70 years | 26 (12%) | 83 (38.6%) |
| Age ≥ 70 years | 10 (4.6%) | 96 (44.4%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marković, F.; Hochmair, M.; Müser, N.; Fabikan, H.; Rodriguez, V.M.; Janzic, U.; Stjepanović, M.; Kontić, M. The Efficacy of First-Line Pembrolizumab Monotherapy in Patients with Metastatic NSCLC Aged ≥70 Years with High PD-L1 (TPS ≥ 50%) Expression: A Multicenter Real-World Study. Cancers 2025, 17, 2190. https://doi.org/10.3390/cancers17132190
Marković F, Hochmair M, Müser N, Fabikan H, Rodriguez VM, Janzic U, Stjepanović M, Kontić M. The Efficacy of First-Line Pembrolizumab Monotherapy in Patients with Metastatic NSCLC Aged ≥70 Years with High PD-L1 (TPS ≥ 50%) Expression: A Multicenter Real-World Study. Cancers. 2025; 17(13):2190. https://doi.org/10.3390/cancers17132190
Chicago/Turabian StyleMarković, Filip, Maximilian Hochmair, Nino Müser, Hannah Fabikan, Vania Mikaela Rodriguez, Urska Janzic, Mihailo Stjepanović, and Milica Kontić. 2025. "The Efficacy of First-Line Pembrolizumab Monotherapy in Patients with Metastatic NSCLC Aged ≥70 Years with High PD-L1 (TPS ≥ 50%) Expression: A Multicenter Real-World Study" Cancers 17, no. 13: 2190. https://doi.org/10.3390/cancers17132190
APA StyleMarković, F., Hochmair, M., Müser, N., Fabikan, H., Rodriguez, V. M., Janzic, U., Stjepanović, M., & Kontić, M. (2025). The Efficacy of First-Line Pembrolizumab Monotherapy in Patients with Metastatic NSCLC Aged ≥70 Years with High PD-L1 (TPS ≥ 50%) Expression: A Multicenter Real-World Study. Cancers, 17(13), 2190. https://doi.org/10.3390/cancers17132190








