The Emerging Role of Extracellular Vesicle-Derived lncRNAs and circRNAs in Tumor and Mesenchymal Stem Cells: The Biological Functions and Potential for Clinical Application
Simple Summary
Abstract
1. Introduction
2. Biological Characteristics of EVs
2.1. Biogenesis of EVs
2.2. Release of EVs
2.3. Uptake of EVs
3. Commonalities in the Mechanisms of lncRNAs and circRNAs
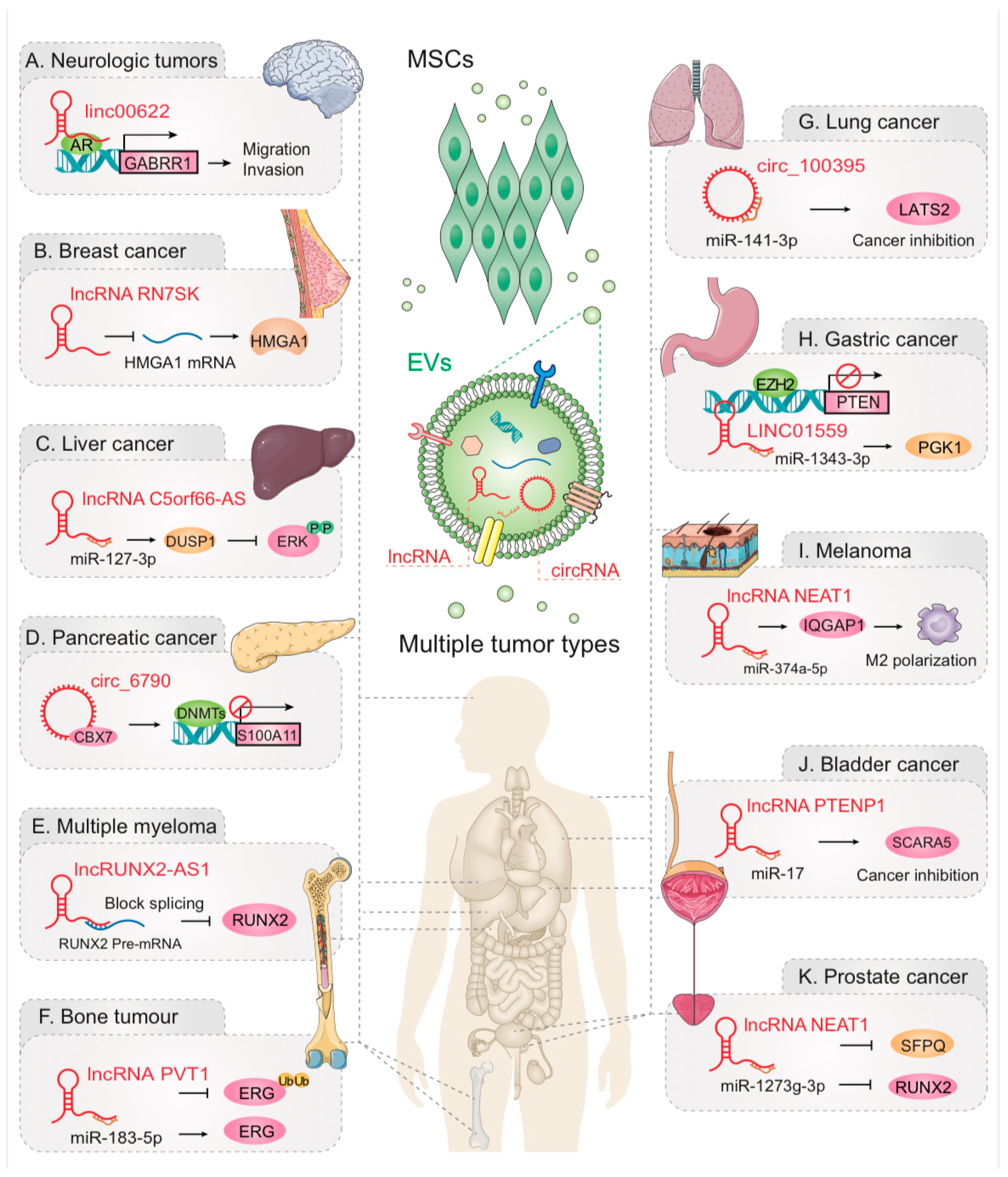
4. EV-lncRNAs and EV-circRNAs Act Between MSCs and Tumors
4.1. Nervous System Neoplasm
4.2. Breast Cancer
4.3. Liver Cancer
4.4. Pancreatic Cancer (PC)
4.5. Multiple Myeloma (MM)
4.6. Bone Tumor
4.7. Lung Cancer
4.8. Gastric Cancer
4.9. Other Tumors
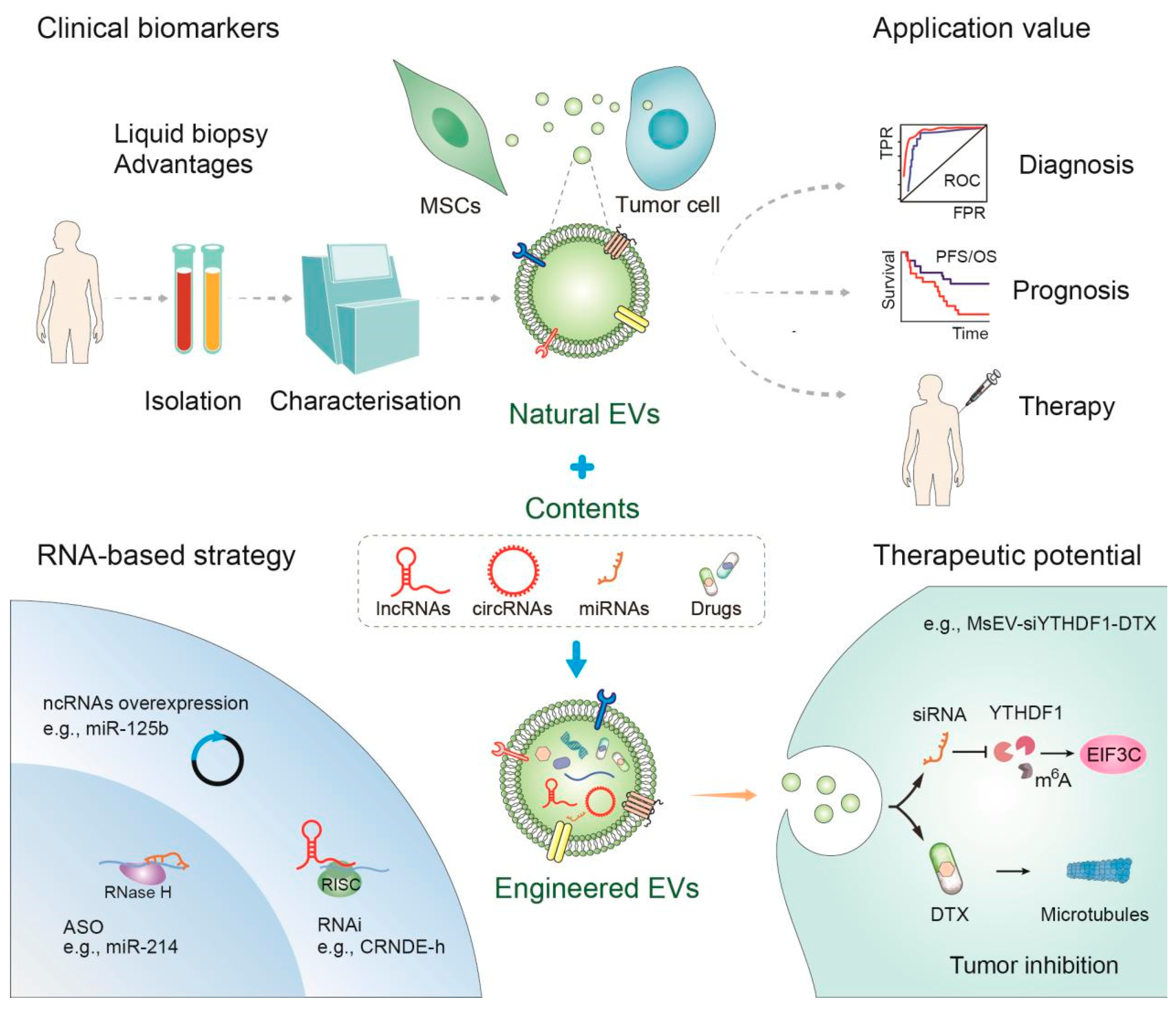
5. Clinical Application of EVs
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, H.; Yue, B. Effects of Various Antimicrobial Agents on Multi-Directional Differentiation Potential of Bone Marrow-Derived Mesenchymal Stem Cells. World J. Stem Cells 2019, 11, 322–336. [Google Scholar] [CrossRef]
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal Stem Cells in Health and Disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Piatetzky-Shapiro, I.I.; Petrakova, K.V. Osteogenesis in Transplants of Bone Marrow Cells. J. Embryol. Exp. Morphol. 1966, 16, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Saini, A.; Kalsan, M.; Kumar, N.; Chandra, R. Describing the Stem Cell Potency: The Various Methods of Functional Assessment and In Silico Diagnostics. Front. Cell Dev. Biol. 2016, 4, 134. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal Stem Cell Perspective: Cell Biology to Clinical Progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef]
- Farini, A.; Sitzia, C.; Erratico, S.; Meregalli, M.; Torrente, Y. Clinical Applications of Mesenchymal Stem Cells in Chronic Diseases. Stem Cells Int. 2014, 2014, 306573. [Google Scholar] [CrossRef]
- Aheget, H.; Tristán-Manzano, M.; Mazini, L.; Cortijo-Gutierrez, M.; Galindo-Moreno, P.; Herrera, C.; Martin, F.; Marchal, J.A.; Benabdellah, K. Exosome: A New Player in Translational Nanomedicine. J. Clin. Med. 2020, 9, 2380. [Google Scholar] [CrossRef]
- Han, C.; Sun, X.; Liu, L.; Jiang, H.; Shen, Y.; Xu, X.; Li, J.; Zhang, G.; Huang, J.; Lin, Z.; et al. Exosomes and Their Therapeutic Potentials of Stem Cells. Stem Cells Int. 2016, 2016, 7653489. [Google Scholar] [CrossRef]
- Mishra, P.J.; Mishra, P.J.; Humeniuk, R.; Medina, D.J.; Alexe, G.; Mesirov, J.P.; Ganesan, S.; Glod, J.W.; Banerjee, D. Carcinoma-Associated Fibroblast-like Differentiation of Human Mesenchymal Stem Cells. Cancer Res. 2008, 68, 4331–4339. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal Information for Studies of Extracellular Vesicles (MISEV2023): From Basic to Advanced Approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- O’Brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA Delivery by Extracellular Vesicles in Mammalian Cells and Its Applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef]
- Pegtel, D.M.; Gould, S.J. Exosomes. Annu. Rev. Biochem. 2019, 88, 487–514. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, L.; Huang, X.; Zhang, S.; Cao, W.; Che, F.; Zhu, Y.; Dai, J. Recent Progress of Exosomes in Multiple Myeloma: Pathogenesis, Diagnosis, Prognosis and Therapeutic Strategies. Cancers 2021, 13, 1635. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, S.W.; Nguyen, J. Exosomes as Therapeutics: The Implications of Molecular Composition and Exosomal Heterogeneity. J. Control Release 2016, 228, 179–190. [Google Scholar] [CrossRef]
- EL Andaloussi, S.; Mäger, I.; Breakefield, X.O.; Wood, M.J.A. Extracellular Vesicles: Biology and Emerging Therapeutic Opportunities. Nat. Rev. Drug Discov. 2013, 12, 347–357. [Google Scholar] [CrossRef]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of Secretion and Uptake of Exosomes and Other Extracellular Vesicles for Cell-to-Cell Communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef]
- Mimeault, M.; Batra, S.K. Molecular Biomarkers of Cancer Stem/Progenitor Cells Associated with Progression, Metastases, and Treatment Resistance of Aggressive Cancers. Cancer Epidemiol. Biomark. Prev. 2014, 23, 234–254. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Greening, D.W.; Zhu, H.-J.; Takahashi, N.; Simpson, R.J. Extracellular Vesicle Isolation and Characterization: Toward Clinical Application. J. Clin. Investig. 2016, 126, 1152–1162. [Google Scholar] [CrossRef]
- Corrado, C.; Raimondo, S.; Chiesi, A.; Ciccia, F.; De Leo, G.; Alessandro, R. Exosomes as Intercellular Signaling Organelles Involved in Health and Disease: Basic Science and Clinical Applications. Int. J. Mol. Sci. 2013, 14, 5338–5366. [Google Scholar] [CrossRef]
- Lo Cicero, A.; Stahl, P.D.; Raposo, G. Extracellular Vesicles Shuffling Intercellular Messages: For Good or for Bad. Curr. Opin. Cell Biol. 2015, 35, 69–77. [Google Scholar] [CrossRef]
- Simons, M.; Raposo, G. Exosomes–Vesicular Carriers for Intercellular Communication. Curr. Opin. Cell Biol. 2009, 21, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Bukong, T.N.; Momen-Heravi, F.; Kodys, K.; Bala, S.; Szabo, G. Exosomes from Hepatitis C Infected Patients Transmit HCV Infection and Contain Replication Competent Viral RNA in Complex with Ago2-miR122-HSP90. PLoS Pathog. 2014, 10, e1004424. [Google Scholar] [CrossRef]
- Fleming, A.; Sampey, G.; Chung, M.-C.; Bailey, C.; van Hoek, M.L.; Kashanchi, F.; Hakami, R.M. The Carrying Pigeons of the Cell: Exosomes and Their Role in Infectious Diseases Caused by Human Pathogens. Pathog. Dis. 2014, 71, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Nolte-’t Hoen, E.; Cremer, T.; Gallo, R.C.; Margolis, L.B. Extracellular Vesicles and Viruses: Are They Close Relatives? Proc. Natl. Acad. Sci. USA 2016, 113, 9155–9161. [Google Scholar] [CrossRef]
- Liu, C.-G.; Chen, J.; Goh, R.M.W.-J.; Liu, Y.-X.; Wang, L.; Ma, Z. The Role of Tumor-Derived Extracellular Vesicles Containing Noncoding RNAs in Mediating Immune Cell Function and Its Implications from Bench to Bedside. Pharmacol. Res. 2023, 191, 106756. [Google Scholar] [CrossRef]
- Théry, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, Biogenesis and Function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-Mediated Transfer of mRNAs and microRNAs Is a Novel Mechanism of Genetic Exchange between Cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Gusachenko, O.N.; Zenkova, M.A.; Vlassov, V.V. Nucleic Acids in Exosomes: Disease Markers and Intercellular Communication Molecules. Biochemistry 2013, 78, 1–7. [Google Scholar] [CrossRef]
- Sadik, N.; Cruz, L.; Gurtner, A.; Rodosthenous, R.S.; Dusoswa, S.A.; Ziegler, O.; Van Solinge, T.S.; Wei, Z.; Salvador-Garicano, A.M.; Gyorgy, B.; et al. Extracellular RNAs: A New Awareness of Old Perspectives. Methods Mol. Biol. 2018, 1740, 1–15. [Google Scholar] [CrossRef]
- Nemeth, K.; Bayraktar, R.; Ferracin, M.; Calin, G.A. Non-Coding RNAs in Disease: From Mechanisms to Therapeutics. Nat. Rev. Genet. 2024, 25, 211–232. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.; Chen, J. Linc00261 Inhibited High-Grade Serous Ovarian Cancer Progression through miR-552-ATG10-EMT Axis. Comput. Math. Methods Med. 2022, 2022, 9450353. [Google Scholar] [CrossRef]
- Slack, F.J.; Chinnaiyan, A.M. The Role of Non-Coding RNAs in Oncology. Cell 2019, 179, 1033–1055. [Google Scholar] [CrossRef] [PubMed]
- Skotland, T.; Hessvik, N.P.; Sandvig, K.; Llorente, A. Exosomal Lipid Composition and the Role of Ether Lipids and Phosphoinositides in Exosome Biology. J. Lipid Res. 2019, 60, 9–18. [Google Scholar] [CrossRef]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle Formation during Reticulocyte Maturation. Association of Plasma Membrane Activities with Released Vesicles (Exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Abdelmohsen, K.; Mustapic, M.; Kapogiannis, D.; Gorospe, M. RNA in Extracellular Vesicles. Wiley Interdiscip. Rev. RNA 2017, 8, e1413. [Google Scholar] [CrossRef] [PubMed]
- Lara, P.; Chan, A.B.; Cruz, L.J.; Quest, A.F.G.; Kogan, M.J. Exploiting the Natural Properties of Extracellular Vesicles in Targeted Delivery towards Specific Cells and Tissues. Pharmaceutics 2020, 12, 1022. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Shao, H.; Im, H.; Castro, C.M.; Breakefield, X.; Weissleder, R.; Lee, H. New Technologies for Analysis of Extracellular Vesicles. Chem. Rev. 2018, 118, 1917–1950. [Google Scholar] [CrossRef]
- Henne, W.M.; Buchkovich, N.J.; Emr, S.D. The ESCRT Pathway. Dev. Cell 2011, 21, 77–91. [Google Scholar] [CrossRef]
- Hanson, P.I.; Cashikar, A. Multivesicular Body Morphogenesis. Annu. Rev. Cell Dev. Biol. 2012, 28, 337–362. [Google Scholar] [CrossRef] [PubMed]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brügger, B.; Simons, M. Ceramide Triggers Budding of Exosome Vesicles into Multivesicular Endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef]
- Qiu, Y.; Li, P.; Zhang, Z.; Wu, M. Insights Into Exosomal Non-Coding RNAs Sorting Mechanism and Clinical Application. Front. Oncol. 2021, 11, 664904. [Google Scholar] [CrossRef]
- Lei, Y.; Guo, W.; Chen, B.; Chen, L.; Gong, J.; Li, W. Tumor-released lncRNA H19 Promotes Gefitinib Resistance via Packaging into Exosomes in Non-small Cell Lung Cancer. Oncol. Rep. 2018, 40, 3438–3446. [Google Scholar] [CrossRef] [PubMed]
- Squadrito, M.L.; Baer, C.; Burdet, F.; Maderna, C.; Gilfillan, G.D.; Lyle, R.; Ibberson, M.; De Palma, M. Endogenous RNAs Modulate microRNA Sorting to Exosomes and Transfer to Acceptor Cells. Cell Rep. 2014, 8, 1432–1446. [Google Scholar] [CrossRef]
- Koppers-Lalic, D.; Hackenberg, M.; Bijnsdorp, I.V.; van Eijndhoven, M.A.J.; Sadek, P.; Sie, D.; Zini, N.; Middeldorp, J.M.; Ylstra, B.; de Menezes, R.X.; et al. Nontemplated Nucleotide Additions Distinguish the Small RNA Composition in Cells from Exosomes. Cell Rep. 2014, 8, 1649–1658. [Google Scholar] [CrossRef]
- Stoorvogel, W.; Strous, G.J.; Geuze, H.J.; Oorschot, V.; Schwartz, A.L. Late Endosomes Derive from Early Endosomes by Maturation. Cell 1991, 65, 417–427. [Google Scholar] [CrossRef]
- Sahu, R.; Kaushik, S.; Clement, C.C.; Cannizzo, E.S.; Scharf, B.; Follenzi, A.; Potolicchio, I.; Nieves, E.; Cuervo, A.M.; Santambrogio, L. Microautophagy of Cytosolic Proteins by Late Endosomes. Dev. Cell 2011, 20, 131–139. [Google Scholar] [CrossRef]
- Liu, J.; Ren, L.; Li, S.; Li, W.; Zheng, X.; Yang, Y.; Fu, W.; Yi, J.; Wang, J.; Du, G. The Biology, Function, and Applications of Exosomes in Cancer. Acta Pharm. Sin. B 2021, 11, 2783–2797. [Google Scholar] [CrossRef]
- Stenmark, H. Rab GTPases as Coordinators of Vesicle Traffic. Nat. Rev. Mol. Cell Biol. 2009, 10, 513–525. [Google Scholar] [CrossRef]
- Vanlandingham, P.A.; Ceresa, B.P. Rab7 Regulates Late Endocytic Trafficking Downstream of Multivesicular Body Biogenesis and Cargo Sequestration. J. Biol. Chem. 2009, 284, 12110–12124. [Google Scholar] [CrossRef] [PubMed]
- Rocha, N.; Kuijl, C.; van der Kant, R.; Janssen, L.; Houben, D.; Janssen, H.; Zwart, W.; Neefjes, J. Cholesterol Sensor ORP1L Contacts the ER Protein VAP to Control Rab7-RILP-P150 Glued and Late Endosome Positioning. J. Cell Biol. 2009, 185, 1209–1225. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Trajkovic, K.; Tsunemi, T.; Krainc, D. Parkin Modulates Endosomal Organization and Function of the Endo-Lysosomal Pathway. J. Neurosci. 2016, 36, 2425–2437. [Google Scholar] [CrossRef] [PubMed]
- Zylbersztejn, K.; Galli, T. Vesicular Traffic in Cell Navigation. FEBS J. 2011, 278, 4497–4505. [Google Scholar] [CrossRef]
- Fader, C.M.; Sánchez, D.G.; Mestre, M.B.; Colombo, M.I. TI-VAMP/VAMP7 and VAMP3/Cellubrevin: Two v-SNARE Proteins Involved in Specific Steps of the Autophagy/Multivesicular Body Pathways. Biochim. Biophys. Acta 2009, 1793, 1901–1916. [Google Scholar] [CrossRef]
- Verweij, F.J.; Bebelman, M.P.; Jimenez, C.R.; Garcia-Vallejo, J.J.; Janssen, H.; Neefjes, J.; Knol, J.C.; de Goeij-de Haas, R.; Piersma, S.R.; Baglio, S.R.; et al. Quantifying Exosome Secretion from Single Cells Reveals a Modulatory Role for GPCR Signaling. J. Cell Biol. 2018, 217, 1129–1142. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, D.; Jin, F.; Bian, Z.; Li, L.; Liang, H.; Li, M.; Shi, L.; Pan, C.; Zhu, D.; et al. Pyruvate Kinase Type M2 Promotes Tumour Cell Exosome Release via Phosphorylating Synaptosome-Associated Protein 23. Nat. Commun. 2017, 8, 14041. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Teng, F.; Fussenegger, M. Shedding Light on Extracellular Vesicle Biogenesis and Bioengineering. Adv. Sci. 2020, 8, 2003505. [Google Scholar] [CrossRef]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R.F. Routes and Mechanisms of Extracellular Vesicle Uptake. J. Extracell. Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef]
- Nazarenko, I.; Rana, S.; Baumann, A.; McAlear, J.; Hellwig, A.; Trendelenburg, M.; Lochnit, G.; Preissner, K.T.; Zöller, M. Cell Surface Tetraspanin Tspan8 Contributes to Molecular Pathways of Exosome-Induced Endothelial Cell Activation. Cancer Res. 2010, 70, 1668–1678. [Google Scholar] [CrossRef]
- Rana, S.; Yue, S.; Stadel, D.; Zöller, M. Toward Tailored Exosomes: The Exosomal Tetraspanin Web Contributes to Target Cell Selection. Int. J. Biochem. Cell Biol. 2012, 44, 1574–1584. [Google Scholar] [CrossRef] [PubMed]
- Desrochers, L.M.; Bordeleau, F.; Reinhart-King, C.A.; Cerione, R.A.; Antonyak, M.A. Microvesicles Provide a Mechanism for Intercellular Communication by Embryonic Stem Cells during Embryo Implantation. Nat. Commun. 2016, 7, 11958. [Google Scholar] [CrossRef] [PubMed]
- Antonyak, M.A.; Li, B.; Boroughs, L.K.; Johnson, J.L.; Druso, J.E.; Bryant, K.L.; Holowka, D.A.; Cerione, R.A. Cancer Cell-Derived Microvesicles Induce Transformation by Transferring Tissue Transglutaminase and Fibronectin to Recipient Cells. Proc. Natl. Acad. Sci. USA 2011, 108, 4852–4857. [Google Scholar] [CrossRef]
- Zhao, W.; Shan, B.; He, D.; Cheng, Y.; Li, B.; Zhang, C.; Duan, C. Recent Progress in Characterizing Long Noncoding RNAs in Cancer Drug Resistance. J. Cancer 2019, 10, 6693–6702. [Google Scholar] [CrossRef] [PubMed]
- Pathania, A.S.; Challagundla, K.B. Exosomal Long Non-Coding RNAs: Emerging Players in the Tumor Microenvironment. Mol. Ther. Nucleic Acids 2021, 23, 1371–1383. [Google Scholar] [CrossRef]
- Hewson, C.; Morris, K.V. Form and Function of Exosome-Associated Long Non-Coding RNAs in Cancer. Curr. Top. Microbiol. Immunol. 2016, 394, 41–56. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, L.; Yu, F.; Zhang, Y.; Li, P.; Wang, K. The Functional Roles of Exosomal Long Non-Coding RNAs in Cancer. Cell Mol. Life Sci. 2019, 76, 2059–2076. [Google Scholar] [CrossRef]
- Deng, H.; Zhang, J.; Shi, J.; Guo, Z.; He, C.; Ding, L.; Tang, J.H.; Hou, Y. Role of Long Non-Coding RNA in Tumor Drug Resistance. Tumour Biol. 2016, 37, 11623–11631. [Google Scholar] [CrossRef]
- Liu, C.-G.; Li, J.; Xu, Y.; Li, W.; Fang, S.-X.; Zhang, Q.; Xin, H.-W.; Ma, Z. Long Non-Coding RNAs and Circular RNAs in Tumor Angiogenesis: From Mechanisms to Clinical Significance. Mol. Ther. Oncolytics 2021, 22, 336–354. [Google Scholar] [CrossRef]
- Kristensen, L.S.; Andersen, M.S.; Stagsted, L.V.W.; Ebbesen, K.K.; Hansen, T.B.; Kjems, J. The Biogenesis, Biology and Characterization of Circular RNAs. Nat. Rev. Genet. 2019, 20, 675–691. [Google Scholar] [CrossRef]
- Li, R.; Jiang, J.; Shi, H.; Qian, H.; Zhang, X.; Xu, W. CircRNA: A Rising Star in Gastric Cancer. Cell Mol. Life Sci. 2020, 77, 1661–1680. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Su, Y.; Zhong, S.; Cong, L.; Liu, B.; Yang, J.; Tao, Y.; He, Z.; Chen, C.; Jiang, Y. Exosomes: Key Players in Cancer and Potential Therapeutic Strategy. Signal Transduct. Target. Ther. 2020, 5, 145. [Google Scholar] [CrossRef]
- Tsai, M.-C.; Manor, O.; Wan, Y.; Mosammaparast, N.; Wang, J.K.; Lan, F.; Shi, Y.; Segal, E.; Chang, H.Y. Long Noncoding RNA as Modular Scaffold of Histone Modification Complexes. Science 2010, 329, 689–693. [Google Scholar] [CrossRef]
- Heo, J.B.; Sung, S. Vernalization-Mediated Epigenetic Silencing by a Long Intronic Noncoding RNA. Science 2011, 331, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhao, G.; Yan, X.; Lv, Z.; Yin, H.; Zhang, S.; Song, W.; Li, X.; Li, L.; Du, Z.; et al. A Novel FLI1 Exonic Circular RNA Promotes Metastasis in Breast Cancer by Coordinately Regulating TET1 and DNMT1. Genome Biol. 2018, 19, 218. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Wang, L.; Li, C. Circ_0006790 Carried by Bone Marrow Mesenchymal Stem Cell-Derived Exosomes Regulates S100A11 DNA Methylation through Binding to CBX7 in Pancreatic Ductal Adenocarcinoma. Am. J. Cancer Res. 2022, 12, 1934–1959. [Google Scholar]
- Guo, M.; Li, D.; Feng, Y.; Li, M.; Yang, B. Adipose-Derived Stem Cell-Derived Extracellular Vesicles Inhibit Neuroblastoma Growth by Regulating GABBR1 Activity through LINC00622-Mediated Transcription Factor AR. J. Leukoc. Biol. 2022, 111, 19–32. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.-O.; Chen, T.; Xiang, J.-F.; Yin, Q.-F.; Xing, Y.-H.; Zhu, S.; Yang, L.; Chen, L.-L. Circular Intronic Long Noncoding RNAs. Mol. Cell 2013, 51, 792–806. [Google Scholar] [CrossRef]
- Salmena, L.; Poliseno, L.; Tay, Y.; Kats, L.; Pandolfi, P.P. A ceRNA Hypothesis: The Rosetta Stone of a Hidden RNA Language? Cell 2011, 146, 353–358. [Google Scholar] [CrossRef]
- Tay, Y.; Rinn, J.; Pandolfi, P.P. The Multilayered Complexity of ceRNA Crosstalk and Competition. Nature 2014, 505, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Li, S.; Zhang, F.; Xi, Y.; Wang, L.; Bi, Y.; Li, D. Long Non-Coding RNA NEAT1 Promotes Non-Small Cell Lung Cancer Progression through Regulation of miR-377-3p-E2F3 Pathway. Oncotarget 2016, 7, 51784–51814. [Google Scholar] [CrossRef]
- Zhou, R.; Chen, K.K.; Zhang, J.; Xiao, B.; Huang, Z.; Ju, C.; Sun, J.; Zhang, F.; Lv, X.-B.; Huang, G. The Decade of Exosomal Long RNA Species: An Emerging Cancer Antagonist. Mol. Cancer 2018, 17, 75. [Google Scholar] [CrossRef]
- Gong, C.; Li, Z.; Ramanujan, K.; Clay, I.; Zhang, Y.; Lemire-Brachat, S.; Glass, D.J. A Long Non-Coding RNA, LncMyoD, Regulates Skeletal Muscle Differentiation by Blocking IMP2-Mediated mRNA Translation. Dev. Cell 2015, 34, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Yang, G.; Wang, X.; Liu, J.; Lu, Z.; Wang, Q.; Xu, B.; Liu, Z.; Li, J. CircBACH1 (Hsa_circ_0061395) Promotes Hepatocellular Carcinoma Growth by Regulating P27 Repression via HuR. J. Cell Physiol. 2020, 235, 6929–6941. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, B.S.; Zhou, A.; Lin, K.; Zheng, S.; Lu, Z.; Chen, Y.; Sulman, E.P.; Xie, K.; Bögler, O.; et al. m6A Demethylase ALKBH5 Maintains Tumorigenicity of Glioblastoma Stem-like Cells by Sustaining FOXM1 Expression and Cell Proliferation Program. Cancer Cell 2017, 31, 591–606.e6. [Google Scholar] [CrossRef]
- Yang, Y.; Fan, X.; Mao, M.; Song, X.; Wu, P.; Zhang, Y.; Jin, Y.; Yang, Y.; Chen, L.-L.; Wang, Y.; et al. Extensive Translation of Circular RNAs Driven by N6-Methyladenosine. Cell Res. 2017, 27, 626–641. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.-H.; Abdelmohsen, K.; Kim, J.; Yang, X.; Martindale, J.L.; Tominaga-Yamanaka, K.; White, E.J.; Orjalo, A.V.; Rinn, J.L.; Kreft, S.G.; et al. Scaffold Function of Long Non-Coding RNA HOTAIR in Protein Ubiquitination. Nat. Commun. 2013, 4, 2939. [Google Scholar] [CrossRef]
- Wang, P.; Xue, Y.; Han, Y.; Lin, L.; Wu, C.; Xu, S.; Jiang, Z.; Xu, J.; Liu, Q.; Cao, X. The STAT3-Binding Long Noncoding RNA Lnc-DC Controls Human Dendritic Cell Differentiation. Science 2014, 344, 310–313. [Google Scholar] [CrossRef]
- Du, W.W.; Fang, L.; Yang, W.; Wu, N.; Awan, F.M.; Yang, Z.; Yang, B.B. Induction of Tumor Apoptosis through a Circular RNA Enhancing Foxo3 Activity. Cell Death Differ. 2017, 24, 357–370. [Google Scholar] [CrossRef]
- Anderson, D.M.; Anderson, K.M.; Chang, C.-L.; Makarewich, C.A.; Nelson, B.R.; McAnally, J.R.; Kasaragod, P.; Shelton, J.M.; Liou, J.; Bassel-Duby, R.; et al. A Micropeptide Encoded by a Putative Long Noncoding RNA Regulates Muscle Performance. Cell 2015, 160, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Legnini, I.; Di Timoteo, G.; Rossi, F.; Morlando, M.; Briganti, F.; Sthandier, O.; Fatica, A.; Santini, T.; Andronache, A.; Wade, M.; et al. Circ-ZNF609 Is a Circular RNA That Can Be Translated and Functions in Myogenesis. Mol. Cell 2017, 66, 22–37.e9. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-Z.; Chen, M.; Chen, D.; Gao, X.-C.; Zhu, S.; Huang, H.; Hu, M.; Zhu, H.; Yan, G.-R. A Peptide Encoded by a Putative lncRNA HOXB-AS3 Suppresses Colon Cancer Growth. Mol. Cell 2017, 68, 171–184.e6. [Google Scholar] [CrossRef]
- Huang, X.; Li, Z.; Zhang, Q.; Wang, W.; Li, B.; Wang, L.; Xu, Z.; Zeng, A.; Zhang, X.; Zhang, X.; et al. Circular RNA AKT3 Upregulates PIK3R1 to Enhance Cisplatin Resistance in Gastric Cancer via miR-198 Suppression. Mol. Cancer 2019, 18, 71. [Google Scholar] [CrossRef]
- Hao, S.-C.; Ma, H.; Niu, Z.-F.; Sun, S.-Y.; Zou, Y.-R.; Xia, H.-C. hUC-MSCs Secreted Exosomes Inhibit the Glioma Cell Progression through PTENP1/miR-10a-5p/PTEN Pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 10013–10023. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, S.; Mohammadi-Yeganeh, S.; Kiani, J.; Hashemi, S.M.; Koochaki, A.; Sharifi, K.; Ghanbarian, H. Exosomal Delivery of 7SK Long Non-Coding RNA Suppresses Viability, Proliferation, Aggressiveness and Tumorigenicity in Triple Negative Breast Cancer Cells. Life Sci. 2023, 322, 121646. [Google Scholar] [CrossRef]
- Sun, Z.; Hu, J.; Ren, W.; Fang, Y.; Hu, K.; Yu, H.; Liao, D.; Liu, S.; Zhou, L.; He, T.; et al. LncRNA SNHG3 Regulates the BMSC Osteogenic Differentiation in Bone Metastasis of Breast Cancer by Modulating the miR-1273g-3p/BMP3 Axis. Biochem. Biophys. Res. Commun. 2022, 594, 117–123. [Google Scholar] [CrossRef]
- Gu, H.; Yan, C.; Wan, H.; Wu, L.; Liu, J.; Zhu, Z.; Gao, D. Mesenchymal Stem Cell-Derived Exosomes Block Malignant Behaviors of Hepatocellular Carcinoma Stem Cells through a lncRNA C5orf66-AS1/microRNA-127-3p/DUSP1/ERK Axis. Hum. Cell 2021, 34, 1812–1829. [Google Scholar] [CrossRef]
- Xu, G.; Ban, K.; Mu, H.; Wang, B. Human Umbilical Cord Mesenchymal Stem Cells-Derived Exosomal lncRNA FAM99B Represses Hepatocellular Carcinoma Cell Malignancy. Mol. Biotechnol. 2024, 66, 1389–1401. [Google Scholar] [CrossRef]
- Yao, X.; Mao, Y.; Wu, D.; Zhu, Y.; Lu, J.; Huang, Y.; Guo, Y.; Wang, Z.; Zhu, S.; Li, X.; et al. Exosomal Circ_0030167 Derived from BM-MSCs Inhibits the Invasion, Migration, Proliferation and Stemness of Pancreatic Cancer Cells by Sponging miR-338-5p and Targeting the Wif1/Wnt8/β-Catenin Axis. Cancer Lett. 2021, 512, 38–50. [Google Scholar] [CrossRef]
- Wu, R.; Su, Z.; Zhao, L.; Pei, R.; Ding, Y.; Li, D.; Zhu, S.; Xu, L.; Zhao, W.; Zhou, W. Extracellular Vesicle-Loaded Oncogenic lncRNA NEAT1 from Adipose-Derived Mesenchymal Stem Cells Confers Gemcitabine Resistance in Pancreatic Cancer via miR-491-5p/Snail/SOCS3 Axis. Stem Cells Int. 2023, 2023, 6510571. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Jiang, P.; Li, J.; Peng, M.; Zhao, X.; Zhang, X.; Chen, K.; Zhang, Y.; Liu, H.; Gan, L.; et al. Tumor-Derived Exosomal Lnc-Sox2ot Promotes EMT and Stemness by Acting as a ceRNA in Pancreatic Ductal Adenocarcinoma. Oncogene 2018, 37, 3822–3838. [Google Scholar] [CrossRef]
- Li, B.; Xu, H.; Han, H.; Song, S.; Zhang, X.; Ouyang, L.; Qian, C.; Hong, Y.; Qiu, Y.; Zhou, W.; et al. Exosome-Mediated Transfer of lncRUNX2-AS1 from Multiple Myeloma Cells to MSCs Contributes to Osteogenesis. Oncogene 2018, 37, 5508–5519. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Yuan, H.; Liu, S.; Hu, Z.; Xiao, H. Exosome-Transmitted LINC00461 Promotes Multiple Myeloma Cell Proliferation and Suppresses Apoptosis by Modulating microRNA/BCL-2 Expression. Cytotherapy 2019, 21, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Qin, P.; Zhang, D.; Cui, X.; Gao, J.; Yu, Z.; Chai, Y.; Wang, J.; Li, J. Long Non-Coding RNA PVT1 Encapsulated in Bone Marrow Mesenchymal Stem Cell-Derived Exosomes Promotes Osteosarcoma Growth and Metastasis by Stabilizing ERG and Sponging miR-183-5p. Aging 2019, 11, 9581–9596. [Google Scholar] [CrossRef]
- Zhu, G.; Xia, Y.; Zhao, Z.; Li, A.; Li, H.; Xiao, T. LncRNA XIST from the Bone Marrow Mesenchymal Stem Cell Derived Exosome Promotes Osteosarcoma Growth and Metastasis through miR-655/ACLY Signal. Cancer Cell Int. 2022, 22, 330. [Google Scholar] [CrossRef]
- Li, F.; Chen, X.; Shang, C.; Ying, Q.; Zhou, X.; Zhu, R.; Lu, H.; Hao, X.; Dong, Q.; Jiang, Z. Bone Marrow Mesenchymal Stem Cells-Derived Extracellular Vesicles Promote Proliferation, Invasion and Migration of Osteosarcoma Cells via the lncRNA MALAT1/miR-143/NRSN2/Wnt/β-Catenin Axis. Onco Targets Ther. 2021, 14, 737–749. [Google Scholar] [CrossRef]
- Feng, D.; Li, Z.; Yang, L.; Liang, H.; He, H.; Liu, L.; Zhang, W. BMSC-EV-Derived lncRNA NORAD Facilitates Migration, Invasion, and Angiogenesis in Osteosarcoma Cells by Regulating CREBBP via Delivery of miR-877-3p. Oxid. Med. Cell Longev. 2022, 2022, 8825784. [Google Scholar] [CrossRef]
- He, H.; Ding, M.; Li, T.; Zhao, W.; Zhang, L.; Yin, P.; Zhang, W. Bone Mesenchymal Stem Cell-Derived Extracellular Vesicles Containing NORAD Promote Osteosarcoma by miR-30c-5p. Lab. Investig. 2022, 102, 826–837. [Google Scholar] [CrossRef]
- Shi, Z.; Wang, K.; Xing, Y.; Yang, X. CircNRIP1 Encapsulated by Bone Marrow Mesenchymal Stem Cell-Derived Extracellular Vesicles Aggravates Osteosarcoma by Modulating the miR-532-3p/AKT3/PI3K/AKT Axis. Front. Oncol. 2021, 11, 658139. [Google Scholar] [CrossRef]
- Huang, L.; Xiong, J.; Fu, J.; Zhou, Z.; Yu, H.; Xu, J.; Wu, L.; Cao, K. Bone Marrow Mesenchymal Stem Cell-Derived Exosomal LINC00847 Inhibits the Proliferation, Migration, and Invasion of Ewing Sarcoma. J. Clin. Transl. Res. 2022, 8, 563–576. [Google Scholar]
- Zhang, C.; Cao, J.; Lv, W.; Mou, H. CircRNA_100395 Carried by Exosomes From Adipose-Derived Mesenchymal Stem Cells Inhibits the Malignant Transformation of Non-Small Cell Lung Carcinoma Through the miR-141-3p-LATS2 Axis. Front. Cell Dev. Biol. 2021, 9, 663147. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Bo, X.; Yi, X.; Xiao, X.; Zheng, Q.; Ma, L.; Li, B. Exosome-Transferred LINC01559 Promotes the Progression of Gastric Cancer via PI3K/AKT Signaling Pathway. Cell Death Dis. 2020, 11, 723. [Google Scholar] [CrossRef] [PubMed]
- Ba, L.; Xue, C.; Li, X.; Zhang, M.; Yang, Y.; Han, Q.; Sun, Z.; Zhao, R.C. Gastric Cancer Cell-Derived Exosomes Can Regulate the Biological Functions of Mesenchymal Stem Cells by Inducing the Expression of Circular RNA Circ_0004303. Stem Cells Dev. 2021, 30, 830–842. [Google Scholar] [CrossRef]
- Liang, Z.F.; Zhang, Y.; Guo, W.; Chen, B.; Fang, S.; Qian, H. Gastric Cancer Stem Cell-Derived Exosomes Promoted Tobacco Smoke-Triggered Development of Gastric Cancer by Inducing the Expression of Circ670. Med. Oncol. 2022, 40, 24. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ma, S.; Ye, Z.; Zheng, Y.; Zheng, Z.; Liu, X.; Zhou, X. NEAT1 in Bone Marrow Mesenchymal Stem Cell-Derived Extracellular Vesicles Promotes Melanoma by Inducing M2 Macrophage Polarization. Cancer Gene Ther. 2022, 29, 1228–1239. [Google Scholar] [CrossRef]
- Mo, C.; Huang, B.; Zhuang, J.; Jiang, S.; Guo, S.; Mao, X. LncRNA Nuclear-Enriched Abundant Transcript 1 Shuttled by Prostate Cancer Cells-Secreted Exosomes Initiates Osteoblastic Phenotypes in the Bone Metastatic Microenvironment via miR-205-5p/Runt-Related Transcription Factor 2/Splicing Factor Proline- and Glutamine-Rich/Polypyrimidine Tract-Binding Protein 2 Axis. Clin. Transl. Med. 2021, 11, e493. [Google Scholar] [CrossRef]
- Liu, S.-C.; Cao, Y.-H.; Chen, L.-B.; Kang, R.; Huang, Z.-X.; Lu, X.-S. BMSC-Derived Exosomal lncRNA PTENP1 Suppresses the Malignant Phenotypes of Bladder Cancer by Upregulating SCARA5 Expression. Cancer Biol. Ther. 2022, 23, 1–13. [Google Scholar] [CrossRef]
- Yuan, Z.; Xiong, B.; Liu, L.; Lu, Y.; Liu, Y.; Wang, G.; Qian, Y.; Diao, B.; Tu, M. Exosomal Circ_0037104 Derived from Hu-MSCs Inhibits Cholangiocarcinoma Progression by Sponging miR-620 and Targeting AFAP1. J. Biochem. Mol. Toxicol. 2024, 38, e23656. [Google Scholar] [CrossRef]
- Song, Y.; Hu, L.; Cheng, J.; Li, Z.; Zheng, J. LncRNA SNHG5 Induces CAFs-like Phenotype and Autophagy of AML-MSCs via PTBP1/ATG5 Axis to Confer Chemoresistance of AML Cells. Cell Signal 2025, 128, 111625. [Google Scholar] [CrossRef]
- Huertas-Castaño, C.; Gómez-Muñoz, M.A.; Pardal, R.; Vega, F.M. Hypoxia in the Initiation and Progression of Neuroblastoma Tumours. Int. J. Mol. Sci. 2019, 21, 39. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.-H.; Xiao, L.-M.; Liu, Y.; Chen, L.-K.; Zheng, S.-Y.; Zeng, E.-M.; Li, D.-H. The lncRNA HOXA11-AS Promotes Glioma Cell Growth and Metastasis by Targeting miR-130a-5p/HMGB2. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 241–252. [Google Scholar] [CrossRef]
- Westphal, M.; Lamszus, K. The Neurobiology of Gliomas: From Cell Biology to the Development of Therapeutic Approaches. Nat. Rev. Neurosci. 2011, 12, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Grassmann, F.; He, W.; Eriksson, M.; Gabrielson, M.; Hall, P.; Czene, K. Interval Breast Cancer Is Associated with Other Types of Tumors. Nat. Commun. 2019, 10, 4648. [Google Scholar] [CrossRef]
- Castaldo, R.; Pane, K.; Nicolai, E.; Salvatore, M.; Franzese, M. The Impact of Normalization Approaches to Automatically Detect Radiogenomic Phenotypes Characterizing Breast Cancer Receptors Status. Cancers 2020, 12, 518. [Google Scholar] [CrossRef] [PubMed]
- Käkönen, S.-M.; Mundy, G.R. Mechanisms of Osteolytic Bone Metastases in Breast Carcinoma. Cancer 2003, 97, 834–839. [Google Scholar] [CrossRef]
- Tahara, R.K.; Brewer, T.M.; Theriault, R.L.; Ueno, N.T. Bone Metastasis of Breast Cancer. Adv. Exp. Med. Biol. 2019, 1152, 105–129. [Google Scholar] [CrossRef]
- Shimada, S.; Mogushi, K.; Akiyama, Y.; Furuyama, T.; Watanabe, S.; Ogura, T.; Ogawa, K.; Ono, H.; Mitsunori, Y.; Ban, D.; et al. Comprehensive Molecular and Immunological Characterization of Hepatocellular Carcinoma. EBioMedicine 2019, 40, 457–470. [Google Scholar] [CrossRef]
- Karadag Soylu, N. Update on Hepatocellular Carcinoma: A Brief Review from Pathologist Standpoint. J. Gastrointest. Cancer 2020, 51, 1176–1186. [Google Scholar] [CrossRef]
- Alzahrani, F.A.; El-Magd, M.A.; Abdelfattah-Hassan, A.; Saleh, A.A.; Saadeldin, I.M.; El-Shetry, E.S.; Badawy, A.A.; Alkarim, S. Potential Effect of Exosomes Derived from Cancer Stem Cells and MSCs on Progression of DEN-Induced HCC in Rats. Stem Cells Int. 2018, 2018, 8058979. [Google Scholar] [CrossRef]
- Venkat, S.; Alahmari, A.A.; Feigin, M.E. Drivers of Gene Expression Dysregulation in Pancreatic Cancer. Trends Cancer 2021, 7, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Bachet, J.-B.; Ayav, A.; Huguet, F.; Lambert, A.; Caramella, C.; Maréchal, R.; Van Laethem, J.-L.; Ducreux, M. Current Standards and New Innovative Approaches for Treatment of Pancreatic Cancer. Eur. J. Cancer 2016, 57, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Lennon, A.M.; Wolfgang, C.L.; Canto, M.I.; Klein, A.P.; Herman, J.M.; Goggins, M.; Fishman, E.K.; Kamel, I.; Weiss, M.J.; Diaz, L.A.; et al. The Early Detection of Pancreatic Cancer: What Will It Take to Diagnose and Treat Curable Pancreatic Neoplasia? Cancer Res. 2014, 74, 3381–3389. [Google Scholar] [CrossRef]
- Ettari, R.; Zappalà, M.; Grasso, S.; Musolino, C.; Innao, V.; Allegra, A. Immunoproteasome-Selective and Non-Selective Inhibitors: A Promising Approach for the Treatment of Multiple Myeloma. Pharmacol. Ther. 2018, 182, 176–192. [Google Scholar] [CrossRef]
- Allegra, A.; Sant’antonio, E.; Penna, G.; Alonci, A.; D’Angelo, A.; Russo, S.; Cannavò, A.; Gerace, D.; Musolino, C. Novel Therapeutic Strategies in Multiple Myeloma: Role of the Heat Shock Protein Inhibitors. Eur. J. Haematol. 2011, 86, 93–110. [Google Scholar] [CrossRef]
- Allegra, A.; Innao, V.; Allegra, A.G.; Pulvirenti, N.; Pugliese, M.; Musolino, C. Antitumorigenic Action of Nelfinavir: Effects on Multiple Myeloma and Hematologic Malignancies (Review). Oncol. Rep. 2020, 43, 1729–1736. [Google Scholar] [CrossRef]
- Allegra, A.; Penna, G.; Alonci, A.; Russo, S.; Greve, B.; Innao, V.; Minardi, V.; Musolino, C. Monoclonal Antibodies: Potential New Therapeutic Treatment against Multiple Myeloma. Eur. J. Haematol. 2013, 90, 441–468. [Google Scholar] [CrossRef]
- Caserta, S.; Innao, V.; Musolino, C.; Allegra, A. Immune Checkpoint Inhibitors in Multiple Myeloma: A Review of the Literature. Pathol. Res. Pract. 2020, 216, 153114. [Google Scholar] [CrossRef]
- Jawad, M.U.; Cheung, M.C.; Clarke, J.; Koniaris, L.G.; Scully, S.P. Osteosarcoma: Improvement in Survival Limited to High-Grade Patients Only. J. Cancer Res. Clin. Oncol. 2011, 137, 597–607. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Jewell, B.E.; Gingold, J.; Lu, L.; Zhao, R.; Wang, L.L.; Lee, D.-F. Osteosarcoma: Molecular Pathogenesis and iPSC Modeling. Trends Mol. Med. 2017, 23, 737–755. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Wu, L.; Huang, L.; Wu, C.; Liu, Z.; Deng, W.; Ma, S.; Zhou, Z.; Yu, H.; Cao, K. LncRNA FOXP4-AS1 Promotes Progression of Ewing Sarcoma and Is Associated With Immune Infiltrates. Front. Oncol. 2021, 11, 718876. [Google Scholar] [CrossRef]
- Bunn, P.A.; Shepherd, F.A.; Sandler, A.; Le Chevalier, T.; Belani, C.P.; Kosmidis, P.A.; Scagliotti, G.V.; Giaccone, G. Ongoing and Future Trials of Biologic Therapies in Lung Cancer. Lung Cancer 2003, 41 (Suppl. 1), S175–S186. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, X.; Zhu, R.; Han, Q.; Zhao, R.C. Lung Cancer Exosomes Initiate Global Long Non-Coding RNA Changes in Mesenchymal Stem Cells. Int. J. Oncol. 2016, 48, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Lin, W.M.; Fisher, D.E. Signaling and Immune Regulation in Melanoma Development and Responses to Therapy. Annu. Rev. Pathol. 2017, 12, 75–102. [Google Scholar] [CrossRef]
- Liu, Q.; Das, M.; Liu, Y.; Huang, L. Targeted Drug Delivery to Melanoma. Adv. Drug Deliv. Rev. 2018, 127, 208–221. [Google Scholar] [CrossRef]
- Guan, H.; Peng, R.; Fang, F.; Mao, L.; Chen, Z.; Yang, S.; Dai, C.; Wu, H.; Wang, C.; Feng, N.; et al. Tumor-Associated Macrophages Promote Prostate Cancer Progression via Exosome-Mediated miR-95 Transfer. J. Cell Physiol. 2020, 235, 9729–9742. [Google Scholar] [CrossRef]
- Melton, L.J.; Lieber, M.M.; Atkinson, E.J.; Achenbach, S.J.; Zincke, H.; Therneau, T.M.; Khosla, S. Fracture Risk in Men with Prostate Cancer: A Population-Based Study. J. Bone Miner. Res. 2011, 26, 1808–1815. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Abouelkheir, R.T.; Abdelhamid, A.; Abou El-Ghar, M.; El-Diasty, T. Imaging of Bladder Cancer: Standard Applications and Future Trends. Medicina 2021, 57, 220. [Google Scholar] [CrossRef] [PubMed]
- Tsimberidou, A.M.; Fountzilas, E.; Bleris, L.; Kurzrock, R. Transcriptomics and Solid Tumors: The next Frontier in Precision Cancer Medicine. Semin. Cancer Biol. 2022, 84, 50–59. [Google Scholar] [CrossRef]
- Abd El Gwad, A.; Matboli, M.; El-Tawdi, A.; Habib, E.K.; Shehata, H.; Ibrahim, D.; Tash, F. Role of Exosomal Competing Endogenous RNA in Patients with Hepatocellular Carcinoma. J. Cell Biochem. 2018, 119, 8600–8610. [Google Scholar] [CrossRef]
- Yeo, R.W.Y.; Lai, R.C.; Zhang, B.; Tan, S.S.; Yin, Y.; Teh, B.J.; Lim, S.K. Mesenchymal Stem Cell: An Efficient Mass Producer of Exosomes for Drug Delivery. Adv. Drug Deliv. Rev. 2013, 65, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Yuan, Z.; Weng, J.; Pei, D.; Du, X.; He, C.; Lai, P. Challenges and Advances in Clinical Applications of Mesenchymal Stromal Cells. J. Hematol. Oncol. 2021, 14, 24. [Google Scholar] [CrossRef]
- Grange, C.; Tapparo, M.; Collino, F.; Vitillo, L.; Damasco, C.; Deregibus, M.C.; Tetta, C.; Bussolati, B.; Camussi, G. Microvesicles Released from Human Renal Cancer Stem Cells Stimulate Angiogenesis and Formation of Lung Premetastatic Niche. Cancer Res. 2011, 71, 5346–5356. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, J.; Miekus, K.; Kucia, M.; Zhang, J.; Reca, R.; Dvorak, P.; Ratajczak, M.Z. Embryonic Stem Cell-Derived Microvesicles Reprogram Hematopoietic Progenitors: Evidence for Horizontal Transfer of mRNA and Protein Delivery. Leukemia 2006, 20, 847–856. [Google Scholar] [CrossRef]
- Yan, L.; Wu, X. Exosomes Produced from 3D Cultures of Umbilical Cord Mesenchymal Stem Cells in a Hollow-Fiber Bioreactor Show Improved Osteochondral Regeneration Activity. Cell Biol. Toxicol. 2020, 36, 165–178. [Google Scholar] [CrossRef]
- Zhang, S.; Chu, W.C.; Lai, R.C.; Lim, S.K.; Hui, J.H.P.; Toh, W.S. Exosomes Derived from Human Embryonic Mesenchymal Stem Cells Promote Osteochondral Regeneration. Osteoarthr. Cartil. 2016, 24, 2135–2140. [Google Scholar] [CrossRef]
- Lo Sicco, C.; Reverberi, D.; Balbi, C.; Ulivi, V.; Principi, E.; Pascucci, L.; Becherini, P.; Bosco, M.C.; Varesio, L.; Franzin, C.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles as Mediators of Anti-Inflammatory Effects: Endorsement of Macrophage Polarization. Stem Cells Transl. Med. 2017, 6, 1018–1028. [Google Scholar] [CrossRef]
- Tian, Y.; Li, S.; Song, J.; Ji, T.; Zhu, M.; Anderson, G.J.; Wei, J.; Nie, G. A Doxorubicin Delivery Platform Using Engineered Natural Membrane Vesicle Exosomes for Targeted Tumor Therapy. Biomaterials 2014, 35, 2383–2390. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Badawi, M.; Pomeroy, S.; Sutaria, D.S.; Xie, Z.; Baek, A.; Jiang, J.; Elgamal, O.A.; Mo, X.; Perle, K.L.; et al. Comprehensive Toxicity and Immunogenicity Studies Reveal Minimal Effects in Mice Following Sustained Dosing of Extracellular Vesicles Derived from HEK293T Cells. J. Extracell. Vesicles 2017, 6, 1324730. [Google Scholar] [CrossRef]
- Ankrum, J.A.; Ong, J.F.; Karp, J.M. Mesenchymal Stem Cells: Immune Evasive, Not Immune Privileged. Nat. Biotechnol. 2014, 32, 252–260. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer Nanomedicine: Progress, Challenges and Opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef]
- Jain, R.K.; Stylianopoulos, T. Delivering Nanomedicine to Solid Tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Dabrowska, S.; Andrzejewska, A.; Lukomska, B.; Janowski, M. Neuroinflammation as a Target for Treatment of Stroke Using Mesenchymal Stem Cells and Extracellular Vesicles. J. Neuroinflamm. 2019, 16, 178. [Google Scholar] [CrossRef]
- Wolfram, J.; Yang, Y.; Shen, J.; Moten, A.; Chen, C.; Shen, H.; Ferrari, M.; Zhao, Y. The Nano-Plasma Interface: Implications of the Protein Corona. Colloids Surf. B Biointerfaces 2014, 124, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Chen, J.; Wang, S.; Fu, F.; Zhu, X.; Wu, C.; Liu, Z.; Zhong, G.; Lin, J. A Nanodrug Consisting Of Doxorubicin And Exosome Derived From Mesenchymal Stem Cells For Osteosarcoma Treatment In Vitro. Int. J. Nanomed. 2019, 14, 8603–8610. [Google Scholar] [CrossRef]
- Joo, H.S.; Suh, J.H.; Lee, H.J.; Bang, E.S.; Lee, J.M. Current Knowledge and Future Perspectives on Mesenchymal Stem Cell-Derived Exosomes as a New Therapeutic Agent. Int. J. Mol. Sci. 2020, 21, 727. [Google Scholar] [CrossRef]
- Wei, W.; Ao, Q.; Wang, X.; Cao, Y.; Liu, Y.; Zheng, S.G.; Tian, X. Mesenchymal Stem Cell-Derived Exosomes: A Promising Biological Tool in Nanomedicine. Front. Pharmacol. 2020, 11, 590470. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Zhang, X.; Li, X. Exosomes Derived from Mesenchymal Stem Cells. Int. J. Mol. Sci. 2014, 15, 4142–4157. [Google Scholar] [CrossRef] [PubMed]
- Motawi, T.M.K.; Sabry, D.; Maurice, N.W.; Rizk, S.M. Role of Mesenchymal Stem Cells Exosomes Derived microRNAs; miR-136, miR-494 and miR-495 in Pre-Eclampsia Diagnosis and Evaluation. Arch. Biochem. Biophys. 2018, 659, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, V.; Sengupta, S.; Lazo, A.; Woods, P.; Nolan, A.; Bremer, N. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19. Stem Cells Dev. 2020, 29, 747–754. [Google Scholar] [CrossRef]
- Trivedi, M.; Talekar, M.; Shah, P.; Ouyang, Q.; Amiji, M. Modification of Tumor Cell Exosome Content by Transfection with Wt-P53 and microRNA-125b Expressing Plasmid DNA and Its Effect on Macrophage Polarization. Oncogenesis 2016, 5, e250. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, Q.; Liu, J.; Dong, M. Leukemia Stem Cell-Released Microvesicles Promote the Survival and Migration of Myeloid Leukemia Cells and These Effects Can Be Inhibited by MicroRNA34a Overexpression. Stem Cells Int. 2016, 2016, 9313425. [Google Scholar] [CrossRef]
- Lou, G.; Chen, L.; Xia, C.; Wang, W.; Qi, J.; Li, A.; Zhao, L.; Chen, Z.; Zheng, M.; Liu, Y. MiR-199a-Modified Exosomes from Adipose Tissue-Derived Mesenchymal Stem Cells Improve Hepatocellular Carcinoma Chemosensitivity through mTOR Pathway. J. Exp. Clin. Cancer Res. 2020, 39, 4. [Google Scholar] [CrossRef]
- Lou, G.; Song, X.; Yang, F.; Wu, S.; Wang, J.; Chen, Z.; Liu, Y. Exosomes Derived from miR-122-Modified Adipose Tissue-Derived MSCs Increase Chemosensitivity of Hepatocellular Carcinoma. J. Hematol. Oncol. 2015, 8, 122. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, X.; Wang, Y.; Ren, F.; Sun, D.; Yan, Y.; Kong, X.; Bu, J.; Liu, M.; Xu, S. circRNA-002178 Act as a ceRNA to Promote PDL1/PD1 Expression in Lung Adenocarcinoma. Cell Death Dis. 2020, 11, 32. [Google Scholar] [CrossRef]
- Yin, Y.; Cai, X.; Chen, X.; Liang, H.; Zhang, Y.; Li, J.; Wang, Z.; Chen, X.; Zhang, W.; Yokoyama, S.; et al. Tumor-Secreted miR-214 Induces Regulatory T Cells: A Major Link between Immune Evasion and Tumor Growth. Cell Res. 2014, 24, 1164–1180. [Google Scholar] [CrossRef]
- Sun, J.; Jia, H.; Bao, X.; Wu, Y.; Zhu, T.; Li, R.; Zhao, H. Tumor Exosome Promotes Th17 Cell Differentiation by Transmitting the lncRNA CRNDE-h in Colorectal Cancer. Cell Death Dis. 2021, 12, 123. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Busch, D.J.; Vershel, C.P.; Stachowiak, J.C. Multifunctional Transmembrane Protein Ligands for Cell-Specific Targeting of Plasma Membrane-Derived Vesicles. Small 2016, 12, 3837–3848. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Chen, X.; Zhang, S.; Fang, J.; Chen, M.; Xu, Y.; Chen, X. Mesenchymal Stem Cells as a Double-Edged Sword in Tumor Growth: Focusing on MSC-Derived Cytokines. Cell Mol. Biol. Lett. 2021, 26, 3. [Google Scholar] [CrossRef]
- Huang, X.; Wu, W.; Jing, D.; Yang, L.; Guo, H.; Wang, L.; Zhang, W.; Pu, F.; Shao, Z. Engineered Exosome as Targeted lncRNA MEG3 Delivery Vehicles for Osteosarcoma Therapy. J. Control Release 2022, 343, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, D.; Huang, W.; Yang, N.; Yuan, Q.; Yang, Y. Aptamer-Functionalized Field-Effect Transistor Biosensors for Disease Diagnosis and Environmental Monitoring. Exploration 2023, 3, 20210027. [Google Scholar] [CrossRef]
- Tan, F.; Li, X.; Wang, Z.; Li, J.; Shahzad, K.; Zheng, J. Clinical Applications of Stem Cell-Derived Exosomes. Signal Transduct. Target. Ther. 2024, 9, 17. [Google Scholar] [CrossRef]
- Sadeghi, S.; Tehrani, F.R.; Tahmasebi, S.; Shafiee, A.; Hashemi, S.M. Exosome Engineering in Cell Therapy and Drug Delivery. Inflammopharmacology 2023, 31, 145–169. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.L.; Yao, J.L.; Wang, K.; Ai, H. Human Umbilical Cord Mesenchymal Stem Cell-Derived Extracellular Vesicles Inhibit Endometrial Cancer Cell Proliferation and Migration through Delivery of Exogenous miR-302a. Stem Cells Int. 2019, 2019, 8108576. [Google Scholar] [CrossRef]
- Bagheri, E.; Abnous, K.; Farzad, S.A.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Targeted Doxorubicin-Loaded Mesenchymal Stem Cells-Derived Exosomes as a Versatile Platform for Fighting against Colorectal Cancer. Life Sci. 2020, 261, 118369. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Pachler, K.; Lener, T.; Streif, D.; Dunai, Z.A.; Desgeorges, A.; Feichtner, M.; Öller, M.; Schallmoser, K.; Rohde, E.; Gimona, M. A Good Manufacturing Practice-Grade Standard Protocol for Exclusively Human Mesenchymal Stromal Cell-Derived Extracellular Vesicles. Cytotherapy 2017, 19, 458–472. [Google Scholar] [CrossRef] [PubMed]
- Varderidou-Minasian, S.; Lorenowicz, M.J. Mesenchymal Stromal/Stem Cell-Derived Extracellular Vesicles in Tissue Repair: Challenges and Opportunities. Theranostics 2020, 10, 5979–5997. [Google Scholar] [CrossRef] [PubMed]
- de Abreu, R.C.; Fernandes, H.; da Costa Martins, P.A.; Sahoo, S.; Emanueli, C.; Ferreira, L. Native and Bioengineered Extracellular Vesicles for Cardiovascular Therapeutics. Nat. Rev. Cardiol. 2020, 17, 685–697. [Google Scholar] [CrossRef] [PubMed]


| ncRNA | Source Cells | Target Cells | EVs Types | Expression | Function | Mechanism | Refs |
|---|---|---|---|---|---|---|---|
| circ_6790 | MSCs | PDAC | Exosomes | Up | Cancer inhibition | Chromatin regulation | [77] |
| LINC00622 | MSCs | Neuroblastoma | EVs | Up | Cancer inhibition | Transcriptional regulation | [78] |
| lncRNA PTENP1 | MSCs | Gliomas | Exosomes | Down | Cancer inhibition | Sponging miRNA | [95] |
| LncRNA RN7SK | MSCs | TNBC | Exosomes | Up | Cancer inhibition | Transcriptional regulation | [96] |
| lncRNA-SNHG3 | MSCs | Breast cancer | Exosomes | Up | Cancer promotion | Sponging miRNA | [97] |
| lncRNA C5orf66 | MSCs | HCC | Exosomes | Up | Cancer inhibition | Sponging miRNA | [98] |
| lncRNA FAM99B | MSCs | HCC | Exosomes | Down | Cancer inhibition | — | [99] |
| circ_0030167 | MSCs | PC | Exosomes | Up | Cancer inhibition | Sponging miRNA | [100] |
| lncRNA NEAT1 | MSCs | PC | EVs | Up | Cancer promotion | Sponging miRNA | [101] |
| lncRNA-Sox2ot | Cancer cells | PDAC | Exosomes | Up | Cancer promotion | Sponging miRNA | [102] |
| lncRUNX2-AS1 | Cancer cells | MSCs | Exosomes | Up | Cancer inhibition | Transcriptional regulation | [103] |
| LINC00461 | MSCs | MM | Exosomes | Up | Cancer promotion | Sponging miRNA | [104] |
| lncRNA PVT1 | MSCs | Osteosarcoma | Exosomes | Up | Cancer promotion | Sponging miRNA | [105] |
| lncRNA XIST | MSCs | Osteosarcoma | Exosomes | Up | Cancer promotion | Sponging miRNA | [106] |
| lncRNA MALAT1 | MSCs | Osteosarcoma | EVs | Up | Cancer promotion | Sponging miRNA | [107] |
| lncRNA NORAD | MSCs | Osteosarcoma | EVs | Up | Cancer promotion | Sponging miRNA | [108] |
| lncRNA NORAD | MSCs | Osteosarcoma | Exosomes | Up | Cancer promotion | Sponging miRNA | [109] |
| circNRIP1 | MSCs | Osteosarcoma | EVs | Up | Cancer promotion | Sponging miRNA | [110] |
| LINC00847 | MSCs | Ewing’s sarcoma | EVs | Down | Cancer inhibition | Sponging miRNA | [111] |
| CircRNA_100395 | MSCs | Lung cancer | Exosomes | Down | Cancer inhibition | Sponging miRNA | [112] |
| LINC01559 | MSCs | Gastric cancer | Exosomes | Up | Cancer promotion | Sponging miRNA | [113] |
| Circ_00004303 | Cancer cells | MSCs | Exosomes | Up | Cancer promotion | Sponging miRNA | [114] |
| circ670 | Cancer stem cells | Gastric cancer | Exosomes | Up | Cancer promotion | — | [115] |
| lncRNA NEAT1 | MSCs | Melanoma | EVs | Up | Cancer promotion | Sponging miRNA | [116] |
| LncRNA NEAT1 | Cancer cells | MSCs | EVs | Up | Cancer promotion | Sponging miRNA | [117] |
| lncRNA PTENP1 | MSCs | Bladder cancer | Exosomes | Up | Cancer inhibition | Sponging miRNA | [118] |
| circ_0037104 | MSCs | Cholangiocarcinoma | Exosomes | Down | Cancer inhibition | Sponging miRNA | [119] |
| lncRNA SNHG5 | Cancer cells | Acute myeloid leukemia | Exosomes | Up | Cancer promotion | mRNA stability | [120] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.-W.; Liu, C.-G.; Kirby, J.A.; Chu, C.; Zang, D.; Chen, J. The Emerging Role of Extracellular Vesicle-Derived lncRNAs and circRNAs in Tumor and Mesenchymal Stem Cells: The Biological Functions and Potential for Clinical Application. Cancers 2025, 17, 2186. https://doi.org/10.3390/cancers17132186
Luo Y-W, Liu C-G, Kirby JA, Chu C, Zang D, Chen J. The Emerging Role of Extracellular Vesicle-Derived lncRNAs and circRNAs in Tumor and Mesenchymal Stem Cells: The Biological Functions and Potential for Clinical Application. Cancers. 2025; 17(13):2186. https://doi.org/10.3390/cancers17132186
Chicago/Turabian StyleLuo, Ya-Wen, Chen-Guang Liu, Jane Allyn Kirby, Chen Chu, Dan Zang, and Jun Chen. 2025. "The Emerging Role of Extracellular Vesicle-Derived lncRNAs and circRNAs in Tumor and Mesenchymal Stem Cells: The Biological Functions and Potential for Clinical Application" Cancers 17, no. 13: 2186. https://doi.org/10.3390/cancers17132186
APA StyleLuo, Y.-W., Liu, C.-G., Kirby, J. A., Chu, C., Zang, D., & Chen, J. (2025). The Emerging Role of Extracellular Vesicle-Derived lncRNAs and circRNAs in Tumor and Mesenchymal Stem Cells: The Biological Functions and Potential for Clinical Application. Cancers, 17(13), 2186. https://doi.org/10.3390/cancers17132186






