Caspofungin for Primary Antifungal Prophylaxis in Acute Myeloid Leukemia: A Real-Life Study from an Academic Center
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Definitions
2.3. Diagnostic Work-Up
2.4. Statistical Analysis
3. Results
3.1. Study Population
3.2. PAP Strategy
3.3. Characteristics of Posa-Group Versus Caspo-Group
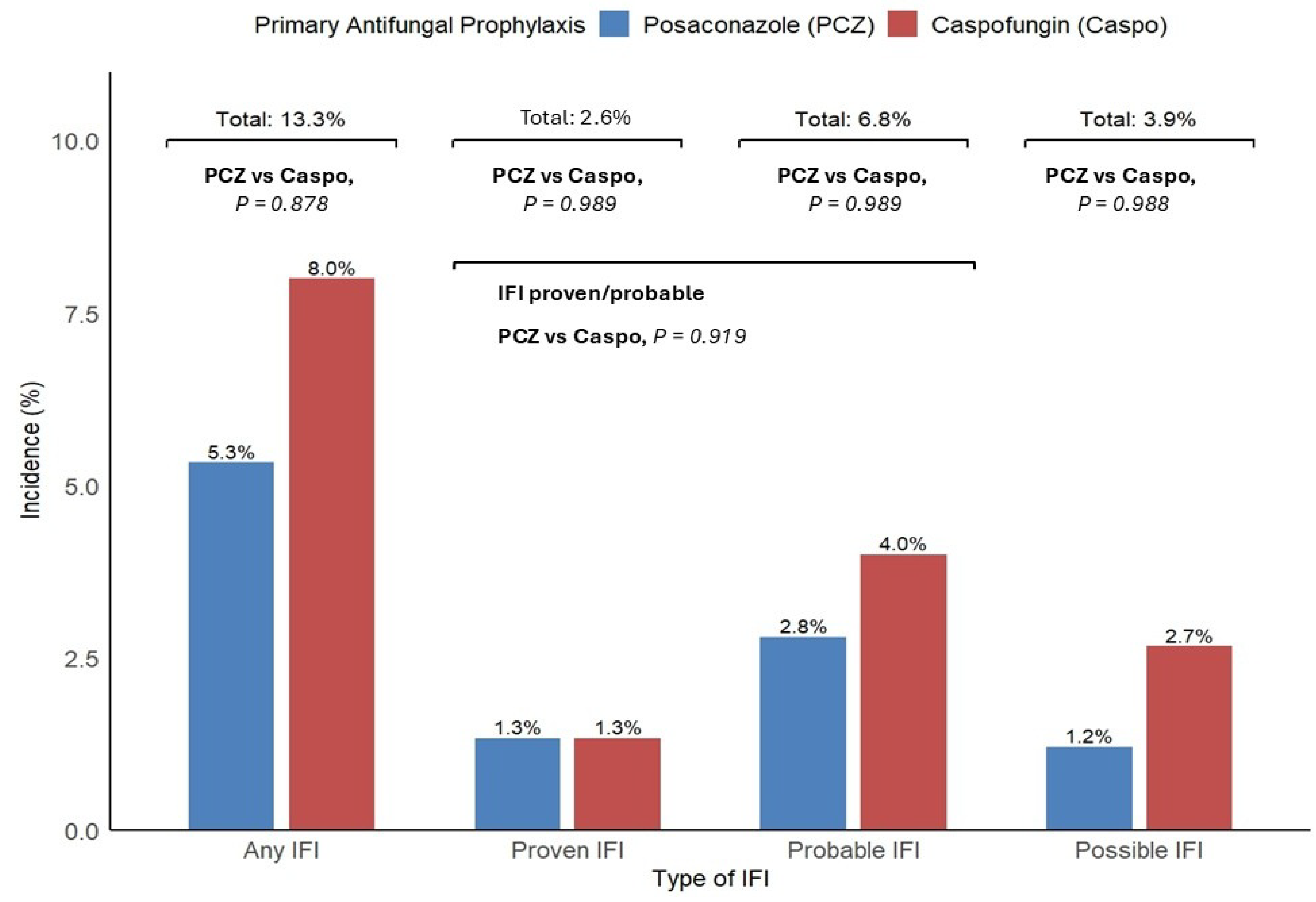
3.4. IFI Incidence, Association with Type of Prophylaxis, Clinical Course, and Safety
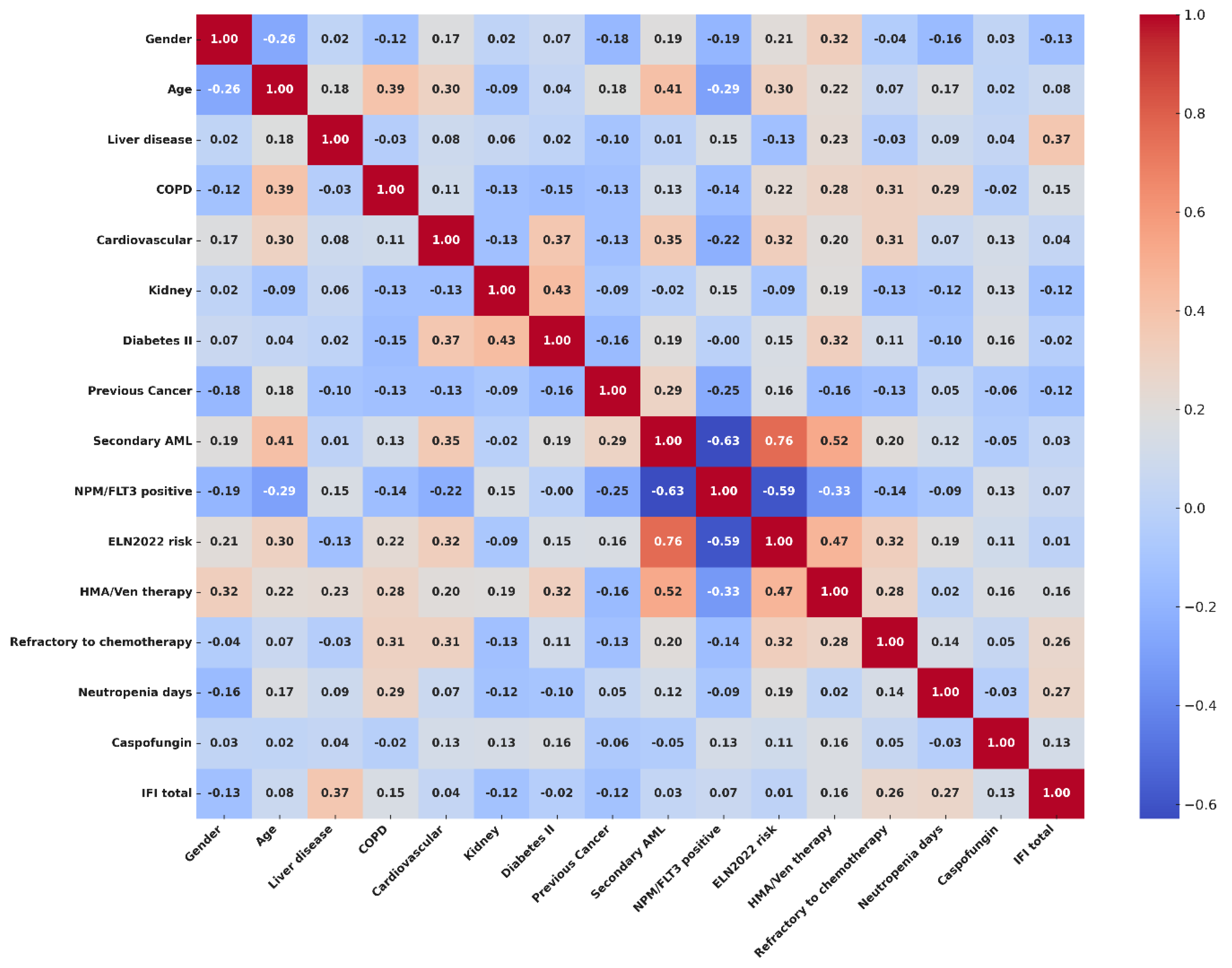
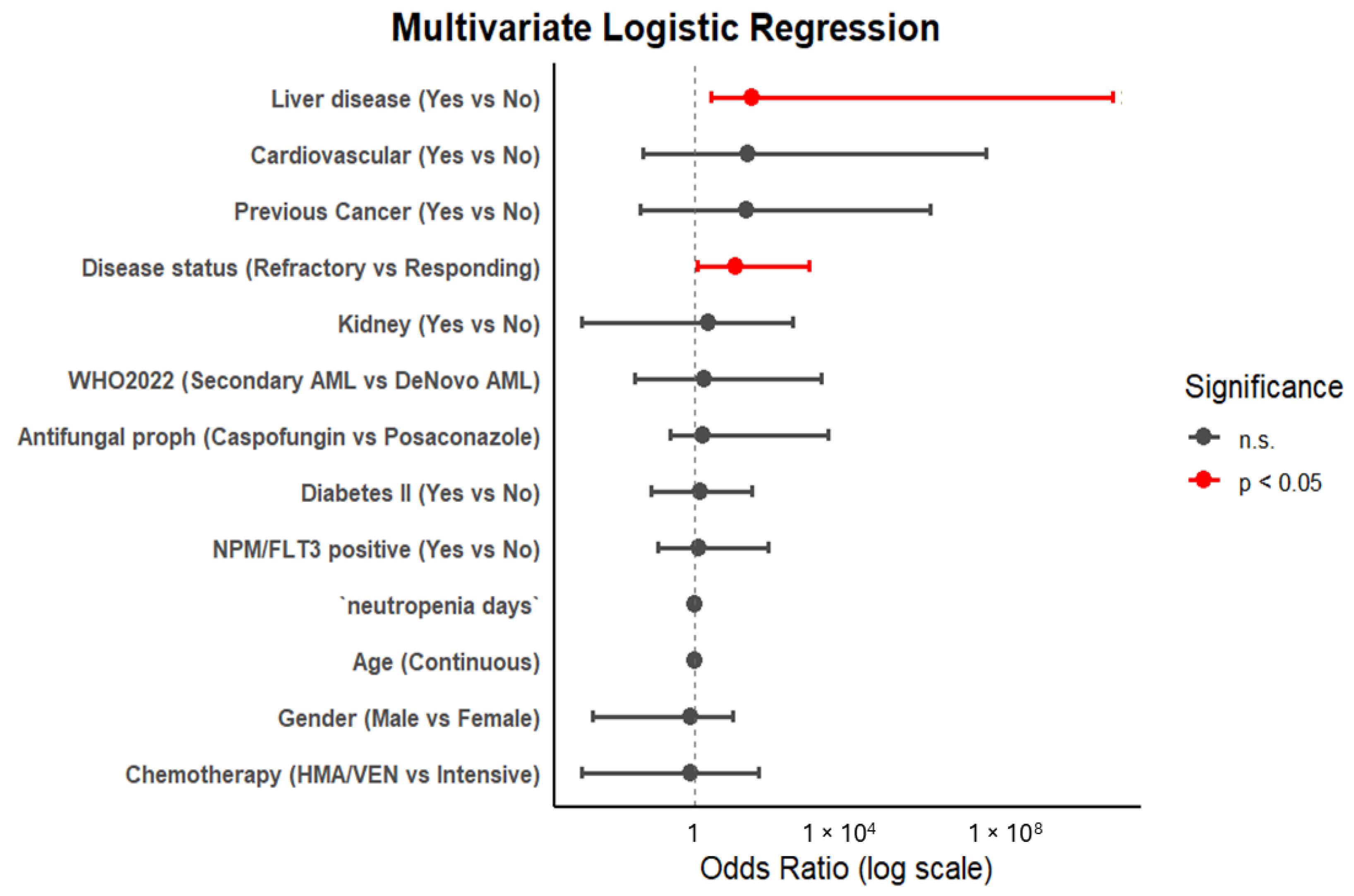
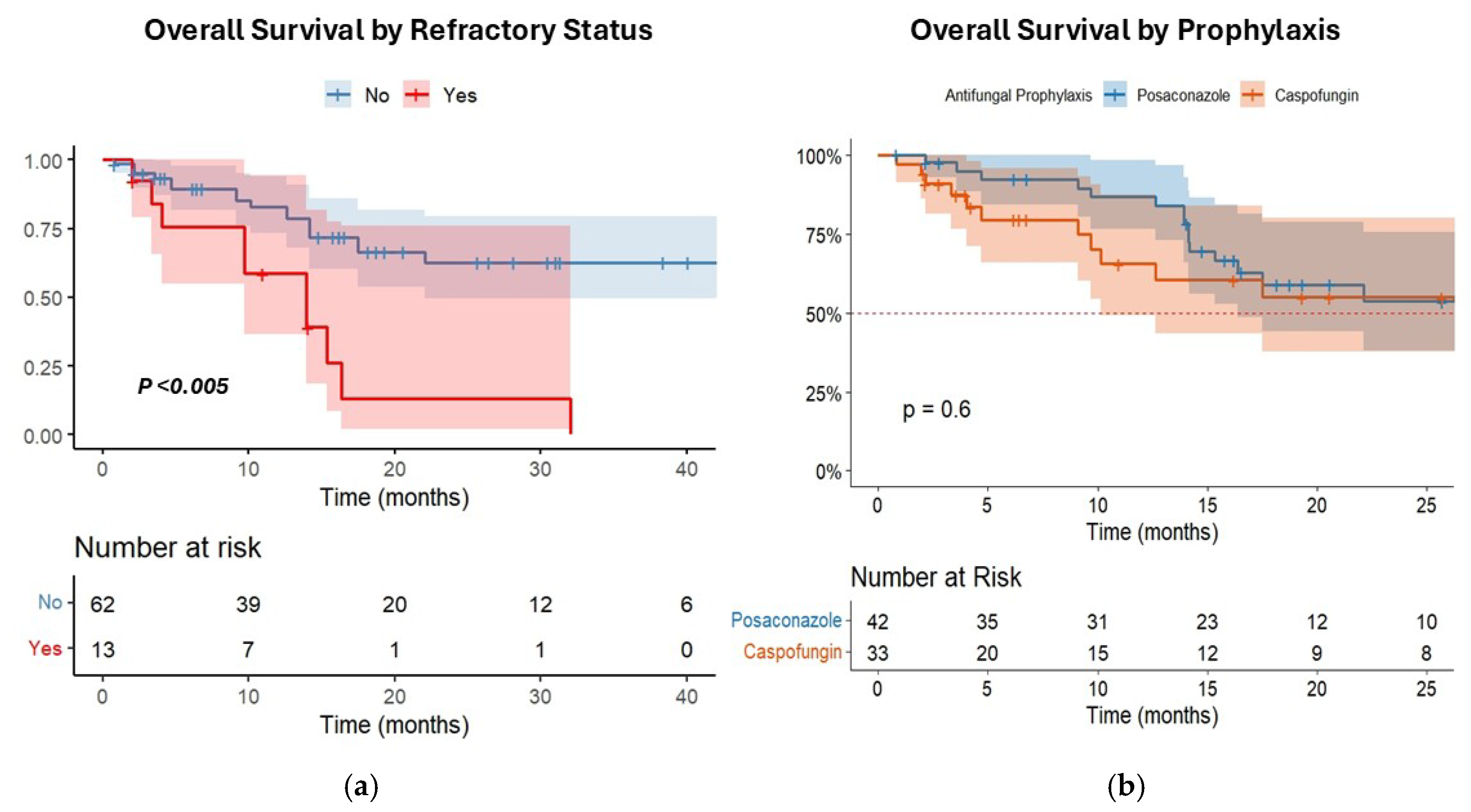
3.5. Risk Factors Associated with IFI Incidence and Survival Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Logan, C.; Koura, D.; Taplitz, R. Updates in infection risk and management in acute leukemia. Hematol. Am. Soc. Hematol. Educ. Program 2020, 2020, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Halpern, A.B.; Lyman, G.H.; Walsh, T.J.; Kontoyiannis, D.P.; Walter, R.B. Primary antifungal prophylaxis during curative-intent therapy for acute myeloid leukemia. Blood 2015, 126, 2790–2797. [Google Scholar] [CrossRef]
- Young, J.A.H.; Andes, D.R.; Ardura, M.I.; Arrieta, A.; Bow, E.J.; Chandrasekar, P.H.; Chen, S.C.A.; Hammond, S.P.; Husain, S.; Koo, S.; et al. Modeling Invasive Aspergillosis Risk for the Application of Prophylaxis Strategies. Open Forum Infect. Dis. 2024, 11, ofae082. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Veiga, R.; Montesinos, P.; Boluda, B.; Lorenzo, I.; Martínez-Cuadrón, D.; Salavert, M.; Pemán, J.; Calvillo, P.; Cano, I.; Acuña, E.; et al. Incidence and outcome of invasive fungal disease after front-line intensive chemotherapy in patients with acute myeloid leukemia: Impact of antifungal prophylaxis. Ann. Hematol. 2019, 98, 2081–2088. [Google Scholar] [CrossRef] [PubMed]
- Del Principe, M.I.; Dragonetti, G.; Verga, L.; Candoni, A.; Marchesi, F.; Cattaneo, C.; Delia, M.; Potenza, L.; Farina, F.; Ballanti, S.; et al. ‘Real-life’ analysis of the role of antifungal prophylaxis in preventing invasive aspergillosis in AML patients undergoing consolidation therapy: Sorveglianza Epidemiologica Infezioni nelle Emopatie (SEIFEM) 2016 study. J. Antimicrob. Chemother. 2019, 74, 1062–1068. [Google Scholar] [CrossRef]
- Dragonetti, G.; Criscuolo, M.; Fianchi, L.; Pagano, L. Invasive aspergillosis in acute myeloid leukemia: Are we making progress in reducing mortality? Med. Mycol. 2017, 55, 82–86. [Google Scholar] [CrossRef]
- Pagano, L.; Caira, M.; Candoni, A.; Offidani, M.; Fianchi, L.; Martino, B.; Pastore, D.; Picardi, M.; Bonini, A.; Chierichini, A.; et al. The epidemiology of fungal infections in patients with hematologic malignancies: The SEIFEM-2004 study. Haematologica 2006, 91, 1068–1075. [Google Scholar] [CrossRef]
- Coussement, J.; Lindsay, J.; Teh, B.W.; Slavin, M. Choice and duration of antifungal prophylaxis and treatment in high-risk haematology patients. Curr. Opin. Infect. Dis. 2021, 34, 297–306. [Google Scholar] [CrossRef]
- Copley, M.S.; Waldron, M.; Athans, V.; Welch, S.C.; Brizendine, K.D.; Cober, E.; Siebenaller, C. Itraconazole vs posaconazole for antifungal prophylaxis in patients with acute myeloid leukemia undergoing intensive chemotherapy: A retrospective study. Int. J. Antimicrob. Agents 2020, 55, 105886. [Google Scholar] [CrossRef]
- Soldi, R.L.; Bernardes Coelho, Y.N.; Paranhos, R.L.; Da Silva, M.J.B. The impact of antifungal prophylaxis in patients diagnosed with acute leukemias undergoing induction chemotherapy: A systematic review and meta-analysis. Clin. Exp. Med. 2023, 23, 3231–3249. [Google Scholar] [CrossRef]
- Tragiannidis, A.; Dokos, C.; Lehrnbecher, T.; Groll, A.H. Antifungal chemoprophylaxis in children and adolescents with haematological malignancies and following allogeneic haematopoietic stem cell transplantation: Review of the literature and options for clinical practice. Drugs 2012, 72, 685–704. [Google Scholar] [CrossRef] [PubMed]
- Cornely, O.A.; Maertens, J.; Winston, D.J.; Perfect, J.; Ullmann, A.J.; Walsh, T.J.; Helfgott, D.; Holowiecki, J.; Stockelberg, D.; Goh, Y.T.; et al. Posaconazole vs fluconazole or itraconazole prophylaxis in patients with neutropenia. N. Engl. J. Med. 2007, 356, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Panagopoulou, P.; Roilides, E. Evaluating posaconazole, its pharmacology, efficacy and safety for the prophylaxis and treatment of fungal infections. Expert. Opin. Pharmacother. 2022, 23, 175–199. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Ferreira, A.R.; Märtson, A.G.; De Boer, A.; Wardill, R.H.; Alffenaar, J.W.; Harmsen, H.J.M.; Tissing, W.J.E. Does chemotherapy-induced gastrointestinal mucositis affect the bioavailability and efficacy of anti-infective drugs? Biomedicines 2021, 9, 1389. [Google Scholar] [CrossRef]
- Stone, R.M.; Mandrekar, S.J.; Sanford, B.L.; Laumann, K.; Geyer, S.; Bloomfield, C.D.; Thiede, C.; Prior, T.W.; Döhner, K.; Marcucci, G.; et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N. Engl. J. Med. 2017, 377, 454–464. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef]
- Stemler, J.; De Jonge, N.; Skoetz, N.; Sinkó, J.; Brüggemann, R.J.; Busca, A.; Ben-Ami, R.; Ráčil, Z.; Piechotta, V.; Lewis, R.; et al. Antifungal prophylaxis in adult patients with acute myeloid leukemia treated with novel targeted therapies: A systematic review and expert consensus recommendation from the European Hematology Association. Lancet Haematol. 2022, 9, e361–e373. [Google Scholar] [CrossRef]
- Agarwal, S.K.; DiNardo, C.D.; Potluri, J.; Dunbar, M.; Kantarjian, H.M.; Humerickhouse, R.A.; Wong, S.L.; Menon, R.M.; Konopleva, M.Y.; Salem, A.H. Management of venetoclax-posaconazole interaction in acute myeloid leukemia patients: Evaluation of dose adjustments. Clin. Ther. 2017, 39, 359–367. [Google Scholar] [CrossRef]
- Stemler, J.; Koehler, P.; Maurer, C.; Müller, C.; Cornely, O.A. Antifungal prophylaxis and novel drugs in acute myeloid leukemia: The midostaurin and posaconazole dilemma. Ann. Hematol. 2020, 99, 1429–1440. [Google Scholar] [CrossRef]
- Zaoutis, T.E.; Jafri, H.S.; Huang, L.M.; Locatelli, F.; Barzilai, A.; Ebell, W.; Steinbach, W.J.; Bradley, J.; Lieberman, J.M.; Hsiao, C.C.; et al. A prospective, multicenter study of caspofungin for the treatment of documented Candida or Aspergillus infections in pediatric patients. Pediatrics 2009, 123, 877–884. [Google Scholar] [CrossRef]
- Ngai, A.L.; Bourque, M.R.; Lupinacci, R.J.; Strohmaier, K.M.; Kartsonis, N.A. Overview of safety experience with caspofungin in clinical trials conducted over the first 15 years: A brief report. Int. J. Antimicrob. Agents. 2011, 38, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Hashemian, S.M.; Farhadi, T.; Velayati, A.A. Caspofungin: A review of its characteristics, activity, and use in intensive care units. Expert. Rev. Anti Infect. Ther. 2020, 18, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, C.; Monte, S.; Algarotti, A.; Audisio, E.; Borlenghi, E.; Campiotti, L.; Cerqui, E.; Fanizza, C.; Giuliani, R.; Micò, C.; et al. A randomized comparison of caspofungin versus antifungal prophylaxis according to investigator policy in acute leukaemia patients undergoing induction chemotherapy (PROFIL-C study). J. Antimicrob. Chemother. 2011, 66, 2140–2145. [Google Scholar] [CrossRef]
- Wang, J.F.; Xue, Y.; Zhu, X.B.; Fan, H. Efficacy and safety of echinocandins versus triazoles for the prophylaxis and treatment of fungal infections: A meta-analysis of randomized controlled trials. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 651–659. [Google Scholar] [CrossRef]
- Walsh, T.J.; Teppler, H.; Donowitz, G.R.; Maertens, J.; Baden, L.R.; Dmoszynska, A.; Cornely, O.A.; Bourque, M.R.; Lupinacci, R.J.; Sable, C.A.; et al. Caspofungin versus liposomal amphotericin B for empirical antifungal therapy in patients with persistent fever and neutropenia. N. Engl. J. Med. 2004, 351, 1391–1402. [Google Scholar] [CrossRef]
- Dvorak, C.C.; Fisher, B.T.; Esbenshade, A.J.; Nieder, M.L.; Alexander, S.; Steinbach, W.J.; Dang, H.; Villaluna, D.; Chen, L.; Skeens, M.; et al. A randomized trial of caspofungin vs. triazoles prophylaxis for invasive fungal disease in pediatric allogeneic hematopoietic cell transplant. J. Pediatr. Infect. Dis. Soc. 2021, 10, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Maertens, J.; Lodewyck, T.; Donnelly, J.; Chantepie, S.; Robin, C.; Blijlevens, N.; Turlure, P.; Selleslag, D.; Baron, F.; Aoun, M.; et al. Empiric vs preemptive antifungal strategy in high-risk neutropenic patients on fluconazole prophylaxis: A randomized trial of the European Organization for Research and Treatment of Cancer. Clin. Infect. Dis. 2023, 76, 674–682. [Google Scholar] [CrossRef]
- Fisher, B.; Zaoutis, T.; Dvorak, C.; Nieder, M.; Zerr, D.; Wingard, J.; Callahan, C.; Villaluna, D.; Chen, L.; Dang, H.; et al. Effect of caspofungin vs fluconazole prophylaxis on invasive fungal disease among children and young adults with acute myeloid leukemia: A randomized clinical trial. JAMA 2019, 322, 1673–1681. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, R.; Bai, C.; Song, X.; Liu, Y. Caspofungin for prophylaxis and treatment of fungal infections in adolescents and adults: A meta-analysis of randomized controlled trials. Pharmazie 2012, 67, 267–273. [Google Scholar] [CrossRef]
- Stemler, J.; Mellinghoff, S.; Khodamoradi, Y.; Sprute, R.; Classen, A.; Zapke, S.; Hoenigl, M.; Krause, R.; Schmidt-Hieber, M.; Heinz, W.; et al. Primary prophylaxis of invasive fungal diseases in patients with haematological malignancies: 2022 update of the recommendations of the Infectious Diseases Working Party (AGIHO) of the German Society for Haematology and Medical Oncology. J. Antimicrob. Chemother. 2023, 78, 1813–1826. [Google Scholar] [CrossRef]
- Baden, L.; Swaminathan, S.; Almyroudis, N.; Angarone, M.; Baluch, A.; Barros, N.; Buss, B.; Cohen, S.; Cooper, B.; Dulanto Chiang, A.; et al. Prevention and treatment of cancer-related infections, version 3.2024, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2024, 22, 617–644. [Google Scholar] [CrossRef]
- Pagano, L.; Maschmeyer, G.; Lamoth, F.; Blennow, O.; Xhaard, A.; Spadea, M.; Busca, A.; Cordonnier, C.; Maertens, J.; ECIL. Primary antifungal prophylaxis in hematological malignancies. Updated clinical practice guidelines by the European Conference on Infections in Leukemia (ECIL). Leukemia 2025. [Google Scholar] [CrossRef] [PubMed]
- Van Burik, J.; Ratanatharathorn, V.; Stepan, D.; Miller, C.; Lipton, J.; Vesole, D.; Bunin, N.; Wall, D.; Hiemenz, J.; Satoi, Y.; et al. Micafungin versus fluconazole for prophylaxis against invasive fungal infections during neutropenia in patients undergoing hematopoietic stem cell transplantation. Clin. Infect. Dis. 2004, 39, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.; Chen, S.; Kauffman, C.; Steinbach, W.; Baddley, J.; Verweij, P.; Clancy, C.; Wingard, J.; Lockhart, S.; Groll, A.; et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef]
- Khoury, J.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.; et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: Myeloid and histiocytic/dendritic neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.P.; Borowitz, M.J.; Calvo, K.R.; Kvasnicka, H.M.; Wang, S.A.; Bagg, A.; Barbui, T.; Branford, S.; et al. International Consensus Classification of myeloid neoplasms and acute leukemias: Integrating morphologic, clinical, and genomic data. Blood 2022, 140, 1200–1228. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Wei, A.; Appelbaum, F.; Craddock, C.; DiNardo, C.; Dombret, H.; Ebert, B.; Fenaux, P.; Godley, L.; Hasserjian, R.; et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef]
- Alexander, B.; Johnson, M.; Bresnik, M.; Anupindi, V.; Pizzicato, L.; DeKoven, M.; Lovelace, B.; Coleman, C. Real-world antifungal therapy patterns across the continuum of care in United States adults with invasive aspergillosis. J. Fungi. 2024, 10, 876. [Google Scholar] [CrossRef]
- Della Pepa, R.; Picardi, M.; Sorà, F.; Stamouli, M.; Busca, A.; Candoni, A.; Delia, M.; Fanci, R.; Perriello, V.; Zancanella, M.; et al. Successful management of chronic disseminated candidiasis in hematologic patients treated with high-dose liposomal amphotericin B: A retrospective study of the SEIFEM registry. Support. Care Cancer 2016, 24, 3839–3845. [Google Scholar] [CrossRef]
- Sharma, A.; Mohamad, B.; Soubani, A.O. Epidemiology and inpatient outcomes of invasive aspergillosis in patients with liver failure and cirrhosis. J. Fungi 2025, 11, 334. [Google Scholar] [CrossRef]
- Del Principe, M.; Seidel, D.; Criscuolo, M.; Dargenio, M.; Rácil, Z.; Piedimonte, M.; Marchesi, F.; Nadali, G.; Koehler, P.; Fracchiolla, N.; et al. Clinical features and prognostic factors of Magnusiomyces (Saprochaete) infections in haematology. A multicentre study of SEIFEM/Fungiscope. Mycoses 2023, 66, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Hoenigl, M.; Salmanton-García, J.; Walsh, T.; Nucci, M.; Neoh, C.; Jenks, J.; Lackner, M.; Sprute, R.; Al-Hatmi, A.; Bassetti, M.; et al. Global guideline for the diagnosis and management of rare mold infection: An initiative of the European Confederation of Medical Mycology in cooperation with ISHAM and ASM. Lancet Infect. Dis. 2021, 21, e246–e257. [Google Scholar] [CrossRef] [PubMed]
- Aldoss, I.; Dadwal, S.; Zhang, J.; Tegtmeier, B.; Mei, M.; Arslan, S.; Al Malki, M.; Salhotra, A.; Ali, H.; Aribi, A.; et al. Invasive fungal infections in acute myeloid leukemia treated with venetoclax and hypomethylating agents. Blood Adv. 2019, 3, 4043–4049. [Google Scholar] [CrossRef] [PubMed]
- Candoni, A.; Lazzarotto, D.; Papayannidis, C.; Piccini, M.; Nadali, G.; Dargenio, M.; Riva, M.; Fracchiolla, N.; Mellillo, L.; Dragonetti, G.; et al. Prospective multicenter study on infectious complications and clinical outcome of 230 unfit acute myeloid leukemia patients receiving first-line therapy with hypomethylating agents alone or in combination with venetoclax. Am. J. Hematol. 2023, 98, E80–E83. [Google Scholar] [CrossRef]
| Caspofungin | Posaconazole | p | |
|---|---|---|---|
| Male/female, n | 16/17 | 19/23 | 0.928 |
| Age, median (range) | 61 (26–75) | 60 (31–74) | 0.928 |
| Comorbidities, median (range) | 1 (0–4) | 1 (0–4) | 0.919 |
| Type II diabetes, n (%) | 9 (27%) | 7 (16%) | 0.619 |
| Cardiovascular disease, n (%) | 7 (21%) | 5 (11%) | 0.616 |
| AML with defining genetic abnormalities, n (%) | 20 (61%) | 22 (54%) | 0.787 |
| AML MDS-related, n (%) | 5 (15%) | 7 (16%) | 0.928 |
| AML defined by differentiation, n (%) | 2 (6%) | 4 (9.5%) | 0.919 |
| AML post-MPN, n (%) | 3 (9%) | 4 (9.5%) | 0.919 |
| MDS/AML TP53 mutation, n (%) | 2 (6%) | 3 (7%) | 0.919 |
| Therapy-related AML, n (%) | 1 (3%) | 2 (4%) | 0.919 |
| ELN2022 low, n (%) | 7 (22%) | 15 (35%) | 0.204 |
| ELN2022 intermediate, n (%) | 13 (39%) | 12 (30%) | 0.765 |
| ELN 2022 high, n (%) | 13 (39%) | 15 (35%) | 0.760 |
| NPM1 pos, n (%) | 14 (42%) | 12 (30%) | 0.606 |
| FLT3 pos, n (%) | 11 (33%) | 7 (16%) | 0.325 |
| IDA-Flag, n (%) | 1 (3%) | 3 (7%) | 0.628 |
| CPX-351, n (%) | 4 (12%) | 7 (16%) | 0.675 |
| 3 + 7, n (%) | 1 (3%) | 15 (36%) | <0.001 |
| 3 + 7+GO, n (%) | 5 (15%) | 10 (25%) | 0.406 |
| 3 + 7+midostaurin, n (%) | 12 (36%) | 0 | <0.005 |
| HMA/venetoclax, n (%) | 10 (31%) | 7 (16%) | 0.411 |
| Days of neutropenia, median (range) | 22 (13–68) | 26 (12–131) | 0.928 |
| Refractory status after chemotherapy, n (%) | 6 (18%) | 7 (16%) | 0.919 |
| Univariate Analysis | ||||
|---|---|---|---|---|
| Covariate | Comparison | HR | 95% CI | p Value |
| Age | Continuous (per year increase) | 1.036 | 1.003–1.070 | 0.031 |
| Gender | Male vs. Female | 0.97 | 0.454–2.071 | 0.937 |
| Liver disease | Yes vs. No | 1.376 | 0.411–4.604 | 0.605 |
| COPD | Yes vs. No | 1.181 | 0.491–2.844 | 0.710 |
| Cardiovascular disease | Yes vs. No | 2.353 | 0.979–5.658 | 0.076 |
| Kidney disease | Yes vs. No | 0.0 | 0–INF | 0.998 |
| Type II diabetes | Yes vs. No | 1.095 | 0.411–2.916 | 0.855 |
| Previous cancer | Yes vs. No | 2.105 | 0.49–9.044 | 0.317 |
| WHO2022 diagnosis | Secondary AML vs. De Novo AML | 2.953 | 1.361–6.408 | 0.006 |
| NPM status | Positive vs. Negative | 0.173 | 0.122–1.027 | 0.074 |
| FLT3 status | Positive vs. Negative | 1.353 | 0.122–1.027 | 0.004 |
| ELN2022 risk | High vs. Low/Intermediate | 4.184 | 1.899–9.215 | <0.001 |
| Chemotherapy regimen | HMA/Ven vs. Intensive | 2.124 | 0.952–4.743 | 0.036 |
| Disease status | Refractory vs. Responding | 4.590 | 2.067–10.192 | 0.017 |
| PAP | Caspofungin vs. Posaconazole | 1.23 | 0.568–2.662 | 0.559 |
| Neutropenia duration | Continuous (per day increase) | 1.032 | 1.005–1.058 | 0.019 |
| HSCT | Yes vs. No | 0.334 | 0.126–0.884 | 0.027 |
| IFI | Proven/Probable vs. None | 3.032 | 1.023–8.985 | 0.045 |
| Multivariate Analysis | ||||
| Age | Continuous (per year increase) | 1.15 | 1.054–1.254 | 0.002 |
| Disease status | Refractory vs. Responding | 5.009 | 1.503–11.695 | 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grimaldi, F.; Memoli, M.; Avilia, S.; Causa, C.; Giannattasio, M.L.; Conversano, I.; Lisi, D.; D’Angelo, D.; Iannotta, R.; Schiano Moriello, N.; et al. Caspofungin for Primary Antifungal Prophylaxis in Acute Myeloid Leukemia: A Real-Life Study from an Academic Center. Cancers 2025, 17, 2184. https://doi.org/10.3390/cancers17132184
Grimaldi F, Memoli M, Avilia S, Causa C, Giannattasio ML, Conversano I, Lisi D, D’Angelo D, Iannotta R, Schiano Moriello N, et al. Caspofungin for Primary Antifungal Prophylaxis in Acute Myeloid Leukemia: A Real-Life Study from an Academic Center. Cancers. 2025; 17(13):2184. https://doi.org/10.3390/cancers17132184
Chicago/Turabian StyleGrimaldi, Francesco, Mara Memoli, Simona Avilia, Carlangela Causa, Maria Luisa Giannattasio, Italia Conversano, Dario Lisi, Daniela D’Angelo, Raffaella Iannotta, Nicola Schiano Moriello, and et al. 2025. "Caspofungin for Primary Antifungal Prophylaxis in Acute Myeloid Leukemia: A Real-Life Study from an Academic Center" Cancers 17, no. 13: 2184. https://doi.org/10.3390/cancers17132184
APA StyleGrimaldi, F., Memoli, M., Avilia, S., Causa, C., Giannattasio, M. L., Conversano, I., Lisi, D., D’Angelo, D., Iannotta, R., Schiano Moriello, N., Viceconte, G., Zappulo, E., Gentile, I., Picardi, M., & Pane, F. (2025). Caspofungin for Primary Antifungal Prophylaxis in Acute Myeloid Leukemia: A Real-Life Study from an Academic Center. Cancers, 17(13), 2184. https://doi.org/10.3390/cancers17132184








