A Review of Emerging Immunotherapeutic Strategies for IDH-Mutant Glioma
Simple Summary
Abstract
1. Introduction
2. IDH-Mutant Glioma (IMG)
2.1. Immune Landscape of IMG
2.2. Current Therapeutic Approaches for IMG
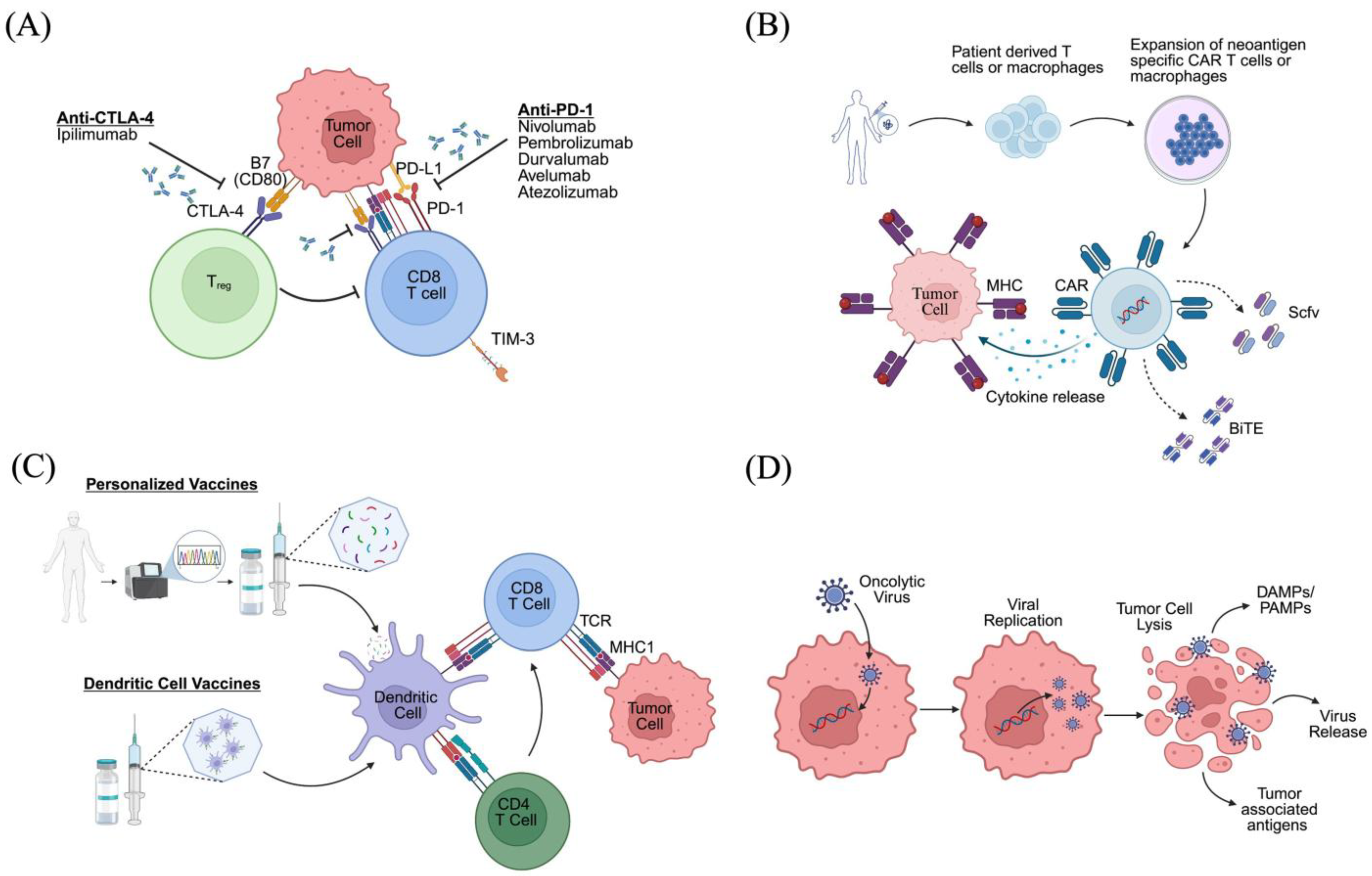
2.2.1. Immune Checkpoint Inhibitors
2.2.2. CAR T Cell Therapy
2.2.3. Myeloid Redirection and CAR Macrophages
2.2.4. Tumor Vaccines
2.2.5. Oncolytic Viruses
2.3. Future Directions for Immunotherapy in IMG
2.3.1. Optimizing Modulation of the TME
2.3.2. Enhancing Antigen-Specific T Cell Responses and Exploring Novel Antigenic Targets
2.3.3. Patient Selection, Stratification, and Immune Biomarker Selection
2.3.4. Prioritization of Experimental Immunotherapies in IMG
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.J.; Gonzalez Castro, L.N.; McBrayer, S.; Weller, M.; Cloughesy, T.; Portnow, J.; Andronesi, O.; Barnholtz-Sloan, J.S.; Baumert, B.G.; Berger, M.S.; et al. Isocitrate dehydrogenase (IDH) mutant gliomas: A Society for Neuro-Oncology (SNO) consensus review on diagnosis, management, and future directions. Neuro-Oncol. 2023, 25, 4–25. [Google Scholar] [CrossRef]
- Haddad, A.F.; Young, J.S.; Oh, J.Y.; Okada, H.; Aghi, M.K. The immunology of low-grade gliomas. Neurosurg. Focus 2022, 52, E2. [Google Scholar] [CrossRef] [PubMed]
- Oberheim Bush, N.A.; Chang, S. Treatment Strategies for Low-Grade Glioma in Adults. J. Oncol. Pract. 2016, 12, 1235–1241. [Google Scholar] [CrossRef]
- Alshiekh Nasany, R.; de la Fuente, M.I. Therapies for IDH-Mutant Gliomas. Curr. Neurol. Neurosci. Rep. 2023, 23, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Lazarev, S.; Sindhu, K.K. Vorasidenib: A new hope or a false promise for patients with low-grade glioma? Nat. Rev. Clin. Oncol. 2024, 21, 1–2. [Google Scholar] [CrossRef]
- Richardson, L.G.; Miller, J.J.; Kitagawa, Y.; Wakimoto, H.; Choi, B.D.; Curry, W.T. Implications of IDH mutations on immunotherapeutic strategies for malignant glioma. Neurosurg. Focus 2022, 52, E6. [Google Scholar] [CrossRef]
- Suryadevara, C.M.; Verla, T.; Sanchez-Perez, L.; Reap, E.A.; Choi, B.D.; Fecci, P.E.; Sampson, J.H. Immunotherapy for malignant glioma. Surg. Neurol. Int. 2015, 6, S68–S77. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. The Microenvironmental Landscape of Brain Tumors. Cancer Cell 2017, 31, 326–341. [Google Scholar] [CrossRef]
- Ott, M.; Prins, R.M.; Heimberger, A.B. The immune landscape of common CNS malignancies: Implications for immunotherapy. Nat. Rev. Clin. Oncol. 2021, 18, 729–744. [Google Scholar] [CrossRef]
- Thorsson, V.; Gibbs, D.L.; Brown, S.D.; Wolf, D.; Bortone, D.S.; Ou Yang, T.-H.; Porta-Pardo, E.; Gao, G.F.; Plaisier, C.L.; Eddy, J.A.; et al. The Immune Landscape of Cancer. Immunity 2018, 48, 812–830.e14. [Google Scholar] [CrossRef] [PubMed]
- Richardson, L.G.; Choi, B.D.; Curry, W.T. (R)-2-hydroxyglutarate drives immune quiescence in the tumor microenvironment of IDH-mutant gliomas. Transl. Cancer Res. 2019, 8, S167–S170. [Google Scholar] [CrossRef] [PubMed]
- Kohanbash, G.; Carrera, D.A.; Shrivastav, S.; Ahn, B.J.; Jahan, N.; Mazor, T.; Chheda, Z.S.; Downey, K.M.; Watchmaker, P.B.; Beppler, C.; et al. Isocitrate dehydrogenase mutations suppress STAT1 and CD8+ T cell accumulation in gliomas. J. Clin. Invest. 2017, 127, 1425–1437. [Google Scholar] [CrossRef] [PubMed]
- Berghoff, A.S.; Kiesel, B.; Widhalm, G.; Wilhelm, D.; Rajky, O.; Kurscheid, S.; Kresl, P.; Wöhrer, A.; Marosi, C.; Hegi, M.E.; et al. Correlation of immune phenotype with IDH mutation in diffuse glioma. Neuro-Oncol. 2017, 19, 1460–1468. [Google Scholar] [CrossRef]
- Richardson, L.G.; Nieman, L.T.; Stemmer-Rachamimov, A.O.; Zheng, X.S.; Stafford, K.; Nagashima, H.; Miller, J.J.; Kiyokawa, J.; Ting, D.T.; Wakimoto, H.; et al. IDH-mutant gliomas harbor fewer regulatory T cells in humans and mice. Oncoimmunology 2020, 9, 1806662. [Google Scholar] [CrossRef]
- Sayour, E.J.; McLendon, P.; McLendon, R.; De Leon, G.; Reynolds, R.; Kresak, J.; Sampson, J.H.; Mitchell, D.A. Increased proportion of FoxP3+ regulatory T cells in tumor infiltrating lymphocytes is associated with tumor recurrence and reduced survival in patients with glioblastoma. Cancer Immunol. Immunother. CII 2015, 64, 419–427. [Google Scholar] [CrossRef]
- Amankulor, N.M.; Kim, Y.; Arora, S.; Kargl, J.; Szulzewsky, F.; Hanke, M.; Margineantu, D.H.; Rao, A.; Bolouri, H.; Delrow, J.; et al. Mutant IDH1 regulates the tumor-associated immune system in gliomas. Genes Dev. 2017, 31, 774–786. [Google Scholar] [CrossRef]
- Friebel, E.; Kapolou, K.; Unger, S.; Núñez, N.G.; Utz, S.; Rushing, E.J.; Regli, L.; Weller, M.; Greter, M.; Tugues, S.; et al. Single-Cell Mapping of Human Brain Cancer Reveals Tumor-Specific Instruction of Tissue-Invading Leukocytes. Cell 2020, 181, 1626–1642.e20. [Google Scholar] [CrossRef] [PubMed]
- Mathewson, N.D.; Ashenberg, O.; Tirosh, I.; Gritsch, S.; Perez, E.M.; Marx, S.; Jerby-Arnon, L.; Chanoch-Myers, R.; Hara, T.; Richman, A.R.; et al. Inhibitory CD161 receptor identified in glioma-infiltrating T cells by single-cell analysis. Cell 2021, 184, 1281–1298.e26. [Google Scholar] [CrossRef] [PubMed]
- Klemm, F.; Maas, R.R.; Bowman, R.L.; Kornete, M.; Soukup, K.; Nassiri, S.; Brouland, J.-P.; Iacobuzio-Donahue, C.A.; Brennan, C.; Tabar, V.; et al. Interrogation of the Microenvironmental Landscape in Brain Tumors Reveals Disease-Specific Alterations of Immune Cells. Cell 2020, 181, 1643–1660.e17. [Google Scholar] [CrossRef]
- Grewal, E.P.; Richardson, L.G.K.; Sun, J.; Ramapriyan, R.; Martinez-Lage, M.; Miller, J.J.; Cahill, D.P.; Choi, B.D.; Curry, W.T. Suppression of antitumor immune signatures and upregulation of VEGFA as IDH-mutant gliomas progress to higher grade. Neurosurg. Focus 2024, 56, E2. [Google Scholar] [CrossRef]
- Han, S.; Liu, Y.; Cai, S.J.; Qian, M.; Ding, J.; Larion, M.; Gilbert, M.R.; Yang, C. IDH mutation in glioma: Molecular mechanisms and potential therapeutic targets. Br. J. Cancer 2020, 122, 1580–1589. [Google Scholar] [CrossRef] [PubMed]
- Hodges, T.R.; Choi, B.D.; Bigner, D.D.; Yan, H.; Sampson, J.H. Isocitrate dehydrogenase 1: What it means to the neurosurgeon. J. Neurosurg. 2013, 118, 1176–1180. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.D.; Curry, W.T. IDH mutational status and the immune system in gliomas: A tale of two tumors? Transl. Cancer Res. 2017, 6, S1253–S1256. [Google Scholar] [CrossRef]
- Bunse, L.; Pusch, S.; Bunse, T.; Sahm, F.; Sanghvi, K.; Friedrich, M.; Alansary, D.; Sonner, J.K.; Green, E.; Deumelandt, K.; et al. Suppression of antitumor T cell immunity by the oncometabolite (R)-2-hydroxyglutarate. Nat. Med. 2018, 24, 1192–1203. [Google Scholar] [CrossRef]
- Friedrich, M.; Sankowski, R.; Bunse, L.; Kilian, M.; Green, E.; Ramallo Guevara, C.; Pusch, S.; Poschet, G.; Sanghvi, K.; Hahn, M.; et al. Tryptophan metabolism drives dynamic immunosuppressive myeloid states in IDH-mutant gliomas. Nat. Cancer 2021, 2, 723–740. [Google Scholar] [CrossRef]
- Zhang, X.; Rao, A.; Sette, P.; Deibert, C.; Pomerantz, A.; Kim, W.J.; Kohanbash, G.; Chang, Y.; Park, Y.; Engh, J.; et al. IDH mutant gliomas escape natural killer cell immune surveillance by downregulation of NKG2D ligand expression. Neuro-Oncol. 2016, 18, 1402–1412. [Google Scholar] [CrossRef]
- Lapeyre-Prost, A.; Terme, M.; Pernot, S.; Pointet, A.-L.; Voron, T.; Tartour, E.; Taieb, J. Chapter Seven—Immunomodulatory Activity of VEGF in Cancer. In International Review of Cell and Molecular Biology; Galluzzi, L., Ed.; Academic Press: Palm Bay, FL, USA, 2017; Volume 330, pp. 295–342. [Google Scholar]
- Chinot, O.L.; Wick, W.; Mason, W.; Henriksson, R.; Saran, F.; Nishikawa, R.; Carpentier, A.F.; Hoang-Xuan, K.; Kavan, P.; Cernea, D.; et al. Bevacizumab plus Radiotherapy–Temozolomide for Newly Diagnosed Glioblastoma. N. Engl. J. Med. 2014, 370, 709–722. [Google Scholar] [CrossRef]
- Gilbert, M.R.; Dignam, J.J.; Armstrong, T.S.; Wefel, J.S.; Blumenthal, D.T.; Vogelbaum, M.A.; Colman, H.; Chakravarti, A.; Pugh, S.; Won, M.; et al. A Randomized Trial of Bevacizumab for Newly Diagnosed Glioblastoma. N. Engl. J. Med. 2014, 370, 699–708. [Google Scholar] [CrossRef]
- Bent, M.J.v.D.; Klein, M.; Smits, M.; Reijneveld, J.C.; French, P.J.; Clement, P.; Vos, F.Y.F.d.; Wick, A.; Mulholland, P.J.; Taphoorn, M.J.B.; et al. Bevacizumab and temozolomide in patients with first recurrence of WHO grade II and III glioma, without 1p/19q co-deletion (TAVAREC): A randomised controlled phase 2 EORTC trial. Lancet Oncol. 2018, 19, 1170–1179. [Google Scholar] [CrossRef]
- Wick, W.; Gorlia, T.; Bendszus, M.; Taphoorn, M.; Sahm, F.; Harting, I.; Brandes, A.A.; Taal, W.; Domont, J.; Idbaih, A.; et al. Lomustine and Bevacizumab in Progressive Glioblastoma. N. Engl. J. Med. 2017, 377, 1954–1963. [Google Scholar] [CrossRef]
- Robert, C.; Thomas, L.; Bondarenko, I.; O’Day, S.; Weber, J.; Garbe, C.; Lebbe, C.; Baurain, J.-F.; Testori, A.; Grob, J.-J.; et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 2011, 364, 2517–2526. [Google Scholar] [CrossRef]
- Marin-Acevedo, J.A.; Kimbrough, E.O.; Lou, Y. Next generation of immune checkpoint inhibitors and beyond. J. Hematol. Oncol.J Hematol Oncol 2021, 14, 1–29. [Google Scholar] [CrossRef]
- Current Oncology | Free Full-Text | Immune Checkpoint Inhibitors in Cancer Therapy. Available online: https://www.mdpi.com/1718-7729/29/5/247 (accessed on 27 August 2024).
- Reardon, D.A.; Brandes, A.A.; Omuro, A.; Mulholland, P.; Lim, M.; Wick, A.; Baehring, J.; Ahluwalia, M.S.; Roth, P.; Bähr, O.; et al. Effect of Nivolumab vs Bevacizumab in Patients With Recurrent Glioblastoma: The CheckMate 143 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1003–1010. [Google Scholar] [CrossRef]
- Lowther, D.E.; Goods, B.A.; Lucca, L.E.; Lerner, B.A.; Raddassi, K.; van Dijk, D.; Hernandez, A.L.; Duan, X.; Gunel, M.; Coric, V.; et al. PD-1 marks dysfunctional regulatory T cells in malignant gliomas. JCI Insight 2016, 1, e85935. [Google Scholar] [CrossRef]
- Filley, A.C.; Henriquez, M.; Dey, M. Recurrent glioma clinical trial, CheckMate-143: The game is not over yet. Oncotarget 2017, 8, 91779–91794. [Google Scholar] [CrossRef]
- Ramapriyan, R.; Sun, J.; Curry, A.; Richardson, L.G.; Ramesh, T.; Gaffey, M.A.; Gedeon, P.C.; Gerstner, E.R.; Curry, W.T.; Choi, B.D. The Role of Antibody-Based Therapies in Neuro-Oncology. Antibodies Basel Switz. 2023, 12, 74. [Google Scholar] [CrossRef]
- Pirozzi, C.J.; Yan, H. The implications of IDH mutations for cancer development and therapy. Nat. Rev. Clin. Oncol. 2021, 18, 645–661. [Google Scholar] [CrossRef]
- Gallus, M.; Kwok, D.; Lakshmanachetty, S.; Yamamichi, A.; Okada, H. Immunotherapy Approaches in Isocitrate-Dehydrogenase-Mutant Low-Grade Glioma. Cancers 2023, 15, 3726. [Google Scholar] [CrossRef]
- Yan, D.; Li, W.; Liu, Q.; Yang, K. Advances in Immune Microenvironment and Immunotherapy of Isocitrate Dehydrogenase Mutated Glioma. Front. Immunol. 2022, 13. [Google Scholar] [CrossRef]
- Kadiyala, P.; Carney, S.V.; Gauss, J.C.; Garcia-Fabiani, M.B.; Haase, S.; Alghamri, M.S.; Núñez, F.J.; Liu, Y.; Yu, M.; Taher, A.; et al. Inhibition of 2-hydroxyglutarate elicits metabolic reprogramming and mutant IDH1 glioma immunity in mice. J. Clin. Invest. 2021, 131, e139542. [Google Scholar] [CrossRef]
- Bordry, N.; Broggi, M.A.S.; de Jonge, K.; Schaeuble, K.; Gannon, P.O.; Foukas, P.G.; Danenberg, E.; Romano, E.; Baumgaertner, P.; Fankhauser, M.; et al. Lymphatic vessel density is associated with CD8+ T cell infiltration and immunosuppressive factors in human melanoma. Oncoimmunology 2018, 7, e1462878. [Google Scholar] [CrossRef]
- Marofi, F.; Motavalli, R.; Safonov, V.A.; Thangavelu, L.; Yumashev, A.V.; Alexander, M.; Shomali, N.; Chartrand, M.S.; Pathak, Y.; Jarahian, M.; et al. CAR T cells in solid tumors: Challenges and opportunities. Stem Cell Res. Ther. 2021, 12, 81. [Google Scholar] [CrossRef]
- Ramapriyan, R.; Vykunta, V.S.; Vandecandelaere, G.; Richardson, L.G.K.; Sun, J.; Curry, W.T.; Choi, B.D. Altered cancer metabolism and implications for next-generation CAR T-cell therapies. Pharmacol. Ther. 2024, 259, 108667. [Google Scholar] [CrossRef]
- Choi, B.D.; Yu, X.; Castano, A.P.; Bouffard, A.A.; Schmidts, A.; Larson, R.C.; Bailey, S.R.; Boroughs, A.C.; Frigault, M.J.; Leick, M.B.; et al. CAR-T cells secreting BiTEs circumvent antigen escape without detectable toxicity. Nat. Biotechnol. 2019, 37, 1049–1058. [Google Scholar] [CrossRef]
- Lee, D.W.; Kochenderfer, J.N.; Stetler-Stevenson, M.; Cui, Y.K.; Delbrook, C.; Feldman, S.A.; Fry, T.J.; Orentas, R.; Sabatino, M.; Shah, N.N.; et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: A phase 1 dose-escalation trial. Lancet 2015, 385, 517–528. [Google Scholar] [CrossRef]
- Park, J.H.; Rivière, I.; Gonen, M.; Wang, X.; Sénéchal, B.; Curran, K.J.; Sauter, C.; Wang, Y.; Santomasso, B.; Mead, E.; et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 449–459. [Google Scholar] [CrossRef]
- Akhavan, D.; Alizadeh, D.; Wang, D.; Weist, M.R.; Shepphird, J.K.; Brown, C.E. CAR T cells for brain tumors: Lessons learned and road ahead. Immunol. Rev. 2019, 290, 60–84. [Google Scholar] [CrossRef]
- Choi, B.D.; Gerstner, E.R.; Frigault, M.J.; Leick, M.B.; Mount, C.W.; Balaj, L.; Nikiforow, S.; Carter, B.S.; Curry, W.T.; Gallagher, K.; et al. Intraventricular CARv3-TEAM-E T Cells in Recurrent Glioblastoma. N. Engl. J. Med. 2024, 390, 1290–1298. [Google Scholar] [CrossRef]
- Bagley, S.J.; Logun, M.; Fraietta, J.A.; Wang, X.; Desai, A.S.; Bagley, L.J.; Nabavizadeh, A.; Jarocha, D.; Martins, R.; Maloney, E.; et al. Intrathecal bivalent CAR T cells targeting EGFR and IL13Rα2 in recurrent glioblastoma: Phase 1 trial interim results. Nat. Med. 2024, 30, 1320–1329. [Google Scholar] [CrossRef]
- Brown, C.E.; Hibbard, J.C.; Alizadeh, D.; Blanchard, M.S.; Natri, H.M.; Wang, D.; Ostberg, J.R.; Aguilar, B.; Wagner, J.R.; Paul, J.A.; et al. Locoregional delivery of IL-13Rα2-targeting CAR-T cells in recurrent high-grade glioma: A phase 1 trial. Nat. Med. 2024, 30, 1001–1012. [Google Scholar] [CrossRef]
- Senhaji, N.; Louati, S.; Chbani, L.; El Fatemi, H.; Hammas, N.; Mikou, K.; Maaroufi, M.; Benzagmout, M.; Boujraf, S.; El Bardai, S.; et al. EGFR Amplification and IDH Mutations in Glioblastoma Patients of the Northeast of Morocco. BioMed Res. Int. 2017, 2017, 8045859. [Google Scholar] [CrossRef]
- Flavahan, W.A.; Drier, Y.; Liau, B.B.; Gillespie, S.M.; Venteicher, A.S.; Stemmer-Rachamimov, A.O.; Suvà, M.L.; Bernstein, B.E. Insulator dysfunction and oncogene activation in IDH mutant gliomas. Nature 2016, 529, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Wang, J.; Wen, X.; Xu, B.; Que, Y.; Yu, K.; Xu, L.; Zhao, J.; Pan, Q.; Zhou, P.; et al. Chimeric antigen receptor-modified T-cell therapy for platelet-derived growth factor receptor α-positive rhabdomyosarcoma. Cancer 2020, 126, 2093–2100. [Google Scholar] [CrossRef] [PubMed]
- Montoya, M.; Gallus, M.; Phyu, S.; Haegelin, J.; de Groot, J.; Okada, H. A Roadmap of CAR-T-Cell Therapy in Glioblastoma: Challenges and Future Perspectives. Cells 2024, 13, 726. [Google Scholar] [CrossRef] [PubMed]
- Pant, A.; Lim, M. CAR-T Therapy in GBM: Current Challenges and Avenues for Improvement. Cancers 2023, 15, 1249. [Google Scholar] [CrossRef]
- Suryadevara, C.M.; Desai, R.; Abel, M.L.; Riccione, K.A.; Batich, K.A.; Shen, S.H.; Chongsathidkiet, P.; Gedeon, P.C.; Elsamadicy, A.A.; Snyder, D.J.; et al. Temozolomide lymphodepletion enhances CAR abundance and correlates with antitumor efficacy against established glioblastoma. Oncoimmunology 2018, 7, e1434464. [Google Scholar] [CrossRef]
- Goff, S.L.; Morgan, R.A.; Yang, J.C.; Sherry, R.M.; Robbins, P.F.; Restifo, N.P.; Feldman, S.A.; Lu, Y.-C.; Lu, L.; Zheng, Z.; et al. Pilot Trial of Adoptive Transfer of Chimeric Antigen Receptor-transduced T Cells Targeting EGFRvIII in Patients With Glioblastoma. J. Immunother. Hagerstown Md 1997 2019, 42, 126–135. [Google Scholar] [CrossRef]
- Lin, Q.; Ba, T.; Ho, J.; Chen, D.; Cheng, Y.; Wang, L.; Xu, G.; Xu, L.; Zhou, Y.; Wei, Y.; et al. First-in-Human Trial of EphA2-Redirected CAR T-Cells in Patients With Recurrent Glioblastoma: A Preliminary Report of Three Cases at the Starting Dose. Front. Oncol. 2021, 11, 694941. [Google Scholar] [CrossRef]
- Tazhibi, M.; McQuillan, N.; Wei, H.-J.; Gallitto, M.; Bendau, E.; Webster Carrion, A.; Berg, X.; Kokossis, D.; Zhang, X.; Zhang, Z.; et al. Focused ultrasound-mediated blood–brain barrier opening is safe and feasible with moderately hypofractionated radiotherapy for brainstem diffuse midline glioma. J. Transl. Med. 2024, 22, 320. [Google Scholar] [CrossRef]
- Geurts, M.; Preusser, M. Locoregional delivery of chimeric antigen receptor-T cells: Breaking the spell in glioblastoma? Neuro-Oncol. 2024, 26, 1177–1180. [Google Scholar] [CrossRef]
- Grosskopf, A.K.; Labanieh, L.; Klysz, D.D.; Roth, G.A.; Xu, P.; Adebowale, O.; Gale, E.C.; Jons, C.K.; Klich, J.H.; Yan, J.; et al. Delivery of CAR-T cells in a transient injectable stimulatory hydrogel niche improves treatment of solid tumors. Sci. Adv. 2022, 8, eabn8264. [Google Scholar] [CrossRef] [PubMed]
- Grewal, E.P.; Richardson, L.G.K.; Sun, J.; Ramapriyan, R.; Martinez-Lage, M.; Miller, J.J.; Carter, B.S.; Cahill, D.P.; Curry, W.T.; Choi, B.D. Mutant IDH Modulates Suppressive Myeloid Populations in Malignant Glioma. Clin. Cancer Res. 2024, 30, 4068–4076. [Google Scholar] [CrossRef]
- Wang, J.; Li, S.; Lan, Y.; Liu, X.; Li, W. Glioma-associated macrophages: Unraveling their dual role in the microenvironment and therapeutic implications. Curr. Med. 2024, 3, 4. [Google Scholar] [CrossRef]
- Tang, F.; Wang, Y.; Zeng, Y.; Xiao, A.; Tong, A.; Xu, J. Tumor-associated macrophage-related strategies for glioma immunotherapy. Npj Precis. Oncol. 2023, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lei, A.; Yu, H.; Lu, S.; Lu, H.; Ding, X.; Tan, T.; Zhang, H.; Zhu, M.; Tian, L.; Wang, X.; et al. A second-generation M1-polarized CAR macrophage with antitumor efficacy. Nat. Immunol. 2024, 25, 102–116. [Google Scholar] [CrossRef]
- Chen, C.; Jing, W.; Chen, Y.; Wang, G.; Abdalla, M.; Gao, L.; Han, M.; Shi, C.; Li, A.; Sun, P.; et al. Intracavity generation of glioma stem cell–specific CAR macrophages primes locoregional immunity for postoperative glioblastoma therapy. Sci. Transl. Med. 2022, 14, eabn1128. [Google Scholar] [CrossRef]
- Gao, L.; Shi, C.; Yang, Z.; Jing, W.; Han, M.; Zhang, J.; Zhang, C.; Tang, C.; Dong, Y.; Liu, Y.; et al. Convection-enhanced delivery of nanoencapsulated gene locoregionally yielding ErbB2/Her2-specific CAR-macrophages for brainstem glioma immunotherapy. J. Nanobiotechnology 2023, 21, 56. [Google Scholar] [CrossRef]
- Capper, D.; Weissert, S.; Balss, J.; Habel, A.; Meyer, J.; Jäger, D.; Ackermann, U.; Tessmer, C.; Korshunov, A.; Zentgraf, H.; et al. Characterization of R132H mutation-specific IDH1 antibody binding in brain tumors. Brain Pathol. Zurich Switz. 2010, 20, 245–254. [Google Scholar] [CrossRef]
- Watanabe, T.; Nobusawa, S.; Kleihues, P.; Ohgaki, H. IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas. Am. J. Pathol. 2009, 174, 1149–1153. [Google Scholar] [CrossRef]
- Pellegatta, S.; Valletta, L.; Corbetta, C.; Patanè, M.; Zucca, I.; Riccardi Sirtori, F.; Bruzzone, M.G.; Fogliatto, G.; Isacchi, A.; Pollo, B.; et al. Effective immuno-targeting of the IDH1 mutation R132H in a murine model of intracranial glioma. Acta Neuropathol. Commun. 2015, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Platten, M.; Bunse, L.; Wick, A.; Bunse, T.; Le Cornet, L.; Harting, I.; Sahm, F.; Sanghvi, K.; Tan, C.L.; Poschke, I.; et al. A vaccine targeting mutant IDH1 in newly diagnosed glioma. Nature 2021, 592, 463–468. [Google Scholar] [CrossRef]
- Azad, T.; Rezaei, R.; Singaravelu, R.; Pelin, A.; Boulton, S.; Petryk, J.; Onsu, K.A.; Martin, N.T.; Hoskin, V.; Ghahremani, M.; et al. Synthetic virology approaches to improve the safety and efficacy of oncolytic virus therapies. Nat. Commun. 2023, 14, 3035. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Zinkus-Boltz, J.; Dickinson, B.C. Evolution of a split RNA polymerase as a versatile biosensor platform. Nat. Chem. Biol. 2017, 13, 432–438. [Google Scholar] [CrossRef]
- Everts, A.; Bergeman, M.; McFadden, G.; Kemp, V. Simultaneous Tumor and Stroma Targeting by Oncolytic Viruses. Biomedicines 2020, 8, 474. [Google Scholar] [CrossRef] [PubMed]
- Twumasi-Boateng, K.; Pettigrew, J.L.; Kwok, Y.Y.E.; Bell, J.C.; Nelson, B.H. Oncolytic viruses as engineering platforms for combination immunotherapy. Nat. Rev. Cancer 2018, 18, 419–432. [Google Scholar] [CrossRef]
- Mokhtarpour, K.; Akbarzadehmoallemkolaei, M.; Rezaei, N. A viral attack on brain tumors: The potential of oncolytic virus therapy. J. Neurovirol. 2024, 30, 1–22. [Google Scholar] [CrossRef]
- Romanishin, A.; Vasilev, A.; Khasanshin, E.; Evtekhov, A.; Pusynin, E.; Rubina, K.; Kakotkin, V.; Agapov, M.; Semina, E. Oncolytic viral therapy for gliomas: Advances in the mechanisms and approaches to delivery. Virology 2024, 593, 110033. [Google Scholar] [CrossRef]
- Andtbacka, R.H.I.; Kaufman, H.L.; Collichio, F.; Amatruda, T.; Senzer, N.; Chesney, J.; Delman, K.A.; Spitler, L.E.; Puzanov, I.; Agarwala, S.S.; et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J. Clin. Oncol. 2015, 33, 2780–2788. [Google Scholar] [CrossRef]
- Alberts, P.; Tilgase, A.; Rasa, A.; Bandere, K.; Venskus, D. The advent of oncolytic virotherapy in oncology: The Rigvir® story. Eur. J. Pharmacol. 2018, 837, 117–126. [Google Scholar] [CrossRef]
- Todo, T.; Ito, H.; Ino, Y.; Ohtsu, H.; Ota, Y.; Shibahara, J.; Tanaka, M. Intratumoral oncolytic herpes virus G47∆ for residual or recurrent glioblastoma: A phase 2 trial. Nat. Med. 2022, 28, 1630–1639. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, S.; Fukuhara, H.; Todo, T. Oncolytic virus therapy in Japan: Progress in clinical trials and future perspectives. Jpn. J. Clin. Oncol. 2019, 49, 201–209. [Google Scholar] [CrossRef]
- Galanis, E.; Dooley, K.E.; Keith Anderson, S.; Kurokawa, C.B.; Carrero, X.W.; Uhm, J.H.; Federspiel, M.J.; Leontovich, A.A.; Aderca, I.; Viker, K.B.; et al. Carcinoembryonic antigen-expressing oncolytic measles virus derivative in recurrent glioblastoma: A phase 1 trial. Nat. Commun. 2024, 15, 493. [Google Scholar] [CrossRef] [PubMed]
- Ling, A.L.; Solomon, I.H.; Landivar, A.M.; Nakashima, H.; Woods, J.K.; Santos, A.; Masud, N.; Fell, G.; Mo, X.; Yilmaz, A.S.; et al. Clinical trial links oncolytic immunoactivation to survival in glioblastoma. Nature 2023, 623, 157–166. [Google Scholar] [CrossRef]
- Notarangelo, G.; Spinelli, J.B.; Perez, E.M.; Baker, G.J.; Kurmi, K.; Elia, I.; Stopka, S.A.; Baquer, G.; Lin, J.-R.; Golby, A.J.; et al. Oncometabolite d-2HG alters T cell metabolism to impair CD8+ T cell function. Science 2022, 377, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Supper, V.M.; Donner, H.; Birocchi, F.; Bratt, A.; Escobar, G.; Kann, M.C.; Park, S.; Martin, G.; Korell, F.; Takei, H.; et al. Secretion of a VEGF-blocking scFv enhances CAR T-cell potency. Cancer Immunol. Res. 2025. [Google Scholar] [CrossRef]
- Tamura, R.; Tanaka, T.; Morimoto, Y.; Kuranari, Y.; Yamamoto, Y.; Takei, J.; Murayama, Y.; Yoshida, K.; Sasaki, H. Alterations of the tumor microenvironment in glioblastoma following radiation and temozolomide with or without bevacizumab. Ann. Transl. Med. 2020, 8, 297. [Google Scholar] [CrossRef]
- Beltzig, L.; Schwarzenbach, C.; Leukel, P.; Frauenknecht, K.B.M.; Sommer, C.; Tancredi, A.; Hegi, M.E.; Christmann, M.; Kaina, B. Senescence Is the Main Trait Induced by Temozolomide in Glioblastoma Cells. Cancers 2022, 14, 2233. [Google Scholar] [CrossRef]
- Chen, M.; Linstra, R.; van Vugt, M.A.T.M. Genomic instability, inflammatory signaling and response to cancer immunotherapy. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188661. [Google Scholar] [CrossRef]
- Amor, C.; Fernández-Maestre, I.; Chowdhury, S.; Ho, Y.-J.; Nadella, S.; Graham, C.; Carrasco, S.E.; Nnuji-John, E.; Feucht, J.; Hinterleitner, C.; et al. Prophylactic and long-lasting efficacy of senolytic CAR T cells against age-related metabolic dysfunction. Nat. Aging 2024, 4, 336–349. [Google Scholar] [CrossRef]
- Hu, Z.; Leet, D.E.; Allesøe, R.L.; Oliveira, G.; Li, S.; Luoma, A.M.; Liu, J.; Forman, J.; Huang, T.; Iorgulescu, J.B.; et al. Personal neoantigen vaccines induce persistent memory T cell responses and epitope spreading in patients with melanoma. Nat. Med. 2021, 27, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Weenink, B.; Draaisma, K.; Ooi, H.Z.; Kros, J.M.; Sillevis Smitt, P.A.E.; Debets, R.; French, P.J. Low-grade glioma harbors few CD8 T cells, which is accompanied by decreased expression of chemo-attractants, not immunogenic antigens. Sci. Rep. 2019, 9, 14643. [Google Scholar] [CrossRef]
- van Hijfte, L.; Geurts, M.; de Heer, I.; Vallentgoed, W.; Balcioglu, E.; Debets, R.; French, P. TMIC-39. PERIVASCULAR CONTAINMENT OF T CELLS IN IDH-MUTANT ASTROCYTOMA ASSOCIATES WITH HIGH ABUNDANCE OF GEMISTOCYTIC TUMOR CELLS AND IMMUNE STIMULATORY MICROGLIA. Neuro-Oncol. 2023, 25, v286–v287. [Google Scholar] [CrossRef]
- Schaettler, M.O.; Desai, R.; Wang, A.Z.; Livingstone, A.J.; Kobayashi, D.K.; Coxon, A.T.; Bowman-Kirigin, J.A.; Liu, C.J.; Li, M.; Bender, D.E.; et al. TCR-engineered adoptive cell therapy effectively treats intracranial murine glioblastoma. J. Immunother. Cancer 2023, 11, e006121. [Google Scholar] [CrossRef]
- Krämer, C.; Kilian, M.; Chih, Y.-C.; Kourtesakis, A.; Hoffmann, D.C.; Boschert, T.; Koopmann, P.; Sanghvi, K.; De Roia, A.; Jung, S.; et al. NLGN4X TCR transgenic T cells to treat gliomas. Neuro-Oncol. 2024, 26, 266–278. [Google Scholar] [CrossRef]
- Zhao, B.; Xia, Y.; Yang, F.; Wang, Y.; Wang, Y.; Wang, Y.; Dai, C.; Wang, Y.; Ma, W. Molecular landscape of IDH-mutant astrocytoma and oligodendroglioma grade 2 indicate tumor purity as an underlying genomic factor. Mol. Med. 2022, 28, 34. [Google Scholar] [CrossRef]
- Makarevic, A.; Rapp, C.; Dettling, S.; Reuss, D.; Jungk, C.; Abdollahi, A.; von Deimling, A.; Unterberg, A.; Herold-Mende, C.; Warta, R. Increased Radiation-Associated T-Cell Infiltration in Recurrent IDH-Mutant Glioma. Int. J. Mol. Sci. 2020, 21, 7801. [Google Scholar] [CrossRef]
- Leon-Ferre, R.A.; Jonas, S.F.; Salgado, R.; Loi, S.; de Jong, V.; Carter, J.M.; Nielsen, T.O.; Leung, S.; Riaz, N.; Chia, S.; et al. Tumor-Infiltrating Lymphocytes in Triple-Negative Breast Cancer. JAMA 2024, 331, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Qiu, X.; Sun, P.; Ye, Y.; Huang, Q.; Kong, L.; Lu, J.J. Association of IDH mutation and 1p19q co-deletion with tumor immune microenvironment in lower-grade glioma. Mol. Ther.—Oncolytics 2021, 21, 288–302. [Google Scholar] [CrossRef]
- Guerra, G.; Kachuri, L.; Wendt, G.; Hansen, H.M.; Mack, S.J.; Molinaro, A.M.; Rice, T.; Bracci, P.; Wiencke, J.K.; Kasahara, N.; et al. The immunogenetics of viral antigen response is associated with subtype-specific glioma risk and survival. Am. J. Hum. Genet. 2022, 109, 1105–1116. [Google Scholar] [CrossRef]
- Otsuji, R.; Fujioka, Y.; Hata, N.; Kuga, D.; Hatae, R.; Sangatsuda, Y.; Nakamizo, A.; Mizoguchi, M.; Yoshimoto, K. Liquid Biopsy for Glioma Using Cell-Free DNA in Cerebrospinal Fluid. Cancers 2024, 16, 1009. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | IDH-Mutant Glioma | GBM (IDH-Wildtype) |
|---|---|---|
| Prognosis | Better median overall survival (ranges from 21 months to over a decade by grade and entity) | Worse median overall survival (roughly 11 to 15 months) |
| Tumor growth | Slower growing | More aggressive and faster growing |
| Metabolic profile | Produces 2-hydroxyglutarate Altered tryptophan metabolism leading to immunosuppression Decreased glycolysis, increased glutaminolysis | Increased glycolysis |
| Epigenetic profile | Global DNA hypermethylation | Less extensive DNA methylation |
| Tumor mutational burden | Generally low | Generally low |
| Known tumor antigens | IDH1-R132H (shared neoantigen); potential TAAs include EGFRvIII, PDGFRA | Many TAAs including EGFR, EGFRvIII, HER2, B7-H3, PTPRZ1, IL-13Rα2 |
| Immune cell infiltration | Lower overall immune cell infiltration | Higher overall immune cell infiltration |
| CD8+ T cells | Reduced CD8+ T cell accumulation and 2-HG-mediated suppression of effector function | Higher CD8+ T cell infiltration |
| Regulatory T cells | Fewer regulatory T cells | More regulatory T cells |
| Myeloid cells | Fewer myeloid cells overall, with grade-dependent increase | Increased myeloid cells, including M2 TAMs and MDSCs |
| NK cell evasion | Downregulation of NKG2D ligands (ULBP1 and ULBP3) | Relatively higher expression of NKG2D ligands |
| VEGF expression | Specific increased VEGF expression in higher-grade tumors | High VEGF expression globally |
| NCT No. | Institution | Phase | Treatment Regimen | Status |
|---|---|---|---|---|
| NCT03576612 | Johns Hopkins | I | GMCI + Nivolumab (anti-PD-1) + Radiation Therapy + TMZ | Completed |
| NCT03991832 | University Health Network, Toronto | II | Durvalumab (anti-PD-L1) + Olaparib (PARPi) | Recruiting |
| NCT03925246 | Hôpitaux de Paris | II | Nivolumab | Completed |
| NCT04056910 | University of Pittsburgh Medical Center | II | Nivolumab + Ivosidenib | Completed |
| NCT02968940 | NYU Langone Health | II | Avelumab (anti-PD-L1) + HFRT | Completed |
| NCT03718767 | National Institutes of Health Clinical Center | II | Nivolumab | Recruiting |
| NCT03557359 | Columbia University Medical Center | II | Nivolumab | Active, not recruiting |
| NCT04160494 | Duke University | I | Atezolizumab (anti-PD-L1) + D2C7-IT | Active, not recruiting |
| NCT03684811 | Novo Nordisk A/S | I/II | Nivolumab + FT-2102 + Azacitidine + Gemcitabine + Cisplatin | Completed |
| NCT03893903 | German Cancer Research Center | I | IDH1-R132H-specific vaccine + Avelumab | Active, not recruiting |
| NCT05484622 | Institut de Recherches Internationales Servier | I | Vorasidenib + Pembrolizumab | Recruiting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tazhibi, M.; Grewal, E.P.; Ramapriyan, R.; Richardson, L.G.K.; Vandecandelaere, G.; Kalaw, A.; Kotlarz, P.; Steuart, S.J.; Sun, J.; Gaffey, M.; et al. A Review of Emerging Immunotherapeutic Strategies for IDH-Mutant Glioma. Cancers 2025, 17, 2178. https://doi.org/10.3390/cancers17132178
Tazhibi M, Grewal EP, Ramapriyan R, Richardson LGK, Vandecandelaere G, Kalaw A, Kotlarz P, Steuart SJ, Sun J, Gaffey M, et al. A Review of Emerging Immunotherapeutic Strategies for IDH-Mutant Glioma. Cancers. 2025; 17(13):2178. https://doi.org/10.3390/cancers17132178
Chicago/Turabian StyleTazhibi, Masih, Eric P. Grewal, Rishab Ramapriyan, Leland G. K. Richardson, Gust Vandecandelaere, Adrian Kalaw, Parker Kotlarz, Samuel J. Steuart, Jing Sun, Matthew Gaffey, and et al. 2025. "A Review of Emerging Immunotherapeutic Strategies for IDH-Mutant Glioma" Cancers 17, no. 13: 2178. https://doi.org/10.3390/cancers17132178
APA StyleTazhibi, M., Grewal, E. P., Ramapriyan, R., Richardson, L. G. K., Vandecandelaere, G., Kalaw, A., Kotlarz, P., Steuart, S. J., Sun, J., Gaffey, M., Cahill, D. P., Miller, J. J., Curry, W. T., & Choi, B. D. (2025). A Review of Emerging Immunotherapeutic Strategies for IDH-Mutant Glioma. Cancers, 17(13), 2178. https://doi.org/10.3390/cancers17132178





