Characterization of Melanoma in Hungary Based on a Retrospective Single-Center Study Between 2001 and 2018
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection and Data Collection
2.2. Statistical Analysis
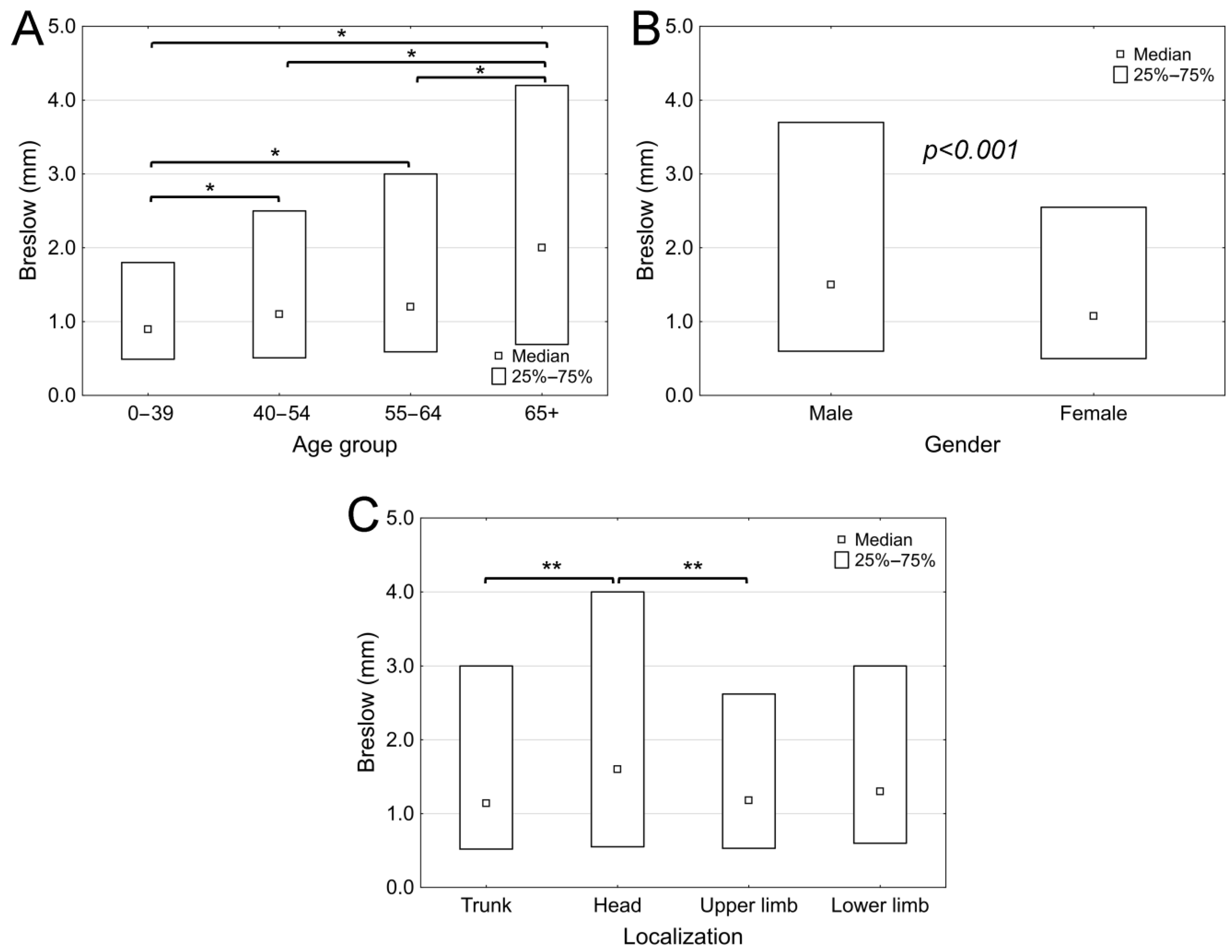
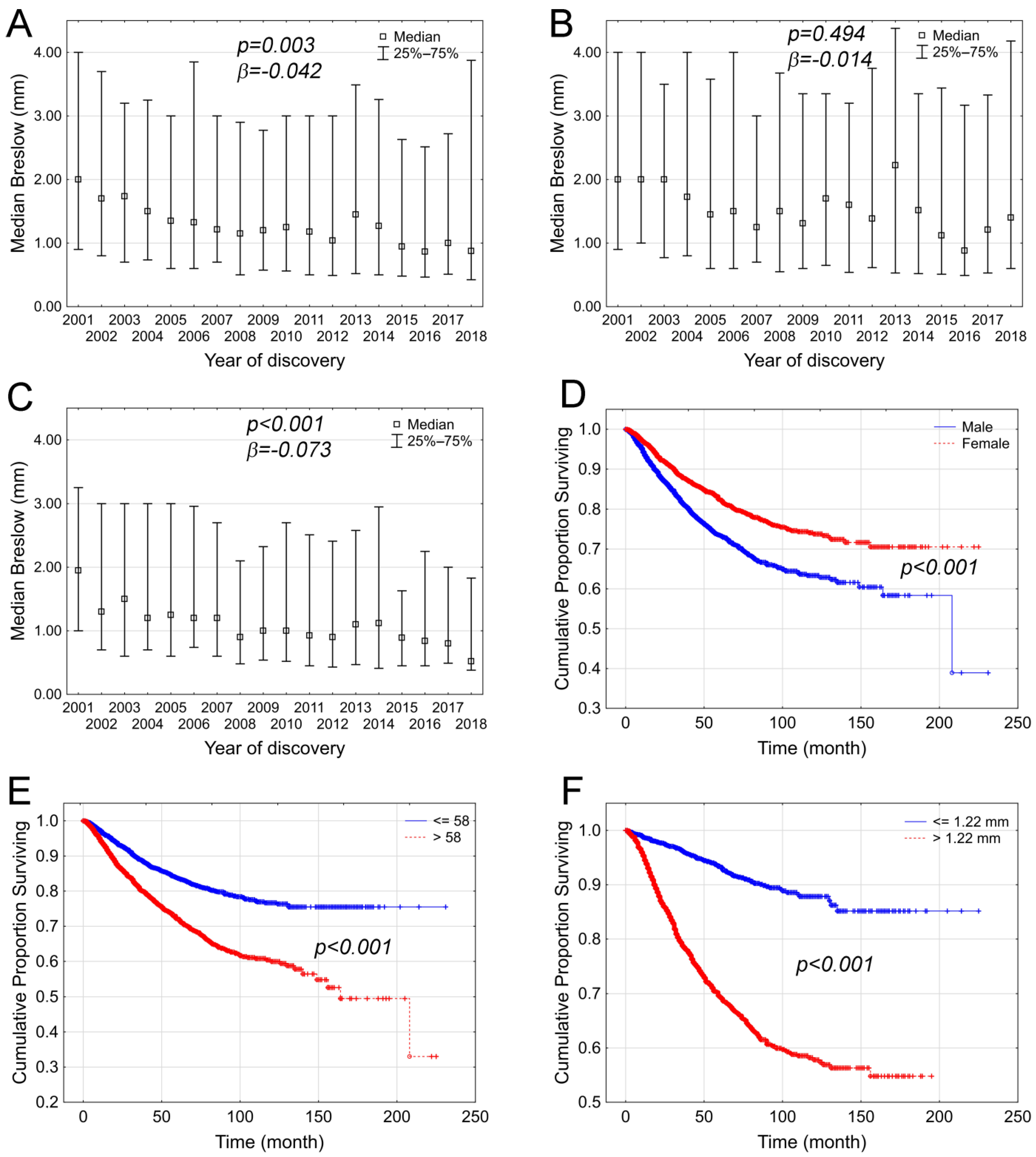
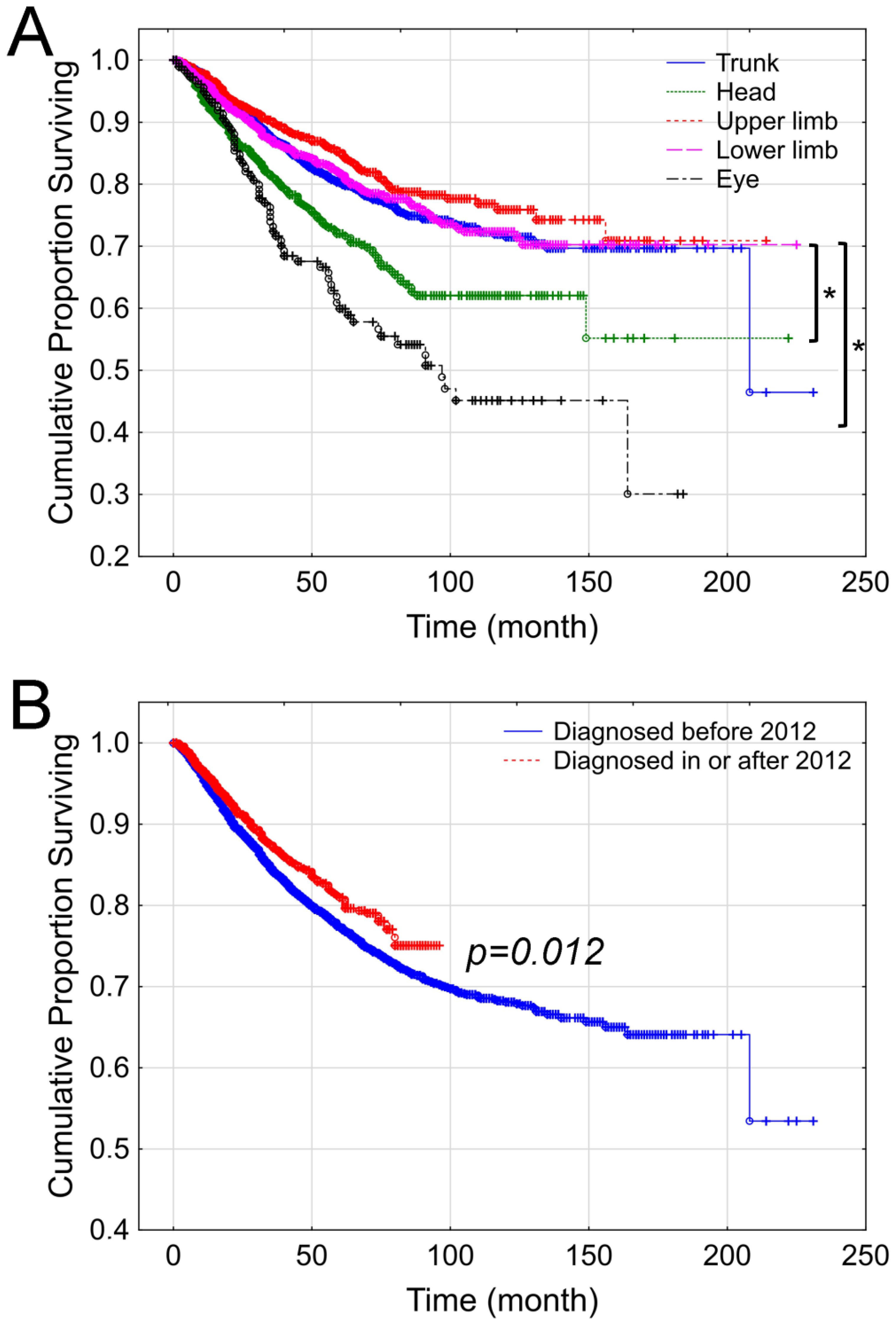
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nikolaou, V.; Stratigos, A.J. Emerging trends in the epidemiology of melanoma. Br. J. Dermatol. 2014, 170, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Waseh, S.; Lee, J.B. Advances in melanoma: Epidemiology, diagnosis, and prognosis. Front. Med. 2023, 10, 1268479. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Singh, D.; Laversanne, M.; Vignat, J.; Vaccarella, S.; Meheus, F.; Cust, A.E.; de Vries, E.; Whiteman, D.C.; Bray, F. Global Burden of Cutaneous Melanoma in 2020 and Projections to 2040. JAMA Dermatol. 2022, 158, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Rahib, L.; Wehner, M.R.; Matrisian, L.M.; Nead, K.T. Estimated Projection of US Cancer Incidence and Death to 2040. JAMA Netw. Open 2021, 4, e214708. [Google Scholar] [CrossRef]
- Lazovich, D.; Vogel, R.I.; Berwick, M.; Weinstock, M.A.; Anderson, K.E.; Warshaw, E.M. Indoor tanning and risk of melanoma: A case-control study in a highly exposed population. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1557–1568. [Google Scholar] [CrossRef]
- Li, Y.; Wu, J.; Cao, Z. Childhood sunburn and risk of melanoma and non-melanoma skin cancer: A Mendelian randomization study. Environ. Sci. Pollut. Res. Int. 2023, 30, 122011–122023. [Google Scholar] [CrossRef]
- Puckett, Y.; Wilson, A.M.; Farci, F.; Thevenin, C. Melanoma Pathology. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Parrag, P.; Wéber, A.; Liszkay, G.; Nagy, P.; Kásler, M.; Polgár, C.; Kenessey, I. Hungarian situation of melanoma incidence and mortality in the first two decades of 21st century. Magy. Onkol. 2022, 66, 94–99. [Google Scholar]
- Liszkay, G.; Kiss, Z.; Gyulai, R.; Oláh, J.; Holló, P.; Emri, G.; Csejtei, A.; Kenessey, I.; Benedek, A.; Polányi, Z.; et al. Changing Trends in Melanoma Incidence and Decreasing Melanoma Mortality in Hungary Between 2011 and 2019: A Nationwide Epidemiological Study. Front. Oncol. 2020, 10, 612459. [Google Scholar] [CrossRef]
- Kenessey, I.; Nagy, P.; Polgar, C. The Hungarian situation of cancer epidemiology in the second decade of the 21st century. Magy. Onkol. 2022, 66, 175–184. [Google Scholar]
- Welch, H.G.; Mazer, B.L.; Adamson, A.S. The Rapid Rise in Cutaneous Melanoma Diagnoses. N. Engl. J. Med. 2021, 384, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Bolick, N.L.; Geller, A.C. Epidemiology of Melanoma. Hematol. Oncol. Clin. N. Am. 2021, 35, 57–72. [Google Scholar] [CrossRef] [PubMed]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Barsouk, A. Epidemiology of Melanoma. Med. Sci. 2021, 9, 63. [Google Scholar] [CrossRef]
- Janka, E.A.; Ványai, B.; Dajnoki, Z.; Szabó, I.L.; Reibl, D.; Komka, I.; Blasszauer, C.; Várvölgyi, T.; Szegedi, A.; Emri, G. Regional variability of melanoma incidence and prevalence in Hungary. Epidemiological impact of ambient UV radiation and socioeconomic factors. Eur. J. Cancer Prev. 2022, 31, 377–384. [Google Scholar] [CrossRef]
- Balatoni, T.; Liszkay, G.; Miklós, Z.; Kásler, M. Epidemiology of malignant melanoma (Clinical experience at the National Institute of Oncology in Hungary). Orv. Hetil. 2011, 152, 1000–1006. [Google Scholar] [CrossRef]
- Raimondi, S.; Suppa, M.; Gandini, S. Melanoma Epidemiology and Sun Exposure. Acta Derm. Venereol. 2020, 100, adv00136. [Google Scholar] [CrossRef]
- Fears, T.R.; Sagebiel, R.W.; Halpern, A.; Elder, D.E.; Holly, E.A.; Guerry, D.t.; Tucker, M.A. Sunbeds and sunlamps: Who used them and their risk for melanoma. Pigment. Cell Melanoma Res. 2011, 24, 574–581. [Google Scholar] [CrossRef]
- Ghazawi, F.M.; Cyr, J.; Darwich, R.; Le, M.; Rahme, E.; Moreau, L.; Netchiporouk, E.; Zubarev, A.; Roshdy, O.; Glassman, S.J.; et al. Cutaneous malignant melanoma incidence and mortality trends in Canada: A comprehensive population-based study. J. Am. Acad. Dermatol. 2019, 80, 448–459. [Google Scholar] [CrossRef]
- Önefäldt, D.; Zommorodi, S.; Falk Delgado, A. Location of cutaneous malignant melanoma in Sweden 2004-2018—Mortality and sex differences. J. Plast. Reconstr. Aesthet. Surg. 2022, 75, 3398–3405. [Google Scholar] [CrossRef]
- Gandini, S.; Sera, F.; Cattaruzza, M.S.; Pasquini, P.; Picconi, O.; Boyle, P.; Melchi, C.F. Meta-analysis of risk factors for cutaneous melanoma: II. Sun exposure. Eur. J. Cancer 2005, 41, 45–60. [Google Scholar] [CrossRef]
- Buja, A.; Rugge, M.; Damiani, G.; Zorzi, M.; De Toni, C.; Vecchiato, A.; Del Fiore, P.; Spina, R.; Baldo, V.; Brazzale, A.R.; et al. Sex Differences in Cutaneous Melanoma: Incidence, Clinicopathological Profile, Survival, and Costs. J. Womens Health 2022, 31, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Saaiq, M.; Zalaudek, I.; Rao, B.; Lee, Y.; Rudnicka, L.; Czuwara, J.; Giuffrida, R.; Wollina, U.; Jafferany, M.; Lotti, T.; et al. A brief synopsis on scalp melanoma. Dermatol. Ther. 2020, 33, e13795. [Google Scholar] [CrossRef] [PubMed]
- Lovasik, B.P.; Sharma, I.; Russell, M.C.; Carlson, G.W.; Delman, K.A.; Rizzo, M. Invasive Scalp Melanoma: Role for Enhanced Detection Through Professional Training. Ann. Surg. Oncol. 2016, 23, 4049–4057. [Google Scholar] [CrossRef] [PubMed]
- Rastrelli, M.; Tropea, S.; Rossi, C.R.; Alaibac, M. Melanoma: Epidemiology, risk factors, pathogenesis, diagnosis and classification. In Vivo 2014, 28, 1005–1011. [Google Scholar]
- Larsen, A.C.; Dahmcke, C.M.; Dahl, C.; Siersma, V.D.; Toft, P.B.; Coupland, S.E.; Prause, J.U.; Guldberg, P.; Heegaard, S. A Retrospective Review of Conjunctival Melanoma Presentation, Treatment, and Outcome and an Investigation of Features Associated With BRAF Mutations. JAMA Ophthalmol. 2015, 133, 1295–1303. [Google Scholar] [CrossRef]
- Berk-Krauss, J.; Stein, J.A.; Weber, J.; Polsky, D.; Geller, A.C. New Systematic Therapies and Trends in Cutaneous Melanoma Deaths Among US Whites, 1986–2016. Am. J. Public Health 2020, 110, 731–733. [Google Scholar] [CrossRef]
- Várnai, M.; Kiss, Z.; Gyulai, R.; Oláh, J.; Holló, P.; Emri, G.; Csejtei, A.; Kenessey, I.; Benedek, A.; Polányi, Z.; et al. Improving Quality Indicator of Melanoma Management—Change of Melanoma Mortality-to-Incidence Rate Ratio Based on a Hungarian Nationwide Retrospective Study. Front. Oncol. 2021, 11, 745550. [Google Scholar] [CrossRef]
| Total Number of Patients (N; %) | 6267 (100%) |
| Age (years) (median, range) | 58 (7–94) |
| Gender | |
| Male | 2970 (47.4%) |
| Female | 3297 (52.6%) |
| Breslow (mm) (median, range) | 1.22 (0.03–50) |
| Follow-up (months) (median, range) | 38 (0–231) |
| Status (N; %) | |
| Alive | 5122 (81.7%) |
| Dead | 1145 (18.3%) |
| Location (N; %) | |
| Trunk | 2776 (45.3%) |
| Head and neck region | 690 (11.3%) |
| Upper extremities | 1061 (17.3%) |
| Lower extremities | 1320 (21.5%) |
| Uveal | 194 (3.2%) |
| Occult | 44 (0.7%) |
| Mucosal | 11 (0.2%) |
| Genital region | 25 (0.4%) |
| Anal region | 10 (0.2%) |
| Histological subtype (N; %) | |
| Superficial spreading | 2216 (35.4%) |
| Nodular | 798 (12.7%) |
| Lentigo melanoma maligna | 152 (2.4%) |
| Ocular | 193 (3.1%) |
| Mucosal | 33 (0.5%) |
| Other | 24 (0.4%) |
| No data available | 2800 (44.7%) |
| Exulceration of primary (N; %) | |
| No | 1183 (18.8%) |
| Yes | 603 (9.6%) |
| No data available | 4481 (71.5%) |
| Clark level (N; %) | |
| I | 455 (7.3%) |
| II | 1044 (16.7%) |
| III | 1829 (29.2%) |
| IV | 1901 (30.3%) |
| V | 314 (5%) |
| No data available | 724 (11.6%) |
| Male | Female | p | |
|---|---|---|---|
| Total number of patients | 2970 (47.4%) | 3297 (52.6%) | |
| Age (in years) (median; range) | 60.5 (7–94) | 55 (10–93) | p < 0.001 |
| Breslow (mm) (median; range) | 1.5 (0.05–45) | 1.075 (0.03–50) | p < 0.001 |
| Localization (N, %) | p < 0.001 | ||
| Trunk | 1574 (54.4%) | 1202 (37.1%) | |
| Head and neck region | 383 (13.2%) | 307 (9.5%) | |
| Upper extremities | 474 (16.4%) | 587 (18.1%) | |
| Lower extremities | 348 (12.2%) | 971 (30.0%) | |
| Eye | 79 (2.7%) | 115 (3.6%) | |
| Occult | 25 (0.9%) | 19 (0.6%) | |
| Mucosa | 4 (0.1%) | 7 (0.2%) | |
| Genital region | 2 (0.1%) | 23 (0.7%) | |
| Anal region | 5 (0.2%) | 5 (0.2%) | |
| Exulceration (N; %) | p < 0.001 | ||
| No | 529 (17.8%) | 654 (19.8%) | |
| Yes | 328 (11.0%) | 275 (8.3%) | |
| No data available | 2113 (71.1%) | 2368 (71.8%) |
| RR (95% CI) | p | |
|---|---|---|
| Age | 1.013 (1.005–1.022) | 0.002 |
| Gender (female—reference: male) | 0.787 (0.606–1.022) | 0.073 |
| Breslow | 1.217 (1.21–1.225) | <0.001 |
| Location (lower extremities—reference: upper extremities) | 1.142 (0822–1.586) | 0.912 |
| (head and neck—reference: upper extremities) | 1.27 (0.794–2.032) | 0.462 |
| (trunk—reference: upper extremities) | 1.103 (0.785–1.549) | 0.906 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gubán, R.; Parrag, P.; Kispál, M.T.; Czirbesz, K.; Danyi, T.; Kenessey, I.; Liszkay, G. Characterization of Melanoma in Hungary Based on a Retrospective Single-Center Study Between 2001 and 2018. Cancers 2025, 17, 2171. https://doi.org/10.3390/cancers17132171
Gubán R, Parrag P, Kispál MT, Czirbesz K, Danyi T, Kenessey I, Liszkay G. Characterization of Melanoma in Hungary Based on a Retrospective Single-Center Study Between 2001 and 2018. Cancers. 2025; 17(13):2171. https://doi.org/10.3390/cancers17132171
Chicago/Turabian StyleGubán, Renáta, Petra Parrag, Mihály Tamás Kispál, Kata Czirbesz, Tímea Danyi, István Kenessey, and Gabriella Liszkay. 2025. "Characterization of Melanoma in Hungary Based on a Retrospective Single-Center Study Between 2001 and 2018" Cancers 17, no. 13: 2171. https://doi.org/10.3390/cancers17132171
APA StyleGubán, R., Parrag, P., Kispál, M. T., Czirbesz, K., Danyi, T., Kenessey, I., & Liszkay, G. (2025). Characterization of Melanoma in Hungary Based on a Retrospective Single-Center Study Between 2001 and 2018. Cancers, 17(13), 2171. https://doi.org/10.3390/cancers17132171







