Analysis of Factors Affecting the Diagnostic Efficacy of Frozen Sections for Tumor Spread Through Air Spaces in Lung Adenocarcinoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Specimen Processing and Baseline Data Collection
2.3. Records of Pathological Indicators
2.4. Statistical Methods
3. Results
3.1. Baseline Characteristic Data and Diagnostic Efficacy of Frozen Sections for Invasive Adenocarcinoma
3.2. Efficacy of Frozen Section STAS Diagnosis
3.3. Logistic Regression for Screening Factors Affecting the Diagnosis of Frozen STAS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| WHO | World Health Organization |
| CT | Computed tomography |
| RFS | Recurrence-free survival |
| OS | Overall survival |
| OCT | Optimal cutting temperature |
| STAS | Tumor spread through air spaces |
| H&E | Hematoxylin and eosin |
| STAKS | Spread through a knife surface |
| CT | Computed tomography |
| GGO | Ground glass opacity |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- De Giglio, A.; Di Federico, A.; Gelsomino, F.; Ardizzoni, A. Prognostic relevance of pleural invasion for resected NSCLC patients undergoing adjuvant treatments: A propensity score-matched analysis of SEER database. Lung Cancer 2021, 161, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.L.; Ocampo, P.S.S.; Xia, Y.; Zhong, H.; Russell, P.A.; Minami, Y.; Cooper, W.A.; Yoshida, A.; Bubendorf, L.; Papotti, M.; et al. A Grading System for Invasive Pulmonary Adenocarcinoma: A Proposal from the International Association for the Study of Lung Cancer Pathology Committee. J. Thorac. Oncol. 2020, 15, 1599–1610. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yi, E.; Bae, M.K.; Cho, S.; Chung, J.H.; Jheon, S.; Kim, K. Pathological prognostic factors of recurrence in early stage lung adenocarcinoma. ANZ J. Surg. 2018, 88, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Brambilla, E.; Burke, A.P.; Marx, A.; Nicholson, A.G. Introduction to The 2015 World Health Organization Classification of Tumors of the Lung, Pleura, Thymus, and Heart. J. Thorac. Oncol. 2015, 10, 1240–1242. [Google Scholar] [CrossRef] [PubMed]
- Warth, A.; Beasley, M.B.; Mino-Kenudson, M. Breaking New Ground: The Evolving Concept of Spread through Air Spaces (STAS). J. Thorac. Oncol. 2017, 12, 176–178. [Google Scholar] [CrossRef] [PubMed]
- Kadota, K.; Nitadori, J.I.; Sima, C.S.; Ujiie, H.; Rizk, N.P.; Jones, D.R.; Adusumilli, P.S.; Travis, W.D. Tumor Spread through Air Spaces is an Important Pattern of Invasion and Impacts the Frequency and Location of Recurrences after Limited Resection for Small Stage I Lung Adenocarcinomas. J. Thorac. Oncol. 2015, 10, 806–814. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ding, Y.; Chen, Y.; Wen, H.; Li, J.; Chen, J.; Xu, M.; Geng, H.; You, L.; Pan, X.; Sun, D. Pretreatment prediction of tumour spread through air spaces in clinical stage I non-small-cell lung cancer. Eur. J. Cardio-Thorac. Surg. 2022, 62, ezac248. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Toyokawa, G.; Yamada, Y.; Tagawa, T.; Oda, Y. Significance of spread through air spaces in early-stage lung adenocarcinomas undergoing limited resection. Thorac. Cancer 2018, 9, 1255–1261. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, L.; Yang, Y.; Ma, P.; Zheng, B.; Liu, W.; Zhang, Z.; Ding, N.; Liu, L.; Mao, Y.; Lv, N. Spread through air spaces predicts a worse survival in patients with stage I adenocarcinomas > 2 cm after radical lobectomy. J. Thorac. Dis. 2018, 10, 5308–5317, Erratum in J. Thorac. Dis. 2019, 11, E80–E81. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kadota, K.; Kushida, Y.; Kagawa, S.; Ishikawa, R.; Ibuki, E.; Inoue, K.; Go, T.; Yokomise, H.; Ishii, T.; Kadowaki, N.; et al. Limited Resection Is Associated with a Higher Risk of Locoregional Recurrence than Lobectomy in Stage I Lung Adenocarcinoma With Tumor Spread Through Air Spaces. Am. J. Surg. Pathol. 2019, 43, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Masai, K.; Sakurai, H.; Sukeda, A.; Suzuki, S.; Asakura, K.; Nakagawa, K.; Asamura, H.; Watanabe, S.I.; Motoi, N.; Hiraoka, N. Prognostic Impact of Margin Distance and Tumor Spread Through Air Spaces in Limited Resection for Primary Lung Cancer. J. Thorac. Oncol. 2017, 12, 1788–1797. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Xie, H.; Su, H.; She, Y.; Zhu, E.; Fan, Z.; Zhou, F.; Ren, Y.; Xie, D.; Zheng, H.; et al. Tumor Spread through Air Spaces Affects the Recurrence and Overall Survival in Patients with Lung Adenocarcinoma > 2 to 3 cm. J. Thorac. Oncol. 2017, 12, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Walts, A.E.; Marchevsky, A.M. Current Evidence Does Not Warrant Frozen Section Evaluation for the Presence of Tumor Spread Through Alveolar Spaces. Arch. Pathol. Lab. Med. 2018, 142, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, T.; Kameda, K.; Lu, S.; Bott, M.J.; Tan, K.S.; Montecalvo, J.; Chang, J.C.; Rekhtman, N.; Jones, D.R.; Travis, W.D.; et al. Lobectomy Is Associated with Better Outcomes than Sublobar Resection in Spread through Air Spaces (STAS)-Positive T1 Lung Adenocarcinoma: A Propensity Score-Matched Analysis. J. Thorac. Oncol. 2019, 14, 87–98. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, F.; Villalba, J.A.; Sayo, T.M.S.; Narula, N.; Pass, H.; Mino-Kenudson, M.; Moreira, A.L. Assessment of the feasibility of frozen sections for the detection of spread through air spaces (STAS) in pulmonary adenocarcinoma. Mod. Pathol. 2022, 35, 210–217. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Villalba, J.A.; Shih, A.R.; Sayo, T.M.S.; Kunitoki, K.; Hung, Y.P.; Ly, A.; Kem, M.; Hariri, L.P.; Muniappan, A.; Gaissert, H.A.; et al. Accuracy and Reproducibility of Intraoperative Assessment on Tumor Spread Through Air Spaces in Stage 1 Lung Adenocarcinomas. J. Thorac. Oncol. 2021, 16, 619–629. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Metovic, J.; Falco, E.C.; Vissio, E.; Santoro, F.; Delsedime, L.; Massa, F.; Pittaro, A.; Osella-Abate, S.; Cassoni, P.; Volante, M.; et al. Gross Specimen Handling Procedures Do Not Impact the Occurrence of Spread Through Air Spaces (STAS) in Lung Cancer. Am. J. Surg. Pathol. 2021, 45, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Su, H.; Zhu, E.; Gu, C.; Zhao, S.; She, Y.; Ren, Y.; Xie, D.; Zheng, H.; Wu, C.; et al. Morphological Subtypes of Tumor Spread Through Air Spaces in Non-Small Cell Lung Cancer: Prognostic Heterogeneity and Its Underlying Mechanism. Front. Oncol. 2021, 11, 608353. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Blaauwgeers, H.; Russell, P.A.; Jones, K.D.; Radonic, T.; Thunnissen, E. Pulmonary loose tumor tissue fragments and spread through air spaces (STAS): Invasive pattern or artifact? A critical review. Lung Cancer 2018, 123, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Myung, J.K.; Choe, G.; Chung, D.H.; Seo, J.W.; Jheon, S.; Lee, C.T.; Chung, J.H. A simple inflation method for frozen section diagnosis of minute precancerous lesions of the lung. Lung Cancer 2008, 59, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Blaauwgeers, H.; Flieder, D.; Warth, A.; Harms, A.; Monkhorst, K.; Witte, B.; Thunnissen, E. A Prospective Study of Loose Tissue Fragments in Non-Small Cell Lung Cancer Resection Specimens: An Alternative View to “Spread Through Air Spaces”. Am. J. Surg. Pathol. 2017, 41, 1226–1230. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, J.; Nakajima, T.; Suzuki, H.; Nagato, K.; Iwata, T.; Yoshida, S.; Fukuyo, M.; Ota, S.; Nakatani, Y.; Yoshino, I. Impact of free tumor clusters on prognosis after resection of pulmonary adenocarcinoma. J. Thorac. Cardiovasc. Surg. 2016, 152, 64–72.e1. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Zheng, Q.; Deng, C.; Fu, Z.; Shen, X.; Jin, Y.; Yang, Y.; Qian, B.; Yuan, C.; Wang, W.; et al. Prediction of spread through air spaces (STAS) by intraoperative frozen section for cT1N0M0 invasive lung adenocarcinoma: A multi-centre observational study (ECTOP-1016). Ann. Surg. 2025, 281, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Y.; Feng, M.; Yang, S.; Zhou, L.; Ge, D.; Lu, S.; Liu, L.; Shan, F.; Zhang, Z. Radiomics nomograms of tumours and peritumoral regions for pre-operative prediction of spread through air spaces in lung adenocarcinoma. Transl. Oncol. 2020, 13, 100820. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; He, W.; Chen, C.; Tan, F.; Chen, J.; Yang, L.; Chen, D.; Xia, L. A CT-based deep learning model for preoperative prediction of spread through air spaces in clinical stage I lung adenocarcinoma. Front. Oncol. 2025, 14, 1482965. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Y.; Lyu, D.; Hu, L.; Wu, J.; Duan, S.; Zhou, T.; Tu, W.; Xiao, Y.; Fan, L.; Liu, S. CT-based intratumoral and peritumoral radiomics nomograms for the preoperative prediction of spread through air spaces in clinical stage IA non-small cell lung cancer. J. Imaging Inform. Med. 2024, 37, 520–535. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Feng, Y.; Ding, H.; Huang, X.; Zhang, Y.; Lu, M.; Zhang, T.; Wang, H.; Chen, Y.; Mao, Q.; Xia, W.; et al. Deep learning-based detection and semi-quantitative model for spread through air spaces (STAS) in lung adenocarcinoma. npj Precis. Oncol. 2024, 8, 173. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, S.; Liu, X.; Jiang, C.; Kang, W.; Pan, Y.; Tang, X.; Luo, Y.; Gong, J. CT-based super-resolution deep learning models with attention mechanisms for predicting spread through air spaces of solid or part-solid lung adenocarcinoma. Acad. Radiol. 2024, 31, 2601–2609. [Google Scholar] [CrossRef] [PubMed]
- Tasnim, S.; Raja, S.; Mukhopadhyay, S.; Blackstone, E.H.; Toth, A.J.; Barron, J.O.; Raymond, D.P.; Bribriesco, A.C.; Schraufnagel, D.P.; Murthy, S.C.; et al. Preoperative predictors of spread through air spaces in lung cancer: A narrative review. J. Thorac. Cardiovasc. Surg. 2024, 168, 660–669. [Google Scholar] [CrossRef] [PubMed]


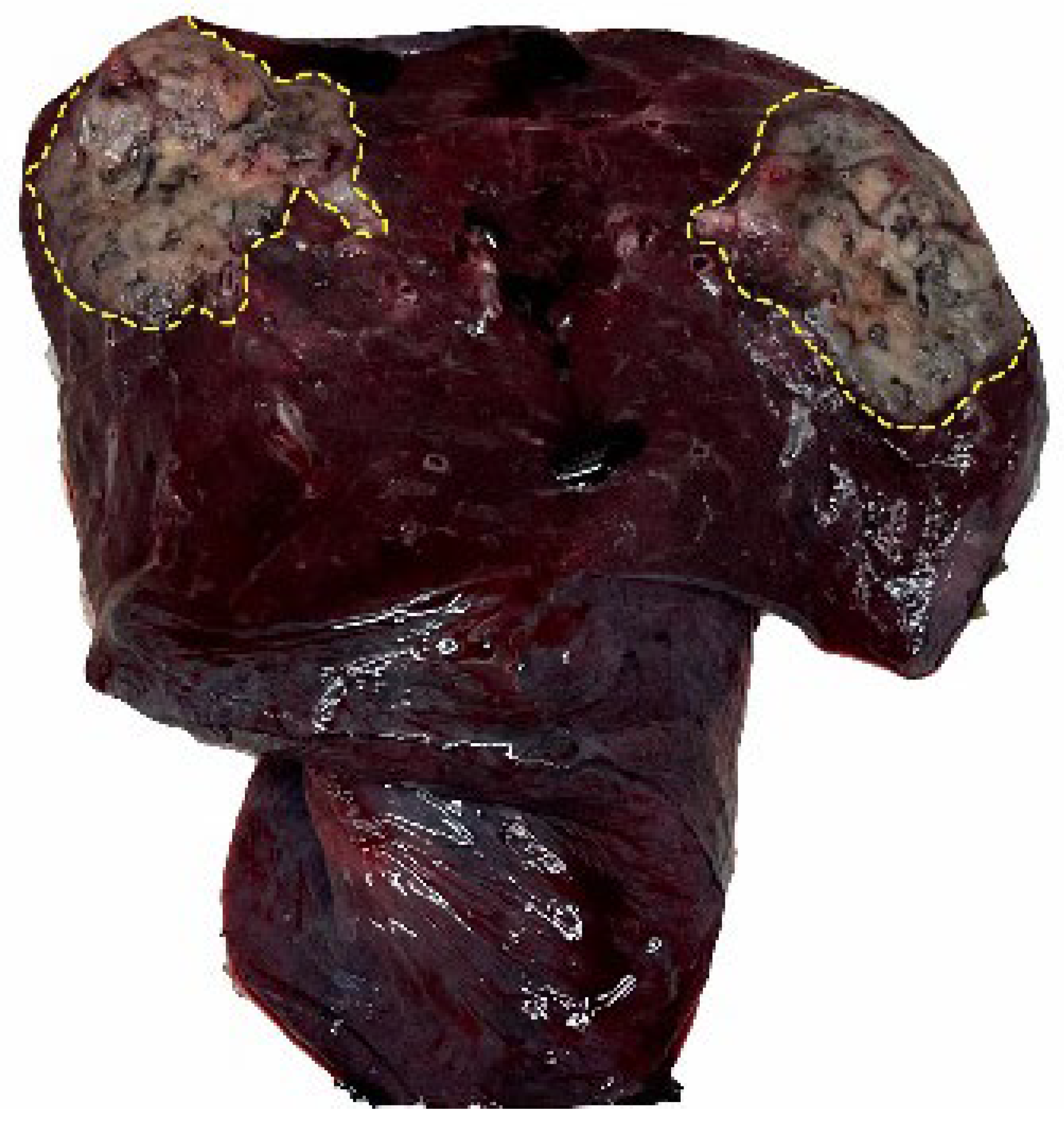
| Benign | Malignant | Total | The Diagnostic Accuracy of Benign and Malignant | ||
|---|---|---|---|---|---|
| Evaluability of STAS in paraffin sections | No | 6 | 127 | 133 | Non-evaluable group 95.5% vs. evaluable group 95.2% |
| Yes | 25 | 491 | 516 | p value = 0.872 | |
| Total | 31 | 618 | 649 | ||
| Gender, n (%) | Male | 246 (47.70) |
|---|---|---|
| Female | 270 (52.30) | |
| Age (years) | Mean | 62.16 |
| Median | 63 | |
| Standard deviation | 8.04 | |
| Range | 18–80 | |
| Frozen sections, n (%) | Benign | 29 (5.60) |
| Malignant | 487 (94.40) | |
| Type of surgery, n (%) | Lobectomy | 489 (94.80) |
| Segmentectomy | 27 (5.20) | |
| Number of frozen sections, n (%) | 1 | 406 (78.70) |
| 1+ | 110 (21.30) | |
| Tumor boundary, n (%) | Not clear | 167 (32.40) |
| Clear | 349 (67.60) | |
| Diameter of paraffin section, D1 (cm) | Mean | 2.19 |
| Standard deviation | 0.88 | |
| Diameter of frozen section, D2 (cm) | Mean | 2.12 |
| Standard deviation | 0.47 | |
| Distance between the tumor boundary and the tissue edge under the digital biopsy scanner (μm), d | Mean | 2285.19 |
| Standard deviation | 543.44 | |
| Number of alveoli from the outer edge of the specimen under digital section scanner, n (%) | 5–10 | 60 (11.60) |
| 10+ | 456 (88.40) | |
| Pathological stage, n (%) | IA | 259 (50.20) |
| IB | 184 (35.70) | |
| IIA | 2 (0.40) | |
| IIB | 29 (5.60) | |
| IIIA | 39 (7.60) | |
| IIIB | 3 (0.60) | |
| T stage, n (%) | 1 | 282 (54.70) |
| 2 | 214 (41.50) | |
| 3 | 16 (3.10) | |
| 4 | 4 (0.80) | |
| STAS on frozen sections, n (%) | No | 341 (66.10) |
| Yes | 175 (33.90) | |
| STAS on paraffin sections, n (%) | No | 321 (62.20) |
| Yes | 195 (37.80) |
| Paraffin Section | Total | Diagnostic Test Performance of STAS on Frozen Sections | |||
|---|---|---|---|---|---|
| Frozen section | STAS− | STAS+ | Sensitivity = 55.4%, NPV = 74.5% | ||
| STAS− | 254 | 87 | 341 | Specificity = 79.1%, PPV = 61.7% | |
| STAS+ | 67 | 108 | 175 | Accuracy = 70.2% | |
| Total | 321 | 195 | 516 | k = 0.35 | |
| Univariate Analysis | |||
|---|---|---|---|
| p Value | HR | 95% Confidence Interval | |
| Gender | 0.145 | 0.755 | 0.517–1.102 |
| Age | 0.156 | 0.983 | 0.959–1.007 |
| Type of surgery | 0.98 | 0.989 | 0.423–2.311 |
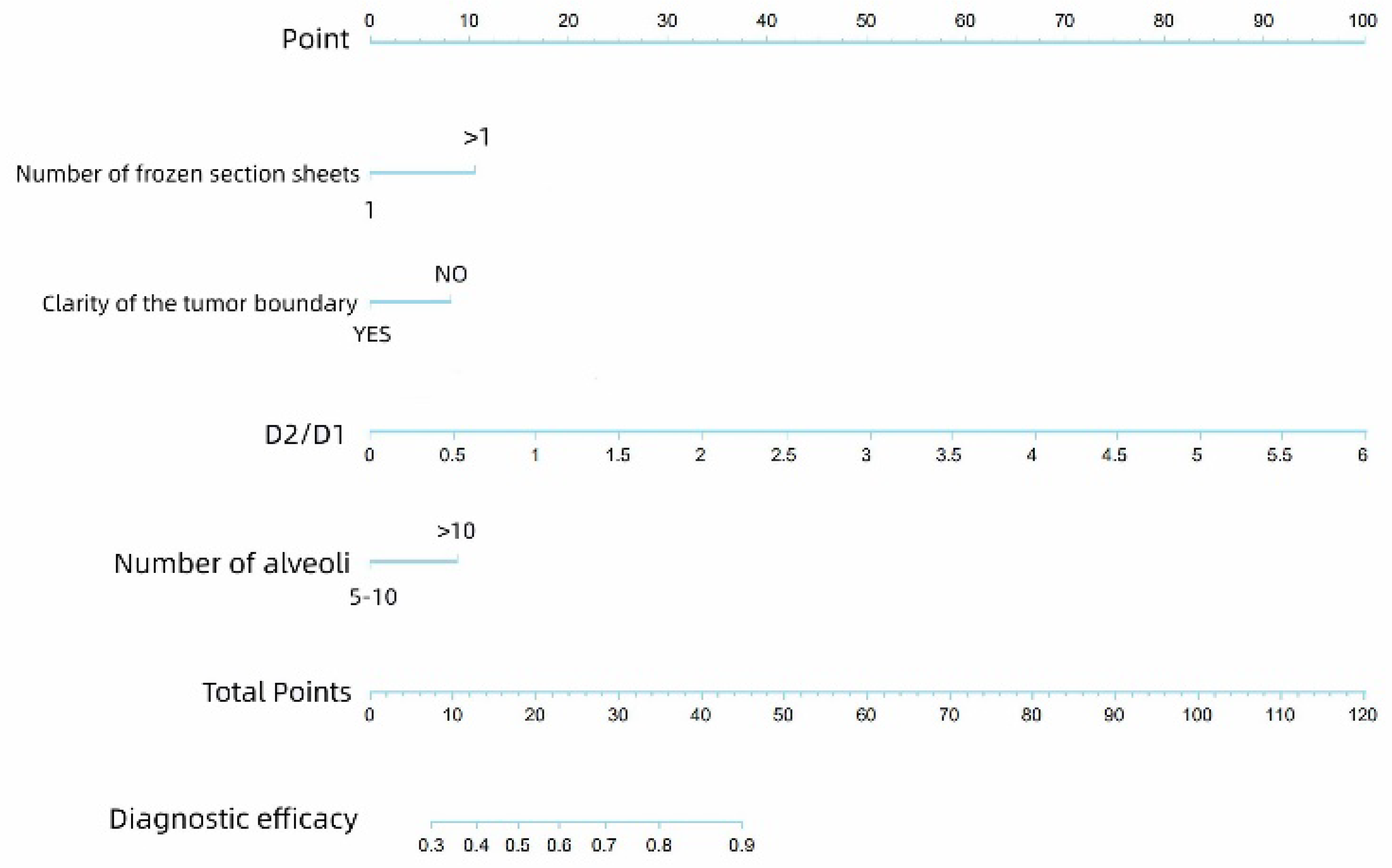
| Number of frozen sections | 0.00 | 3.025 | 1.713–5.341 |
| Tumor boundary | 0.00 | 0.218 | 0.130–0.365 |
| Diameter of paraffin section, D1 | 0.00 | 0.567 | 0.453–0.709 |
| Diameter of frozen section, D2 | 0.222 | 1.282 | 0.860–1.192 |
| D2/D1 | 0.00 | 7.283 | 3.813–13.913 |
| Number of alveoli | 0.001 | 2.477 | 1.434–4.279 |
| d | 0.918 | 1 | 1.000–1.000 |
| d/D2 | 0.931 | 0.816 | 0.008–83.141 |
| Pathological stage | 0.068 | ||
| T stage | 0.422 | 0.88 | 0.645–1.202 |
| Multivariate Analysis | |||
|---|---|---|---|
| p Value | HR | 95% Confidence Interval | |
| Number of frozen sections | 0.004 | 2.381 | 1.314–4.317 |
| Clarity of tumor boundary | 0.041 | 0.503 | 0.260–0.972 |
| Diameter of paraffin section, D1 | 0.483 | 1.171 | 0.753–1.820 |
| D2/D1 ratio | 0.002 | 3.697 | 1.630–8.389 |
| Number of alveoli | 0.016 | 2.034 | 1.143–3.618 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Ding, Y.; Ren, J.; Li, J.; Wang, K.; Sun, S.; Zhang, W.; Xu, M.; Jing, Y.; Gao, G.; et al. Analysis of Factors Affecting the Diagnostic Efficacy of Frozen Sections for Tumor Spread Through Air Spaces in Lung Adenocarcinoma. Cancers 2025, 17, 2168. https://doi.org/10.3390/cancers17132168
Liu X, Ding Y, Ren J, Li J, Wang K, Sun S, Zhang W, Xu M, Jing Y, Gao G, et al. Analysis of Factors Affecting the Diagnostic Efficacy of Frozen Sections for Tumor Spread Through Air Spaces in Lung Adenocarcinoma. Cancers. 2025; 17(13):2168. https://doi.org/10.3390/cancers17132168
Chicago/Turabian StyleLiu, Xin, Yun Ding, Jie Ren, Jiuzhen Li, Kai Wang, Shuai Sun, Weiran Zhang, Meilin Xu, Yuhao Jing, Guozheng Gao, and et al. 2025. "Analysis of Factors Affecting the Diagnostic Efficacy of Frozen Sections for Tumor Spread Through Air Spaces in Lung Adenocarcinoma" Cancers 17, no. 13: 2168. https://doi.org/10.3390/cancers17132168
APA StyleLiu, X., Ding, Y., Ren, J., Li, J., Wang, K., Sun, S., Zhang, W., Xu, M., Jing, Y., Gao, G., Zong, W., & Sun, D. (2025). Analysis of Factors Affecting the Diagnostic Efficacy of Frozen Sections for Tumor Spread Through Air Spaces in Lung Adenocarcinoma. Cancers, 17(13), 2168. https://doi.org/10.3390/cancers17132168





