Targeting DAMPs by Aspirin Inhibits Head and Neck Cancer Stem Cells and Stimulates Radio-Sensitization to Proton Therapy
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Reagents
2.2. RNA Isolation and Real Time PCR
2.3. Western Blot Analysis
2.4. ELISA
2.5. ALDH Activity
2.6. Immunocytochemistry
2.7. Proliferation Assays
2.8. Clinical Specimens and Immunohistochemistry
2.9. Fluorescent Immunohistochemistry
2.10. Migration Assay
2.11. Proton Irradiation
2.12. Statistics
3. Results
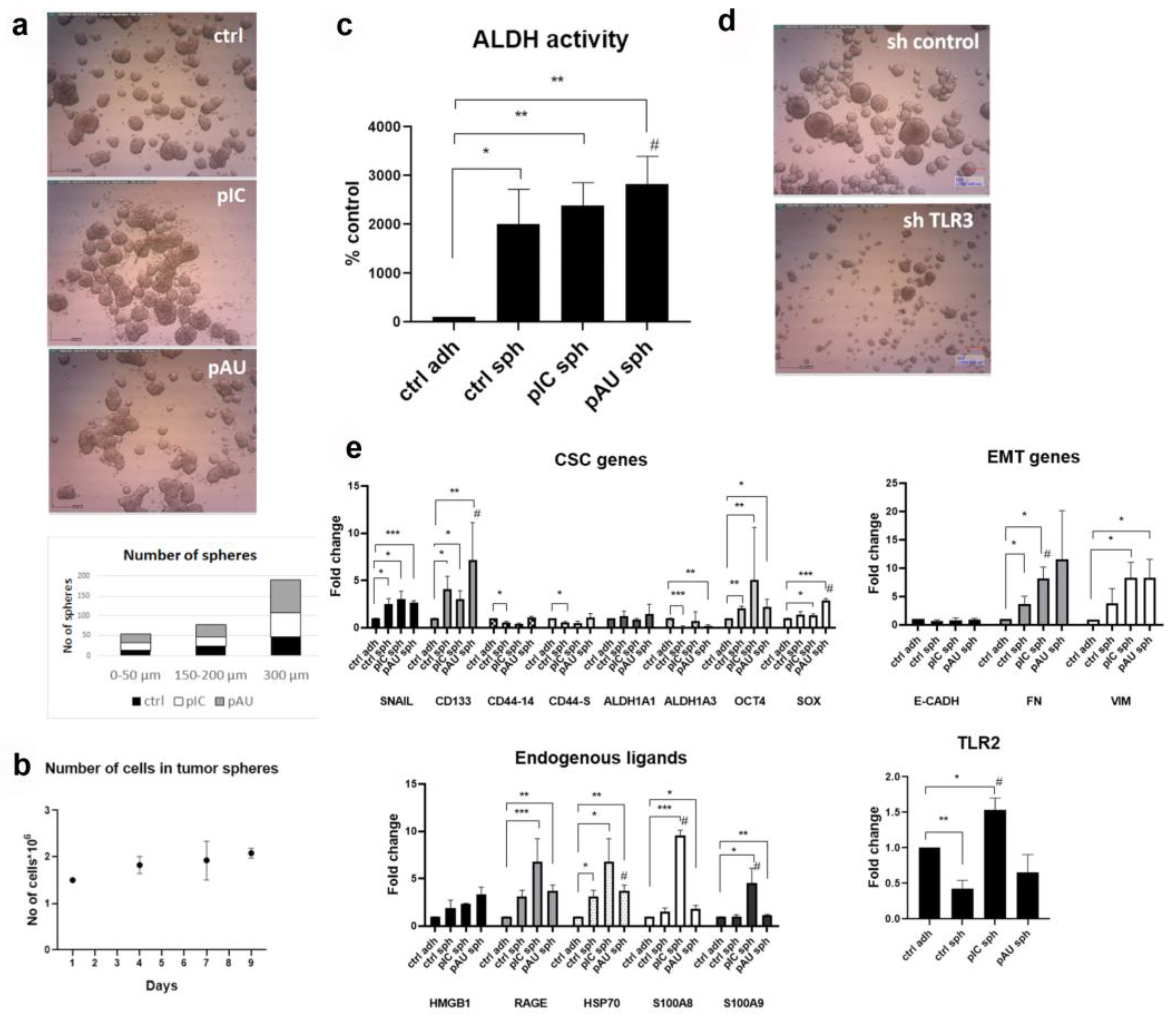
3.1. TLR3 Activation Increases the Stemness of HNSCC Tumor Spheres
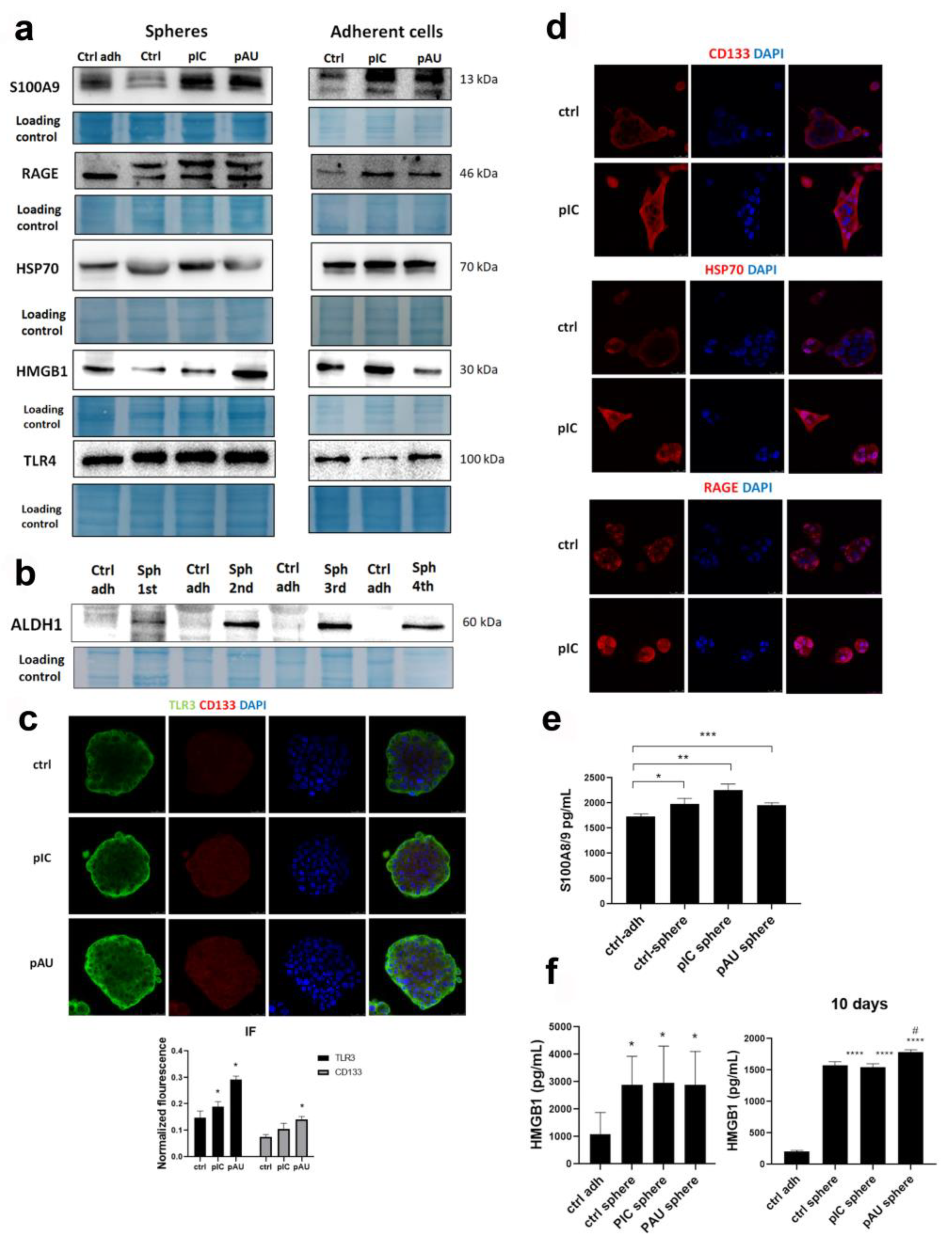
3.2. TLR3 Activation Induces the Expression of DAMPs and Their Release into the Microenvironment
3.3. TLR3 Activation Increases Cell Migration, Which Can Be Abolished by Aspirin and Metformin
3.4. Immunohistochemistry of Patients’ HNSCC Tissue Shows Strong Expression of TLR3 Which Co-Localizes with CD133, ALDHA1, and DAMPs
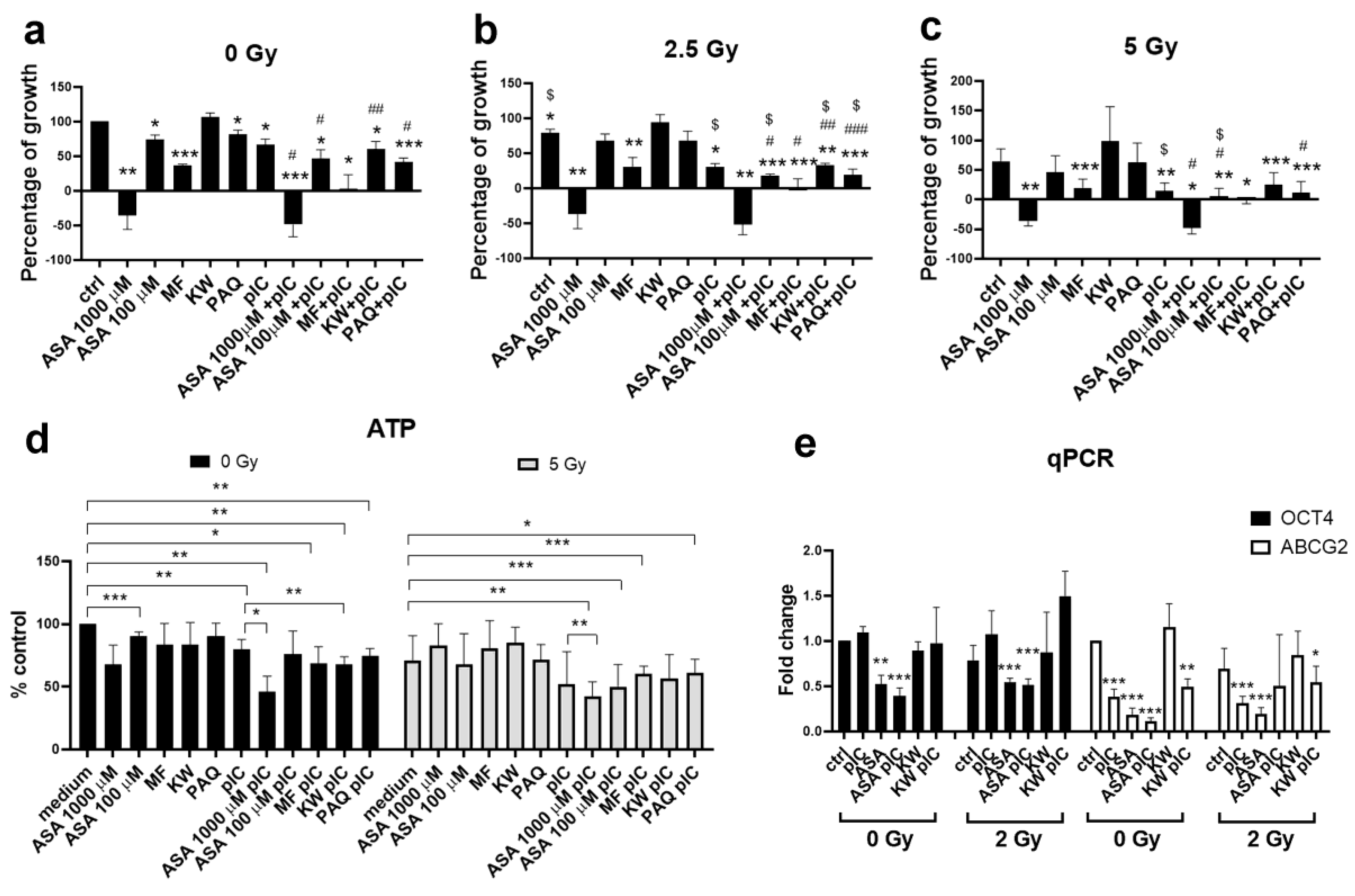
3.5. Gamma Irradiation in Combination with Aspirin and Poly (I:C) Reduces the Survival of Adherent Tumor Cells
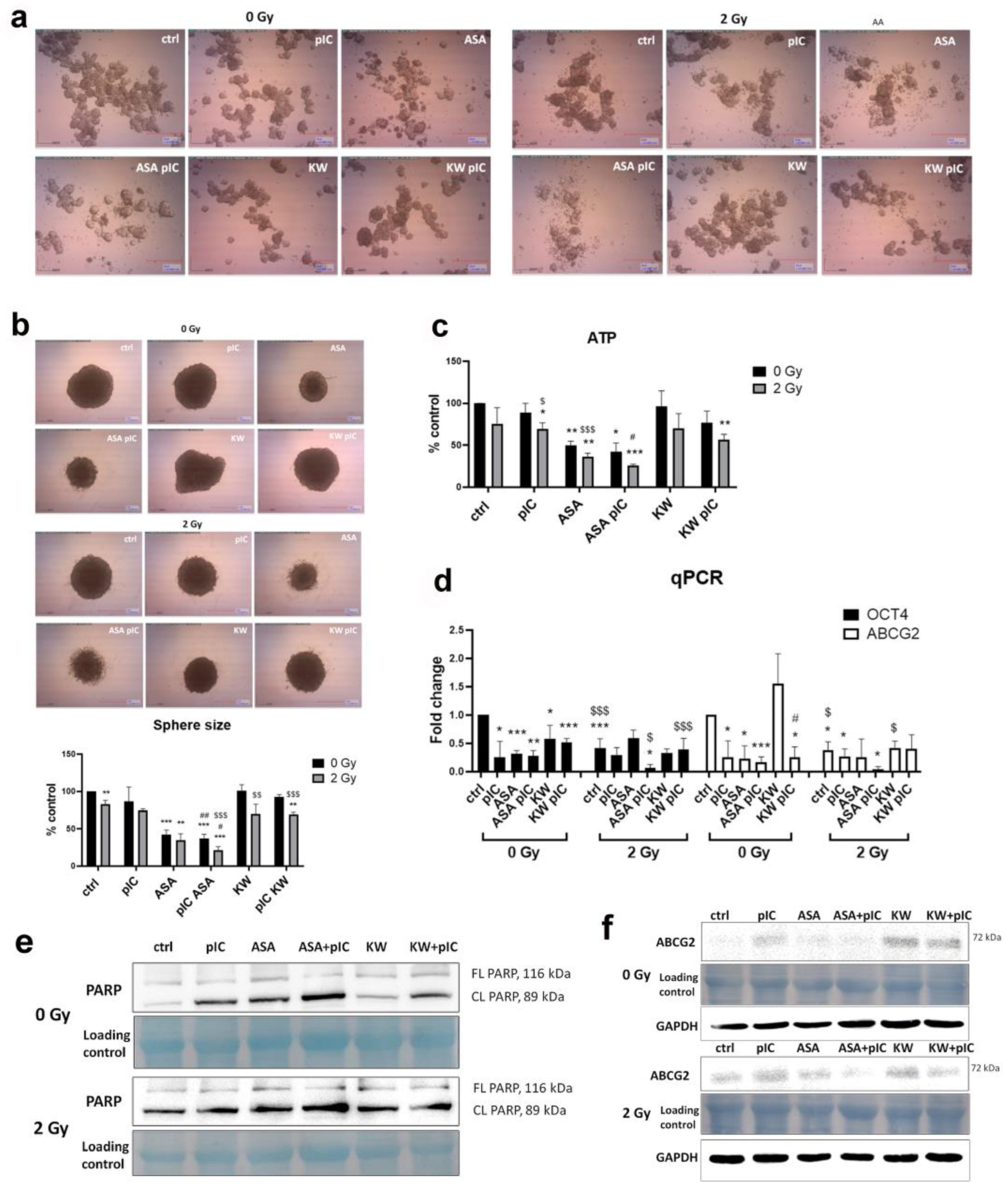
3.6. The Combination of Aspirin and Proton Irradiation Effectively Eradicates Cancer Stem Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALDH | aldehyde dehydrogenase |
| ASA | acetylsalicylic acid |
| BAX | BCL2-associated X protein |
| BCL2 | B cell lymphoma 2 |
| CSCs | Cancer stem cells |
| DAMPs | damage-associated molecular patterns |
| DSB | DNA double-stranded breaks |
| EMT | epithelial-to-mesenchymal transition |
| HNSCC | head and neck squamous cell carcinoma |
| HSP70 | heat shock protein 70 |
| KW | kahweol |
| MF | metformin |
| PAQ | paquinimod |
| RAGE | receptor for advanced glycation end products |
| SAA | serum amyloid A |
| TIGIT | T cell immunoglobulin and ITAM domain |
| TLR3 | Toll-like receptor 3 |
| TRAF6 | TNF receptor-associated factor 6 |
References
- Ortiz, R.C.; Amor, N.G.; Saito, L.M.; Santesso, M.R.; Lopes, N.M.; Buzo, R.F.; Fonseca, A.C.; Amaral-Silva, G.K.; Moyses, R.A.; Rodini, C.O. CSC(high)E-cadherin(low) immunohistochemistry panel predicts poor prognosis in oral squamous cell carcinoma. Sci. Rep. 2024, 14, 10583. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, M.H.; Sorensen, M.K.; Tramm, T.; Alsner, J.; Sorensen, B.S.; Maare, C.; Johansen, J.; Primdahl, H.; Bratland, A.; Kristensen, C.A.; et al. Tumor volume and cancer stem cell expression as prognostic markers for high-dose loco-regional failure in head and neck squamous cell carcinoma—A DAHANCA 19 study. Radiother. Oncol. 2024, 193, 110149. [Google Scholar] [CrossRef]
- Li, Y.; Lin, C.; Chu, Y.; Wei, Z.; Ding, Q.; Gu, S.; Deng, H.; Liao, Q.; Shen, Z. Characterization of Cancer Stem Cells in Laryngeal Squamous Cell Carcinoma by Single-cell RNA Sequencing. Genom. Proteom. Bioinform. 2024, 22, qzae056. [Google Scholar] [CrossRef] [PubMed]
- Salaun, B.; Coste, I.; Rissoan, M.C.; Lebecque, S.J.; Renno, T. TLR3 can directly trigger apoptosis in human cancer cells. J. Immunol. 2006, 176, 4894–4901. [Google Scholar] [CrossRef]
- Matijevic, T.; Pavelic, J. The dual role of TLR3 in metastatic cell line. Clin. Exp. Metastasis 2011, 28, 701–712. [Google Scholar] [CrossRef]
- Veyrat, M.; Durand, S.; Classe, M.; Glavan, T.M.; Oker, N.; Kapetanakis, N.I.; Jiang, X.; Gelin, A.; Herman, P.; Casiraghi, O.; et al. Stimulation of the toll-like receptor 3 promotes metabolic reprogramming in head and neck carcinoma cells. Oncotarget 2016, 7, 82580–82593. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Matijevic Glavan, T.; Cipak Gasparovic, A.; Verillaud, B.; Busson, P.; Pavelic, J. Toll-like receptor 3 stimulation triggers metabolic reprogramming in pharyngeal cancer cell line through Myc, MAPK, and HIF. Mol. Carcinog. 2017, 56, 1214–1226. [Google Scholar] [CrossRef]
- Paone, A.; Galli, R.; Gabellini, C.; Lukashev, D.; Starace, D.; Gorlach, A.; De Cesaris, P.; Ziparo, E.; Del Bufalo, D.; Sitkovsky, M.V.; et al. Toll-like receptor 3 regulates angiogenesis and apoptosis in prostate cancer cell lines through hypoxia-inducible factor 1 alpha. Neoplasia 2010, 12, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gu, Y.; Han, Y.; Zhang, Q.; Jiang, Z.; Zhang, X.; Huang, B.; Xu, X.; Zheng, J.; Cao, X. Tumor Exosomal RNAs Promote Lung Pre-metastatic Niche Formation by Activating Alveolar Epithelial TLR3 to Recruit Neutrophils. Cancer Cell 2016, 30, 243–256. [Google Scholar] [CrossRef]
- Vasiljevic, T.; Tarle, M.; Hat, K.; Luksic, I.; Mikulandra, M.; Busson, P.; Matijevic Glavan, T. Necrotic Cells from Head and Neck Carcinomas Release Biomolecules That Are Activating Toll-like Receptor 3. Int. J. Mol. Sci. 2023, 24, 15269. [Google Scholar] [CrossRef]
- Zapletal, E.; Vasiljevic, T.; Busson, P.; Glavan, T.M. Dialog beyond the Grave: Necrosis in the Tumor Microenvironment and Its Contribution to Tumor Growth. Int. J. Mol. Sci. 2023, 24, 5278. [Google Scholar] [CrossRef] [PubMed]
- Leemans, C.R.; Snijders, P.J.F.; Brakenhoff, R.H. The molecular landscape of head and neck cancer. Nat. Rev. Cancer 2018, 18, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.E.W.; Soulieres, D.; Le Tourneau, C.; Dinis, J.; Licitra, L.; Ahn, M.J.; Soria, A.; Machiels, J.P.; Mach, N.; Mehra, R.; et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): A randomised, open-label, phase 3 study. Lancet 2019, 393, 156–167. [Google Scholar] [CrossRef]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulieres, D.; Tahara, M.; de Castro, G., Jr.; Psyrri, A.; Baste, N.; Neupane, P.; Bratland, A.; et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef]
- Lui, V.W.; Hedberg, M.L.; Li, H.; Vangara, B.S.; Pendleton, K.; Zeng, Y.; Lu, Y.; Zhang, Q.; Du, Y.; Gilbert, B.R.; et al. Frequent mutation of the PI3K pathway in head and neck cancer defines predictive biomarkers. Cancer Discov. 2013, 3, 761–769. [Google Scholar] [CrossRef]
- Ritter, A.; Levyn, H.; Shah, J. Recent advances in head and neck surgical oncology. J. Surg. Oncol. 2024, 129, 32–39. [Google Scholar] [CrossRef]
- Park, Y.M.; Jung, C.M.; Cha, D.; Kim, S.H. The long-term oncological and functional outcomes of transoral robotic surgery in patients with hypopharyngeal cancer. Oral. Oncol. 2017, 71, 138–143. [Google Scholar] [CrossRef]
- Derfi, K.V.; Vasiljevic, T.; Dragicevic, T.; Glavan, T.M. Mithramycin targets head and neck cancer stem cells by inhibiting Sp1 and UFMylation. Cancer Cell Int. 2024, 24, 412. [Google Scholar] [CrossRef]
- Jaksic, M.; Provatas, G.; Mihalic, I.B.; Crnjac, A.; Cosic, D.; Dunatov, T.; Romanenko, O.; Siketic, Z. The dual ion beam microprobe. Nucl. Instrum. Meth B 2023, 539, 120–126. [Google Scholar] [CrossRef]
- Matijevic, T.; Marjanovic, M.; Pavelic, J. Functionally active toll-like receptor 3 on human primary and metastatic cancer cells. Scand. J. Immunol. 2009, 70, 18–24. [Google Scholar] [CrossRef]
- Yang, H.; Pellegrini, L.; Napolitano, A.; Giorgi, C.; Jube, S.; Preti, A.; Jennings, C.J.; De Marchis, F.; Flores, E.G.; Larson, D.; et al. Aspirin delays mesothelioma growth by inhibiting HMGB1-mediated tumor progression. Cell Death Dis. 2015, 6, e1786. [Google Scholar] [CrossRef]
- Zhou, Z.; Tang, Y.; Jin, X.; Chen, C.; Lu, Y.; Liu, L.; Shen, C. Metformin Inhibits Advanced Glycation End Products-Induced Inflammatory Response in Murine Macrophages Partly through AMPK Activation and RAGE/NFkappaB Pathway Suppression. J. Diabetes Res. 2016, 2016, 4847812. [Google Scholar] [CrossRef]
- Schelbergen, R.F.; Geven, E.J.; van den Bosch, M.H.; Eriksson, H.; Leanderson, T.; Vogl, T.; Roth, J.; van de Loo, F.A.; Koenders, M.I.; van der Kraan, P.M.; et al. Prophylactic treatment with S100A9 inhibitor paquinimod reduces pathology in experimental collagenase-induced osteoarthritis. Ann. Rheum. Dis. 2015, 74, 2254–2258. [Google Scholar] [CrossRef] [PubMed]
- Abi Zamer, B.; El-Huneidi, W.; Eladl, M.A.; Muhammad, J.S. Ins and Outs of Heat Shock Proteins in Colorectal Carcinoma: Its Role in Carcinogenesis and Therapeutic Perspectives. Cells 2021, 10, 2862. [Google Scholar] [CrossRef] [PubMed]
- Mikulandra, M.; Kobescak, A.; Verillaud, B.; Busson, P.; Matijevic Glavan, T. Radio-sensitization of head and neck cancer cells by a combination of poly(I:C) and cisplatin through downregulation of survivin and c-IAP2. Cell Oncol 2019, 42, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Yang, W.; Li, L.; Liu, H.; Tan, Y.; Ooi, S.; Chi, L.; Filion, L.G.; Figeys, D.; Wang, L. Beta-Catenin and NF-kappaB co-activation triggered by TLR3 stimulation facilitates stem cell-like phenotypes in breast cancer. Cell Death Differ. 2015, 22, 298–310. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.C.; Wu, L.; Yu, G.T.; Zhang, W.F.; Huang, C.F.; Sun, Z.J. TRAF6 regulates tumour metastasis through EMT and CSC phenotypes in head and neck squamous cell carcinoma. J. Cell Mol. Med. 2018, 22, 1337–1349. [Google Scholar] [CrossRef]
- Umemura, N.; Zhu, J.; Mburu, Y.K.; Forero, A.; Hsieh, P.N.; Muthuswamy, R.; Kalinski, P.; Ferris, R.L.; Sarkar, S.N. Defective NF-kappaB signaling in metastatic head and neck cancer cells leads to enhanced apoptosis by double-stranded RNA. Cancer Res. 2012, 72, 45–55. [Google Scholar] [CrossRef]
- Mori, K.; Yanagita, M.; Hasegawa, S.; Kubota, M.; Yamashita, M.; Yamada, S.; Kitamura, M.; Murakami, S. Necrosis-induced TLR3 Activation Promotes TLR2 Expression in Gingival Cells. J. Dent. Res. 2015, 94, 1149–1157. [Google Scholar] [CrossRef]
- Di Lorenzo, A.; Bolli, E.; Ruiu, R.; Ferrauto, G.; Di Gregorio, E.; Avalle, L.; Savino, A.; Poggio, P.; Merighi, I.F.; Riccardo, F.; et al. Toll-like receptor 2 promotes breast cancer progression and resistance to chemotherapy. Oncoimmunology 2022, 11, 2086752. [Google Scholar] [CrossRef] [PubMed]
- Conti, L.; Lanzardo, S.; Arigoni, M.; Antonazzo, R.; Radaelli, E.; Cantarella, D.; Calogero, R.A.; Cavallo, F. The noninflammatory role of high mobility group box 1/Toll-like receptor 2 axis in the self-renewal of mammary cancer stem cells. FASEB J. 2013, 27, 4731–4744. [Google Scholar] [CrossRef]
- Li, X.; Xu, F.; Wang, R.; Shen, L.; Luo, B.; Zhou, S.; Zhang, J.; Zhang, Z.; Cao, Z.; Zhan, K.; et al. Aspirin enhances radio/chemo-therapy sensitivity in C. elegans by inducing germ cell apoptosis and suppresses RAS overactivated tumorigenesis via mtROS-mediated DNA damage and MAPK pathway. Biochem. Biophys. Res. Commun. 2024, 735, 150828. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, Q.; Meng, K.; Yang, X.; Ma, B.; Su, C.; Duan, X. Aspirin Inhibits Colorectal Cancer via the TIGIT-BCL2-BAX pathway in T Cells. Int. J. Med. Sci. 2024, 21, 1990–1999. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, H.; Ji, Y.; Nie, F.; Wang, R.; Han, W. Effects of aspirin on colon cancer using quantitative proteomic analysis. Cancer Pathog. Ther. 2024, 2, 121–131. [Google Scholar] [CrossRef]
- Ausina, P.; Branco, J.R.; Demaria, T.M.; Esteves, A.M.; Leandro, J.G.B.; Ochioni, A.C.; Mendonca, A.P.M.; Palhano, F.L.; Oliveira, M.F.; Abou-Kheir, W.; et al. Acetylsalicylic acid and salicylic acid present anticancer properties against melanoma by promoting nitric oxide-dependent endoplasmic reticulum stress and apoptosis. Sci. Rep. 2020, 10, 19617. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Fujita, K.; Gong, J.; Nakahara, M.; Iwama, H.; Liu, S.; Yoneyama, H.; Morishita, A.; Nomura, T.; Tani, J.; et al. Aspirin inhibits hepatocellular carcinoma cell proliferation in vitro and in vivo via inducing cell cycle arrest and apoptosis. Oncol. Rep. 2020, 44, 457–468. [Google Scholar] [CrossRef]
- Ren, G.; Ma, Y.; Wang, X.; Zheng, Z.; Li, G. Aspirin blocks AMPK/SIRT3-mediated glycolysis to inhibit NSCLC cell proliferation. Eur. J. Pharmacol. 2022, 932, 175208. [Google Scholar] [CrossRef]
- Rezania, M.A.; Eghtedari, A.; Taha, M.F.; Ardekani, A.M.; Javeri, A. A novel role for aspirin in enhancing the reprogramming function of miR-302/367 cluster and breast tumor suppression. J. Cell Biochem. 2022, 123, 1077–1090. [Google Scholar] [CrossRef]
- Xu, R.; Yan, Y.; Zheng, X.; Zhang, H.; Chen, W.; Li, H.; Dong, Z. Aspirin suppresses breast cancer metastasis to lung by targeting anoikis resistance. Carcinogenesis 2022, 43, 104–114. [Google Scholar] [CrossRef]
- Chen, J.; Xu, R.; Xia, J.; Huang, J.; Su, B.; Wang, S. Aspirin inhibits hypoxia-mediated lung cancer cell stemness and exosome function. Pathol. Res. Pract. 2019, 215, 152379. [Google Scholar] [CrossRef]
- Susan, M.; Macasoi, I.; Pinzaru, I.; Dehelean, C.; Ilia, I.; Susan, R.; Ionita, I. In Vitro Assessment of the Synergistic Effect of Aspirin and 5-Fluorouracil in Colorectal Adenocarcinoma Cells. Curr. Oncol. 2023, 30, 6197–6219. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zhang, N.Q.; Xu, C.J.; Huang, W.Q.; Li, D.X.; Li, J.; Yao, L.L.; Sundquist, K.; Sundquist, J.; Jiang, S.H.; et al. Dipyridamole enhances the anti-cancer ability of aspirin against colorectal cancer by inducing apoptosis in an unfolded protein response-dependent manner. Cell Oncol 2023, 46, 953–967. [Google Scholar] [CrossRef]
- Zhou, H.; Yun, X.; Shu, Y.; Xu, K. Aspirin increases the efficacy of gemcitabine in pancreatic cancer by modulating the PI3K/AKT/mTOR signaling pathway and reversing epithelial-mesenchymal transition. Oncol. Lett. 2023, 25, 101. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhu, Y.; Yu, L.; Li, Y.; Guo, J.; Cai, J.; Liu, L.; Wang, Z. Aspirin inhibits tumor progression and enhances cisplatin sensitivity in epithelial ovarian cancer. PeerJ 2021, 9, e11591. [Google Scholar] [CrossRef]
- Liu, Z.; Cui, L.; Wang, J.; Zhao, W.; Teng, Y. Aspirin boosts the synergistic effect of EGFR/p53 inhibitors on lung cancer cells by regulating AKT/mTOR and p53 pathways. Cell Biochem. Funct. 2024, 42, e3902. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Yan, Y.; Chen, M.; Luo, G.; Hao, J.; Pan, J.; Hu, S.; Guo, P.; Li, W.; Wang, R.; et al. Aspirin enhances the sensitivity of colon cancer cells to cisplatin by abrogating the binding of NF-kappaB to the COX-2 promoter. Aging 2020, 12, 611–627. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Zheng, W.; Fan, H.; Deng, G.; Lu, S.H.; Jiang, W.; Yu, X. Aspirin enhances the therapeutic efficacy of cisplatin in oesophageal squamous cell carcinoma by inhibition of putative cancer stem cells. Br. J. Cancer 2021, 125, 826–838. [Google Scholar] [CrossRef]
- Palazzolo, G.; Mollica, H.; Lusi, V.; Rutigliani, M.; Di Francesco, M.; Pereira, R.C.; Filauro, M.; Paleari, L.; DeCensi, A.; Decuzzi, P. Modulating the Distant Spreading of Patient-Derived Colorectal Cancer Cells via Aspirin and Metformin. Transl. Oncol. 2020, 13, 100760. [Google Scholar] [CrossRef]
- Pozzoli, G.; Marei, H.E.; Althani, A.; Boninsegna, A.; Casalbore, P.; Marlier, L.; Lanzilli, G.; Zonfrillo, M.; Petrucci, G.; Rocca, B.; et al. Aspirin inhibits cancer stem cells properties and growth of glioblastoma multiforme through Rb1 pathway modulation. J. Cell Physiol. 2019, 234, 15459–15471. [Google Scholar] [CrossRef]
- Maity, G.; De, A.; Das, A.; Banerjee, S.; Sarkar, S.; Banerjee, S.K. Aspirin blocks growth of breast tumor cells and tumor-initiating cells and induces reprogramming factors of mesenchymal to epithelial transition. Lab. Investig. 2015, 95, 702–717. [Google Scholar] [CrossRef]
- Saha, S.; Mukherjee, S.; Khan, P.; Kajal, K.; Mazumdar, M.; Manna, A.; Mukherjee, S.; De, S.; Jana, D.; Sarkar, D.K.; et al. Aspirin Suppresses the Acquisition of Chemoresistance in Breast Cancer by Disrupting an NFkappaB-IL6 Signaling Axis Responsible for the Generation of Cancer Stem Cells. Cancer Res. 2016, 76, 2000–2012. [Google Scholar] [CrossRef]
- Cook, N.R.; Lee, I.M.; Zhang, S.M.; Moorthy, M.V.; Buring, J.E. Alternate-day, low-dose aspirin and cancer risk: Long-term observational follow-up of a randomized trial. Ann. Intern. Med. 2013, 159, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Lam, A.; Hao, Z.; Yiu, K.; Chan, S.; Chan, F.; Sung, J.; Tsoi, K. Long-term use of low-dose aspirin for cancer prevention: A 20-year longitudinal cohort study of 1,506,525 Hong Kong residents. Int. J. Cancer 2025, 156, 2330–2339. [Google Scholar] [CrossRef]
- Sikavi, D.R.; Wang, K.; Ma, W.; Drew, D.A.; Ogino, S.; Giovannucci, E.L.; Cao, Y.; Song, M.; Nguyen, L.H.; Chan, A.T. Aspirin Use and Incidence of Colorectal Cancer According to Lifestyle Risk. JAMA Oncol. 2024, 10, 1354–1361. [Google Scholar] [CrossRef]
- Cao, Y.Q.; Tan, A.H. Aspirin might reduce the incidence of breast cancer An updated meta-analysis of 38 observational studies. Medicine 2020, 99, e21917. [Google Scholar] [CrossRef]
- Algra, A.M.; Rothwell, P.M. Effects of regular aspirin on long-term cancer incidence and metastasis: A systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol. 2012, 13, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, M.; Girish, G.V.; Ghosh, R.; Chakraborty, S.; Sinha, A.K. Acetyl salicylic acid (aspirin) improves synthesis of maspin and lowers incidence of metastasis in breast cancer patients. Cancer Sci. 2010, 101, 2105–2109, Erratum in Cancer Sci. 2010, 101, 2676. [Google Scholar] [CrossRef] [PubMed]
- Miller, I.S.; Khan, S.; Shiels, L.P.; Das, S.; AC, O.F.; Connor, K.; Lafferty, A.; Moran, B.; Isella, C.; Loadman, P.; et al. Implementing subtype-specific pre-clinical models of breast cancer to study pre-treatment aspirin effects. Cancer Med. 2022, 11, 3820–3836. [Google Scholar] [CrossRef]
- Bains, S.J.; Mahic, M.; Myklebust, T.A.; Smastuen, M.C.; Yaqub, S.; Dorum, L.M.; Bjornbeth, B.A.; Moller, B.; Brudvik, K.W.; Tasken, K. Aspirin As Secondary Prevention in Patients With Colorectal Cancer: An Unselected Population-Based Study. J. Clin. Oncol. 2016, 34, 2501–2508. [Google Scholar] [CrossRef]
- McNeil, J.J.; Gibbs, P.; Orchard, S.G.; Lockery, J.E.; Bernstein, W.B.; Cao, Y.; Ford, L.; Haydon, A.; Kirpach, B.; Macrae, F.; et al. Effect of Aspirin on Cancer Incidence and Mortality in Older Adults. J. Natl. Cancer Inst. 2021, 113, 258–265. [Google Scholar] [CrossRef]
- Tyagi, A.; Vishnoi, K.; Kaur, H.; Srivastava, Y.; Roy, B.G.; Das, B.C.; Bharti, A.C. Cervical cancer stem cells manifest radioresistance: Association with upregulated AP-1 activity. Sci. Rep. 2017, 7, 4781. [Google Scholar] [CrossRef] [PubMed]
- Hoque, S.; Dhar, R.; Kar, R.; Mukherjee, S.; Mukherjee, D.; Mukerjee, N.; Nag, S.; Tomar, N.; Mallik, S. Cancer stem cells (CSCs): Key player of radiotherapy resistance and its clinical significance. Biomarkers 2023, 28, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Mal, A.; Bukhari, A.B.; Singh, R.K.; Kapoor, A.; Barai, A.; Deshpande, I.; Wadasadawala, T.; Ray, P.; Sen, S.; De, A. EpCAM-Mediated Cellular Plasticity Promotes Radiation Resistance and Metastasis in Breast Cancer. Front. Cell Dev. Biol. 2020, 8, 597673. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, S.H.; Fang, B.; Gillin, M.; Mohan, R.; Chang, J.Y. Therapy-resistant cancer stem cells have differing sensitivity to photon versus proton beam radiation. J. Thorac. Oncol. 2013, 8, 1484–1491. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, R.; Zhang, Q.; Luo, H.; Wu, X.; Du, T.; Chen, Y.; Tan, M.; Liu, Z.; Sun, S.; et al. Biological effects of cancer stem cells irradiated by charged particle: A systematic review of in vitro studies. J. Cancer Res. Clin. Oncol. 2023, 149, 6625–6638. [Google Scholar] [CrossRef] [PubMed]
- Nuyts, S.; Bollen, H.; Ng, S.P.; Corry, J.; Eisbruch, A.; Mendenhall, W.M.; Smee, R.; Strojan, P.; Ng, W.T.; Ferlito, A. Proton Therapy for Squamous Cell Carcinoma of the Head and Neck: Early Clinical Experience and Current Challenges. Cancers 2022, 14, 2587. [Google Scholar] [CrossRef]
- Hsieh, K.; Hotca, A.E.; Dickstein, D.R.; Lehrer, E.J.; Hsieh, C.; Gupta, V.; Sindhu, K.K.; Liu, J.T.; Reed, S.H.; Chhabra, A.; et al. Adjuvant Reirradiation With Proton Therapy in Head and Neck Squamous Cell Carcinoma. Adv. Radiat. Oncol. 2024, 9, 101418. [Google Scholar] [CrossRef]
- Mumaw, D.A.; Hazy, A.J.; Vayntraub, A.; Quinn, T.J.; Salari, K.; Chang, J.H.; Kalman, N.; Katz, S.; Urbanic, J.; Press, R.H.; et al. Low contralateral failure rate with unilateral proton beam radiotherapy for oropharyngeal squamous cell carcinoma: A multi-institutional prospective study from the proton collaborative group. Radiother. Oncol. 2024, 190, 109977. [Google Scholar] [CrossRef]

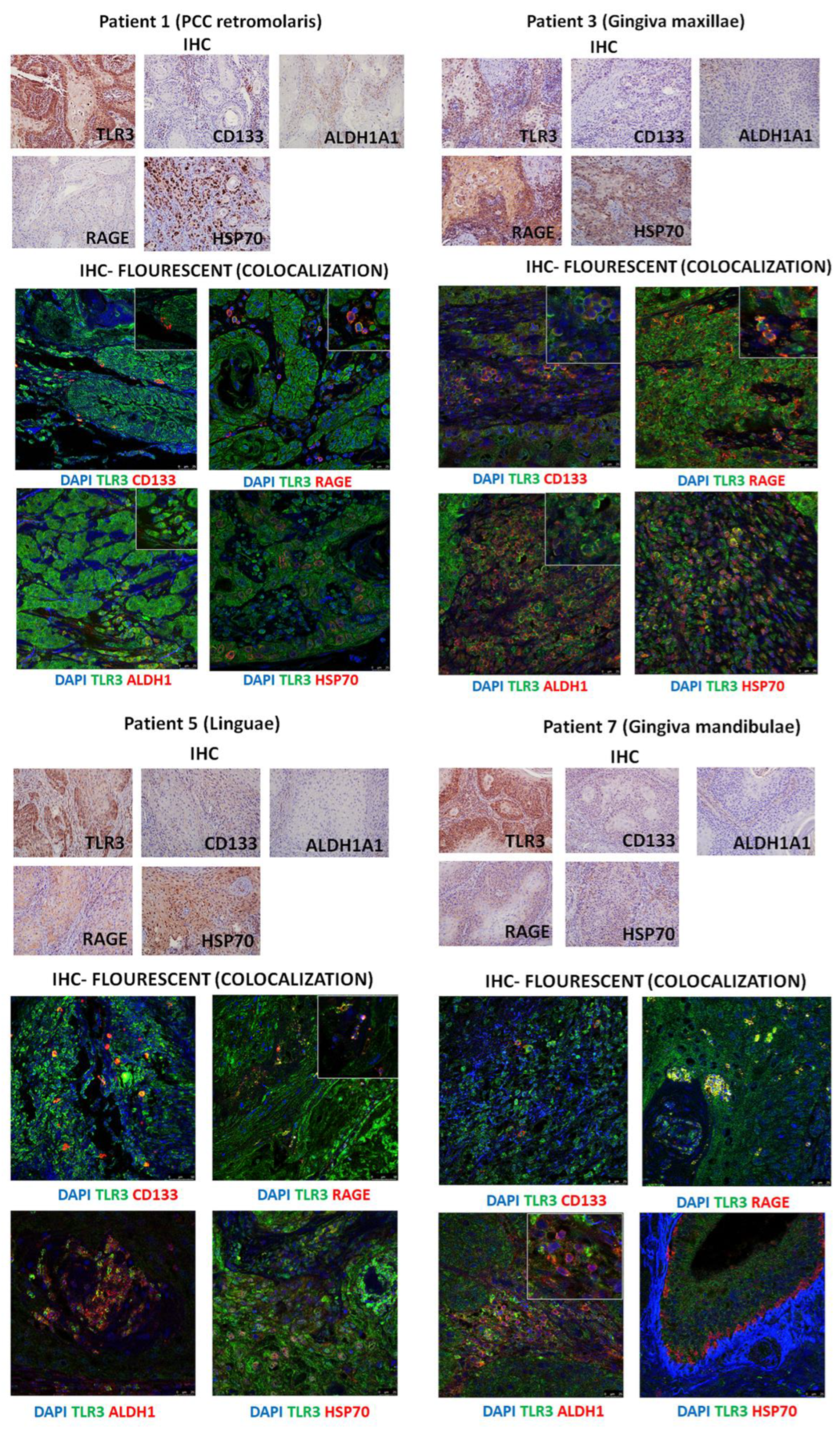
| Patient No | pN Status | No. of Metastases | ENE | PVI | LVI | PNI | Bone Invasion | SPT | Histopathological Subtype | TNM | HPV | Alcohol | Tobacco |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | pN+ | 3 | Yes | Yes | No | Yes | Yes | No | retromolar | T4aN0M0 | No | Yes | Yes |
| 2 | pN0 | 0 | No | No | No | No | No | No | gingiva mandibulae | T4aN3bM0 | No | Yes | Yes |
| 3 | pN+ | 1 | Yes | Yes | No | No | No | No | gingiva maxillae | T4aN0M0 | No | Yes | Yes |
| 4 | pN+ | 3 | Yes | No | No | Yes | No | No | linguae | T4aN3bM0 | No | Yes | Yes |
| 5 | pN+ | 3 | No | No | Yes | No | No | No | linguae | T4aN3bM0 | No | Yes | Yes |
| 6 | pN0 | 0 | No | No | No | Yes | Yes | No | retromolar | T3N2bM0 | No | Yes | Yes |
| 7 | pN0 | 0 | No | No | No | No | Yes | Yes | gingiva mandibulae | T4aN0M0 | No | Yes | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasiljevic, T.; Zapletal, E.; Tarle, M.; Bozicevic Mihalic, I.; Gouasmia, S.; Provatas, G.; Vukovic Djerfi, K.; Müller, D.; Hat, K.; Luksic, I.; et al. Targeting DAMPs by Aspirin Inhibits Head and Neck Cancer Stem Cells and Stimulates Radio-Sensitization to Proton Therapy. Cancers 2025, 17, 2157. https://doi.org/10.3390/cancers17132157
Vasiljevic T, Zapletal E, Tarle M, Bozicevic Mihalic I, Gouasmia S, Provatas G, Vukovic Djerfi K, Müller D, Hat K, Luksic I, et al. Targeting DAMPs by Aspirin Inhibits Head and Neck Cancer Stem Cells and Stimulates Radio-Sensitization to Proton Therapy. Cancers. 2025; 17(13):2157. https://doi.org/10.3390/cancers17132157
Chicago/Turabian StyleVasiljevic, Tea, Emilija Zapletal, Marko Tarle, Iva Bozicevic Mihalic, Sabrina Gouasmia, Georgios Provatas, Kristina Vukovic Djerfi, Danko Müller, Koraljka Hat, Ivica Luksic, and et al. 2025. "Targeting DAMPs by Aspirin Inhibits Head and Neck Cancer Stem Cells and Stimulates Radio-Sensitization to Proton Therapy" Cancers 17, no. 13: 2157. https://doi.org/10.3390/cancers17132157
APA StyleVasiljevic, T., Zapletal, E., Tarle, M., Bozicevic Mihalic, I., Gouasmia, S., Provatas, G., Vukovic Djerfi, K., Müller, D., Hat, K., Luksic, I., & Matijevic Glavan, T. (2025). Targeting DAMPs by Aspirin Inhibits Head and Neck Cancer Stem Cells and Stimulates Radio-Sensitization to Proton Therapy. Cancers, 17(13), 2157. https://doi.org/10.3390/cancers17132157








