Real-World Evidence of Treatment-Free Remission Strategies and Outcomes in Chronic Myeloid Leukemia
Simple Summary
Abstract
1. Introduction
2. Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bourne, G.; Bhatia, R.; Jamy, O. Treatment-Free Remission in Chronic Myeloid Leukemia. J. Clin. Med. 2024, 13, 2567. [Google Scholar] [CrossRef] [PubMed]
- Kantarjian, H.; O’Brien, S.; Jabbour, E.; Garcia-Manero, G.; Quintas-Cardama, A.; Shan, J.; Rios, M.B.; Ravandi, F.; Faderl, S.; Kadia, T.; et al. Improved survival in chronic myeloid leukemia since the introduction of imatinib therapy: A single-institution historical experience. Blood 2012, 119, 1981–1987. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, E.; Oehler, V.G.; Koller, P.B.; Jamy, O.; Lomaia, E.; Hunter, A.M.; Uspenskaya, O.; Samarina, S.; Mukherjee, S.; Cortes, J.E.; et al. Olverembatinib After Failure of Tyrosine Kinase Inhibitors, Including Ponatinib or Asciminib: A Phase 1b Randomized Clinical Trial. JAMA Oncol. 2025, 11, 28–35. [Google Scholar] [CrossRef]
- Kantarjian, H.; Zhai, Y.; Oehler, V.G.; Jamy, O.; Koller, P.B.; Haddad, F.G.; Sasaki, K.; Jabbour, E.J. Olverembatinib in chronic myeloid leukemia-Review of historical development, current status, and future research. Cancer 2025, 131, e35832. [Google Scholar] [CrossRef]
- Cohen, M.H.; Johnson, J.R.; Pazdur, R.U.S. Food and Drug Administration Drug Approval Summary: Conversion of imatinib mesylate (STI571; Gleevec) tablets from accelerated approval to full approval. Clin. Cancer Res. 2005, 11, 12–19. [Google Scholar] [CrossRef]
- Jamy, O.; Godby, R.; Sarmad, R.; Costa, L.J. Survival of chronic myeloid leukemia patients in comparison to the general population in the tyrosine kinase inhibitors era: A US population-based study. Am. J. Hematol. 2021, 96, E265–E268. [Google Scholar] [CrossRef]
- Shah, N.P.; Bhatia, R.; Altman, J.K.; Amaya, M.; Begna, K.H.; Berman, E.; Chan, O.; Clements, J.; Collins, R.H.; Curtin, P.T.; et al. Chronic Myeloid Leukemia, Version 2.2024, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2024, 22, 43–69. [Google Scholar] [CrossRef]
- Jamy, O.; Sarmad, R.; Costa, L. Risk and outcomes of second malignant neoplasms in chronic myeloid leukemia survivors. Leuk. Res. 2019, 82, 1–6. [Google Scholar] [CrossRef]
- Mahon, F.X.; Réa, D.; Guilhot, J.; Guilhot, F.; Huguet, F.; Nicolini, F.; Legros, L.; Charbonnier, A.; Guerci, A.; Varet, B.; et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: The prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010, 11, 1029–1035. [Google Scholar] [CrossRef]
- Clark, R.E.; Polydoros, F.; Apperley, J.F.; Milojkovic, D.; Pocock, C.; Smith, G.; Byrne, J.L.; de Lavallade, H.; O’Brien, S.G.; Coffey, T.; et al. De-escalation of tyrosine kinase inhibitor dose in patients with chronic myeloid leukaemia with stable major molecular response (DESTINY): An interim analysis of a non-randomised, phase 2 trial. Lancet Haematol. 2017, 4, e310–e316. [Google Scholar] [CrossRef]
- Lee, S.E.; Choi, S.Y.; Song, H.Y.; Kim, S.H.; Choi, M.Y.; Park, J.S.; Kim, H.J.; Kim, S.H.; Zang, D.Y.; Oh, S.; et al. Imatinib withdrawal syndrome and longer duration of imatinib have a close association with a lower molecular relapse after treatment discontinuation: The KID study. Haematologica 2016, 101, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Mahon, F.X.; Boquimpani, C.; Kim, D.W.; Benyamini, N.; Clementino, N.C.D.; Shuvaev, V.; Ailawadhi, S.; Lipton, J.H.; Turkina, A.G.; De Paz, R.; et al. Treatment-Free Remission After Second-Line Nilotinib Treatment in Patients with Chronic Myeloid Leukemia in Chronic Phase: Results from a Single-Group, Phase 2, Open-Label Study. Ann. Intern. Med. 2018, 168, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Ross, D.M.; Branford, S.; Seymour, J.F.; Schwarer, A.P.; Arthur, C.; Yeung, D.T.; Dang, P.; Goyne, J.M.; Slader, C.; Filshie, R.J.; et al. Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: Results from the TWISTER study. Blood 2013, 122, 515–522. [Google Scholar] [CrossRef]
- Rousselot, P.; Charbonnier, A.; Cony-Makhoul, P.; Agape, P.; Nicolini, F.E.; Varet, B.; Gardembas, M.; Etienne, G.; Réa, D.; Roy, L.; et al. Loss of major molecular response as a trigger for restarting tyrosine kinase inhibitor therapy in patients with chronic-phase chronic myelogenous leukemia who have stopped imatinib after durable undetectable disease. J. Clin. Oncol. 2014, 32, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Saussele, S.; Richter, J.; Guilhot, J.; Gruber, F.X.; Hjorth-Hansen, H.; Almeida, A.; Janssen, J.; Mayer, J.; Koskenvesa, P.; Panayiotidis, P.; et al. Discontinuation of tyrosine kinase inhibitor therapy in chronic myeloid leukaemia (EURO-SKI): A prespecified interim analysis of a prospective, multicentre, non-randomised, trial. Lancet Oncol. 2018, 19, 747–757. [Google Scholar] [CrossRef]
- Fujisawa, S.; Ueda, Y.; Usuki, K.; Kobayashi, H.; Kondo, E.; Doki, N.; Nakao, T.; Kanda, Y.; Kosugi, N.; Kosugi, H.; et al. Feasibility of the imatinib stop study in the Japanese clinical setting: Delightedly overcome CML expert stop TKI trial (DOMEST Trial). Int. J. Clin. Oncol. 2019, 24, 445–453. [Google Scholar] [CrossRef]
- Kimura, S.; Imagawa, J.; Murai, K.; Hino, M.; Kitawaki, T.; Okada, M.; Tanaka, H.; Shindo, M.; Kumagai, T.; Ikezoe, T.; et al. Treatment-free remission after first-line dasatinib discontinuation in patients with chronic myeloid leukaemia (first-line DADI trial): A single-arm, multicentre, phase 2 trial. Lancet Haematol. 2020, 7, e218–e225. [Google Scholar] [CrossRef]
- Mori, S.; Vagge, E.; le Coutre, P.; Abruzzese, E.; Martino, B.; Pungolino, E.; Elena, C.; Pierri, I.; Assouline, S.; D’Emilio, A.; et al. Age and dPCR can predict relapse in CML patients who discontinued imatinib: The ISAV study. Am. J. Hematol. 2015, 90, 910–914. [Google Scholar] [CrossRef]
- Okada, M.; Imagawa, J.; Tanaka, H.; Nakamae, H.; Hino, M.; Murai, K.; Ishida, Y.; Kumagai, T.; Sato, S.; Ohashi, K.; et al. Final 3-year Results of the Dasatinib Discontinuation Trial in Patients With Chronic Myeloid Leukemia Who Received Dasatinib as a Second-line Treatment. Clin. Lymphoma Myeloma Leuk. 2018, 18, 353–360.e351. [Google Scholar] [CrossRef]
- Hochhaus, A.; Masszi, T.; Giles, F.J.; Radich, J.P.; Ross, D.M.; Gómez Casares, M.T.; Hellmann, A.; Stentoft, J.; Conneally, E.; García-Gutiérrez, V.; et al. Treatment-free remission following frontline nilotinib in patients with chronic myeloid leukemia in chronic phase: Results from the ENESTfreedom study. Leukemia 2017, 31, 1525–1531. [Google Scholar] [CrossRef]
- Imagawa, J.; Tanaka, H.; Okada, M.; Nakamae, H.; Hino, M.; Murai, K.; Ishida, Y.; Kumagai, T.; Sato, S.; Ohashi, K.; et al. Discontinuation of dasatinib in patients with chronic myeloid leukaemia who have maintained deep molecular response for longer than 1 year (DADI trial): A multicentre phase 2 trial. Lancet Haematol. 2015, 2, e528–e535. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, T.; Nakaseko, C.; Nishiwaki, K.; Yoshida, C.; Ohashi, K.; Takezako, N.; Takano, H.; Kouzai, Y.; Murase, T.; Matsue, K.; et al. Dasatinib cessation after deep molecular response exceeding 2 years and natural killer cell transition during dasatinib consolidation. Cancer Sci. 2018, 109, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Rea, D.; Nicolini, F.E.; Tulliez, M.; Guilhot, F.; Guilhot, J.; Guerci-Bresler, A.; Gardembas, M.; Coiteux, V.; Guillerm, G.; Legros, L.; et al. Discontinuation of dasatinib or nilotinib in chronic myeloid leukemia: Interim analysis of the STOP 2G-TKI study. Blood 2017, 129, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Ross, D.M.; Masszi, T.; Gómez Casares, M.T.; Hellmann, A.; Stentoft, J.; Conneally, E.; Garcia-Gutierrez, V.; Gattermann, N.; le Coutre, P.D.; Martino, B.; et al. Durable treatment-free remission in patients with chronic myeloid leukemia in chronic phase following frontline nilotinib: 96-week update of the ENESTfreedom study. J. Cancer Res. Clin. Oncol. 2018, 144, 945–954. [Google Scholar] [CrossRef]
- Shah, N.P.; García-Gutiérrez, V.; Jiménez-Velasco, A.; Larson, S.; Saussele, S.; Rea, D.; Mahon, F.X.; Levy, M.Y.; Gómez-Casares, M.T.; Pane, F.; et al. Dasatinib discontinuation in patients with chronic-phase chronic myeloid leukemia and stable deep molecular response: The DASFREE study. Leuk. Lymphoma 2020, 61, 650–659. [Google Scholar] [CrossRef]
- Campiotti, L.; Suter, M.B.; Guasti, L.; Piazza, R.; Gambacorti-Passerini, C.; Grandi, A.M.; Squizzato, A. Imatinib discontinuation in chronic myeloid leukaemia patients with undetectable BCR-ABL transcript level: A systematic review and a meta-analysis. Eur. J. Cancer 2017, 77, 48–56. [Google Scholar] [CrossRef]
- Chen, K.K.; Du, T.F.; Xiong, P.S.; Fan, G.H.; Yang, W. Discontinuation of Tyrosine Kinase Inhibitors in Chronic Myeloid Leukemia with Losing Major Molecular Response as a Definition for Molecular Relapse: A Systematic Review and Meta-Analysis. Front. Oncol. 2019, 9, 372. [Google Scholar] [CrossRef]
- Hochhaus, A.; Baccarani, M.; Silver, R.T.; Schiffer, C.; Apperley, J.F.; Cervantes, F.; Clark, R.E.; Cortes, J.E.; Deininger, M.W.; Guilhot, F.; et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia 2020, 34, 966–984. [Google Scholar] [CrossRef]
- Breccia, M.; Abruzzese, E.; Stagno, F.; Iurlo, A.; Pene, F.; Attolica, I.; Sportoletti, P.; Pregno, P.; Galimberti, S.; Scappini, B.; et al. First Interim Analysis of the Italian Dante Study: De-Escalation before Treatment-Free Remission in Patients with Chronic Myeloid Leukemia Treated with First-Line Nilotinib. Blood 2021, 138, 1474. [Google Scholar] [CrossRef]
- Clark, R.E.; Polydoros, F.; Apperley, J.F.; Milojkovic, D.; Rothwell, K.; Pocock, C.; Byrne, J.; de Lavallade, H.; Osborne, W.; Robinson, L.; et al. De-escalation of tyrosine kinase inhibitor therapy before complete treatment discontinuation in patients with chronic myeloid leukaemia (DESTINY): A non-randomised, phase 2 trial. Lancet Haematol. 2019, 6, e375–e383. [Google Scholar] [CrossRef]
- Iurlo, A.; Breccia, M.; Stagno, F.; Abruzzese, E.; Pane, F.; Attolico, I.; Sportoletti, P.; Cerrano, M.; Galimberti, S.; Scappini, B.; et al. Full Treatment-Free Remission Outcome in Patients with Chronic Myeloid Leukemia in Chronic Phase Following One Year of Nilotinib De-Escalation: 96-Week Update of Dante Study. Blood 2023, 142, 4534. [Google Scholar] [CrossRef]
- Gener-Ricos, G.; Haddad, F.G.; Sasaki, K.; Issa, G.C.; Skinner, J.; Masarova, L.; Borthakur, G.; Alvarado, Y.; Garcia-Manero, G.; Jabbour, E.; et al. Low-Dose Dasatinib (50 mg Daily) Frontline Therapy in Newly Diagnosed Chronic Phase Chronic Myeloid Leukemia: 5-Year Follow-up Results. Clin. Lymphoma Myeloma Leuk. 2023, 23, 742–748. [Google Scholar] [CrossRef] [PubMed]
| Baseline Characteristics | TFR Cohort (N = 39) | Full Cohort (N = 233) | ||
|---|---|---|---|---|
| Age in Years (Median) | 47 | Range: 20 y–65 y | 54 | Range: 20 y–72 y |
| Gender (%) | ||||
| Female | 69 | 63 | ||
| Race (%) | ||||
| Caucasian | 76 | 72 | ||
| African American | 20 | 26 | ||
| Asian | 4 | 2 | ||
| Vital Status (%) | ||||
| Alive | 89 | 81 | ||
| Deceased | 11 | 11 | ||
| Lost to Follow-up | 0 | 8 | ||
| Standard-Dose Abrupt Stop (N = 21) | Standard-Dose Tapering (N = 11) | Upfront Dose Reduction (N = 7) | ||||
|---|---|---|---|---|---|---|
| Age in Years (Median) | 47 | Range: 20–65 y | 58 | Range: 26–61 y | 46 | Range: 28–61 y |
| Gender (%) | ||||||
| Female | 65 | 73 | 71 | |||
| Race (%) | ||||||
| Caucasian | 73 | 71 | ||||
| African | 65 | 9 | 29 | |||
| American | 20 | |||||
| Asian | 10 | |||||
| TKI prior to TFR (%) | ||||||
| Imatinib | 48 | 10 | 29 | |||
| Dasatinib | 33 | 45 | 71 | |||
| Nilotinib | 19 | 45 | ||||
| CML Related Death (%) | ||||||
| No | 95 | 91 | 100 | |||
| Lost to Follow-up | 5 | 9 | ||||
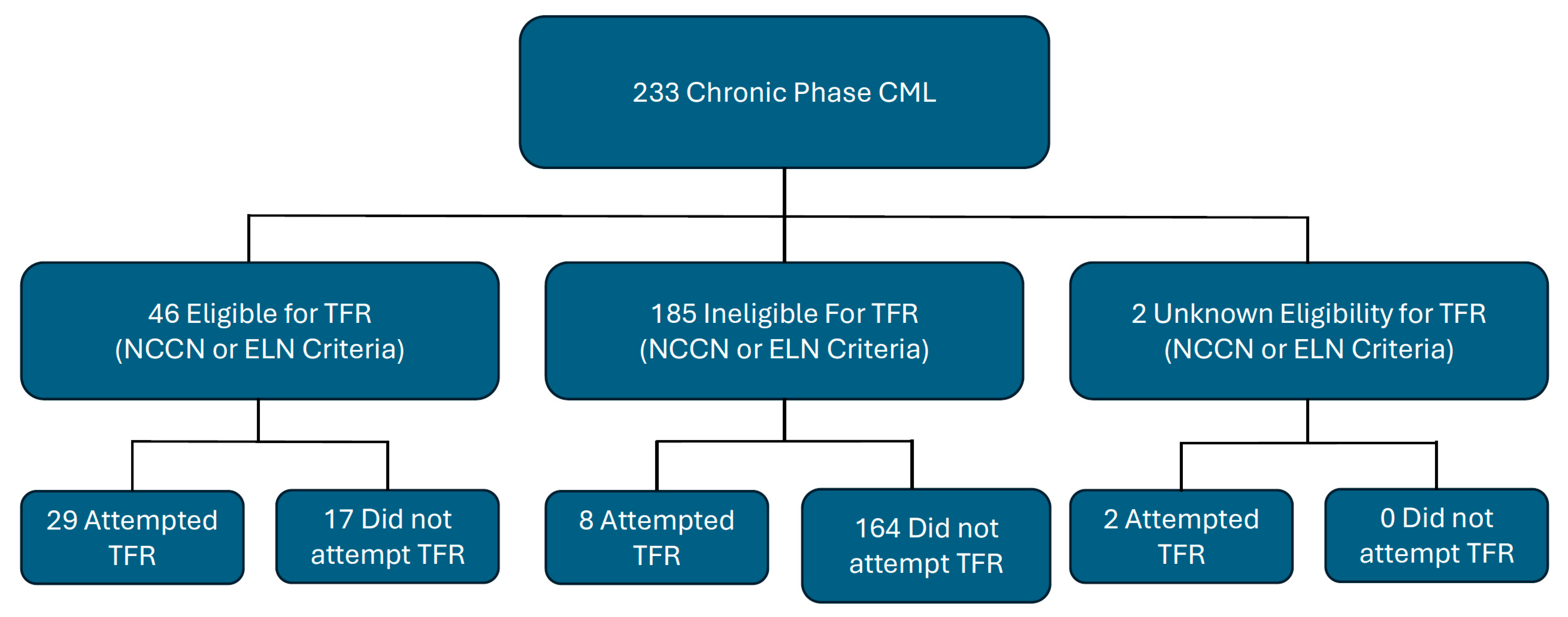
| Total CML-CP Patients Receiving and/or Eligible for TFR (N = 56) | |
|---|---|
| Number Eligible (NCCN and/or ELN) | N = 46 |
| NCCN Criteria (%) | 100 (N = 46) |
| ELN Criteria (%) | 96 (N = 44) |
| Unknown (%) | 4 (N = 2) |
| TFR Eligible and Not Attempted (%) | 30 (N = 17) |
| TFR Attempted | 70 (N = 39) |
| Eligible and Attempted (%) | 74 (N = 29) |
| Ineligible ant Attempted (%) | 21 (N = 8) |
| Unknown Eligibility and Attempted (%) | 5 (N = 2) |
| Time from Diagnosis to TFR Attempt (Months) | Median = 83 (8–257) |
| Total Duration on TKI Prior to TFR (Months) | Median = 87 (23–220) |
| Duration of Deep Response Prior to TFR (Months) | Median = 58 (0–153) |
| MMR Before TFR (%) | |
| MR4 | 16 |
| MR4.5 | 84 |
| Number of TKIs before TFR (%) | |
| 1 | 82 |
| 2 | 13 |
| 3 | 5 |
| Prior Therapy | Median TFR Duration (m) | Median Time to Lose TFR (m) | TFR Lost 0–6 m | TFR Lost 6–12 m | TFR Lost 1–2 y | TFR Lost >2 y | MMR Reobtained | MR4.5 Reobtained | Median Time to Reobtain MMR (m) | Disease Progression/CML-Related Death |
|---|---|---|---|---|---|---|---|---|---|---|
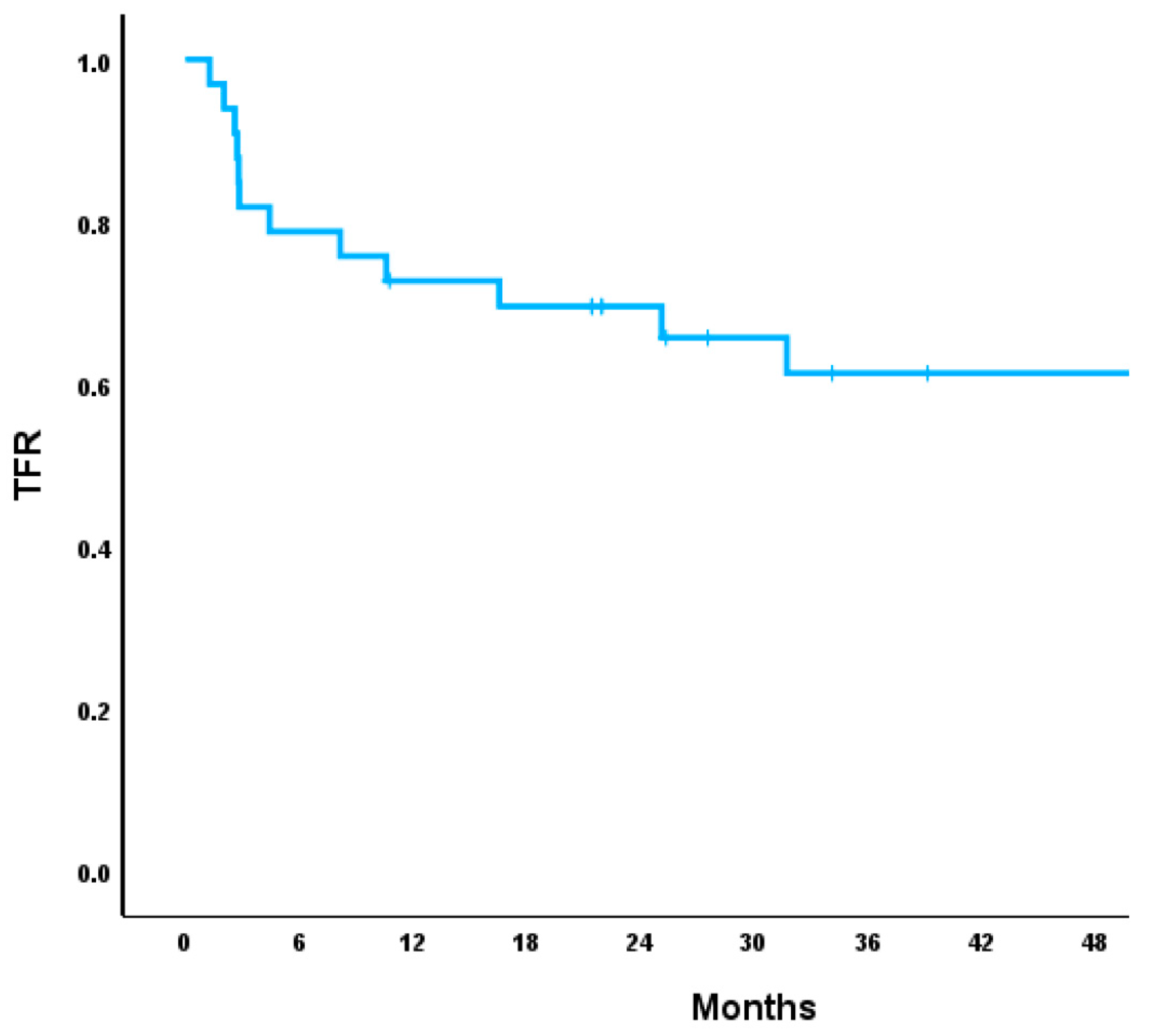
| Initial TFR for All Participants (n = 39) | 14.6 (2.0–78.0) | 5.9 (2.0–50.7) | 55.00% | 16.00% | 8.00% | 21.00% | 100.00% | 100.00% | 3.1 (0.6–8.4) | 0.00% |
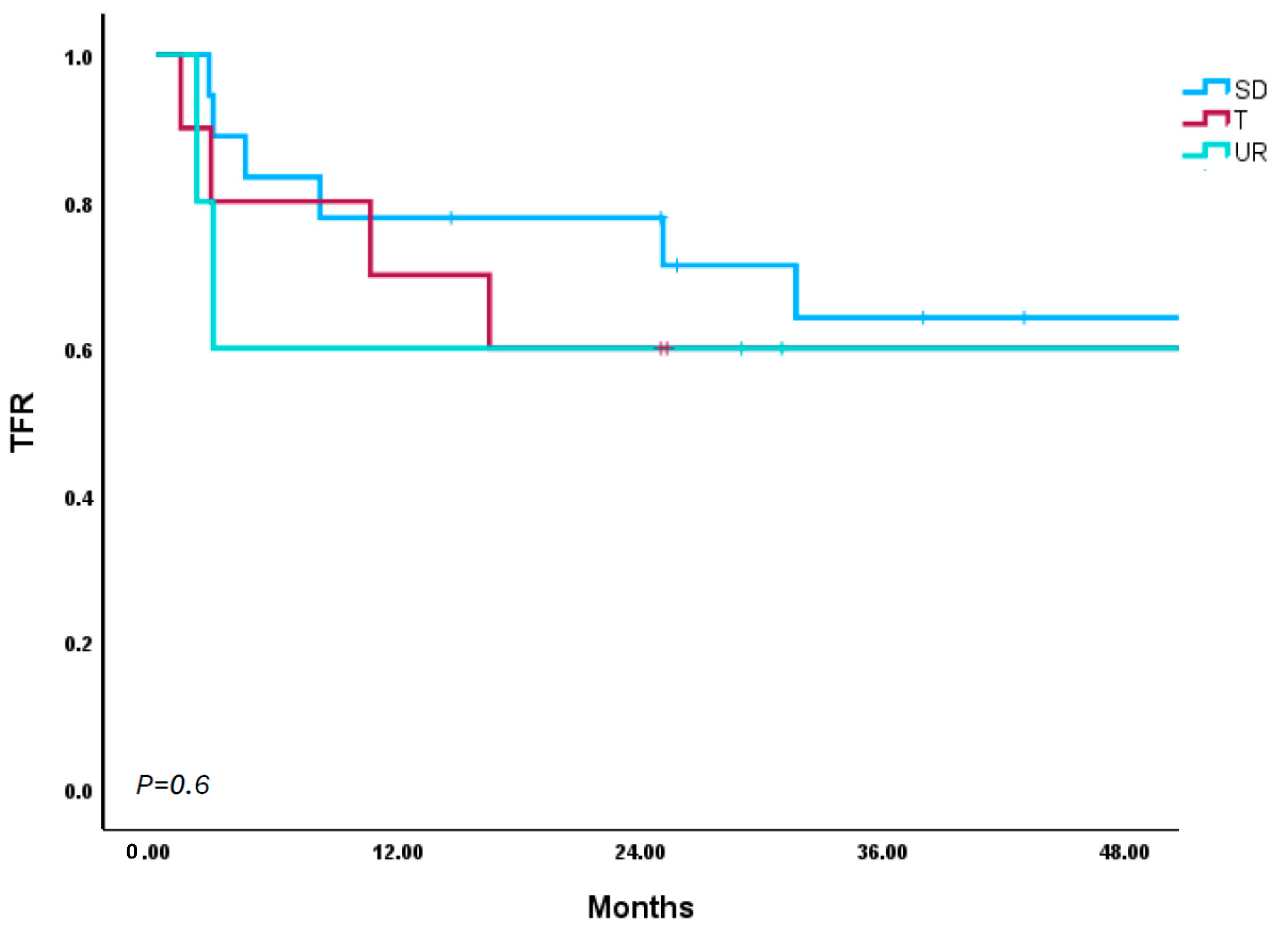
| Standard-Dose Abrupt Cessation (n = 21) | 25.7 (2.9–78.0) | 8.1 (2.6–34.3) | 50.00% | 16.67% | 0.00% | 33.33% | 100.00% | 100.00% | 3.7 (0.8–8.4) | 0.00% |
| Imatinib (n = 10) | 30.3 (3.2–78.0) | 25.1 (2.6–31.7) | 40% | 0.00% | 0.00% | 60% | 100.00% | 100.00% | 5.1 (0.8–6.6) | 0.00% |
| Dasatinib (n = 7) | 9.8 (2.9–45.6) | 7.4 (4.3–13.6) | 100.00% | 0.00% | 0.00% | 0.00% | 100.00% | 100.00% | 3.40 (2.9–5.1) | 0.00% |
| Nilotinib (n = 4) | 9.7 (3.0–39.1) | 5.5 (2.8–8.2) | 50.00% | 50.00% | 0.00% | 0.00% | 100.00% | 100.00% | 3.0 (2.1–4.9) | 0.00% |
| Standard-Dose Taper (n = 11) | 12.9 (2.7–61.6) | 13.6 (2.7–50.7) | 25.00% | 25.00% | 25.00% | 25.00% | 100.00% | 100.00% | 1.2 (0.6–2.8) | 0.00% |
| Imatinib (n = 1) | 11.3 (8.9–25.9) | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 0.00% |
| Dasatinib (n = 5) | 25.6 (11.2–54.0) | 10.6 (8.5–18.5) | N/A | 40% | 20% | 40% | 100.00% | 100.00% | 1.2 (0.8–4.1) | 0.00% |
| Nilotinib (n = 5) | 13.8 (2.73–61.6) | 9.7 (2.7–16.7) | 60.00% | 0.00% | 40.00% | 0.00% | 100.00% | 100.00% | 1.7 (0.6–2.8) | 0.00% |
| Upfront Dose Reduction (n = 7) | 7.3 (2.0–51.6) | 3.4 (2.0–5.7) | 100% | 0.00% | 0.00% | 0.00% | 100.00% | 100.00% | 4.3 (2.5–7.5) | 0.00% |
| Imatinib (n = 2) | 4.0 (2.8–5.1) | 2.83 (2.5–3.3) | 100% | N/A | N/A | N/A | 100.00% | 100.00% | 4.7 (4.3–5.1) | 0.00% |
| Dasatinib (n = 5) | 19.0 (2.0–51.6) | 5.11 (4.5–20.2) | 100% | 0.00% | 0.00% | 0.00% | 100.00% | 100.00% | 2.2 (1.5–6.9) | 0.00% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bourne, G.; Diebold, K.; Bascug, G.; Knapp, J.; Espinoza-Gutarra, M.; Vachhani, P.; Bachiashvili, K.; Rangaraju, S.; Mohty, R.; Bhatia, R.; et al. Real-World Evidence of Treatment-Free Remission Strategies and Outcomes in Chronic Myeloid Leukemia. Cancers 2025, 17, 2148. https://doi.org/10.3390/cancers17132148
Bourne G, Diebold K, Bascug G, Knapp J, Espinoza-Gutarra M, Vachhani P, Bachiashvili K, Rangaraju S, Mohty R, Bhatia R, et al. Real-World Evidence of Treatment-Free Remission Strategies and Outcomes in Chronic Myeloid Leukemia. Cancers. 2025; 17(13):2148. https://doi.org/10.3390/cancers17132148
Chicago/Turabian StyleBourne, Garrett, Kendall Diebold, Greg Bascug, Joshua Knapp, Manuel Espinoza-Gutarra, Pankit Vachhani, Kimo Bachiashvili, Sravanti Rangaraju, Razan Mohty, Ravi Bhatia, and et al. 2025. "Real-World Evidence of Treatment-Free Remission Strategies and Outcomes in Chronic Myeloid Leukemia" Cancers 17, no. 13: 2148. https://doi.org/10.3390/cancers17132148
APA StyleBourne, G., Diebold, K., Bascug, G., Knapp, J., Espinoza-Gutarra, M., Vachhani, P., Bachiashvili, K., Rangaraju, S., Mohty, R., Bhatia, R., & Jamy, O. (2025). Real-World Evidence of Treatment-Free Remission Strategies and Outcomes in Chronic Myeloid Leukemia. Cancers, 17(13), 2148. https://doi.org/10.3390/cancers17132148







