Vaginal Adenocarcinoma: A Review of a Rare Gynecologic Cancer
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Search Strategy
3. Results
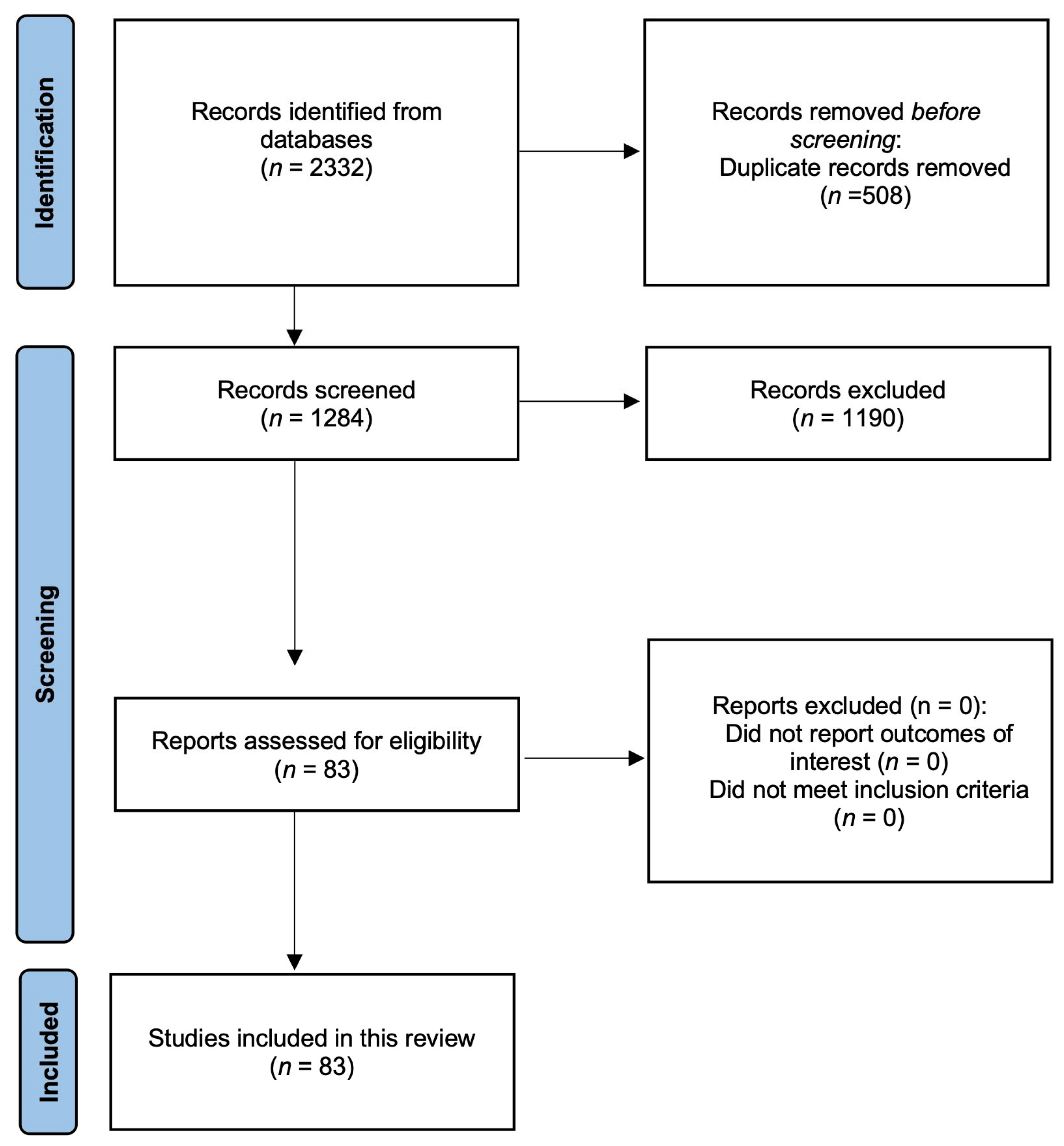
3.1. Screening Results
3.2. Literature Review
4. Discussion
4.1. Epidemiology
4.1.1. Global and Regional Incidence and Prevalence
4.1.2. Demographics (Age Groups, Racial/Ethnic Differences)
4.1.3. Risk Factors and Associated Conditions
4.2. Histological Subtypes and Etiology
4.2.1. Clear Cell Adenocarcinoma (Most Common Type)
4.2.2. Mesonephric Adenocarcinoma
4.2.3. Endometrioid Adenocarcinoma
4.2.4. Serous Carcinoma
4.2.5. HPV Involvement
4.3. Clinical Presentation
4.3.1. Symptoms
4.3.2. Physical Examination Findings
4.3.3. Patterns of Spread (Local Invasion and Lymphatic Spread)
4.4. Diagnostic Evaluation
4.4.1. Pelvic Examination and Colposcopy
4.4.2. Imaging Studies
4.4.3. Immunohistochemical Profiles and Differential Diagnosis
Intestinal-Type Vaginal Adenocarcinoma
- SATB2—highly sensitive for colorectal origin (>90% expression), and its expression increases 2- to 5-fold in intestinal-type tumors compared to Müllerian or cervical adenocarcinomas;
- CDX2—nuclear staining is strong (>80% of cases), and its expression levels are 3- to 10-fold higher than in non-intestinal adenocarcinomas;
- CK20—typically positive (70–90% of cases), and its expression is markedly elevated (5- to 20-fold) compared to gynecologic tumors;
- CEA—cytoplasmic positivity in >70% of cases, and its levels may rise 2- to 4-fold in metastatic intestinal-type tumors [54].
- CK7—absent or faint (<5% of cases), and its expression is reduced by >90% compared to Müllerian tumors;
- PAX8—negative (≤1% of cases), and near-undetectable levels help exclude a Müllerian origin;
- p16—patchy or negative (HPV-independent), and its expression is 50–80% lower than in cervical adenocarcinomas;
- GATA3—negative and absent in intestinal-type tumors but strongly expressed in urothelial/breast cancers [55].
Endometrial and Ovarian Adenocarcinomas
- PAX8—strong nuclear positivity (>95% of cases), and its expression is 5- to 50-fold higher than in colorectal tumors;
- CK7—diffuse cytoplasmic staining (>90%) that is 10- to 30-fold higher than CK20 in these tumors;
- ER/PR—hormone receptors are 2- to 10-fold more abundant in endometrioid subtypes vs. serous/cervical tumors [56].
- SATB2—negative (≤5% of cases), with a >95% reduction compared to colorectal tumors;
- CK20—rarely expressed (<10%), and the levels are >80% lower than in gastrointestinal adenocarcinomas [56].
Cervical Adenocarcinomas
- p16—diffuse strong positivity (HPV-related), and its expression increases 10- to 100-fold in high-risk HPV-associated tumors;
- CEA—focal to diffuse (60–80% of cases) and its levels may rise 2- to 5-fold in endocervical primaries.
- ER/PR—Negative or weak (<10% of cases), with a >90% reduction compared to endometrial tumors;
- Vimentin—typically absent and >70% lower than in endometrial carcinomas [7].
Serous Papillary Adenocarcinomas
- PAX8/WT1—strong nuclear staining (>90%), and WT1 expression is 5- to 20-fold higher than in non-serous tumors;
- p53—aberrant (overexpressed/null) in >80% of cases, and mutant p53 levels may be 10- to 50-fold higher than wild-type levels;
- CA125—elevated in 70–90% of cases, with serum levels often 100-fold above normal in advanced disease [54].
- SATB2/CK20—negative; its expression is >95% lower than in gastrointestinal tumors [57].
4.4.4. Staging (FIGO Classification for Vaginal Cancer)
4.5. Management Strategies
4.5.1. Surgery
4.5.2. Radiation Therapy
4.5.3. Chemoradiotherapy
4.5.4. Emerging Targeted Therapies or Immunotherapy
4.6. Prognosis
4.6.1. Key Prognostic Factors
4.6.2. Stage at Diagnosis
4.6.3. Histologic Comparison
4.6.4. Impact of Treatment
4.6.5. Additional Influencing Factors
4.6.6. Recurrence Patterns and Follow-Up Strategies
Recurrence Patterns
- The recurrence pattern of vaginal adenocarcinoma is highly variable, with no consistent trend. In a study of 320 patients treated with radical vaginal trachelectomy (RVT), 10 (3.1%) experienced recurrence at a mean of 26.1 months post-treatment, despite the absence of identifiable high-risk factors. Recurrence may be local (vaginal vault or cervix), regional (pelvic lymph nodes or adjacent organs), or distant (lungs, liver, or bones) [76].
- Clear cell adenocarcinoma has an overall recurrence rate of ~21%, with a predilection for the lungs, supraclavicular lymph nodes, and pelvis [75].
- Distant metastases in adenocarcinomas frequently involve the lungs, liver, adrenal glands, and bones, with higher rates of peritoneal carcinomatosis compared to squamous cell carcinomas [77].
Survival Rates and Kaplan–Meier Data
- The 5-year overall survival (OS) for early-stage (I–II) vaginal adenocarcinoma ranges from 65–80%, declining to <30% for advanced stages (III–IV) [72];
- For the clear cell subtype, a Registry for Research on Hormonal Transplacental Carcinogenesis (n = 695) reported a 5-year OS of 86.1% for patients with prenatal DES exposure and 81.2% for patients without DES exposure [80].
Imaging and Prognosis
Follow-Up Strategies
- Given the unpredictable recurrence and poor prognosis of metastatic disease, close surveillance is essential.
- The frequency of clinical exams and imaging (e.g., MRI) is every 3 months for the first 2 years, and then biannually up to 5 years [76].
- For high-risk cases, consider tumor markers (e.g., CA125 for serous subtypes) and PET/CT for suspected relapse [77].
4.7. Special Considerations
4.7.1. Young Patients with DES-Related Clear Cell Carcinoma
4.7.2. Fertility Preservation
4.7.3. Psychosocial Impact and Quality of Life
4.8. Research Gaps and Future Directions
4.8.1. Need for Prospective Trials and Molecular Studies
4.8.2. Potential Biomarkers for Early Diagnosis
4.8.3. Personalized Therapeutic Approaches
5. Conclusions and Future Directions
5.1. Summary of Key Points
5.2. Clinical Implications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CT | Computed tomography |
| DFS | Disease-free survival |
| EBRT | External beam radiation therapy |
| EGFR | Epidermal growth factor receptor |
| ER | Estrogen receptor |
| FDA | Food and Drug Administration |
| FIGO | Federation Internationale de Gynecologie et d’Obstetrique |
| HDR | High dose rate |
| MRI | Magnetic resonance imaging |
| NCDB | National Cancer Data Base |
| PET | Positron emission tomography |
| PR | Progesterone receptor |
| QoL | Quality of life |
References
- Rajaram, S.; Maheshwari, A.; Srivastava, A. Staging for Vaginal Cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2015, 29, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Matylevich, O.P.; Isachanka, M.S.; Zubets, O.I.; Mavrichev, S.A.; Shelkovich, S.Y.; Schmeler, K.M. Malignant Neoplasms of the Vagina: A 30-Year Review from the Republic of Belarus. Gynecol. Oncol. Rep. 2023, 50, 101309. [Google Scholar] [CrossRef] [PubMed]
- Hacker, N.F.; Eifel, P.J.; van der Velden, J. Cancer of the Vagina. Int. J. Gynaecol. Obstet. 2015, 131 (Suppl. 2), S84–S87. [Google Scholar] [CrossRef]
- Lee, H.; Kim, H.; Kim, H.-S. Mesonephric Adenocarcinoma of the Vagina Harboring TP53 Mutation. Diagnostics 2022, 12, 119. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Li, L.; Zhu, L.; Lang, J.; Bi, Y. Malignant Transformation of Vaginal Adenosis to Clear Cell Carcinoma Without Prenatal Diethylstilbestrol Exposure: A Case Report and Literature Review. BMC Cancer 2019, 19, 798. [Google Scholar] [CrossRef]
- Baral, S.K.; Biswas, P.; Kaium, M.A.; Islam, M.A.; Dey, D.; Saber, M.A.; Rahaman, T.I.; Emran, T.B.; Hasan, M.N.; Jeong, M.K.; et al. A Comprehensive Discussion in Vaginal Cancer Based on Mechanisms, Treatments, Risk Factors and Prevention. Front. Oncol. 2022, 12, 883805. [Google Scholar] [CrossRef]
- Sabri, A.; Li, C.; Monika, F.; Sharma, A.; Sharma, P. Primary Vaginal Adenocarcinoma of Intestinal-Type: A Case Report of a Rare Tumor with Review of Histology, Differential Diagnosis, and Literature. Cureus 2022, 14, e25298. [Google Scholar] [CrossRef]
- McNall, R.Y.; Nowicki, P.D.; Miller, B.; Billups, C.A.; Liu, T.; Daw, N.C. Adenocarcinoma of the Cervix and Vagina in Pediatric Patients. Pediatr. Blood Cancer 2004, 43, 289–294. [Google Scholar] [CrossRef]
- Zhang, L.-Z.; Huang, L.-Y.; Huang, A.-L.; Liu, J.-X.; Yang, F. Adenoid Cystic Carcinoma of the Vagina: A Case Report. Medicine 2019, 98, e13852. [Google Scholar] [CrossRef]
- Shen, Y.; Meng, X.; Wang, L.; Wang, X.; Chang, H. Advanced Primary Vaginal Squamous Cell Carcinoma: A Case Report and Literature Review. Front. Immunol. 2022, 13, 1007462. [Google Scholar] [CrossRef]
- Porragas-Paseiro, H.S.; Guntupalli, S.; Xiong, J.; Greenwood, A. A Complete Durable Response of Vaginal Clear Cell Carcinoma with Pembrolizumab: A Case Report. Gynecol. Oncol. Rep. 2023, 49, 101160. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, F.; Omodei, A.S.; Ferrari, F.A.; Soleymani Majd, H.; Ardighieri, L.; Vitale, S.G.; Laganà, A.S.; Angioni, S.; Ciravolo, G.; Odicino, F. Diagnosis, Treatment and Prognosis of Mesonephric Adenocarcinoma of the Vagina: A Literature Review and a Case Report. J. Clin. Med. 2023, 12, 4846. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Gu, J.-J.; Qi, Y.; Zhao, W.; Wang, X.-L. Endometrioid Adenocarcinoma of the Rectovaginal Septum with Invasion of the Rectum: A Case Report and Review of Literature. World J. Surg. Oncol. 2019, 17, 206. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Wang, C.; Liu, H.; Xu, Y.; Luan, S.; Xia, B. Endometrioid Adenocarcinoma of the Rectovaginal Septum: A Case Report. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2023, 48, 941–946. [Google Scholar]
- Saijilafu; Gu, Y.-J.; Huang, A.-W.; Xu, C.-F.; Qian, L.-W. Individualized Vaginal Applicator for Stage IIb Primary Vaginal Adenocarcinoma: A Case Report. World J. Clin. Oncol. 2024, 15, 1102–1109. [Google Scholar] [CrossRef]
- Barcellini, A.; Ditto, A.; Mirandola, A.; Roccio, M.; Imparato, S.; Raspagliesi, F.; Orlandi, E. Is a Tailored Strategy Using Proton Beam Radiotherapy for Reirradiation Advantageous for Elderly Women? A Case Report. Tumori 2021, 107, NP67–NP72. [Google Scholar] [CrossRef]
- Plesinac-Karapandzic, V.; Stojanovic Rundic, S.; Jankovic, R.; Nadrljanski, M.; Milovanovic, Z.; Tomasevic, A.; Perisie Jeremic, N. Non-Diethylstilbestrol Exposed Vaginal Adenocarcinoma in Young Patients Associated with Unilateral Renal Agenesis: Two Case Reports and Literature Review. Eur. J. Gynaecol. Oncol. 2017, 38, 157–161. [Google Scholar]
- Kumar, S.; Sucheta; Saklani, B.; Archana; Kapil, R.; Sen, R. Post Hysterectomy Mesonephric Carcinoma: A Case Report and Literature Review. J. Cancer Res. Ther. 2022, 18, 277–279. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, G.; Duan, S.; Li, Q. Primary Clear Cell Adenocarcinoma of the Rectovaginal Septum: A Rare Case Report. Medicine 2023, 102, e33285. [Google Scholar] [CrossRef]
- Haddout, S.; Imami, Y.; Benhessou, M.; Ennachit, M.; El Karroumi, M. Primary Clear Cell Adenocarcinoma of the Vagina Not Associated with Diethylstilbestrol: A Case Report. Int. J. Surg. Case Rep. 2022, 98, 107460. [Google Scholar] [CrossRef]
- Felicelli, C.; Strickland, A.; Wei, J.-J. Primary HPV-Associated Enteric-Type Adenocarcinoma of Vagina: A Case Report. Int. J. Surg. Pathol. 2023, 31, 1393–1397. [Google Scholar] [CrossRef]
- Ugwu, A.O.; Haruna, M.; Okunade, K.S.; Ohazurike, E.; Anorlu, R.I.; Banjo, A.A.F. Primary Vaginal Adenocarcinoma of Intestinal-Type: Case Report of a Rare Gynaecological Tumour. Oxf. Med. Case Rep. 2019, 2019, omz088. [Google Scholar] [CrossRef]
- Mei, L.; Zou, J.; Chen, Q.; Jiang, W.; Chen, Y. Primary Vaginal Clear Cell Adenocarcinoma Accompanied by Herlyn-Werner-Wunderlich Syndrome Without Prenatal Diethylstilbestrol Exposure: A Case Report. Int. J. Clin. Exp. Pathol. 2020, 13, 2784–2787. [Google Scholar] [PubMed]
- Warembourg, S.; Cayrac, M.; Rathat, G.; Rafii, A. Recto-Vaginal Septum Cystadenocarcinoma: A Case Report and Review of the Literature. BMC Women’s. Health 2016, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Kalampokas, E.; Giannis, G.; Alexandrou, P.; Kritikos, E.; Triantafyllidou, O.; Kalampokas, T.; Vlahos, N.; Marinis, S. Small-Cell Neuroendocrine Carcinoma of the Vagina: A Case Report. Int. J. Gynaecol. Obstet. 2023, 161, 678–679. [Google Scholar] [CrossRef]
- Nguyen-Xuan, H.-T.; Montero Macias, R.; Bonsang-Kitzis, H.; Deloménie, M.; Ngô, C.; Koual, M.; Bats, A.-S.; Hivelin, M.; Lécuru, F.; Balaya, V. Use of Fluorescence to Guide Surgical Resection in Vulvo-Vaginal Neoplasia: Two Case Reports. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 101768. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.-G.; Zhang, H. Vaginal Clear Cell Adenocarcinoma in Herlyn-Werner-Wunderlich Syndrome: A Case Report. World J. Clin. Oncol. 2024, 15, 1359–1365. [Google Scholar] [CrossRef]
- Howitt, B.E.; Nucci, M.R. Mesonephric Proliferations of the Female Genital Tract. Pathology 2018, 50, 141–150. [Google Scholar] [CrossRef]
- Furau, G.; Dascau, V.; Furau, C.; Paiusan, L.; Radu, A.; Stanescu, C. Gynecological Cancer Age Groups at the “Dr. Salvator Vuia” Clinical Obstetrics and Gynecology Hospital During the 2000-2009 Period. Maedica 2011, 6, 268–271. [Google Scholar]
- Wu, X.; Matanoski, G.; Chen, V.W.; Saraiya, M.; Coughlin, S.S.; King, J.B.; Tao, X.-G. Descriptive Epidemiology of Vaginal Cancer Incidence and Survival by Race, Ethnicity, and Age in the United States. Cancer 2008, 113, 2873–2882. [Google Scholar] [CrossRef]
- Adams, T.S.; Cuello, M.A. Cancer of the Vagina. Int. J. Gynaecol. Obstet. 2018, 143 (Suppl. 2), 14–21. [Google Scholar] [CrossRef] [PubMed]
- Devita, V.T.; Lawrence, T.S.; Rosenberg, S.A. Cancer of the Cervix, Vagina, and Vulva. In Devita, Hellman, and Rosenberg’s Cancer: Principles and Practice of Oncology; Devita, V.T., Lawrence, T.S., Rosenberg, S.A., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2019; pp. 1171–1210. ISBN 9781496394637. [Google Scholar][Green Version]
- Huang, J.; Chan, S.C.; Pang, W.S.; Mak, F.Y.; Fung, Y.C.; Lok, V.; Zhang, L.; Lin, X.; Lucero-Prisno, D.E., III; Xu, W.; et al. Incidence Distributions, Risk Factors and Trends of Vaginal Cancer: A Global Population-Based Study. BJOG 2024, 131, 1660–1672. [Google Scholar] [CrossRef] [PubMed]
- Herbst, A.L.; Robboy, S.J.; Scully, R.E.; Poskanzer, D.C. Clear-Cell Adenocarcinoma of the Vagina and Cervix in Girls: Analysis of 170 Registry Cases. Am. J. Obstet. Gynecol. 1974, 119, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Herbst, A.L.; Ulfelder, H.; Poskanzer, D.C. Adenocarcinoma of the Vagina. Association of Maternal Stilbestrol Therapy with Tumor Appearance in Young Women. N. Engl. J. Med. 1971, 284, 878–881. [Google Scholar] [CrossRef]
- Herbst, A.L.; Scully, R.E. Adenocarcinoma of the Vagina in Adolescence. A Report of 7 Cases Including 6 Clear-Cell Carcinomas (so-Called Mesonephromas). Cancer 1970, 25, 745–757. [Google Scholar] [CrossRef]
- Robboy, S.J.; Kaufman, R.H.; Prat, J.; Welch, W.R.; Gaffey, T.; Scully, R.E.; Richart, R.; Fenoglio, C.M.; Virata, R.; Tilley, B.C. Pathologic Findings in Young Women Enrolled in the National Cooperative Diethylstilbestrol Adenosis (DESAD) Project. Obstet. Gynecol. 1979, 53, 309–317. [Google Scholar] [CrossRef]
- Dieckmann, W.J.; Davis, M.E.; Rynkiewicz, L.M.; Pottinger, R.E. Does the Administration of Diethylstilbestrol During Pregnancy Have Therapeutic Value? Am. J. Obstet. Gynecol. 1953, 66, 1062–1081. [Google Scholar] [CrossRef]
- Smith, O.W.; Smith, G.V.; Hurwitz, D. Increased Excretion of Pregnanediol in Pregnancy from Diethylstilbestrol with Special Reference to the Prevention of Late Pregnancy Accidents. Am. J. Obstet. Gynecol. 1946, 51, 411–415. [Google Scholar] [CrossRef]
- Smith, O.W. Diethylstilbestrol in the Prevention and Treatment of Complications of Pregnancy. Am. J. Obstet. Gynecol. 1948, 56, 821–834. [Google Scholar]
- Herbst, A.L.; Cole, P.; Norusis, M.J.; Welch, W.R.; Scully, R.E. Epidemiologic Aspects and Factors Related to Survival in 384 Registry Cases of Clear Cell Adenocarcinoma of the Vagina and Cervix. Am. J. Obstet. Gynecol. 1979, 135, 876–886. [Google Scholar] [CrossRef]
- Höhn, A.K.; Brambs, C.E.; Hiller, G.G.R.; May, D.; Schmoeckel, E.; Horn, L.-C. 2020 WHO Classification of Female Genital Tumors. Geburtshilfe Frauenheilkd. 2021, 81, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Salama, A.M.; Momeni-Boroujeni, A.; Vanderbilt, C.; Ladanyi, M.; Soslow, R. Molecular Landscape of Vulvovaginal Squamous Cell Carcinoma: New Insights into Molecular Mechanisms of HPV-Associated and HPV-Independent Squamous Cell Carcinoma. Mod. Pathol. 2022, 35, 274–282. [Google Scholar] [CrossRef]
- Voltaggio, L.; McCluggage, W.G.; Iding, J.S.; Martin, B.; Longacre, T.A.; Ronnett, B.M. A Novel Group of HPV-Related Adenocarcinomas of the Lower Anogenital Tract (Vagina, Vulva, and Anorectum) in Women and Men Resembling HPV-Related Endocervical Adenocarcinomas. Mod. Pathol. 2020, 33, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Kaltenecker, B.; Dunton, C.J.; Tikaria, R. Vaginal Cancer. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar][Green Version]
- Adams, T.S.; Rogers, L.J.; Cuello, M.A. Cancer of the Vagina: 2021 Update. Int. J. Gynaecol. Obstet. 2021, 155 (Suppl. 1), 19–27. [Google Scholar] [CrossRef]
- Paspulati, R.M. Carcinoma of the Vagina and Vulva. In Gynecologic Imaging; Elsevier: Amsterdam, The Netherlands, 2011; pp. 530–544. ISBN 9781437715750. [Google Scholar][Green Version]
- Cardenes, H.R.; Schilder, J.M.; Roth, L.M. Chapter 20: Vagina. In Principles and Practice of Gynecologic Oncology; Barakat, R.R., Markman, M., Randall, M.E., Eds.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2009; pp. 591–622. [Google Scholar][Green Version]
- Betova, T.; Trifonov, R.; Popovska, S.; Yordanov, A.; Karakadieva, K.; Dancheva, Z.; Kostov, S. Primary Vaginal Mucinous Adenocarcinoma of Intestinal Type-Clinical, Radiological and Morphological Aspects. Medicina 2024, 60, 525. [Google Scholar] [CrossRef] [PubMed]
- Akçam, T.İ.; Köse, E.; Kahraman Aydın, S.; Tekneci, A.K.; Büyüktalancı, D.Ö.; Ergönül, A.G.; Özdil, A.; Nart, D.; Turhan, K.; Çakan, A.; et al. Diagnostic Efficacy of Intraoperative Histopathological Examination of Lesions with Unknown Diagnosis Suspicious for Malignancy. Heliyon 2023, 9, e22405. [Google Scholar] [CrossRef]
- Nout, R.A.; Calaminus, G.; Planchamp, F.; Chargari, C.; Lax, S.; Martelli, H.; McCluggage, W.G.; Morice, P.; Pakiz, M.; Schmid, M.P.; et al. ESTRO/ESGO/SIOPe Guidelines for the Management of Patients with Vaginal Cancer. Int. J. Gynecol. Cancer 2023, 33, 1185–1202. [Google Scholar] [CrossRef]
- Gardner, C.S.; Sunil, J.; Klopp, A.H.; Devine, C.E.; Sagebiel, T.; Viswanathan, C.; Bhosale, P.R. Primary Vaginal Cancer: Role of MRI in Diagnosis, Staging and Treatment. Br. J. Radiol. 2015, 88, 20150033. [Google Scholar] [CrossRef]
- Ebrahimi, S.; Lundström, E.; Batasin, S.J.; Hedlund, E.; Stålberg, K.; Ehman, E.C.; Sheth, V.R.; Iranpour, N.; Loubrie, S.; Schlein, A.; et al. Application of PET/MRI in Gynecologic Malignancies. Cancers 2024, 16, 1478. [Google Scholar] [CrossRef]
- Yemelyanova, A.; Gown, A.M.; Wu, L.-S.-F.; Holmes, B.J.; Ronnett, B.M.; Vang, R. PAX8 Expression in Uterine Adenocarcinomas and Mesonephric Proliferations. Int. J. Gynecol. Pathol. 2014, 33, 492–499. [Google Scholar] [CrossRef]
- Hernandez-Caballero, A.I.; Vierkoetter, K.R.; Ahn, H.J.; Shimizu, D.; Terada, K. Novel Immunohistochemical Markers in the Differential Diagnosis of Endocervical and Endometrial Adenocarcinoma: The Added Benefit of CAIX and PAX8. Gynecol. Oncol. Rep. 2020, 33, 100614. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Ahuja, S.; Kalwaniya, D.S. The Evolving Landscape of Immunohistochemistry in Cervical and Uterine Carcinoma in Gynecologic Oncology: Current Status and Future Directions. Obstet. Gynecol. Sci. 2024, 67, 449–466. [Google Scholar] [CrossRef]
- Riva, C.; Fabbri, A.; Facco, C.; Tibiletti, M.G.; Guglielmin, P.; Capella, C. Primary Serous Papillary Adenocarcinoma of the Vagina: A Case Report. Int. J. Gynecol. Pathol. 1997, 16, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Tjalma, W.A.; Monaghan, J.M.; de Barros Lopes, A.; Naik, R.; Nordin, A.J.; Weyler, J.J. The Role of Surgery in Invasive Squamous Carcinoma of the Vagina. Gynecol. Oncol. 2001, 81, 360–365. [Google Scholar] [CrossRef]
- Stock, R.G.; Chen, A.S.; Seski, J. A 30-Year Experience in the Management of Primary Carcinoma of the Vagina: Analysis of Prognostic Factors and Treatment Modalities. Gynecol. Oncol. 1995, 56, 45–52. [Google Scholar] [CrossRef]
- Rubin, S.C.; Young, J.; Mikuta, J.J. Squamous Carcinoma of the Vagina: Treatment, Complications, and Long-Term Follow-Up. Gynecol. Oncol. 1985, 20, 346–353. [Google Scholar] [CrossRef]
- Gadducci, A.; Fabrini, M.G.; Lanfredini, N.; Sergiampietri, C. Squamous Cell Carcinoma of the Vagina: Natural History, Treatment Modalities and Prognostic Factors. Crit. Rev. Oncol. Hematol. 2015, 93, 211–224. [Google Scholar] [CrossRef]
- Yang, J.; Delara, R.; Magrina, J.; Magtibay, P.; Langstraat, C.; Dinh, T.; Karlin, N.; Vora, S.A.; Butler, K. Management and Outcomes of Primary Vaginal Cancer. Gynecol. Oncol. 2020, 159, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Guerri, S.; Perrone, A.M.; Buwenge, M.; Ferioli, M.; Macchia, G.; Tagliaferri, L.; Ferrandina, G.; Galuppi, A.; Andrulli, A.D.; Frakulli, R.; et al. Definitive Radiotherapy in Invasive Vaginal Carcinoma: A Systematic Review. Oncologist 2019, 24, 132–141. [Google Scholar] [CrossRef]
- Frank, S.J.; Jhingran, A.; Levenback, C.; Eifel, P.J. Definitive Radiation Therapy for Squamous Cell Carcinoma of the Vagina. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 138–147. [Google Scholar] [CrossRef]
- Lian, J.; Dundas, G.; Carlone, M.; Ghosh, S.; Pearcey, R. Twenty-Year Review of Radiotherapy for Vaginal Cancer: An Institutional Experience. Gynecol. Oncol. 2008, 111, 298–306. [Google Scholar] [CrossRef]
- Schmid, M.P.; Fokdal, L.; Westerveld, H.; Chargari, C.; Rohl, L.; Morice, P.; Nesvacil, N.; Mazeron, R.; Haie-Meder, C.; Pötter, R.; et al. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group—ACROP: Target Concept for Image Guided Adaptive Brachytherapy in Primary Vaginal Cancer. Radiother. Oncol. 2020, 145, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Tyree, W.C.; Cardenes, H.; Randall, M.; Papiez, L. High-Dose-Rate Brachytherapy for Vaginal Cancer: Learning from Treatment Complications. Int. J. Gynecol. Cancer 2002, 12, 27–31. [Google Scholar] [CrossRef]
- Rajagopalan, M.S.; Xu, K.M.; Lin, J.F.; Sukumvanich, P.; Krivak, T.C.; Beriwal, S. Adoption and Impact of Concurrent Chemoradiation Therapy for Vaginal Cancer: A National Cancer Data Base (NCDB) Study. Gynecol. Oncol. 2014, 135, 495–502. [Google Scholar] [CrossRef]
- Miyamoto, D.T.; Viswanathan, A.N. Concurrent Chemoradiation for Vaginal Cancer. PLoS ONE 2013, 8, e65048. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Lee, K.-A.; Kim, B.-G.; Bae, D.-S.; Lee, J.-W. Vaginal Cancer with Multiple Liver and Pulmonary Metastases That Achieved Long-Term Survival. Obstet. Gynecol. Sci. 2013, 56, 416–419. [Google Scholar] [CrossRef]
- Colombo, N.; Dubot, C.; Lorusso, D.; Caceres, M.V.; Hasegawa, K.; Shapira-Frommer, R.; Tewari, K.S.; Salman, P.; Hoyos Usta, E.; Yañez, E.; et al. Pembrolizumab for Persistent, Recurrent, or Metastatic Cervical Cancer. N. Engl. J. Med. 2021, 385, 1856–1867. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Cai, M.; Zhu, Z. Survival and Prognostic Factors in Primary Vaginal Cancer: An Analysis of 2004-2014 SEER Data. Transl. Cancer Res. 2020, 9, 7091–7102. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, C.L.; Bertoli, H.K.; Sand, F.L.; Kjaer, A.K.; Thomsen, L.T.; Kjaer, S.K. The Prognostic Significance of HPV, p16, and p53 Protein Expression in Vaginal Cancer: A Systematic Review. Acta Obstet. Gynecol. Scand. 2021, 100, 2144–2156. [Google Scholar] [CrossRef]
- Zafar, M.; Sweis, N.; Kapoor, H.; Gantt, G. Advances and Challenges in the Treatment of HPV-Associated Lower Genital Tract Cancers by Immune Checkpoint Blockers: Insights from Basic and Clinical Science. Cancers 2025, 17, 1260. [Google Scholar] [CrossRef]
- Jhingran, A. Updates in the Treatment of Vaginal Cancer. Int. J. Gynecol. Cancer 2022, 32, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Mangler, M.; Lanowska, M.; Köhler, C.; Vercellino, F.; Schneider, A.; Speiser, D. Pattern of Cancer Recurrence in 320 Patients after Radical Vaginal Trachelectomy. Int. J. Gynecol. Cancer 2014, 24, 130–134. [Google Scholar] [CrossRef]
- Miccò, M.; Lupinelli, M.; Mangialardi, M.; Gui, B.; Manfredi, R. Patterns of Recurrent Disease in Cervical Cancer. J. Pers. Med. 2022, 12, 755. [Google Scholar] [CrossRef]
- Creasman, W.T.; Phillips, J.L.; Menck, H.R. The National Cancer Data Base Report on Cancer of the Vagina. Cancer 1998, 83, 1033–1040. [Google Scholar] [CrossRef]
- Matylevich, O.P.; Trukhan, H.V.; Zubets, O.I.; Mavrichev, S.A. Twenty Years’ Experience of Primary Vaginal Cancer Treatment at One Cancer Centre: Does Residence Status Matter? Ecancermedicalscience 2021, 15, 1267. [Google Scholar] [CrossRef] [PubMed]
- Huo, D.; Anderson, D.; Herbst, A.L. Follow-up of Patients with Clear-Cell Adenocarcinoma of the Vagina and Cervix. N. Engl. J. Med. 2018, 378, 1746–1748. [Google Scholar] [CrossRef] [PubMed]
- Huo, D.; Anderson, D.; Palmer, J.R.; Herbst, A.L. Incidence Rates and Risks of Diethylstilbestrol-Related Clear-Cell Adenocarcinoma of the Vagina and Cervix: Update after 40-Year Follow-Up. Gynecol. Oncol. 2017, 146, 566–571. [Google Scholar] [CrossRef]
- Hanselaar, A.G.; Van Leusen, N.D.; De Wilde, P.C.; Vooijs, G.P. Clear Cell Adenocarcinoma of the Vagina and Cervix. A Report of the Central Netherlands Registry with Emphasis on Early Detection and Prognosis. Cancer 1991, 67, 1971–1978. [Google Scholar] [CrossRef]
- Turkgeldi, L.; Cutner, A.; Turkgeldi, E.; Al Chami, A.; Cassoni, A.; Macdonald, N.; Mould, T.; Nichol, A.; Olaitan, A.; Saridogan, E. Laparoscopic Ovarian Transposition and Ovariopexy for Fertility Preservation in Patients Treated with Pelvic Radiotherapy with or Without Chemotherapy. Facts Views Vis. ObGyn 2019, 11, 235–242. [Google Scholar]
- Matthews, K.S.; Numnum, T.M.; Conner, M.G.; Barnes, M., III. Fertility-Sparing Radical Abdominal Trachelectomy for Clear Cell Adenocarcinoma of the Upper Vagina: A Case Report. Gynecol. Oncol. 2007, 105, 820–822. [Google Scholar] [CrossRef]
- Kállay, É.; Müller-Fabian, A.; Dégi, C.L. Fear of Cancer Progression and the Quality of Sexual Life of Female Cancer Patients in Romania. Front. Public Health 2024, 12, 1417681. [Google Scholar] [CrossRef]
- Marano, G.; Mazza, M. Impact of Gynecological Cancers on Women’s Mental Health. World J. Psychiatry 2024, 14, 1294–1300. [Google Scholar] [CrossRef] [PubMed]
- Mohamad Muhit, A.M.; Sy-Cherng Woon, L.; Nik Mhd Nor, N.S.; Sidi, H.; Mohd Kalok, A.H.; Kampan, N.C.; Shafiee, M.N. Sexual Dysfunction Among Gynaecological Cancer Survivors: A Descriptive Cross-Sectional Study in Malaysia. Int. J. Environ. Res. Public Health 2022, 19, 15545. [Google Scholar] [CrossRef] [PubMed]
- Malandrone, F.; Bevilacqua, F.; Merola, M.; Gallio, N.; Ostacoli, L.; Carletto, S.; Benedetto, C. The Impact of Vulvar Cancer on Psychosocial and Sexual Functioning: A Literature Review. Cancers 2021, 14, 63. [Google Scholar] [CrossRef]
- Jefferies, H.; Clifford, C. A Literature Review of the Impact of a Diagnosis of Cancer of the Vulva and Surgical Treatment. J. Clin. Nurs. 2011, 20, 3128–3142. [Google Scholar] [CrossRef] [PubMed]
- Iżycki, D.; Woźniak, K.; Iżycka, N. Consequences of Gynecological Cancer in Patients and Their Partners from the Sexual and Psychological Perspective. Prz. Menopauzalny 2016, 15, 112–116. [Google Scholar] [CrossRef]
- Neppl, T.K.; Senia, J.M.; Donnellan, M.B. Effects of Economic Hardship: Testing the Family Stress Model over Time. J. Fam. Psychol. 2016, 30, 12–21. [Google Scholar] [CrossRef]
- Zhao, J.; Kong, Y.; Xiang, Y.; Yang, J. The Research Landscape of the Quality of Life or Psychological Impact on Gynecological Cancer Patients: A Bibliometric Analysis. Front. Oncol. 2023, 13, 1115852. [Google Scholar] [CrossRef]
- Zhang, Y.; Kwok-Shing Ng, P.; Kucherlapati, M.; Chen, F.; Liu, Y.; Tsang, Y.H.; de Velasco, G.; Jeong, K.J.; Akbani, R.; Hadjipanayis, A.; et al. A Pan-Cancer Proteogenomic Atlas of PI3K/AKT/mTOR Pathway Alterations. Cancer Cell 2017, 31, 820–832.e3. [Google Scholar] [CrossRef]
- de Melo, A.C.; Paulino, E.; Garces, Á.H.I. A Review of mTOR Pathway Inhibitors in Gynecologic Cancer. Oxid. Med. Cell. Longev. 2017, 2017, 4809751. [Google Scholar] [CrossRef]
- Ediriweera, M.K.; Tennekoon, K.H.; Samarakoon, S.R. Role of the PI3K/AKT/mTOR Signaling Pathway in Ovarian Cancer: Biological and Therapeutic Significance. Semin. Cancer Biol. 2019, 59, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Wang, Y.; Zhou, C.; Mei, W.; Zeng, C. PI3K/Akt/mTOR Pathway and Its Role in Cancer Therapeutics: Are We Making Headway? Front. Oncol. 2022, 12, 819128. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.-P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/mismatch Repair-Deficient Cancer: Results from the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Skomedal, H.; Kristensen, G.; Helland, A.; Nesland, J.M.; Kooi, S.; Børresen, A.L.; Holm, R. TP53 Gene Mutations and Protein Accumulation in Primary Vaginal Carcinomas. Br. J. Cancer 1995, 72, 129–133. [Google Scholar] [CrossRef]
- Talia, K.L.; Scurry, J.; Manolitsas, T.; McCluggage, W.G. Primary Vaginal Mucinous Adenocarcinoma of Gastric Type Arising in Adenosis: A Report of 2 Cases, 1 Associated with Uterus Didelphys. Int. J. Gynecol. Pathol. 2012, 31, 184–191. [Google Scholar] [CrossRef]
- Waggoner, S.E.; Anderson, S.M.; Luce, M.C.; Takahashi, H.; Boyd, J. P53 Protein Expression and Gene Analysis in Clear Cell Adenocarcinoma of the Vagina and Cervix. Gynecol. Oncol. 1996, 60, 339–344. [Google Scholar] [CrossRef]
- Chung, T.K.H.; Doran, G.; Cheung, T.-H.; Yim, S.-F.; Yu, M.-Y.; Worley, M.J., Jr.; Elias, K.M.; Thorner, A.R.; Pedamallu, C.S.; Ojesina, A.I.; et al. Dissection of PIK3CA Aberration for Cervical Adenocarcinoma Outcomes. Cancers 2021, 13, 3218. [Google Scholar] [CrossRef]
- Voutsadakis, I.A. PI3KCA Mutations in Uterine Cervix Carcinoma. J. Clin. Med. 2021, 10, 220. [Google Scholar] [CrossRef]
- Passarelli, A.; Carbone, V.; Pignata, S.; Mazzeo, R.; Lorusso, D.; Scambia, G.; Canova, S.; Di Palma, T.; Tasca, G.; Mantiero, M.; et al. Alpelisib for PIK3CA-Mutated Advanced Gynecological Cancers: First Clues of Clinical Activity. Gynecol. Oncol. 2024, 183, 61–67. [Google Scholar] [CrossRef]
- Zeng, X.; Xi, M.-R.; Ma, H.-W. PTEN Gene and AKT/mTOR Pathway in Gynecological Cancers and Cancer Immune Escape. Eur. J. Gynaecol. Oncol. 2022, 43, 19–24. [Google Scholar] [CrossRef]
- Bossler, F.; Hoppe-Seyler, K.; Hoppe-Seyler, F. PI3K/AKT/mTOR Signaling Regulates the Virus/host Cell Crosstalk in HPV-Positive Cervical Cancer Cells. Int. J. Mol. Sci. 2019, 20, 2188. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, A.; Hasanzadeh, M.; Hassanian, S.M.; ShahidSales, S.; Ghayour-Mobarhan, M.; Ferns, G.A.; Avan, A. The Potential Value of the PI3K/Akt/mTOR Signaling Pathway for Assessing Prognosis in Cervical Cancer and as a Target for Therapy. J. Cell. Biochem. 2017, 118, 4163–4169. [Google Scholar] [CrossRef] [PubMed]
- Xing, D.; Fadare, O. Molecular Events in the Pathogenesis of Vulvar Squamous Cell Carcinoma. Semin. Diagn. Pathol. 2021, 38, 50–61. [Google Scholar] [CrossRef]
- Zięba, S.; Kowalik, A.; Zalewski, K.; Rusetska, N.; Goryca, K.; Piaścik, A.; Misiek, M.; Bakuła-Zalewska, E.; Kopczyński, J.; Kowalski, K.; et al. Somatic Mutation Profiling of Vulvar Cancer: Exploring Therapeutic Targets. Gynecol. Oncol. 2018, 150, 552–561. [Google Scholar] [CrossRef]
- Jiang, T.-Y.; Pan, Y.-F.; Wan, Z.-H.; Lin, Y.-K.; Zhu, B.; Yuan, Z.-G.; Ma, Y.-H.; Shi, Y.-Y.; Zeng, T.-M.; Dong, L.-W.; et al. PTEN Status Determines Chemosensitivity to Proteasome Inhibition in Cholangiocarcinoma. Sci. Transl. Med. 2020, 12, eaay0152. [Google Scholar] [CrossRef]
- Cho, Y.S.; Kim, H.R.; Park, S.J.; Chung, S.W.; Ko, Y.G.; Yeo, J.H.; Lee, J.; Kim, S.K.; Choi, J.U.; Kim, S.Y.; et al. Sustained Potentiation of Bystander Killing via PTEN-Loss Driven Macropinocytosis Targeted Peptide-Drug Conjugate Therapy in Metastatic Triple-Negative Breast Cancer. Biomaterials 2022, 289, 121783. [Google Scholar] [CrossRef] [PubMed]
- Owusu-Brackett, N.; Zhao, M.; Akcakanat, A.; Evans, K.W.; Yuca, E.; Dumbrava, E.I.; Janku, F.; Meric-Bernstam, F. Targeting PI3Kβ Alone and in Combination with Chemotherapy or Immunotherapy in Tumors with PTEN Loss. Oncotarget 2020, 11, 969–981. [Google Scholar] [CrossRef]
- González-García, A.; Garrido, A.; Carrera, A.C. Targeting PTEN Regulation by Post Translational Modifications. Cancers 2022, 14, 5613. [Google Scholar] [CrossRef]
- Manrai, P.A.; McHenry, A.; Sun, T.; Santin, A.D.; Ratner, E.; Lin, D.I.; Elvin, J.A.; Hui, P.; Buza, N. Targetable ERBB2/HER2 Mutations in Gynecologic Malignancies: Clinicopathological, Immunohistochemical, and Molecular Correlations. Int. J. Gynecol. Pathol. 2025, 44, 144–154. [Google Scholar] [CrossRef]
- Roussel-Simonin, C.; Blanc-Durand, F.; Bayle, A.; Baldini, C.; Champiat, S.; Ponce Aix, S.; Loriot, Y.; Michels, J.; Leary, A.; Pautier, P.; et al. Outcomes of Patients with Gynecological Tumors Harboring HER2 Defects: A Single Center Study Based on Gustave Roussy Cancer Center’s Molecular Program. ESMO Open 2023, 8, 100856. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Tan, D.; Ray-Coquard, I.; Lee, J.B.; Kim, B.G.; Van Nieuwenhuysen, E.; Huang, R.Y.-J.; Tse, K.Y.; González-Martin, A.; Scott, C.; et al. Phase II Randomized Study of Dostarlimab Alone or with Bevacizumab versus Non-Platinum Chemotherapy in Recurrent Gynecological Clear Cell Carcinoma (DOVE/APGOT-OV7/ENGOT-ov80). J. Gynecol. Oncol. 2025, 36, e51. [Google Scholar] [CrossRef] [PubMed]
| Item | Specifications |
|---|---|
| Period | From January 2016 to 28 April 2025 |
| Database | PubMed, Scopus, Web of Science |
| Search term used | Vaginal adenocarcinoma |
| Inclusion and exclusion criteria | All references were SCI-indexed articles written in English |
| Selection process | Two independent reviewers evaluated the titles and abstracts to determine eligibility |
| Author, Year | Age | Clinical Presentation | Diagnosis | Management | Outcome |
|---|---|---|---|---|---|
| Zhang et al., 2019 [9] | 45 | Vaginal swelling, dyspnea, itching | Adenoid cystic carcinoma | Chemoradiotherapy | Alive at 13 months |
| Shen et al., 2022 [10] | 64 | Vaginal bleeding/discharge | Stage IVA squamous cell carcinoma | Chemo, radiotherapy, immunotherapy, TKIs | Complete remission |
| Porragas-Paseiro et al., 2023 [11] | 38 | Recurrent disease | Clear cell carcinoma | Pembrolizumab | Complete, durable response |
| Ferrari et al., 2023 [12] | 52 | Vaginal bleeding | Mesonephric adenocarcinoma | Surgery, adjuvant therapy | Mean follow-up 6 years, mostly favorable |
| Yang et al., 2019 [13] | 57 | Vaginal bleeding, pelvic pain | Endometrioid adenocarcinoma | Surgery, chemotherapy | No recurrence at 12 months |
| Mu et al., 2023 [14] | 40 | Incessant menstruation, distension | Endometrioid adenocarcinoma | Staged surgery, chemo (6 cycles) | No recurrence at 2 years |
| Saijilafu et al., 2024 [15] | 62 | Postmenopausal bleeding | Stage IIB adenocarcinoma | Chemo + external + intracavitary radiotherapy | Tumor control at 3 months |
| Barcellini et al., 2021 [16] | 80 | Recurrent vaginal tumor | Squamous cell carcinoma | Proton beam therapy | Complete response at 12 months |
| Pang et al., 2019 [5] | 39 | Chronic vaginal pain, bleeding | Clear cell carcinoma (adenosis origin) | Wide excision + chemoradiotherapy | No recurrence at 16 months |
| Plesinac-Karapandzic et al., 2017 [17] | 22 | Vaginal mass | Clear cell/mesonephric carcinoma | Radiotherapy chemotherapy | Disease-free at 11 years, morbidity noted |
| Kumar et al., 2022 [18] | 40 | Post-hysterectomy bleeding | Mesonephric carcinoma | IHC-based diagnosis | Not stated |
| Li et al., 2023 [19] | 55 | Vaginal bleeding | Clear cell carcinoma (rectovaginal septum) | Surgery + chemotherapy | Complete response |
| Haddout et al., 2022 [20] | 60 | Inguinal mass, vaginal lesion | Clear cell carcinoma | Surgery + chemoradiotherapy | Poor prognosis |
| Felicelli et al., 2023 [21] | 63 | Vaginal polypoid mass | HPV-associated enteric-type adenocarcinoma | Surgery | First reported case, outcome not detailed |
| Sabri et al., 2022 [7] | 62 | Vaginal tumor | Intestinal-type adenocarcinoma | Varied treatments | Prognosis depends on multiple factors |
| Ugwu et al., 2019 [22] | 40 | Vaginal mass, bleeding | Intestinal-type adenocarcinoma | Surgery | Recognition key for diagnosis |
| Mei et al., 2020 [23] | 40 | Irregular bleeding | Clear cell carcinoma + HWW syndrome | Surgery | Not stated |
| Warembourg et al., 2016 [24] | 63 | Pelvic pain | Cystadenocarcinoma (rectovaginal) | Surgery + chemotherapy | No recurrence at 36 months |
| Kalampokas et al., 2023 [25] | 79 | Vaginal pressure feeling and bleeding | Small-cell neuroendocrine carcinoma | Surgery | - |
| Nguyen-Xuan et al., 2021 [26] | 44 | Metrorrhagia | Clear cell carcinoma | Fluorescence-guided surgery | Negative margins |
| Lei & Zhang, 2024 [27] | 40 | Irregular bleeding | Clear cell carcinoma + HWW syndrome | Radical surgery, chemotherapy | Lung metastasis at 4 years |
| Symptom | Description | Frequency/Notes | References |
|---|---|---|---|
| Abnormal Vaginal Bleeding | Includes postmenopausal, postcoital, or intermenstrual bleeding | Most common symptom (50–75% of cases) | [10,45] |
| Vaginal Discharge | Persistent or foul-smelling discharge; may suggest tumor necrosis or infection | Common | [10,46] |
| Palpable Mass or Lump | Nodule or thickening, especially in upper third of the vaginal wall | Frequently observed on a physical exam | [17] |
| Pain or Discomfort | Pelvic pain, dyspareunia, rectal pain; more common in advanced stages | Variable | [24,46] |
| Other Symptoms | Itching, burning, urinary symptoms (dysuria, hematuria), constipation, hematuria (if local invasion occurs) | Less common, dependent on tumor spread | [26] |
| Tumor Type | Positive Markers (Fold Change vs. Non-Relevant Tumors) | Negative Markers (Reduction vs. Relevant Tumors) | Diagnostic Utility |
|---|---|---|---|
| Intestinal-Type Vaginal ADC | SATB2 (2–5×), CDX2 (3–10×), CK20 (5–20×), CEA (2–4×) | CK7 (>90% ↓), PAX8 (near-absent), p16 (50–80% ↓) | SATB2/CDX2 confirm an intestinal origin; CK7/PAX8 exclude gynecologic primary tumors. |
| Endometrial/Ovarian ADC | PAX8 (5–50×), CK7 (10–30×), ER/PR (2–10×) | SATB2 (>95% ↓), CK20 (>80% ↓) | PAX8/CK7 + ER/PR confirm a Müllerian origin; SATB2 excludes CRC. |
| Cervical ADC | p16 (10–100×), CEA (2–5×) | ER/PR (>90% ↓), Vimentin (>70% ↓) | p16’s extreme overexpression indicates an HPV-driven etiology. |
| Serous Papillary ADC | PAX8/WT1 (5–20×), p53 (10–50×), CA125 (↑ ↑ serum) | SATB2/CK20 (>95% ↓) | An aberrant WT1/p53 pattern confirms a serous subtype. |
| AJCC Stage | Stage Grouping (TNM) | FIGO Stage | Stage Description |
|---|---|---|---|
| IA | T1a | I | Present only in the vagina and is no larger than 2.0 cm (4/5 inch) (T1a) |
| N0 | No spread to nearby lymph nodes (N0) or to distant sites (M0) | ||
| IB | T1b | I | Present only in the vagina and is larger than 2.0 cm (4/5 inch) (T1b) |
| N0 | No spread to nearby lymph nodes (N0) or to distant sites (M0) | ||
| IIA | T2a | II | Cancer has grown through the vaginal wall, but not as far as the pelvic wall, and is no larger than 2.0 cm (4/5 inch) (T2a) |
| N0 | No spread to nearby lymph nodes (N0) or to distant sites (M0) | ||
| IIB | T2b | II | Cancer has grown through the vaginal wall, but not as far as the pelvic wall, and is larger than 2.0 cm (4/5 inch) (T2b) |
| N0 | No spread to nearby lymph nodes (N0) or to distant sites (M0) | ||
| III | T1 to T3 | III | Cancer can be any size and might be growing into the pelvic wall, and/or growing into the lower one-third of the vagina, and/or has blocked the flow of urine (hydronephrosis), which is causing kidney problems (T1 to T3); has spread to nearby lymph nodes in the pelvis or groin (inguinal) area (N1) but not distant sites (M0) |
| N1 | |||
| OR | |||
| T3 | III | Cancer is growing into the pelvic wall, and/or growing into the lower one-third of the vagina, and/or has blocked the flow of urine (hydronephrosis), which is causing kidney problems (T3) | |
| N0 | No spread to nearby lymph nodes (N0) or to distant sites (M0) | ||
| IVA | T4 | IVA | Cancer is growing into the bladder or rectum or is growing out of the pelvis (T4) |
| Any N | May/may not have spread to lymph nodes in the pelvis or groin (inguinal area) (Any N); has not spread to distant sites (M0) | ||
| IVB | Any T | IVB | Cancer has spread to distant organs such as the lungs or bones (M1). It can be any size and might or might not have grown into nearby structures or organs (Any T) |
| Any N | May/may not have spread to nearby lymph nodes (Any N) | ||
| M1 | May/may not have spread to nearby lymph nodes (Any N) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, M.-K.; Ding, D.-C. Vaginal Adenocarcinoma: A Review of a Rare Gynecologic Cancer. Cancers 2025, 17, 2130. https://doi.org/10.3390/cancers17132130
Hong M-K, Ding D-C. Vaginal Adenocarcinoma: A Review of a Rare Gynecologic Cancer. Cancers. 2025; 17(13):2130. https://doi.org/10.3390/cancers17132130
Chicago/Turabian StyleHong, Mun-Kun, and Dah-Ching Ding. 2025. "Vaginal Adenocarcinoma: A Review of a Rare Gynecologic Cancer" Cancers 17, no. 13: 2130. https://doi.org/10.3390/cancers17132130
APA StyleHong, M.-K., & Ding, D.-C. (2025). Vaginal Adenocarcinoma: A Review of a Rare Gynecologic Cancer. Cancers, 17(13), 2130. https://doi.org/10.3390/cancers17132130







