Assessing Oncologic and Functional Outcomes of 3D Image-Guided Robotic-Assisted Partial Nephrectomy (3D-IGRAPN): A Prospective Study (UroCCR-186)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3D | Three-Dimensional |
| 3D-IGRAPN | 3D Image-Guided Robotic-Assisted Partial Nephrectomy |
| eGFR | Estimated Glomerular Filtration Rate |
| NSS | Nephron-Sparing Surgery |
| SD | Standard Deviation |
| AKI | Acute Kidney Injury |
References
- Ljungberg, B.; Albiges, L.; Abu-Ghanem, Y.; Bedke, J.; Capitanio, U.; Dabestani, S.; Fernández-Pello, S.; Giles, R.-H.; Hofmann, F.; Hora, M.; et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2022 Update. Eur. Urol. 2022, 82, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Hung, A.J.; Cai, J.; Simmons, M.N.; Gill, I.S. “Trifecta” in Partial Nephrectomy. J. Urol. 2013, 189, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.E.; You, J.H.; Kim, D.K.; Rha, K.H.; Lee, S.H. Comparison of Perioperative Outcomes Between Robotic and Laparoscopic Partial Nephrectomy: A Systematic Review and Meta-Analysis. Eur. Urol. 2015, 67, 891–901. [Google Scholar] [CrossRef]
- Masson-Lecomte, A.; Yates, D.R.; Hupertan, V.; Haertig, A.; Chartier-Kastler, E.; Bitker, M.-O.; Vaessen, C.; Rouprêt, M. A Prospective Comparison of the Pathologic and Surgical Outcomes Obtained after Elective Treatment of Renal Cell Carcinoma by Open or Robot-Assisted Partial Nephrectomy. Urol. Oncol. Semin. Orig. Investig. 2013, 31, 924–929. [Google Scholar] [CrossRef]
- Long, J.-A.; Yakoubi, R.; Lee, B.; Guillotreau, J.; Autorino, R.; Laydner, H.; Eyraud, R.; Stein, R.J.; Kaouk, J.H.; Haber, G.-P. Robotic Versus Laparoscopic Partial Nephrectomy for Complex Tumors: Comparison of Perioperative Outcomes. Eur. Urol. 2012, 61, 1257–1262. [Google Scholar] [CrossRef]
- Thakker, P.U.; O’Rourke, T.K.; Hemal, A.K. Technologic Advances in Robot-Assisted Nephron Sparing Surgery: A Narrative Review. Transl. Androl. Urol. 2023, 12, 1184–1198. [Google Scholar] [CrossRef] [PubMed]
- Michiels, C.; Khene, Z.-E.; Prudhomme, T.; Boulenger De Hauteclocque, A.; Cornelis, F.H.; Percot, M.; Simeon, H.; Dupitout, L.; Bensadoun, H.; Capon, G.; et al. 3D-Image Guided Robotic-Assisted Partial Nephrectomy: A Multi-Institutional Propensity Score-Matched Analysis (UroCCR Study 51). World J. Urol. 2021, 41, 303–313. [Google Scholar] [CrossRef]
- EAU 2019—Image Guided Robotic Partial Nephrectomy IGRAPN—Pr Bernhard CHU BX—YouTube. Available online: https://www.youtube.com/watch?v=Lr0kmNG_RuM&list=PLq2kJTeAXamW_UbjAbGGEpj_1Ou6r3GYp&index=14 (accessed on 2 May 2025).
- ERUS 2020—Virtual Reality & 3D Printed IGRAPN—Pr Bernhard CHU BX—YouTube. Available online: https://www.youtube.com/watch?v=T2_2IISHpAc&list=PLq2kJTeAXamW_UbjAbGGEpj_1Ou6r3GYp&index=9 (accessed on 3 May 2025).
- UroCCR: Réseau Français de Recherche sur le Cancer du Rein. Available online: https://host.credim.u-bordeaux.fr/UROCCR/Public/Index.aspx (accessed on 3 May 2025).
- ERUS 2016—Live Surgical Guidance Robotic Partial Nephrectomy—Pr Bernhard CHU BX—YouTube. Available online: https://www.youtube.com/watch?v=jiAw3u1VaeU&list=PLq2kJTeAXamW_UbjAbGGEpj_1Ou6r3GYp&index=21 (accessed on 3 May 2025).
- Brassetti, A.; Anceschi, U.; Bertolo, R.; Ferriero, M.; Tuderti, G.; Capitanio, U.; Larcher, A.; Garisto, J.; Antonelli, A.; Mottire, A.; et al. Surgical quality, cancer control and functional preservation: Introducing a novel trifecta for robot-assisted partial nephrectomy. Minerva Urol Nefrol. 2020, 72, 82–90. [Google Scholar] [CrossRef]
- Khalifeh, A.; Autorino, R.; Hillyer, S.P.; Laydner, H.; Eyraud, R.; Panumatrassamee, K.; Long, J.-A.; Kaouk, J.H. Comparative Outcomes and Assessment of Trifecta in 500 Robotic and Laparoscopic Partial Nephrectomy Cases: A Single Surgeon Experience. J. Urol. 2013, 189, 1236–1242. [Google Scholar] [CrossRef]
- Margue, G.; Ingels, A.; Bensalah, K.; Doumerc, N.; Vaessen, C.; Roupret, M.; Audenet, F.; Mejean, A.; Bruyere, F.; Olivier, J.; et al. Late complications and 5 years outcomes of robotic partial nephrectomy in France: Prospective assessment in the French Kidney Cancer Research Network (UroCCR 10). World J. Urol. 2023, 41, 2281–2288. [Google Scholar] [CrossRef]
- Peyronnet, B.; Seisen, T.; Oger, E.; Vaessen, C.; Grassano, Y.; Benoit, T.; Carrouget, J.; Pradère, B.; Khene, Z.; French Comittee of Urologic Oncology (CCAFU); et al. Comparison of 1800 Robotic and Open Partial Nephrectomies for Renal Tumors. Ann. Surg. Oncol. 2016, 23, 4277–4283. [Google Scholar] [CrossRef] [PubMed]
- Bravi, C.A.; Larcher, A.; Capitanio, U.; Mari, A.; Antonelli, A.; Artibani, W.; Barale, M.; Bertini, R.; Bove, P.; Brunocilla, E.; et al. Perioperative Outcomes of Open, Laparoscopic, and Robotic Partial Nephrectomy: A Prospective Multicenter Observational Study (The RECORd 2 Project). Eur. Urol. Focus 2021, 7, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Grosso, A.-A.; Di Maida, F.; Lambertini, L.; Cadenar, A.; Coco, S.; Ciaralli, E.; Salamone, V.; Vittori, G.; Tuccio, A.; Mari, A.; et al. Three-Dimensional Virtual Model for Robot-Assisted Partial Nephrectomy: A Propensity-Score Matching Analysis with a Contemporary Control Group. World J. Urol. 2024, 42, 338. [Google Scholar] [CrossRef] [PubMed]
- Flammia, R.-S.; Anceschi, U.; Tuderti, G.; Di Maida, F.; Grosso, A.-A.; Lambertini, L.; Mari, A.; Mastroianni, R.; Bove, A.; Capitanio, U.; et al. Development and internal validation of a nomogram predicting 3-year chronic kidney disease upstaging following robot-assisted partial nephrectomy. Int. Urol. Nephrol. 2024, 56, 913–921. [Google Scholar] [CrossRef]
- Pecoraro, A.; Amparore, D.; Checcucci, E.; Piramide, F.; Carbonaro, B.; De Cillis, S.; Granato, S.; Sica, M.; Campi, R.; Fiori, C.; et al. Three-Dimensional Virtual Models Assistance Predicts Higher Rates of “Successful” Minimally Invasive Partial Nephrectomy: An Institutional Analysis across the Available Trifecta Definitions. World J. Urol. 2023, 41, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Porpiglia, F.; Checcucci, E.; Amparore, D.; Piramide, F.; Volpi, G.; Granato, S.; Verri, P.; Manfredi, M.; Bellin, A.; Piazzolla, P.; et al. Three-Dimensional Augmented Reality Robot-Assisted Partial Nephrectomy in Case of Complex Tumours (PADUA ≥ 10): A New Intraoperative Tool Overcoming the Ultrasound Guidance. Eur. Urol. 2020, 78, 229–238. [Google Scholar] [CrossRef]
- Amparore, D.; Pecoraro, A.; Checcucci, E.; Piramide, F.; Verri, P.; De Cillis, S.; Granato, S.; Angusti, T.; Solitro, F.; Veltri, A.; et al. Three-Dimensional Virtual Models’ Assistance During Minimally Invasive Partial Nephrectomy Minimizes the Impairment of Kidney Function. Eur. Urol. Oncol. 2022, 5, 104–108. [Google Scholar] [CrossRef]
- Shirk, J.D.; Thiel, D.D.; Wallen, E.M.; Linehan, J.M.; White, W.M.; Badani, K.K.; Porter, J.R. Effect of 3-Dimensional Virtual Reality Models for Surgical Planning of Robotic-Assisted Partial Nephrectomy on Surgical Outcomes: A Randomized Clinical Trial. JAMA Netw. Open 2019, 2, e1911598. [Google Scholar] [CrossRef]
- Piramide, F.; Kowalewski, K.-F.; Cacciamani, G.; Rivero Belenchon, I.; Taratkin, M.; Carbonara, U.; Marchioni, M.; De Groote, R.; Knipper, S.; Pecoraro, A.; et al. Three-Dimensional Model–Assisted Minimally Invasive Partial Nephrectomy: A Systematic Review with Meta-Analysis of Comparative Studies. Eur. Urol. Oncol. 2022, 5, 640–650. [Google Scholar] [CrossRef]
- Makiyama, K.; Komeya, M.; Tatenuma, T.; Noguchi, G.; Ohtake, S. Patient-specific Simulations and Navigation Systems for Partial Nephrectomy. Int. J. Urol. 2023, 30, 1087–1095. [Google Scholar] [CrossRef]
- Porpiglia, F.; Amparore, D.; Checcucci, E.; Autorino, R.; Manfredi, M.; Iannizzi, G.; Fiori, C. Current Use of Three-Dimensional Model Technology in Urology: A Road Map for Personalised Surgical Planning. Eur. Urol. Focus 2018, 4, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Campi, R.; Grosso, A.A.; Lane, B.R.; Cobelli, O.; Sanguedolce, F.; Hatzichristodoulou, G.; Antonelli, A.; Noyes, S.; Maida, F.; Mari, A.; et al. SIB International Consortium. Impact of Trifecta definition on rates and predictors of “successful” robotic partial nephrectomy for localized renal masses: Results from the Surface-Intermediate-Base Margin Score International Consortium. Minerva Urol Nephrol. 2022, 74, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Margue, G.; Bernhard, J.-C.; Giai, J.; Bouzit, A.; Ricard, S.; Jaffredo, M.; Guillaume, B.; Jambon, E.; Fiard, G.; Bigot, P.; et al. Clinical Trial Protocol for ACCURATE: A CCafU-UroCCR Randomized Trial: Three-dimensional Image-guided Robot-assisted Partial Nephrectomy for Renal Complex Tumor (UroCCR 99). Eur. Urol. Oncol. 2025; in press. [Google Scholar] [CrossRef] [PubMed]
- Khaddad, A.; Bernhard, J.-C.; Margue, G.; Michiels, C.; Ricard, S.; Chandelon, K.; Bladou, F.; Bourdel, N.; Bartoli, A. A Survey of Augmented Reality Methods to Guide Minimally Invasive Partial Nephrectomy. World J. Urol. 2022, 41, 335–343. [Google Scholar] [CrossRef]
- Piana, A.; Amparore, D.; Sica, M.; Volpi, G.; Checcucci, E.; Piramide, F.; De Cillis, S.; Busacca, G.; Scarpelli, G.; Sidoti, F.; et al. Automatic 3D Augmented-Reality Robot-Assisted Partial Nephrectomy Using Machine Learning: Our Pioneer Experience. Cancers 2024, 16, 1047. [Google Scholar] [CrossRef]
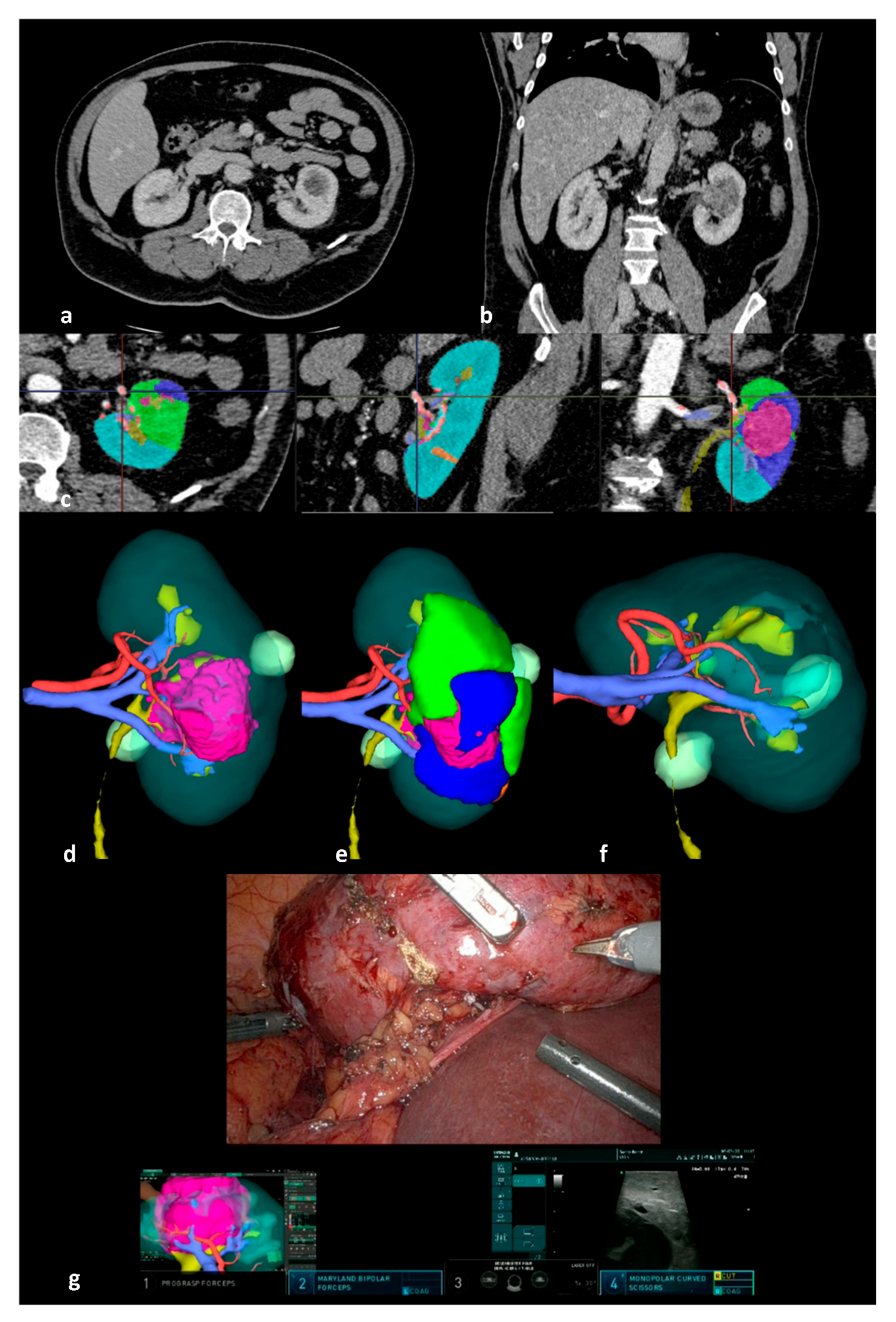
| Patients Characteristics | n = 568 |
| Age at surgery (years), mean (SD) | 59.9 (13.8) |
| Sex, n (%) | |
| Male | 398 (70.1) |
| Female | 170 (29.9) |
| cT stage | |
| 1a | 219 (38.6) |
| 1b | 233 (41) |
| 2a | 61 (10.7) |
| 2b | 17 (3.0) |
| 3a | 14 (2.5) |
| NR | 24 (4.2) |
| Surgery and Tumor Characteristics | n = 586 |
| Preoperative (eGFR mL/min), mean (SD) | 85.6 (22.5) |
| NSS Indication, n (%) | |
| Elective | 473 (80.7) |
| Imperative | 104 (17.7) |
| Relative | 45 (7.7) |
| Multiple tumorectomies, n (%) | 49 (8.4) |
| Tumor size (cm), mean (SD) | 4.9 (2.4) |
| RENAL score, mean (SD) | 8.4 (1.7) |
| Complexity according to RENAL score, n (%) | |
| Low | 93 (15.9) |
| Moderate | 298 (50.8) |
| High | 195 (33.3) |
| Clamping type, n (%) | |
| Main artery | 80 (13.7) |
| Superselective | 151 (25.8) |
| Off-clamp | 353 (60.2) |
| Warm ischemia time in case of main artery clamping (min), mean (SD) | 22.0 (14.5) |
| Renorrhaphy, n (%) | |
| None (sutureless) | 85 (14.5) |
| Parenchymal suture (single layer) | 294 (50.2) |
| Capsular + parenchymal suture (double layer) | 207 (35.3) |
| Blood loss (mL), mean (SD) | 322.3 (360.4) |
| Intra-operative transfusion rate, n (%) | 14 (2.4) |
| Conversion to open surgery, n (%) | 2 (0.3) |
| Conversion to radical nephrectomy, n (%) | 2 (0.3) |
| Operative time (min), mean (SD) | 203.2 (78.1) |
| Malignant lesion, n (%) | 528 (90.1) |
| Histological subtype, n (%) | |
| Clear cell carcinoma | 369 (63.0) |
| Papillary | 62 (10.6) |
| Chromophobe | 54 (9.2) |
| Other malignant tumors | 43 (7.3) |
| Oncocytoma | 44 (7.5) |
| Benign cyst | 9 (1.5) |
| Angiomylipoma | 5 (0.9) |
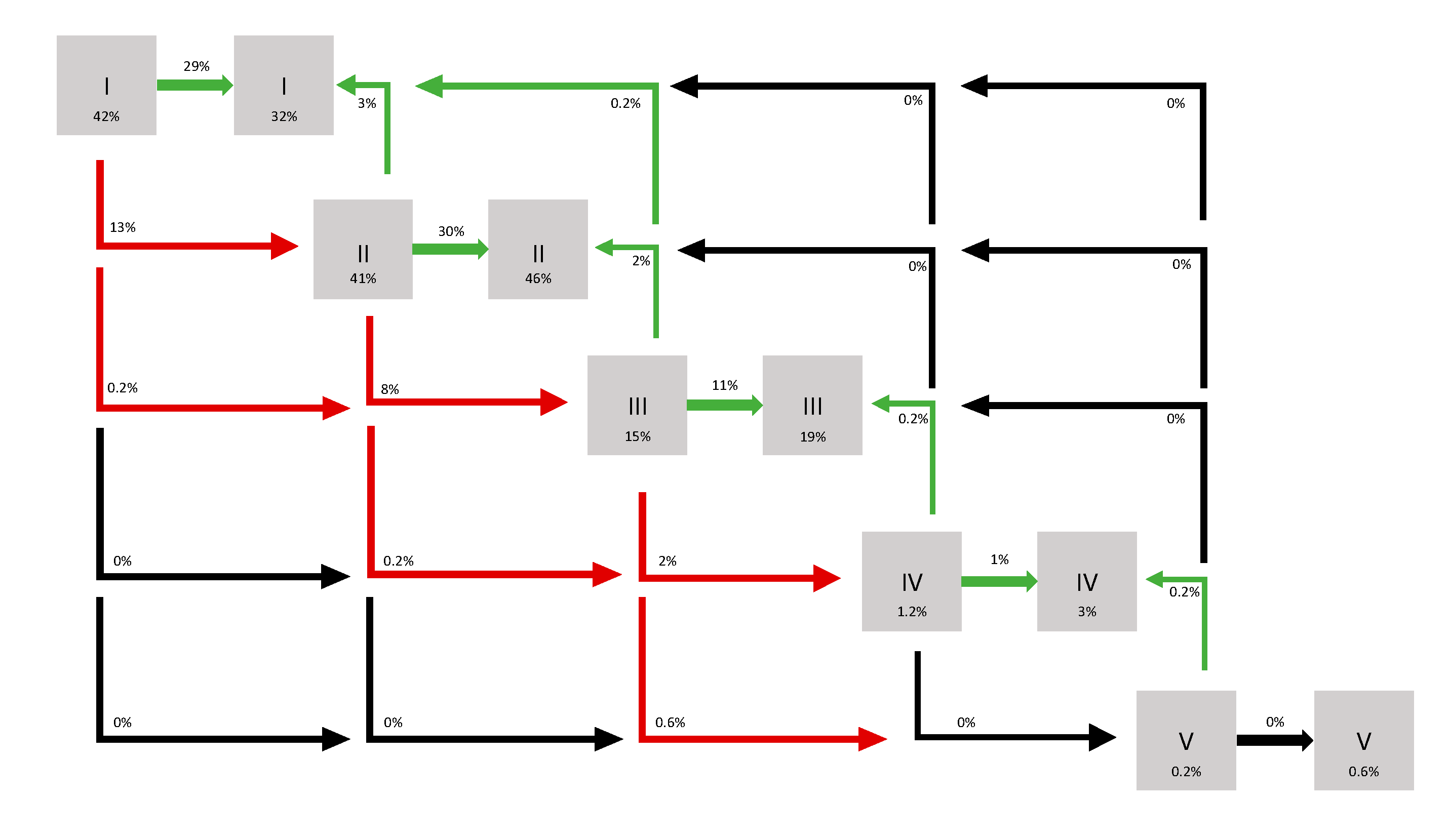
| pT stage | |
| 1a | 231 (39.4) |
| 1b | 119 (20.3) |
| 2a | 22 (3.8) |
| 2b | 3 (0.5) |
| 3a | 168 (28.7) |
| 3b | 1 (0.2) |
| NR | 42 (7.2) |
| Length of hospital stay (days), mean (SD) | 2.1 (4.4) |
| Hospital stay, n (%) | |
| Ambulatory (less than 12 h) | 54 (9.2) |
| Discharge at day 1 | 350 (59.7) |
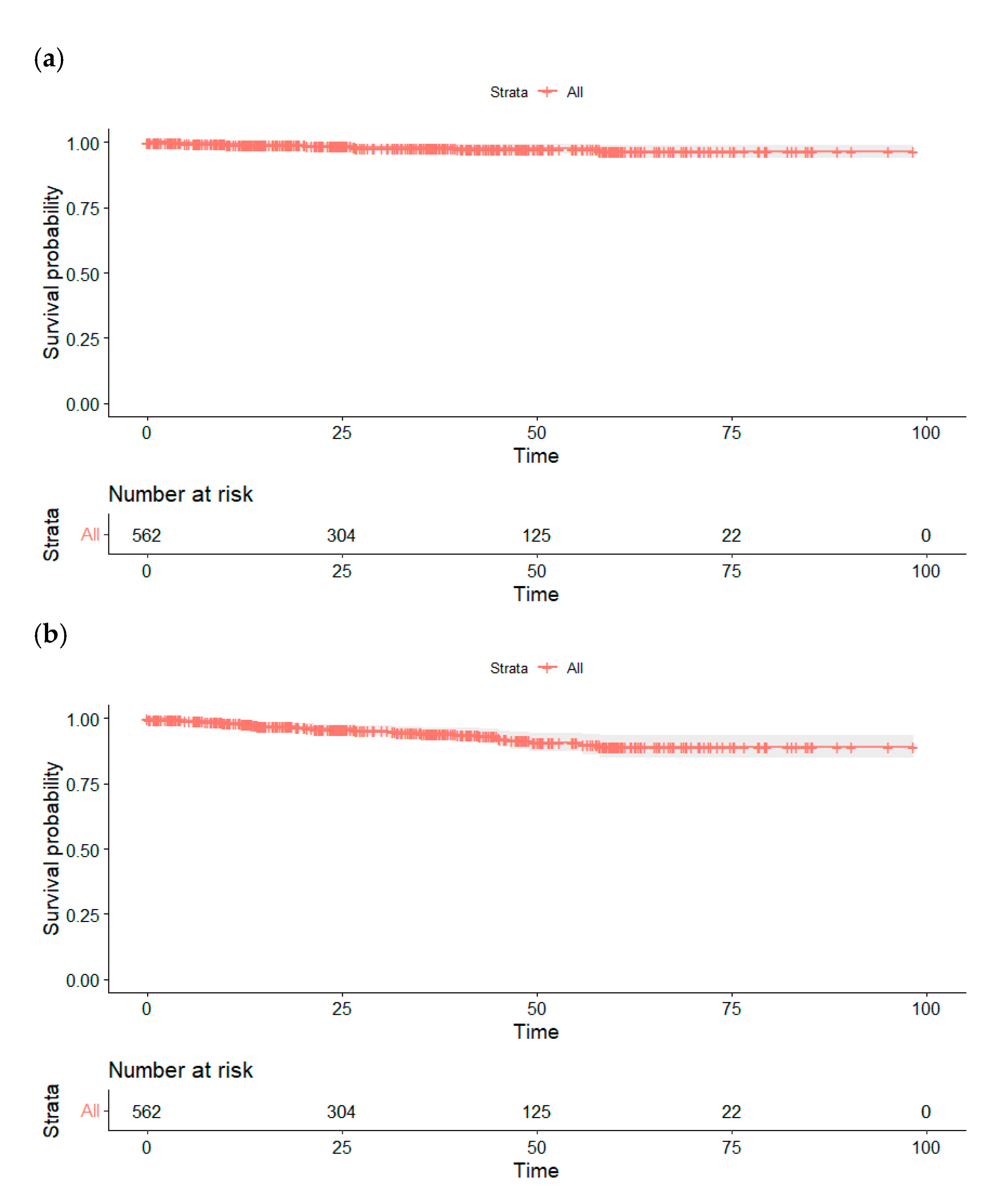
| Preoperative | Day 1 | 3 Months | 6 Months | 9 Months | 12 Months | 24 Months | Last Follow-Up | |
|---|---|---|---|---|---|---|---|---|
| GFR 1 according to CKD-EPI formula (mL/min), mean ± SD 2 | 85.6 ± 22.5 | 60.9 ± 25.74 | 77.3 ± 24.01 | 75.85 ± 25.83 | 72.14 ± 22.05 | 74.18 ± 23.96 | 73.39 ± 26.44 | 76.4 ± 22.9 |
| Change in GFR 1 compared to preoperative (mL/min), mean ± SD 2 | — | −22.55 ± 16.76 | −6.4 ± 26.69 | −10.29 ± 17.86 | −7.62 ± 12.74 | −6.85 ± 14.43 | −8.43 ± 16.07 | −8.9 ± 13.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pitout, A.; Margue, G.; Rubat Baleuri, F.; Khaddad, A.; Pattou, M.; Bladou, F.; Robert, G.; Bernhard, J.-C. Assessing Oncologic and Functional Outcomes of 3D Image-Guided Robotic-Assisted Partial Nephrectomy (3D-IGRAPN): A Prospective Study (UroCCR-186). Cancers 2025, 17, 2127. https://doi.org/10.3390/cancers17132127
Pitout A, Margue G, Rubat Baleuri F, Khaddad A, Pattou M, Bladou F, Robert G, Bernhard J-C. Assessing Oncologic and Functional Outcomes of 3D Image-Guided Robotic-Assisted Partial Nephrectomy (3D-IGRAPN): A Prospective Study (UroCCR-186). Cancers. 2025; 17(13):2127. https://doi.org/10.3390/cancers17132127
Chicago/Turabian StylePitout, Alice, Gaëlle Margue, Federico Rubat Baleuri, Abderrahmane Khaddad, Maxime Pattou, Franck Bladou, Grégoire Robert, and Jean-Christophe Bernhard. 2025. "Assessing Oncologic and Functional Outcomes of 3D Image-Guided Robotic-Assisted Partial Nephrectomy (3D-IGRAPN): A Prospective Study (UroCCR-186)" Cancers 17, no. 13: 2127. https://doi.org/10.3390/cancers17132127
APA StylePitout, A., Margue, G., Rubat Baleuri, F., Khaddad, A., Pattou, M., Bladou, F., Robert, G., & Bernhard, J.-C. (2025). Assessing Oncologic and Functional Outcomes of 3D Image-Guided Robotic-Assisted Partial Nephrectomy (3D-IGRAPN): A Prospective Study (UroCCR-186). Cancers, 17(13), 2127. https://doi.org/10.3390/cancers17132127







