Immune Checkpoint Inhibitors for Metastatic Colorectal Cancer: A Systematic Review
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
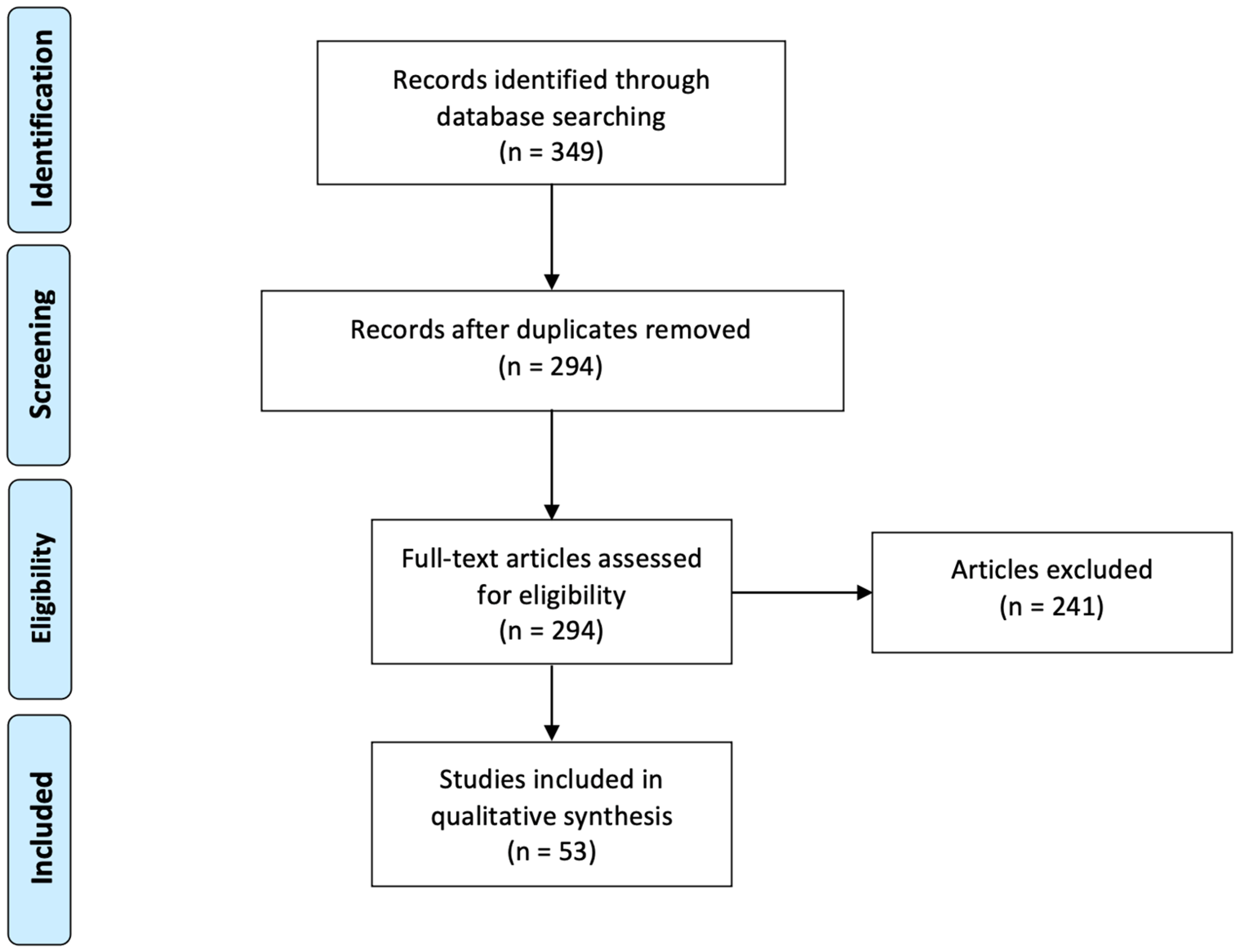
3.1. Inclusion Process
3.2. Immune Checkpoint Inhibitor Monotherapy
3.2.1. Anti-PD1 Monotherapy
3.2.2. Anti-PD-L1 Monotherapy
3.3. Immune Checkpoint Inhibitor (ICI) Combinations
3.3.1. Anti-PD-1 and Anti-CTLA-4 Combinations
3.3.2. Anti-PD-L1 and Anti-CTLA-4 Combinations
3.3.3. A Novel Dual-Target ICI and a Bispecific Antibody
3.4. Checkpoint Inhibitors Combined with Other Monoclonal Antibodies
3.4.1. Anti-PD1 with Other Monoclonal Antibodies
3.4.2. Anti-PDL1 with Other Monoclonal Antibodies
3.5. Immune Checkpoint Inhibitors Combined with Conventional Treatments
3.5.1. Checkpoint Inhibitors with Chemotherapy
Anti-PD1 with Chemotherapy
Anti-PDL1 with Chemotherapy
3.5.2. Checkpoint Inhibitors with Small Molecule Inhibitors
Anti-PD1 with Small Molecule Inhibitors
Anti-PDL1 with Small Molecule Inhibitors
3.5.3. Checkpoints Inhibitors with Radiotherapy
3.5.4. Checkpoints Inhibitors with Other Treatments
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AE | Adverse events |
| CRC | Colorectal cancer |
| CTLA-4 | Cytotoxic T lymphocyte antigen 4 protein |
| DCR | Disease control rate |
| EGFR | Epidermal growth factor receptor |
| ESMO | European Society for Medical Oncology |
| FOLFOX | Fluorouracil leucovorin oxaliplatin |
| FOLFOXIRI | Leucovorin–5-fluorouracil–oxaliplatin–irinotecan |
| HMA | Hypomethylating agents |
| MSI | Microsatellite instability (sub-categorized into MSI-L for Low and MSI-H for High) or d-MMR: deficient mismatch repair |
| MSS | Microsatellite stability or p-MMR: proficient mismatch repair |
| ORR | Objective response rate |
| OS | Overall survival |
| PD-1 | Programmed cell death protein 1 |
| PD-L1 | Programmed death-ligand 1 |
| PFS | Progression-free survival |
| VEGF | Vascular endothelial growth factor |
References
- Saraiva, M.R.; Rosa, I.; Claro, I. Early-onset colorectal cancer: A review of current knowledge. World J. Gastroenterol. 2023, 29, 1289–1303. [Google Scholar] [CrossRef] [PubMed]
- Colorectal Cancer Statistics|WCRF International. Available online: https://www.wcrf.org/cancer-trends/colorectal-cancer-statistics/ (accessed on 17 June 2023).
- Colorectal Cancer—IARC. Available online: https://www.iarc.who.int/cancer-type/colorectal-cancer/ (accessed on 4 September 2023).
- Colorectal Cancer Survival Rates|Colorectal Cancer Prognosis. Available online: https://www.cancer.org/cancer/types/colon-rectal-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 17 June 2023).
- Monoclonal Antibodies (MABs). Available online: https://www.cancerresearchuk.org/about-cancer/treatment/immunotherapy/types/monoclonal-antibodies (accessed on 17 June 2023).
- What Is Immunotherapy?|Cancer.Net. Available online: https://www.cancer.net/navigating-cancer-care/how-cancer-treated/immunotherapy-and-vaccines/what-immunotherapy (accessed on 17 June 2023).
- Berraondo, P.; Sanmamed, M.F.; Ochoa, M.C.; Etxeberria, I.; Aznar, M.A.; Pérez-Gracia, J.L.; Rodríguez-Ruiz, M.E.; Ponz-Sarvise, M.; Castañón, E.; Melero, I. Cytokines in clinical cancer immunotherapy. Br. J. Cancer 2019, 120, 6–15. [Google Scholar] [CrossRef]
- Cancer Research Institute. Adoptive Cell Therapy. Available online: https://www.cancerresearch.org/treatment-types/adoptive-cell-therapy (accessed on 10 August 2024).
- Cancer Vaccines. Available online: https://www.cancerresearch.org/treatment-types/cancer-vaccines (accessed on 10 August 2024).
- Oncolytic Virus Therapy. Available online: https://www.cancerresearch.org/treatment-types/oncolytic-virus-therapy (accessed on 10 August 2024).
- PRISMA Statement. Available online: https://www.prisma-statement.org (accessed on 20 April 2024).
- André, T.; Shiu, K.-K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability–High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef] [PubMed]
- Diaz, L.A.; Shiu, K.-K.; Kim, T.-W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): Final analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 2022, 23, 659–670. [Google Scholar] [CrossRef]
- Bi, F.; Dong, J.; Jin, C.; Niu, Z.; Yang, W.; He, Y.; Yu, D.; Sun, M.; Wang, T.; Yin, X.; et al. Iparomlimab (QL1604) in patients with microsatellite instability-high (MSI-H) or mismatch repair-deficient (dMMR) unresectable or metastatic solid tumors: A pivotal, single-arm, multicenter, phase II trial. J. Hematol. Oncol. 2024, 17, 109. [Google Scholar] [CrossRef]
- Felip, E.; Moreno, V.; Morgensztern, D.; Curigliano, G.; Rutkowski, P.; Trigo, J.M.; Calvo, A.; Kowalski, D.; Cortinovis, D.; Plummer, R.; et al. First-in-human, open-label, phase 1/2 study of the monoclonal antibody programmed cell death protein-1 (PD-1) inhibitor cetrelimab (JNJ-63723283) in patients with advanced cancers. Cancer Chemother. Pharmacol. 2022, 89, 499–514. [Google Scholar] [CrossRef]
- Taieb, J.; Bouche, O.; Andre, T.; Le Malicot, K.; Laurent-Puig, P.; Bez, J.; Toullec, C.; Borg, C.; Randrian, V.; Evesque, L.; et al. Avelumab vs. Standard Second-Line Chemotherapy in Patients With Metastatic Colorectal Cancer and Microsatellite Instability A Randomized Clinical Trial. JAMA Oncol. 2023, 9, 1356–1363. [Google Scholar] [CrossRef]
- Li, J.; Deng, Y.; Zhang, W.; Zhou, A.P.; Guo, W.; Yang, J.; Yuan, Y.; Zhu, L.; Qin, S.; Xiang, S.; et al. Subcutaneous envafolimab monotherapy in patients with advanced defective mismatch repair/microsatellite instability high solid tumors. J. Hematol. Oncol. 2021, 14, 95. Available online: https://pubmed.ncbi.nlm.nih.gov/34154614/ (accessed on 21 March 2024). [CrossRef]
- Eng, C.; Kim, T.W.; Bendell, J.; Argilés, G.; Tebbutt, N.C.; Bartolomeo, M.D.; Falcone, A.; Fakih, M.; Kozloff, M.; Segal, N.H.; et al. Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): A multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2019, 20, 849–861. [Google Scholar] [CrossRef]
- Liu, L.; Mayes, P.A.; Eastman, S.; Shi, H.; Yadavilli, S.; Zhang, T.; Yang, J.; Seestaller-Wehr, L.; Zhang, S.-Y.; Hopson, C.; et al. The BRAF and MEK Inhibitors Dabrafenib and Trametinib: Effects on Immune Function and in Combination with Immunomodulatory Antibodies Targeting PD-1, PD-L1, and CTLA-4. Clin. Cancer Res. 2015, 21, 1639–1651. [Google Scholar] [CrossRef]
- Loi, S.; Dushyanthen, S.; Beavis, P.A.; Salgado, R.; Denkert, C.; Savas, P.; Combs, S.; Rimm, D.L.; Giltnane, J.M.; Estrada, M.V.; et al. RAS/MAPK Activation Is Associated with Reduced Tumor-Infiltrating Lymphocytes in Triple-Negative Breast Cancer: Therapeutic Cooperation Between MEK and PD-1/PD-L1 Immune Checkpoint Inhibitors. Clin. Cancer Res. 2016, 22, 1499–1509. [Google Scholar] [CrossRef] [PubMed]
- Ebert, P.J.R.; Cheung, J.; Yang, Y.; McNamara, E.; Hong, R.; Moskalenko, M.; Gould, S.E.; Maecker, H.; Irving, B.A.; Kim, J.M.; et al. MAP Kinase Inhibition Promotes T Cell and Anti-tumor Activity in Combination with PD-L1 Checkpoint Blockade. Immunity 2016, 44, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Lenz, H.-J.; Van Cutsem, E.; Luisa Limon, M.; Wong, K.Y.M.; Hendlisz, A.; Aglietta, M.; García-Alfonso, P.; Neyns, B.; Luppi, G.; Cardin, D.B.; et al. First-Line Nivolumab Plus Low-Dose Ipilimumab for Microsatellite Instability-High/Mismatch Repair-Deficient Metastatic Colorectal Cancer: The Phase II CheckMate 142 Study. J. Clin. Oncol. 2022, 40, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Andre, T.; Elez, E.; Cutsem, E.V.; Jensen, L.H.; Bennouna, J.; Mendez, G.; Schenker, M.; de la Fouchardiere, C.; Limon, M.L.; Yoshino, T.; et al. Nivolumab plus Ipilimumab in Microsatellite-Instability–High Metastatic Colorectal Cancer. N. Engl. J. Med. 2024, 391, 2014–2026. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Fakih, M.; Gordon, M.S.; Tsimberidou, A.M.; Bullock, A.J.; Wilky, B.A.; Trent, J.C.; Margolin, K.A.; Mahadevan, D.; Balmanoukian, A.S.; et al. Results from a phase 1a/1b study of botensilimab (BOT), a novel innate/adaptive immune activator, plus balstilimab (BAL; anti-PD-1 antibody) in metastatic heavily pretreated microsatellite stable colorectal cancer (MSS CRC). J. Clin. Oncol. 2023, 41, LBA8. [Google Scholar] [CrossRef]
- Bullock, A.J.; Schlechter, B.L.; Fakih, M.G.; Tsimberidou, A.M.; Grossman, J.E.; Gordon, M.S.; Wilky, B.A.; Pimentel, A.; Mahadevan, D.; Balmanoukian, A.S.; et al. Botensilimab plus balstilimab in relapsed/refractory microsatellite stable metastatic colorectal cancer: A phase 1 trial. Nat. Med. 2024, 30, 2558–2567. [Google Scholar] [CrossRef]
- Chen, E.X.; Jonker, D.J.; Loree, J.M.; Kennecke, H.F.; Berry, S.R.; Couture, F.; Ahmad, C.E.; Goffin, J.R.; Kavan, P.; Harb, M.; et al. Effect of Combined Immune Checkpoint Inhibition vs. Best Supportive Care Alone in Patients With Advanced Colorectal Cancer. JAMA Oncol. 2020, 6, 831–838. [Google Scholar] [CrossRef]
- Kanikarla Marie, P.; Haymaker, C.; Parra, E.R.; Kim, Y.U.; Lazcano, R.; Gite, S.; Lorenzini, D.; Wistuba, I.I.; Tidwell, R.S.S.; Song, X.; et al. Pilot Clinical Trial of Perioperative Durvalumab and Tremelimumab in the Treatment of Resectable Colorectal Cancer Liver Metastases. Clin. Cancer Res. 2021, 27, 3039–3049. [Google Scholar] [CrossRef]
- Zhao, Y.; Ma, Y.; Zang, A.; Cheng, Y.; Zhang, Y.; Wang, X.; Chen, Z.; Qu, S.; He, J.; Chen, C.; et al. First-in-human phase I/Ib study of QL1706 (PSB205), a bifunctional PD1/CTLA4 dual blocker, in patients with advanced solid tumors. J. Hematol. Oncol. 2023, 16, 50. [Google Scholar] [CrossRef]
- Frentzas, S.; Austria Mislang, A.R.; Lemech, C.; Nagrial, A.; Underhill, C.; Wang, W.; Wang, Z.M.; Li, B.; Xia, Y.; Coward, J.I.G. Phase 1a dose escalation study of ivonescimab (AK112/SMT112), an anti-PD-1/VEGF-A bispecific antibody, in patients with advanced solid tumors. J. Immunother. Cancer 2024, 12, e008037. [Google Scholar] [CrossRef]
- Geva, R.; Voskoboynik, M.; Dobrenkov, K.; Mayawala, K.; Gwo, J.; Wnek, R.; Chartash, E.; Long, G.V. First-in-human phase 1 study of MK-1248, an anti-glucocorticoid-induced tumor necrosis factor receptor agonist monoclonal antibody, as monotherapy or with pembrolizumab in patients with advanced solid tumors. Cancer 2020, 126, 4926–4935. [Google Scholar] [CrossRef] [PubMed]
- Rahma, O.E.; Tyan, K.; Giobbie-Hurder, A.; Brohl, A.S.; Bedard, P.L.; Renouf, D.J.; Sharon, E.; Streicher, H.; Hathaway, E.; Cunningham, R.; et al. Phase IB study of ziv-aflibercept plus pembrolizumab in patients with advanced solid tumors. J. Immunother. Cancer 2022, 10, e003569. [Google Scholar] [CrossRef] [PubMed]
- Lentz, R.W.; Friedrich, T.J.; Blatchford, P.J.; Jordan, K.R.; Pitts, T.M.; Robinson, H.R.; Davis, S.L.; Kim, S.S.; Leal, A.D.; Lee, M.R.; et al. A Phase II Study of Potentiation of Pembrolizumab with Binimetinib and Bevacizumab in Refractory Microsatellite-Stable Colorectal Cancer. Clin. Cancer Res. 2024, 30, 3768–3778. [Google Scholar] [CrossRef] [PubMed]
- Curigliano, G.; Gelderblom, H.; Mach, N.; Doi, T.; Tai, D.; Forde, P.M.; Sarantopoulos, J.; Bedard, P.L.; Lin, C.-C.; Hodi, F.S.; et al. Phase I/Ib Clinical Trial of Sabatolimab, an Anti-TIM-3 Antibody, Alone and in Combination with Spartalizumab, an Anti-PD-1 Antibody, in Advanced Solid Tumors. Clin. Cancer Res. 2021, 27, 3620–3629. [Google Scholar] [CrossRef]
- Bauer, T.M.; Santoro, A.; Lin, C.-C.; Garrido-Laguna, I.; Joerger, M.; Greil, R.; Spreafico, A.; Yau, T.; Goebeler, M.-E.; Hütter-Krönke, M.L.; et al. Phase I/Ib, open-label, multicenter, dose-escalation study of the anti-TGF-β monoclonal antibody, NIS793, in combination with spartalizumab in adult patients with advanced tumors. J. Immunother. Cancer 2023, 11, e007353. [Google Scholar] [CrossRef]
- Kawazoe, A.; Itahashi, K.; Yamamoto, N.; Kotani, D.; Kuboki, Y.; Taniguchi, H.; Harano, K.; Naito, Y.; Suzuki, M.; Fukutani, M.; et al. TAS-116 (Pimitespib), an Oral HSP90 Inhibitor, in Combination with Nivolumab in Patients with Colorectal Cancer and Other Solid Tumors: An Open-Label, Dose-Finding, and Expansion Phase Ib Trial (EPOC1704). Clin. Cancer Res. 2021, 27, 6709–6715. [Google Scholar] [CrossRef]
- Lemech, C.; Dredge, K.; Bampton, D.; Hammond, E.; Clouston, A.; Waterhouse, N.J.; Stanley, A.C.; Leveque-El Mouttie, L.; Chojnowski, G.M.; Haydon, A.; et al. Phase Ib open-label, multicenter study of pixatimod, an activator of TLR9, in combination with nivolumab in subjects with microsatellite-stable metastatic colorectal cancer, metastatic pancreatic ductal adenocarcinoma and other solid tumors. J. Immunother. Cancer 2023, 11, e006136. [Google Scholar] [CrossRef]
- Li, C.; Ferro, A.; Mhatre, S.K.; Lu, D.; Lawrance, M.; Li, X.; Li, S.; Allen, S.; Desai, J.; Fakih, M.; et al. Hybrid-control arm construction using historical trial data for an early-phase, randomized controlled trial in metastatic colorectal cancer. Commun. Med. 2022, 2, 90. [Google Scholar] [CrossRef]
- Bendell, J.; LoRusso, P.; Overman, M.; Noonan, A.M.; Kim, D.-W.; Strickler, J.H.; Kim, S.-W.; Clarke, S.; George, T.J.; Grimison, P.S.; et al. First-in-human study of oleclumab, a potent, selective anti-CD73 monoclonal antibody, alone or in combination with durvalumab in patients with advanced solid tumors. Cancer Immunol. Immunother. 2023, 72, 2443–2458. [Google Scholar] [CrossRef]
- Patel, S.P.; Alonso-Gordoa, T.; Banerjee, S.; Wang, D.; Naidoo, J.; Standifer, N.E.; Palmer, D.C.; Cheng, L.-Y.; Kourtesis, P.; Ascierto, M.L.; et al. Phase 1/2 study of monalizumab plus durvalumab in patients with advanced solid tumors. J. Immunother. Cancer 2024, 12, e007340. [Google Scholar] [CrossRef]
- Herting, C.J.; Farren, M.R.; Tong, Y.; Liu, Z.; O’Neil, B.; Bekaii-Saab, T.; Noonan, A.; McQuinn, C.; Mace, T.A.; Shaib, W.; et al. A multi-center, single-arm, phase Ib study of pembrolizumab (MK-3475) in combination with chemotherapy for patients with advanced colorectal cancer: HCRN GI14-186. Cancer Immunol. Immunother. 2021, 70, 3337–3348. [Google Scholar] [CrossRef] [PubMed]
- Kuang, C.; Park, Y.; Augustin, R.C.; Lin, Y.; Hartman, D.J.; Seigh, L.; Pai, R.K.; Sun, W.; Bahary, N.; Ohr, J.; et al. Pembrolizumab plus azacitidine in patients with chemotherapy refractory metastatic colorectal cancer: A single-arm phase 2 trial and correlative biomarker analysis. Clin. Epigenetics 2022, 14, 3. [Google Scholar] [CrossRef]
- Lenz, H.-J.; Parikh, A.; Spigel, D.R.; Cohn, A.L.; Yoshino, T.; Kochenderfer, M.; Elez, E.; Shao, S.H.; Deming, D.; Holdridge, R.; et al. Modified FOLFOX6 plus bevacizumab with and without nivolumab for first-line treatment of metastatic colorectal cancer: Phase 2 results from the CheckMate 9X8 randomized clinical trial. J. Immunother. Cancer 2024, 12, e008409. [Google Scholar] [CrossRef] [PubMed]
- Ree, A.H.; Šaltytė Benth, J.; Hamre, H.M.; Kersten, C.; Hofsli, E.; Guren, M.G.; Sorbye, H.; Johansen, C.; Negård, A.; Bjørnetrø, T.; et al. First-line oxaliplatin-based chemotherapy and nivolumab for metastatic microsatellite-stable colorectal cancer—The randomised METIMMOX trial. Br. J. Cancer 2024, 130, 1921–1928. [Google Scholar] [CrossRef] [PubMed]
- Saunders, M.P.; Graham, J.; Cunningham, D.; Plummer, R.; Church, D.; Kerr, R.; Cook, S.; Zheng, S.; La Thangue, N.; Kerr, D. CXD101 and nivolumab in patients with metastatic microsatellite-stable colorectal cancer (CAROSELL): A multicentre, open-label, single-arm, phase II trial. ESMO Open 2022, 7, 100594. [Google Scholar] [CrossRef]
- Patel, M.R.; Falchook, G.S.; Hamada, K.; Makris, L.; Bendell, J.C. A phase 2 trial of trifluridine/tipiracil plus nivolumab in patients with heavily pretreated microsatellite-stable metastatic colorectal cancer. Cancer Med. 2021, 10, 1183–1190. [Google Scholar] [CrossRef]
- Wang, Z.-X.; Peng, J.; Liang, X.; Cheng, Y.; Deng, Y.; Chen, K.; Zhang, M.; Zhang, J.; Wang, W.; Cao, B.; et al. First-line serplulimab in metastatic colorectal cancer: Phase 2 results of a randomized, double-blind, phase 2/3 trial. Med 2024, 5, 1150–1163.e3. [Google Scholar] [CrossRef]
- Antoniotti, C.; Borelli, B.; Rossini, D.; Pietrantonio, F.; Morano, F.; Salvatore, L.; Lonardi, S.; Marmorino, F.; Tamberi, S.; Corallo, S.; et al. AtezoTRIBE: A randomised phase II study of FOLFOXIRI plus bevacizumab alone or in combination with atezolizumab as initial therapy for patients with unresectable metastatic colorectal cancer. BMC Cancer 2020, 20, 683. [Google Scholar] [CrossRef]
- Antoniotti, C.; Rossini, D.; Pietrantonio, F.; Catteau, A.; Salvatore, L.; Lonardi, S.; Boquet, I.; Tamberi, S.; Marmorino, F.; Moretto, R.; et al. Upfront FOLFOXIRI plus bevacizumab with or without atezolizumab in the treatment of patients with metastatic colorectal cancer (AtezoTRIBE): A multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 2022, 23, 876–887. [Google Scholar] [CrossRef]
- Stein, A.; Simnica, D.; Schultheiß, C.; Scholz, R.; Tintelnot, J.; Gökkurt, E.; von Wenserski, L.; Willscher, E.; Paschold, L.; Sauer, M.; et al. PD-L1 targeting and subclonal immune escape mediated by PD-L1 mutations in metastatic colorectal cancer. J. Immunother. Cancer 2021, 9, e002844. [Google Scholar] [CrossRef]
- Kim, D.W.; Tan, E.; Zhou, J.-M.; Schell, M.J.; Martinez, M.; Yu, J.; Carballido, E.; Mehta, R.; Strosberg, J.; Imanirad, I.; et al. A phase 1/2 trial of ibrutinib in combination with pembrolizumab in patients with mismatch repair proficient metastatic colorectal cancer. Br. J. Cancer 2021, 124, 1803–1808. [Google Scholar] [CrossRef] [PubMed]
- Kawazoe, A.; Xu, R.-H.; García-Alfonso, P.; Passhak, M.; Teng, H.-W.; Shergill, A.; Gumus, M.; Qvortrup, C.; Stintzing, S.; Towns, K.; et al. Lenvatinib Plus Pembrolizumab Versus Standard of Care for Previously Treated Metastatic Colorectal Cancer: Final Analysis of the Randomized, Open-Label, Phase III LEAP-017 Study. J. Clin. Oncol. 2024, 42, 2918–2927. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Geva, R.; Chung, H.C.; Lemech, C.; Miller, W.H., Jr.; Hansen, A.R.; Lee, J.-S.; Tsai, F.; Solomon, B.J.; Kim, T.M.; et al. CXCR2 antagonist navarixin in combination with pembrolizumab in select advanced solid tumors: A phase 2 randomized trial. Investig. New Drugs 2024, 42, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Fakih, M.; Sandhu, J.; Lim, D.; Li, X.; Li, S.; Wang, C. Regorafenib, Ipilimumab, and Nivolumab for Patients With Microsatellite Stable Colorectal Cancer and Disease Progression With Prior Chemotherapy. JAMA Oncol. 2023, 9, 627–634. [Google Scholar] [CrossRef]
- Xiao, A.; Li, X.; Wang, C.; Ye, J.; Fakih, M. Updated survival outcome of regorafenib, ipilimumab, and nivolumab in refractory microsatellite stable non-liver metastatic colorectal cancer: A phase I nonrandomized clinical trial. Eur. J. Cancer 2024, 213, 115111. [Google Scholar] [CrossRef]
- Kim, R.D.; Kovari, B.P.; Martinez, M.; Xie, H.; Sahin, I.H.; Mehta, R.; Strosberg, J.; Imanirad, I.; Ghayouri, M.; Kim, Y.-C.; et al. A phase I/Ib study of regorafenib and nivolumab in mismatch repair proficient advanced refractory colorectal cancer. Eur. J. Cancer 2022, 169, 93–102. [Google Scholar] [CrossRef]
- Fukuoka, S.; Hara, H.; Takahashi, N.; Kojima, T.; Kawazoe, A.; Asayama, M.; Yoshii, T.; Kotani, D.; Tamura, H.; Mikamoto, Y.; et al. Regorafenib Plus Nivolumab in Patients With Advanced Gastric or Colorectal Cancer: An Open-Label, Dose-Escalation, and Dose-Expansion Phase Ib Trial (REGONIVO, EPOC1603). J. Clin. Oncol. 2020, 38, 2053–2061. [Google Scholar] [CrossRef]
- Morris, V.K.; Parseghian, C.M.; Escano, M.; Johnson, B.; Raghav, K.P.S.; Dasari, A.; Huey, R.; Overman, M.J.; Willis, J.; Lee, M.S.; et al. Phase I/II trial of encorafenib, cetuximab, and nivolumab in patients with microsatellite stable, BRAFV600E metastatic colorectal cancer. J. Clin. Oncol. 2022, 40, 2372886. [Google Scholar] [CrossRef]
- Wang, F.; He, M.-M.; Yao, Y.-C.; Zhao, X.; Wang, Z.-Q.; Jin, Y.; Luo, H.-Y.; Li, J.-B.; Wang, F.-H.; Qiu, M.-Z.; et al. Regorafenib plus toripalimab in patients with metastatic colorectal cancer: A phase Ib/II clinical trial and gut microbiome analysis. Cell Rep. Med. 2021, 2, 100383. [Google Scholar] [CrossRef]
- Johnson, B.; Haymaker, C.L.; Parra, E.R.; Soto, L.M.S.; Wang, X.; Thomas, J.V.; Dasari, A.; Morris, V.K.; Raghav, K.; Vilar, E.; et al. Phase II study of durvalumab (anti-PD-L1) and trametinib (MEKi) in microsatellite stable (MSS) metastatic colorectal cancer (mCRC). J. Immunother. Cancer 2022, 10, e005332. [Google Scholar] [CrossRef]
- Hernando-Calvo, A.; Han, M.; Ayodele, O.; Wang, B.X.; Bruce, J.P.; Abbas-Aghababazadeh, F.; Vila-Casadesús, M.; Sanz-Garcia, E.; Yang, S.Y.C.; Berman, H.K.; et al. A Phase II, Open-Label, Randomized Trial of Durvalumab With Olaparib or Cediranib in Patients With Mismatch Repair-Proficient Colorectal or Pancreatic Cancer. Clin. Color. Cancer 2024, 23, 272–284.e9. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.; Park, R.; Pathak, H.; Al-Bzour, A.N.; Dai, J.; Phadnis, M.; Al-Rajabi, R.; Kasi, A.; Baranda, J.; Sun, W.; et al. Clinical and biomarker results from a phase II trial of combined cabozantinib and durvalumab in patients with chemotherapy-refractory colorectal cancer (CRC): CAMILLA CRC cohort. Nat. Commun. 2024, 15, 1533. [Google Scholar] [CrossRef]
- Ducreux, M.; Tabernero, J.; Grothey, A.; Arnold, D.; O’Dwyer, P.J.; Gilberg, F.; Abbas, A.; Thakur, M.D.; Prizant, H.; Irahara, N.; et al. Clinical and exploratory biomarker findings from the MODUL trial (Cohorts 1, 3 and 4) of biomarker-driven maintenance therapy for metastatic colorectal cancer. Eur. J. Cancer 2023, 184, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Segal, N.H.; Cercek, A.; Ku, G.; Wu, A.J.; Rimner, A.; Khalil, D.N.; Reidy-Lagunes, D.; Cuaron, J.; Yang, T.J.; Weiser, M.R.; et al. Phase II Single-arm Study of Durvalumab and Tremelimumab with Concurrent Radiotherapy in Patients with Mismatch Repair-proficient Metastatic Colorectal Cancer. Clin. Cancer Res. 2021, 27, 2200–2208. [Google Scholar] [CrossRef] [PubMed]
- Parikh, A.R.; Szabolcs, A.; Allen, J.N.; Clark, J.W.; Wo, J.Y.; Raabe, M.; Thel, H.; Hoyos, D.; Mehta, A.; Arshad, S.; et al. Radiation Therapy Enhances Immunotherapy Response in Microsatellite-stable Colorectal and Pancreatic Adenocarcinoma in a Phase II Trial. Nat. Cancer 2021, 2, 1124–1135. [Google Scholar] [CrossRef]
- Morano, F.; Raimondi, A.; Pagani, F.; Lonardi, S.; Salvatore, L.; Cremolini, C.; Murgioni, S.; Randon, G.; Palermo, F.; Antonuzzo, L.; et al. Temozolomide Followed by Combination With Low-Dose Ipilimumab and Nivolumab in Patients With Microsatellite-Stable, O6-Methylguanine–DNA Methyltransferase–Silenced Metastatic Colorectal Cancer: The MAYA Trial. J. Clin. Oncol. 2022, 40, 1562–1573. [Google Scholar] [CrossRef]
- Haag, G.M.; Springfeld, C.; Grün, B.; Apostolidis, L.; Zschäbitz, S.; Dietrich, M.; Berger, A.-K.; Weber, T.F.; Zoernig, I.; Schaaf, M.; et al. Pembrolizumab and maraviroc in refractory mismatch repair proficient/microsatellite-stable metastatic colorectal cancer—The PICCASSO phase I trial. Eur. J. Cancer 2022, 167, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.; Loo Yau, H.; Chakravarthy, A.; Wang, B.; Shen, S.Y.; Ettayebi, I.; Ishak, C.A.; Bedard, P.L.; Abdul Razak, A.; R Hansen, A.; et al. An open-label, phase II multicohort study of an oral hypomethylating agent CC-486 and durvalumab in advanced solid tumors. J. Immunother. Cancer 2020, 8, e000883. [Google Scholar] [CrossRef]
- Akce, M.; Farran, B.; Switchenko, J.M.; Rupji, M.; Kang, S.; Khalil, L.; Ruggieri-Joyce, A.; Olson, B.; Shaib, W.L.; Wu, C.; et al. Phase II trial of nivolumab and metformin in patients with treatment-refractory microsatellite stable metastatic colorectal cancer. J. Immunother. Cancer 2023, 11, e007235. [Google Scholar] [CrossRef]
- Fakih, M.; Harb, W.; Mahadevan, D.; Babiker, H.; Berlin, J.; Lillie, T.; Krige, D.; Carter, J.; Cox, C.; Patel, M.; et al. Safety and efficacy of the tumor-selective adenovirus enadenotucirev, in combination with nivolumab, in patients with advanced/metastatic epithelial cancer: A phase I clinical trial (SPICE). J. Immunother. Cancer 2023, 11, e006561. [Google Scholar] [CrossRef]
- de Gramont, A.; Figer, A.; Seymour, M.; Homerin, M.; Hmissi, A.; Cassidy, J.; Boni, C.; Cortes-Funes, H.; Cervantes, A.; Freyer, G.; et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J. Clin. Oncol. 2000, 18, 2938–2947. [Google Scholar] [CrossRef]
- Bokemeyer, C.; Bondarenko, I.; Hartmann, J.T.; de Braud, F.; Schuch, G.; Zubel, A.; Celik, I.; Schlichting, M.; Koralewski, P. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: The OPUS study. Ann. Oncol. 2011, 22, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Li, J.; Wang, L.; Xu, J.; Cheng, Y.; Bai, Y.; Li, W.; Xu, N.; Lin, L.-Z.; Wu, Q.; et al. Efficacy and Tolerability of First-Line Cetuximab Plus Leucovorin, Fluorouracil, and Oxaliplatin (FOLFOX-4) Versus FOLFOX-4 in Patients With RAS Wild-Type Metastatic Colorectal Cancer: The Open-Label, Randomized, Phase III TAILOR Trial. J. Clin. Oncol. 2018, 36, 3031–3039. [Google Scholar] [CrossRef] [PubMed]
- Doleschel, D.; Hoff, S.; Koletnik, S.; Rix, A.; Zopf, D.; Kiessling, F.; Lederle, W. Regorafenib enhances anti-PD1 immunotherapy efficacy in murine colorectal cancers and their combination prevents tumor regrowth. J. Exp. Clin. Cancer Res. 2021, 40, 288. [Google Scholar] [CrossRef]
- Morris, V.K.; Guthrie, K.A.; Kopetz, S.; Breakstone, R.; Karasic, T.B.; Hu, Z.I.; Bellasea, S.; Fakih, M.; Gholami, S.; Gold, P.J.; et al. Randomized phase II trial of encorafenib and cetuximab with or without nivolumab for patients with previously treated, microsatellite stable, BRAFV600E metastatic and/or unresectable colorectal cancer: SWOG S2107. J. Clin. Oncol. 2023, 41, TPS265. [Google Scholar] [CrossRef]
- Ngwa, W.; Irabor, O.C.; Schoenfeld, J.D.; Hesser, J.; Demaria, S.; Formenti, S.C. Using immunotherapy to boost the abscopal effect. Nat. Rev. Cancer 2018, 18, 313–322. [Google Scholar] [CrossRef]
- Abushukair, H.; Ababneh, O.; Zaitoun, S.; Saeed, A. Primary and secondary immune checkpoint inhibitors resistance in colorectal cancer: Key mechanisms and ways to overcome resistance. Cancer Treat. Res. Commun. 2022, 33, 100643. [Google Scholar] [CrossRef]
| Authors and Trial Name | Population | MSI/MSS Status (%) | Intervention | Control | Outcome (95% CI) | Safety/Adverse Events | |
|---|---|---|---|---|---|---|---|
| Anti-PD-1 | André et al. [12], Diaz et al. [13] KEYNOTE-177 | N = 852 No prior treatment Randomized 1:1 | 100% MSI-H | Pembrolizumab (200 mg/3 weeks) | Chemotherapy repeated every 2 weeks | ORR: 43.8% (35.8–52) vs. 33.1% (25.8–41.1) Complete response: 11% vs. 4% PFS: 16.5 months (5.4–32.4) vs. 8.2 months (6.1–10.2) OS: not reached (49.2—NR) vs. 36.7 months (27.6—NR), ns | 22% vs. 66% grade ≥ 3 TRAEs (including one patient who died in the chemotherapy group) |
| Bi et al. [14] | N = 38 CRC patients out of a cohort of 60 patients | 100% MSI | Iparomlimab 200 mg/3 weeks | - | ORR: 57.9% (52.9% for liver metastatic patients, 61.9% for non-liver metastatic patients) PFS: median PFS not evaluable, 63.4% at 24 months OS: median OS not evaluable, 65.5% at 24 months | 20.8% grade ≥ 3 TRAEs | |
| Felip et al. [15] | N = 48 patients out of a cohort of 204 patients | 56.3% MSI-H, 25% MSS, others non evaluable (insufficient tumor sample) | Cetrelimab 240 mg/2 weeks | - | ORR: 16.7%, 23.8% in MSI-H CRC PFS: 2.1 months OS: - | 53.9% grade ≥ 3 TRAEs | |
| Anti-PD-L1 | Taieb et al. [16] SAMCO-PRODIGE 54 |
N
= 122 Progression under standard first-line therapy Randomized 1:1 | 100% MSI |
Avelumab (10 mg/kg/2 weeks) | Standard second-line chemotherapy ± targeted agent | ORR: 29.5% vs. 26.2% Estimated PFS at 12 months: 31.2% vs. 19.4% OS: 25.8 months (14.1-NR) vs. 23.4 months (13-NR) | 31.7% vs. 53.1% ≥ 3 TRAEs |
| Li et al. [17] | N = 65 CRC patients out of a cohort of 103 patients with previously treated solid tumors | 100% MSI-H | Envafolimab (150 mg/week) | - | ORR: 43.1% (30.8–56.0) in the CRC subgroup PFS: 7.2 months for the CRC subgroup OS: 87.1% at 12 months for the CRC subgroup | 16% grade ≥ 3 TRAEs | |
| Eng et al. [18] IMblaze370 | N = 363 | 92% MSS 2% MSI |
2 groups: Atezolizumab only Atezolizumab + cobimetinib | Regorafenib | ORR: 2%, 3%, and 2% PFS: 1.94, 1.91, and 2.00 months, respectively OS: 7.1, 8.9, and 8.1 months, respectively | 61% and 31% vs. 58% ≥ 3 TRAEs |
| Authors and Trial Name | Population | MSI/MSS Status (%) | Intervention | Control | Outcome (95% CI) | Safety/Adverse Events | |
|---|---|---|---|---|---|---|---|
| Anti-PD-1 and Anti-CTLA-4 Combinations | Lenz et al. [22] Checkmate 142 | N = 45 | 100% MSI | Nivolumab every 2 weeks + low dose Ipilimumab every 6 weeks | - | ORR: 69% PFS and OS: not reached with minimum follow-up of 24.2 months | 22% grade ≥ 3 TRAEs |
| André et al. [23] Checkmate 8HW | N = 303 Randomized in 2:1 ratio | 84% MSI | Nivolumab (240 mg) + Ipilimumab (1 mg/kg) each 3 weeks | Chemotherapy with or without targeted therapies | PFS at 24 months: 72% vs. 14% | 23% grade ≥ 3 TRAEs vs. 48% | |
| El-Khoueiry et al. [24] | N = 59 | 100% MSS | Botensilimab 1 or 2 mg/kg every 6 weeks + balstilimab 3 mg/kg every 2 weeks | - | ORR: 22% PFS: - OS: 61% at 12 months, median OS not reached | 34% grade ≥ 3 TRAEs | |
| Bullock et al. [25] | N = 148 All pre-treated | 100% MSS | Botensilimab (every 3 or 6 weeks) + balstilimab 3 mg/kg every 2 weeks for 2 years | - | ORR: 17% (10–26%) PFS: 3.5 months (2.7–4.1) OS: 20.9 months (10.6-NR) | 32% grade 3–4 TRAEs No grade 5 TRAE 12% discontinuation rate when botensilimab was given/3 weeks | |
| Anti-PD-L1 and Anti-CTLA-4 | Chen et al. [26] | N = 180 70.5% with liver metastasis All received all available standard systemic therapies | 1.1% MSI 92.2% MSS | Tremelimumab + durvalumab during a median of 12 weeks | Best supportive care | PFS: 1.8 vs. 1.9 months OS: 6.6 vs. 4.1 months | 64% vs. 20% ≥ grade 3 TRAEs |
| Marie et al. [27] | N = 24 | 87.5% MSS 8.3% MSI 4.2% POLE | Tremelimumab and durvalumab 1 dose + adjuvant durvalumab | - | ORR: - PFS: 9.7 months OS: 24.5 months | 22% grade ≥ 3 TRAEs | |
| Dual-target ICI; bispecific Antibody | Zhao et al. [28] | N = 27 CRC patients out of a cohort of 518 patients Metastatic or recurrent | Unspecified | QL1706 (bifunctional PD1/CTLA4 dual blocker) at one of five doses ranging from 0.3 to 10 mg/kg, once every 3 weeks | - | ORR: 7.4% in CRC, 16.9% in the whole cohort PFS: - OS: - Median duration of response: 11.7 months for the whole cohort | 16% grade ≥ 3 TRAEs |
| Frentzas et al. [29] | N = 9 CRC patients out of a cohort of 51 patients | 100% MSS | Ivonescimab (bispecific monoclonal antibody anti-PD1 and anti-VEGF) 0.3, 1, 3, 10, 20, or 30 mg/kg IV every 2 weeks using a 3 + 3 + 3 dose escalation design | - | ORR: 25.5% PFS: - OS: - | 27.5% grade ≥ 3 TRAEs |
| Authors and Trial Name | Population | MSI/MSS (%) | Intervention(s) | Control | Outcome | Safety/Adverse Events | |
|---|---|---|---|---|---|---|---|
| Anti-PD1 with other monoclonal antibodies | Geva et al. [30] | N = 8 CRC patients out of a cohort of 37 patients Group A&B: N = 20 (4 CRC) Group D: N = 17 (4 CRC) | Unspecified | Group D: MK-1248 + pembrolizumab | Group A&B: MK-1248 (anti-GITR antibody) alone | ORR: 18% vs. 0% Partial response: 12% vs. 0% Complete response: 6% vs. 0% No PFS or OS | 45% on monotherapy group vs. 53% on combination therapy grade ≥ 3 TRAEs |
| Rahma et al. [31] | N = 6 | 100% MSS | Ziv-aflibercept + pembrolizumab | PFS: 2.5 months OS: 3.3 months | 58% grade ≥ 3 TRAEs | ||
| Lentz et al. [32] | N = 50 | 100% MSS | Pembrolizumab + binimetinib + bevacizumab | - | ORR: 12.0% (not statistically different than the historical control data of 5%) PFS: 5.9 months OS: 9.3 months | 64% grade ≥ 3 TRAEs | |
| Curigliano et al. [33] | N = 219 133 with Sabatolimab alone 86 with Sabatolimab + spartalizumab All had progressed under or were intolerant to standard therapy | Unspecified | Sabatolimab + spartalizumab | Sabatolimab alone | Partial response: 0% Sabatolimab alone 6% lasting 12–27 months with combination of Sabatolimab + Spartalizumab No ORR, PFS, or OS | 51% grade ≥ 3 TRAEs | |
| Bauer et al. [34] | N = 53 CRC patients out of a cohort of 120 patients 60 patients in dose escalation group, 60 in dose expansion group | 100% MSS for the 53 CRC | NIS793 +/- spartalizumab | 3.5% partial response, 24.2% stable disease, 55.8% disease progression, and 17.5% unreported responses PFS: 1.41 months, regardless of whether patients received single-agent or combination No ORR or OS | 99.2% TRAE of any grade, 57.5% TRAE grade ≥ 3 | ||
| Kawazoe et al. [35] EPOC1704 | N = 29 CRC patients out of a cohort of 44 patients 57% received three or more previous lines of chemotherapy | 97% MSS, 3% MSI | TAS-116 + nivolumab | - | ORR: 16% in CRC subgroup PFS: 3.2 months in CRC subgroup OS: 13.5 months in CRC subgroup | 27% grade ≥ 3 TRAEs | |
| Lemech et al. [36] | N = 25 CRC patients out of a cohort of 58 patients | 100% MSS | 3 + 3 dose escalation Pixatimod + nivolumab | - | 12% partial response, 32% stable disease No ORR, PFS, or OS | 21% TRAE grade ≥ 3, 12% in CRC subgroup | |
| Anti-PDL1 with other monoclonal antibodies | Chen Li et al. [37] MORPHEUS-CRC | N = 28 + 28 from the Imblaze370 study data | 100% MSS | Atezolizumab + isatuximab | Regorafenib | ORR: 0% vs. 0% PFS: 1.4 months vs. 2.8 months OS: 5.1 months vs. 10.2 months | 13% vs. 70% of grade ≥ 3 TRAEs |
| Bendell et al. [38] | N = 42 CRC patients out of a cohort of 192 patients 66 during escalation and 126 during expansion | 100% MSS | Oleclumab + durvalumab | Oleclumab monotherapy | ORR: - PFS: 1.8 months in both, median PFS of 5.4% at 6 months in CRC subgroup OS: 5.6 months in the combination therapy cohort, 6.1 months in the monotherapy cohort, and 7 months for CRC subgroup | 19% TRAE grade ≥3 in CRC subgroup | |
| Patel et al. [39] | N = 40 CRC patients out of a cohort of 140 patients | 100% MSS-CRC | Durvalumab 1500 mg + monalizumab 750 mg | - | ORR: 7.7% in CRC subgroup PFS: 1.9 months in CRC subgroup OS: 10.6 months in CRC subgroup | 5% grade 3/4 TRAEs in CRC subgroup |
| Authors | Population | MSI/MSS (%) | Intervention | Control | Outcome | Safety/Adverse events | |
|---|---|---|---|---|---|---|---|
| Checkpoint inhibitors with chemotherapy | Herting et al. [40] MK-3475 | N = 6 | 66% MSI, 33% unassessed | Pembrolizumab + modified FOLFOX6 | - | ORR: 56.7% PFS: 8.8 months OS: not reached as median follow-up was 19.9 months | 30% grade 4 TRAEs |
| Kuang et al. [41] | N = 30 | Not reported | Pembrolizumab + azacitidine | - | ORR: 3% PFS: 1.9 months OS: 6.3 months | 2.5% grade ≥ 3 TRAEs | |
| Lenz et al. [42] Checkmate 9X8 |
N
= 195 Randomized 2:1 | 93% MSS | Nivolumab + SOC (5-fluorouracil/leucovorin/oxaliplatin/bevacizumab) (N = 127) | SOC (N = 68) | ORR: 60% vs. 46% PFS: 11.9 months vs. 11.9 months OS: 29.2 months vs. not reached | 75% vs. 48% ≥ 3 TRAEs | |
| Ree et al. [43] METIMMOX |
N
= 80 Randomized 1:1 | 100% MSS | Nivolumab + FLOX | FLOX alone | ORR: 47% vs. 65% PFS: 9.2 months vs. 9.2 months If CRP < 5.0 mg/L before Nivolumab (N = 17) PFS 15.8 months OS: 20.7 months vs. 14.6 months | 26% vs. 14% grade ≥ 3 TRAEs | |
| Saunders et al. [44] CAROSELL | N = 55 | 100% MSS | Nivolumab + CDX101 (zabadinostat) | - | ORR 9% achieved partial response and 39% stable disease PFS: 2.1 months OS: 7.0 months | 25% grade ≥3 TRAEs | |
| Patel et al. [45] | N = 18 | 100% MSS | Nivolumab + TAS-102 (trifluridine/tipiracil) | - | ORR: 0% PFS: - OS: - stopped at stage I because no partial or complete response | 72% grade ≥ 3 TRAEs | |
| Wang et al. [46] |
N
= 114 No prior therapy Randomized 1:1 | 95.7% MSS | Serplulimab + HLX04 + XELOX | Placebo + bevacizumab + XELOX | PFS: 17.2 vs. 10.7 months OS: not reached in either group | 65.5% vs. 56.1% ≥ 3 TRAEs | |
| Antoniotti et al. [47,48] AtezoTRIBE | N = 218 Randomized 2:1 All previously untreated | 100% MSS | Atezolizumab + FOLFOXIRI + bevacizumab (N = 145) | FOLFOXIRI + bevacizumab (N = 73) | PFS: 13.1 months vs. 11.5 months OS: - ORR: - | 27% vs. 26% ≥ grade 3 TRAEs | |
| Stein et al. [49] | N = 43 | 100% MSS or MSI-low | Avelumab + mFOLFOX6 + cetuximab | - | ORR: 81% PFS: 11.1 months | 32% grade ≥ 3 infections and neutropenia | |
| Checkpoints inhibitors with small molecule inhibitors | Kim et al. [50] | N = 40 | 100% MSS | Pembrolizumab + ibrutinib | - | ORR: 0% PFS: 1.4 months OS: 6.6 months | 40% grade ≥ 3 TRAEs |
| Kawazoe et al. [51] LEAP-017 |
N
= 480 Randomized 1:1 | 100% MSS or MSI-L | Pembrolizumab + lenvatinib | SOC | ORR: 10.4% vs. 1.7% PFS: 3.8 vs. 3.3 months OS: 9.8 vs. 9.3 months | 58.4% vs. 42.1% ≥ 3 TRAEs | |
| Armstrong et al. [52] | N = 40 CRC patients out of a cohort of 105 patients | 100% MSS | Pembrolizumab (200 mg/3 weeks) + navarixin (30 mg or 100 mg daily) | - | ORR: 2.5% for CRC subgroup PFS: 1.8 months for CRC subgroup with 30 mg and 1.9 months for CRC subgroup with 100 mg OS: 6.5 months (subgroup 30 mg) and 8.0 (subgroup 100 mg) | 25% in navarixin 30 mg and 22% in navarixin 100 mg grade ≥ 3 TRAEs | |
| Fakih et al. [53] | N = 39 | 100% MSS | Nivolumab + regorafenib + ipilimumab | - | ORR: 27.6% PFS: 4 months OS: 20 months | 37.9% grade ≥ 3 TRAEs | |
| Xiao et al. [54] | N = 22 | 100% MSS | Nivolumab + regorafenib + ipilimumab | - | ORR: 36.4% PFS: 5.0 months OS: 27.5 months | 25% of patients discontinued treatment because of TRAEs but there is no clear number of grade ≥3 TRAEs | |
| D.Kim et al. [55] | N = 52 | 100% MSS | Nivolumab + regorafenib | - | ORR: - PFS: 4.3 months OS: 11.1 month | 51% grade ≥ 3 TRAEs | |
| Kukuoka et al. [56] REGONIVO, EPOC160 | N = 25 CRC patients out of a cohort of 50 patients All had received >2 previous lines of chemotherapy | 96% MSS, 4% MSI | Nivolumab + regorafenib | - | ORR: 36% in CRC PFS: 7.9 months in CRC mOS: not reached in CRC One-year OS: 68.0% in CRC | 40% grade ≥ 3 TRAEs | |
| Morris et al. [57] | N = 26 | 100% MSS | Encorafenib (300 mg PO daily) + cetuximab C (500 mg/m2 IV/14 days) + nivolumab (480 mg IV/28 days) | - | ORR: 45% PFS: 7.3 months OS: 11.4 months | 18% grade ≥ 3 TRAEs | |
| Wang et al. [58] | N = 42 | 100% MSS 50% with RAS mutation 4.8% with BRAF mutation | Regorafenib + toripalimab | - | ORR: 15.2% Patients with liver metastasis had a lower response rate (ORR 8,7% vs. 30%) PFS: 2.1 months OS: 15.5 months | 38.5% grade 3 TRAEs, no grade 4 or 5 | |
| Johnson et al. [59] | N = 29 | 100% MSS | Durvalumab + trametinib | - | ORR: 3.4% PFS: 3.2 months OS: The one partial response lasted for 9.3 months | 8% grade ≥ 3 TRAEs | |
| Hernando-Calvo [60] | N = 31 | 100% MSS | Durvalumab + olaparib | Durvalumab + cediranib | ORR: 0 PFS: 2.6 and 2.4 months, respectively, in CRC patients OS: 6.6 months for the two groups of CRC patients | 28% grade 3 TRAEs, no grade 4 | |
| Saeed et al. [61] CAMILLA CRC cohort | N = 29 | 100% MSS | Durvalumab + cabozantinib | - | ORR: 27.6% PFS: 44.83% OS: 9.1 months | 39% grade ≥ 3 TRAEs | |
| Ducreux et al. [62] MODUL trial |
Group 1: N = 60 Group 3: N = 5 Group 4: N = 99 | Group 1: BRAF mutated Group 3: HER2+ Group 4: MSI-H or MSS witBRAFmut or RASmut | . Group 1: Vemurafenib + cetuximab + 5-FU/LV . Group 3: Capecitabine + trastuzumab + pertuzumab . Group 4: Cobimetinib + atezolizumab | Fluoropyrimidine + bevacizumab | PFS: no difference OS: numerically, but not significantly, longer in the intervention arm | 60.0% vs. 27.8% ≥ 3 TRAEs | |
| With radiotherapy | Segal et al. [63] | N = 24 Chemotherapy-refractory MSS mCRC | 100% MSS | Durvalumab + tremelimumab + Radiotherapy | - | ORR: 8.3% PFS: 1.8 months OS: 11.4 months | 25% grade ≥ 3 TRAEs 13% discontinued tremelimumab |
| Parikh et al. [64] | N = 40 CRC patients out of a cohort of 65 patients | 100% MSS | Ipilimumab + nivolumab + Radiotherapy | ORR: 10% (15% for the 27 patients with radiotherapy) in CRC subgroup PFS: 2.4 months in CRC subgroup OS: 7.1 months in CRC subgroup | 70% grade ≥ 3 TRAEs in CRC subgroup 32% discontinued given autoimmune toxicity | ||
| With other treatments | Morano et al. [65] MAYA Trial | N = 135 started the first treatment part; 33 achieved the second treatment part | 100% MSS | First part treatment: two cycles of oral temozolomide Second part: in absence of progression, ipilimumab and nivolumab | - | ORR: 45% PFS: 36% at 8 months median PFS: 7.0 months OS: 18.4 months | 12% grade ≥ 3 TRAEs in the 33 patients (24.4%) who attained the second treatment part |
| Haag et al. [66] PICCASSO | N = 20 | - | Pembrolizumab + maraviroc | - | ORR: 5.3% PFS: 2.1 months OS: 9.83 months | 5% grade ≥ 3 TRAEs | |
| Taylor et al. [67] | N = 28 (19 in A, 9 in B) | 100% MSS | Group A: CC-486 + durvalumab Group B: CC-486+ durvalumab + vitamin C | - | ORR: 0% PFS: 1.9 months OS: 5 months | 18% grade ≥ 3 | |
| Akce et al. [68] | N = 18 Prior chemotherapy (5FU, Oxaliplatin, or Irinotecan) | 100% MSS | Nivolumab + metformin | - | ORR: - PFS: 2.3 months OS: 5.2 months | 44% grade ≥ 3 TRAEs | |
| Fakih et al. [69] SPICE | N = 45 CRC patients out of a cohort of 51 patients | All MSI-L or MSS | Nivolumab + enadenotucirev | - | ORR: 2.4% in CRC PFS: 1.6 months in total cohort (CRC subgroup not mentioned) OS: 16 months in total cohort (CRC not mentioned) | 61% grade ≥ 3 TRAEs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gilson, A.; Tan, V.; Koessler, T.; Meyer, J.; Meurette, G.; Liot, É.; Ris, F.; Delaune, V. Immune Checkpoint Inhibitors for Metastatic Colorectal Cancer: A Systematic Review. Cancers 2025, 17, 2125. https://doi.org/10.3390/cancers17132125
Gilson A, Tan V, Koessler T, Meyer J, Meurette G, Liot É, Ris F, Delaune V. Immune Checkpoint Inhibitors for Metastatic Colorectal Cancer: A Systematic Review. Cancers. 2025; 17(13):2125. https://doi.org/10.3390/cancers17132125
Chicago/Turabian StyleGilson, Alice, Vincent Tan, Thibaud Koessler, Jeremy Meyer, Guillaume Meurette, Émilie Liot, Frédéric Ris, and Vaihere Delaune. 2025. "Immune Checkpoint Inhibitors for Metastatic Colorectal Cancer: A Systematic Review" Cancers 17, no. 13: 2125. https://doi.org/10.3390/cancers17132125
APA StyleGilson, A., Tan, V., Koessler, T., Meyer, J., Meurette, G., Liot, É., Ris, F., & Delaune, V. (2025). Immune Checkpoint Inhibitors for Metastatic Colorectal Cancer: A Systematic Review. Cancers, 17(13), 2125. https://doi.org/10.3390/cancers17132125







